Effect of Mesopore Development on Butane Working Capacity of Biomass-Derived Activated Carbon for Automobile Canister
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterizations
3. Results and Discussion
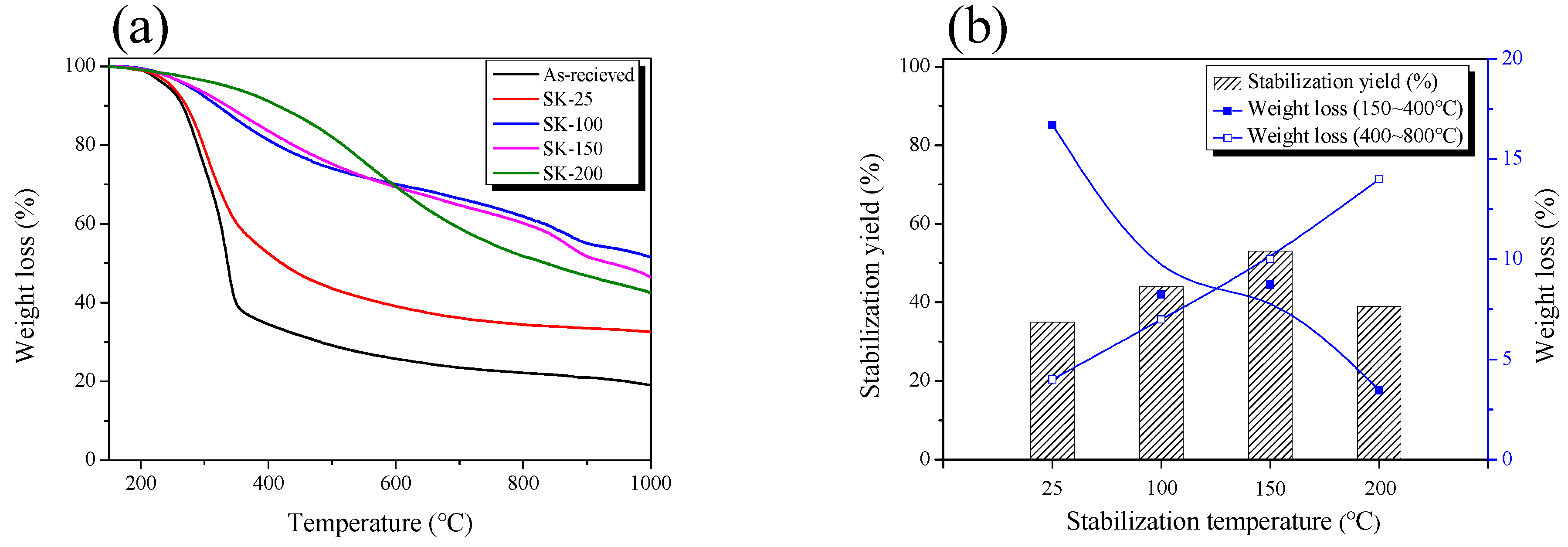
3.1. Thermogravimetric Analysis
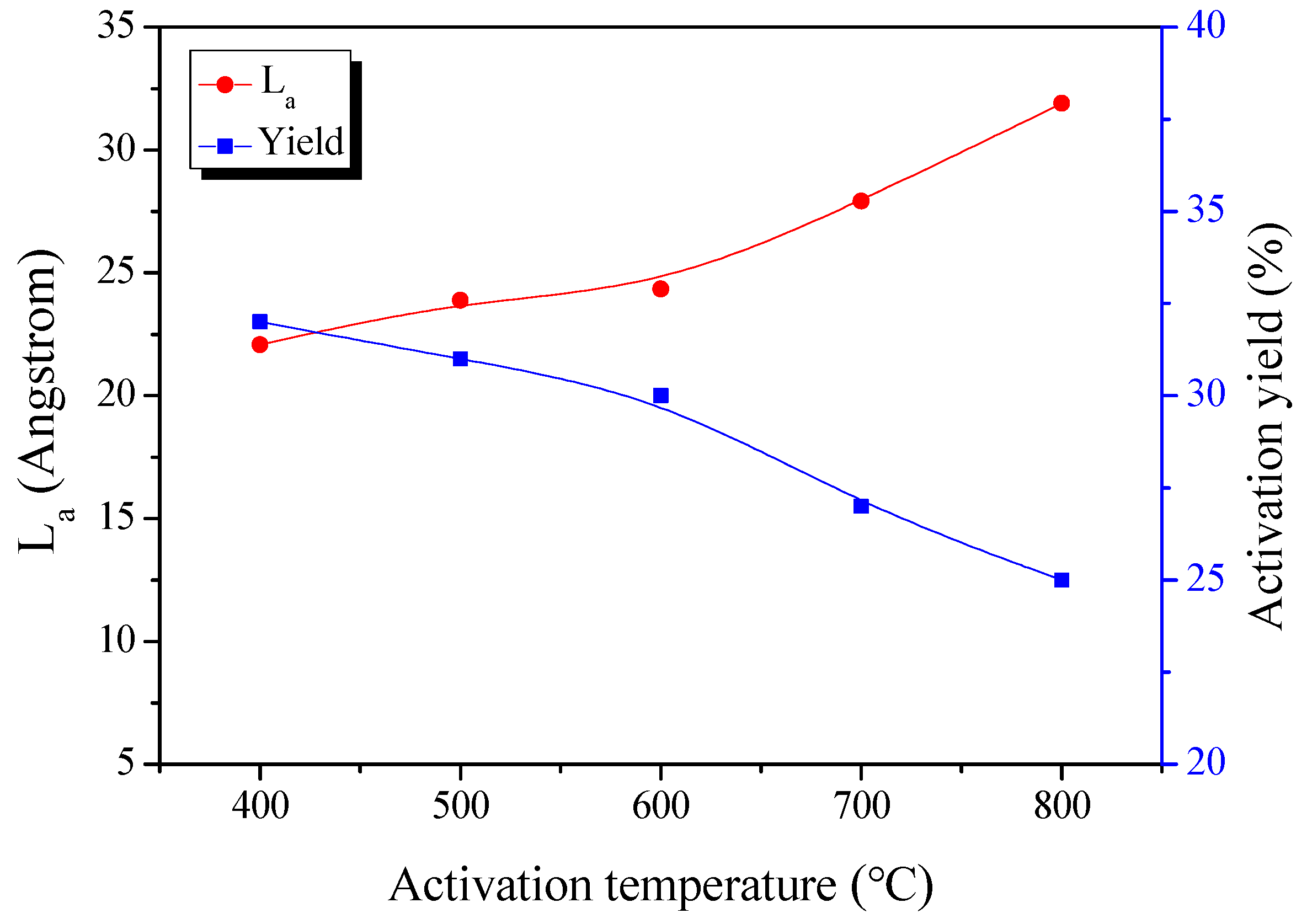
3.2. X-ray Diffraction Analysis
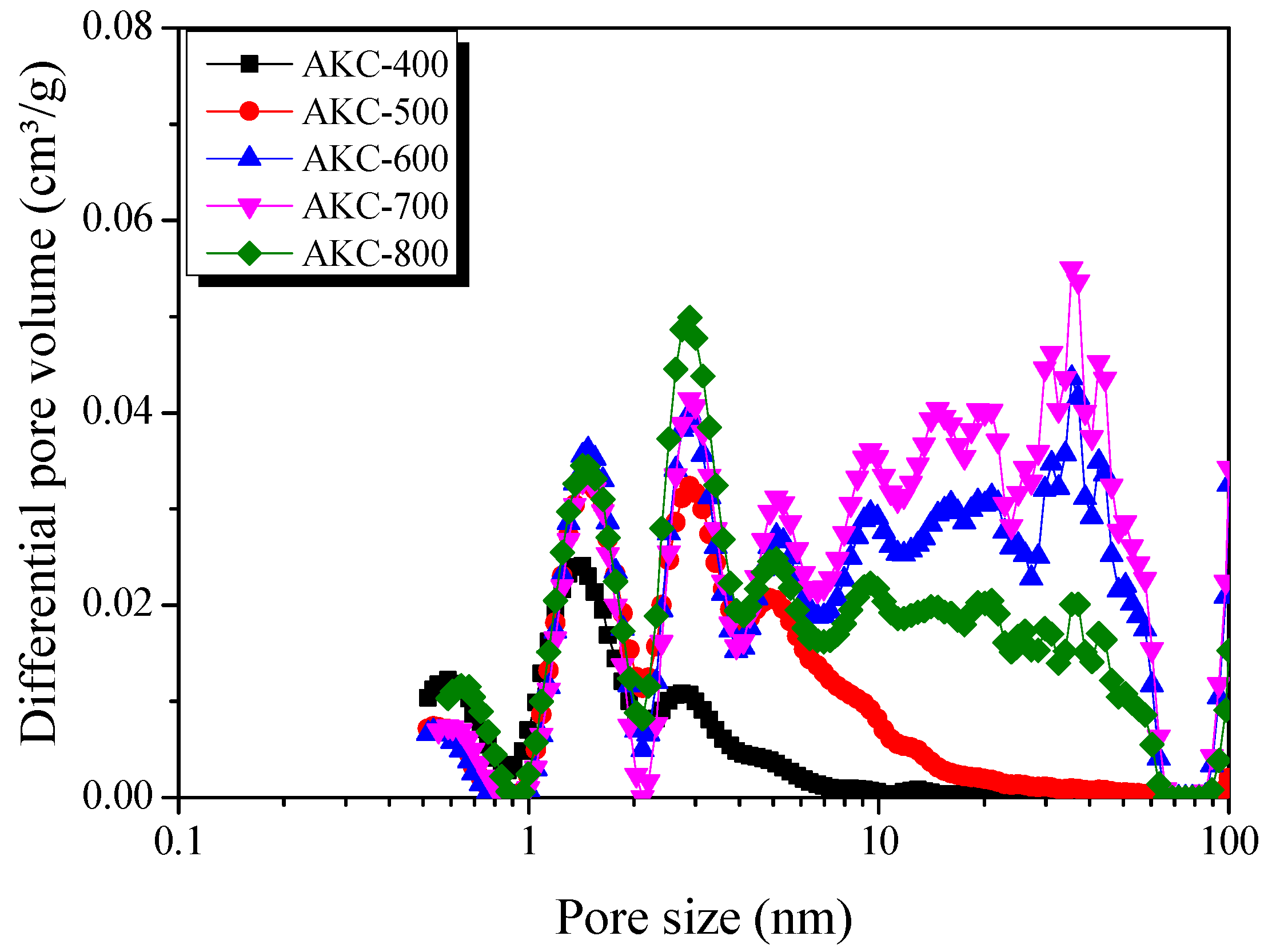
3.3. Adsorption Isotherm and Textural Properties
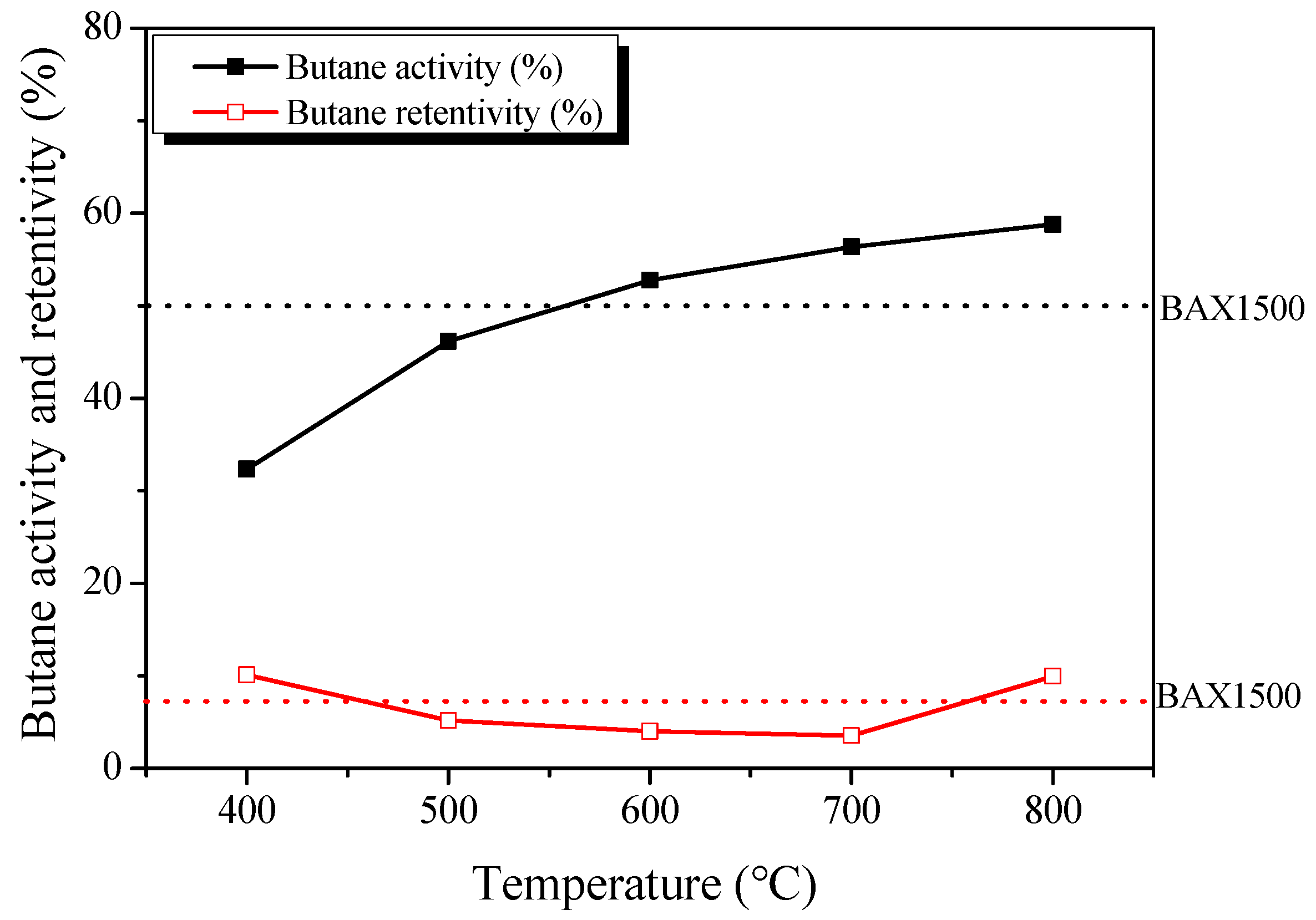
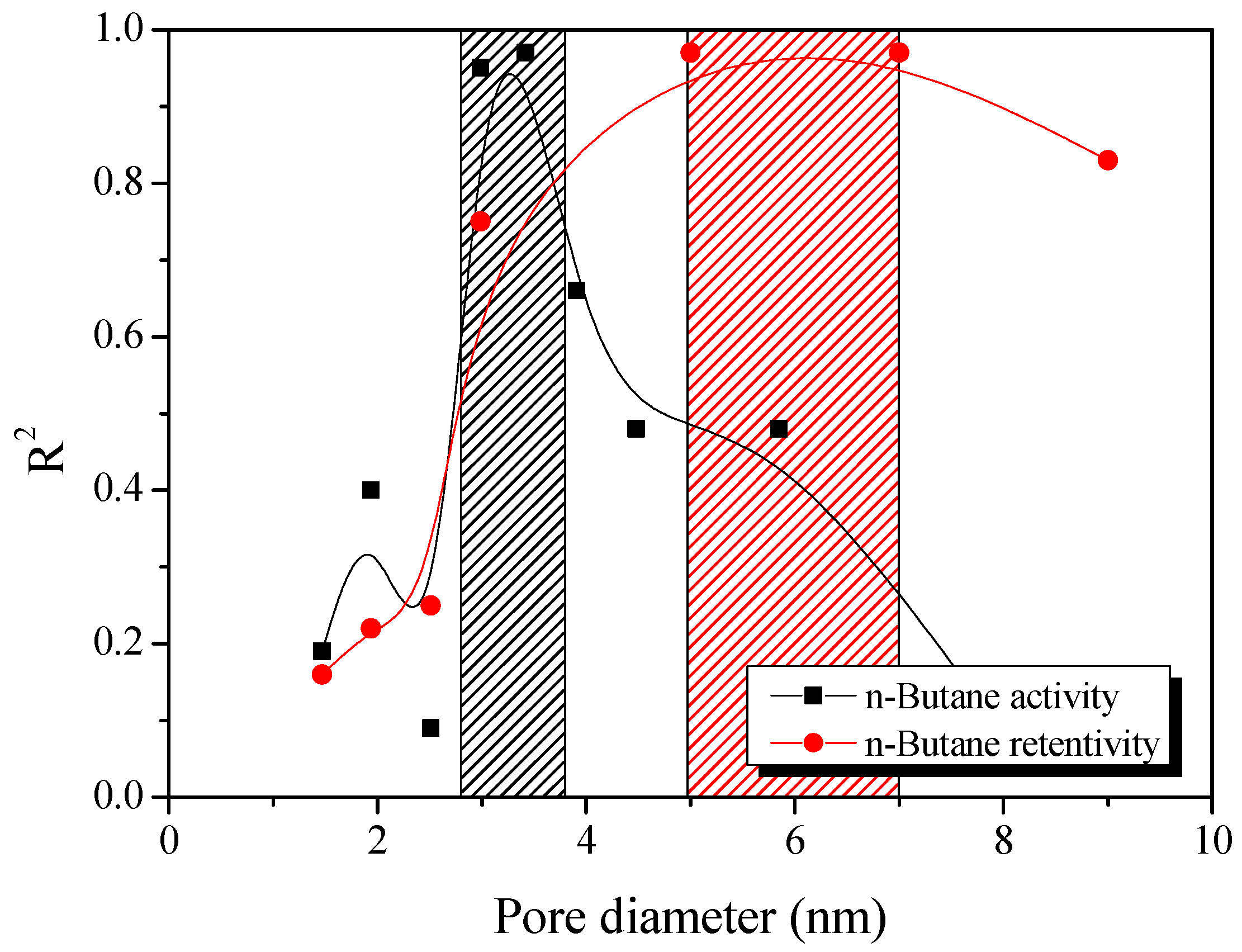
3.4. Butane Working Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- York, R.; Elizabeth, S. Energy transitions or additions? Why a transition from fossil fuels requires more than the growth of renewable energy. Energy Res. Soc. Sci. 2019, 51, 40–43. [Google Scholar] [CrossRef]
- Kinnon, M.M.; Zhu, S.; Carreras-sospedra, M.; Soukup, J.V.; Dabdub, D.; Samuelsen, G.S.; Brouwer, J. Considering future regional air quality impacts of the transportation sector. Energy Policy 2019, 124, 63–80. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Kausar, M.A.; Saeed, M.; Gupta, B.K.; Singh, R.; Ramteke, P.W. Advances in nanomaterials induced biohydrogen production using waste biomass. Bioresour. Technol. 2020, 307, 123094. [Google Scholar] [CrossRef] [PubMed]
- Dahunsi, S.O. Mechanical pretreatment of lignocelluloses for enhanced biogas production: Methane yield prediction from biomass structural components. Bioresour. Technol. 2019, 280, 18–26. [Google Scholar] [CrossRef]
- Vieira, S.; Vetroni, M.; Cristine, A.; Sydney, N.; Moro, C.; Carlos, A.; Francisco, D.; Porto, L.; Vandenberghe, D.S.; Bittencourt, E. Sustainability of sugarcane lignocellulosic biomass pretreatment for the production of bioethanol. Bioresour. Technol. 2020, 299, 122635. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Munuswamy, D.B. Effect of nanoparticle on emission and performance characteristics of a diesel engine fueled with cashew nut shell biodiesel. Energy Sources Part A 2018, 40, 2485–2493. [Google Scholar] [CrossRef]
- Hall, D.O.; House, J.I. Reducing atmospheric CO2 using biomass energy and photobiology. Energy Convers. Manag. 1993, 34, 889–896. [Google Scholar] [CrossRef]
- Thi Bach, T.L.; Keko, H.; Kenji, I. Structural characteristics of cell walls of kenaf (Hibiscus cannabinus L.) and fixation of carbon dioxide. J. Wood Sci. 2003, 49, 255–261. [Google Scholar]
- Saba, N.; Jawaid, M.; Hakeem, K.R.; Paridah, M.T.; Khalina, A.; Alothman, O.Y. Potential of bioenergy production from industrial kenaf (Hibiscus cannabinus L.) based on Malaysian perspective. Renew. Sustain. Energy Rev. 2015, 42, 446–459. [Google Scholar] [CrossRef]
- Wong, Y.H.; Tan, W.Y.; Tan, C.P.; Long, K.; Nyam, K.L. Cytotoxic activity of kenaf (Hibiscus cannabinus L.) seed extract and oil against human cancer cell lines. Asian Pac. J. Trop. Biomed. 2014, 4, 510–515. [Google Scholar] [CrossRef]
- Hanim, H.; Zainuddin, M.; Nawi, M.A.M.; Kasim, M.S. Studies on characteristic of glycerol-kenaf pellet via fluidization drying process toward energy produced. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2020; Volume 2213, p. 020276. [Google Scholar]
- Kipriotis, E.; Heping, X.; Vafeiadakis, T.; Kiprioti, M.; Alexopoulou, E. Ramie and kenaf as feed crops. Ind. Crop. Prod. 2015, 68, 126–130. [Google Scholar] [CrossRef]
- Khiari, B.; Ghouma, I.; Ibn, A.; Amine, A.; Jellali, S. Kenaf stems: Thermal characterization and conversion for biofuel and biochar production. Fuel 2020, 262, 116654. [Google Scholar] [CrossRef]
- Baek, J.; Lee, H.M.; An, H.K.; Kim, B.J. Preparation and characterization of highly mesoporous activated short carbon fibers from kenaf precursors. Carbon Lett. 2019, 29, 393–399. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Khan, R.A.; Islam, M.S. Preparation and characterizations of activated carbon from kenaf fiber for equilibrium adsorption studies of copper from wastewater. Korean J. Chem. Eng. 2012, 29, 1187–1195. [Google Scholar] [CrossRef]
- Shamsuddin, M.S.; Yusoff, N.R.N.; Sulaiman, M.A. Synthesis and characterization of activated carbon produced from kenaf core fiber using H3PO4 activation. Procedia Chem. 2016, 19, 558–565. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Macías-García, A.; Díez, M.A.D.; Ortiz, A.L. Textural and morphological study of activated carbon fibers prepared from kenaf. Microporous Mesoporous Mater. 2008, 111, 523–529. [Google Scholar] [CrossRef]
- California Air Resources Board. Available online: http://ww3.arb.ca.gov/regact/2012/leviiidtc12/leviiifrorev.pdf (accessed on 15 November 2012).
- Reddy, S. Understanding and designing automotive evaporative emission control systems. SAE Tech. Pap. 2012. [Google Scholar] [CrossRef]
- Yamada, H. Contribution of evaporative emissions from gasoline vehicles toward total VOC emissions in Japan. Sci. Total Environ. 2013, 449, 143–149. [Google Scholar] [CrossRef]
- Lee, H.M.; Lee, B.H.; Park, S.J.; An, K.H.; Kim, B.J. Pitch-derived activated carbon fibers for emission control of low-concentration hydrocarbon. Nanomaterials 2019, 9, 1313. [Google Scholar] [CrossRef]
- Biscoe, J.; Warren, B.E. An X-ray study of carbon black. J. Appl. Phys. 1942, 13, 364. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Evans, R.; Tarazona, P. Theory of condensation in narrow capillaries. Phys. Rev. Lett. 1984, 52, 557–560. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Determination of Butane Working Capacity of Activated Carbon; ASTM Standard D5228-16; ASTM: West Conshohocken, PA, USA, 1992. [Google Scholar]
- Jagtoyen, M.; Derbyshire, F. Activated carbon from yellow poplar and white oak by H3PO4 activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J. Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Willianms, P.T.; Besler, S. The influence of temperature and heating rate on the slow pyrolysis of biomass. Renew. Energy 1996, 7, 233–250. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Baek, J.; Lee, H.M.; Roh, J.S.; Lee, H.S.; Kang, H.S.; Kim, B.J. Studies on preparation and applications of polymeric precursor-based activated hard carbons: I. Activation mechanism and microstructure analyses. Microporous Mesoporous Mater. 2016, 219, 258–264. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitor electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lee, H.M.; Lee, B.H.; Kim, B.J. Facile preparation of activated carbon with optimal pore range for high butane working capacity. Carbon Lett. 2020, 30, 297–305. [Google Scholar] [CrossRef]


| Sample | Activation Conditions | SBET (m2/g) | VTotal 1 (cm3/g) | VMicro 2 (cm3/g) | VMeso 3 (cm3/g) | Activation Yield 4 (%) |
|---|---|---|---|---|---|---|
| AKC-400 | 673 K, 1 h | 1260 | 0.68 | 0.44 | 0.24 | 32 |
| AKC-500 | 773 K, 1 h | 1510 | 1.26 | 0.44 | 0.72 | 31 |
| AKC-600 | 873 K, 1 h | 1650 | 2.43 | 0.43 | 2.00 | 30 |
| AKC-700 | 973 K, 1 h | 1620 | 2.77 | 0.39 | 2.38 | 27 |
| AKC-800 | 1073 K, 1 h | 1810 | 2.11 | 0.48 | 1.63 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-H.; Lee, H.-M.; Chung, D.C.; Kim, B.-J. Effect of Mesopore Development on Butane Working Capacity of Biomass-Derived Activated Carbon for Automobile Canister. Nanomaterials 2021, 11, 673. https://doi.org/10.3390/nano11030673
Lee B-H, Lee H-M, Chung DC, Kim B-J. Effect of Mesopore Development on Butane Working Capacity of Biomass-Derived Activated Carbon for Automobile Canister. Nanomaterials. 2021; 11(3):673. https://doi.org/10.3390/nano11030673
Chicago/Turabian StyleLee, Byeong-Hoon, Hye-Min Lee, Dong Chul Chung, and Byung-Joo Kim. 2021. "Effect of Mesopore Development on Butane Working Capacity of Biomass-Derived Activated Carbon for Automobile Canister" Nanomaterials 11, no. 3: 673. https://doi.org/10.3390/nano11030673
APA StyleLee, B.-H., Lee, H.-M., Chung, D. C., & Kim, B.-J. (2021). Effect of Mesopore Development on Butane Working Capacity of Biomass-Derived Activated Carbon for Automobile Canister. Nanomaterials, 11(3), 673. https://doi.org/10.3390/nano11030673







