n-Octadecane/Fumed Silica Phase Change Composite as Building Envelope for High Energy Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of n-Octadecane/Fumed Silica (FS) Phase-Change Composites (PCC)
2.3. Characterization Methods
2.4. Evaluation of the Thermal Performance of n-Octadecane/FS PCC
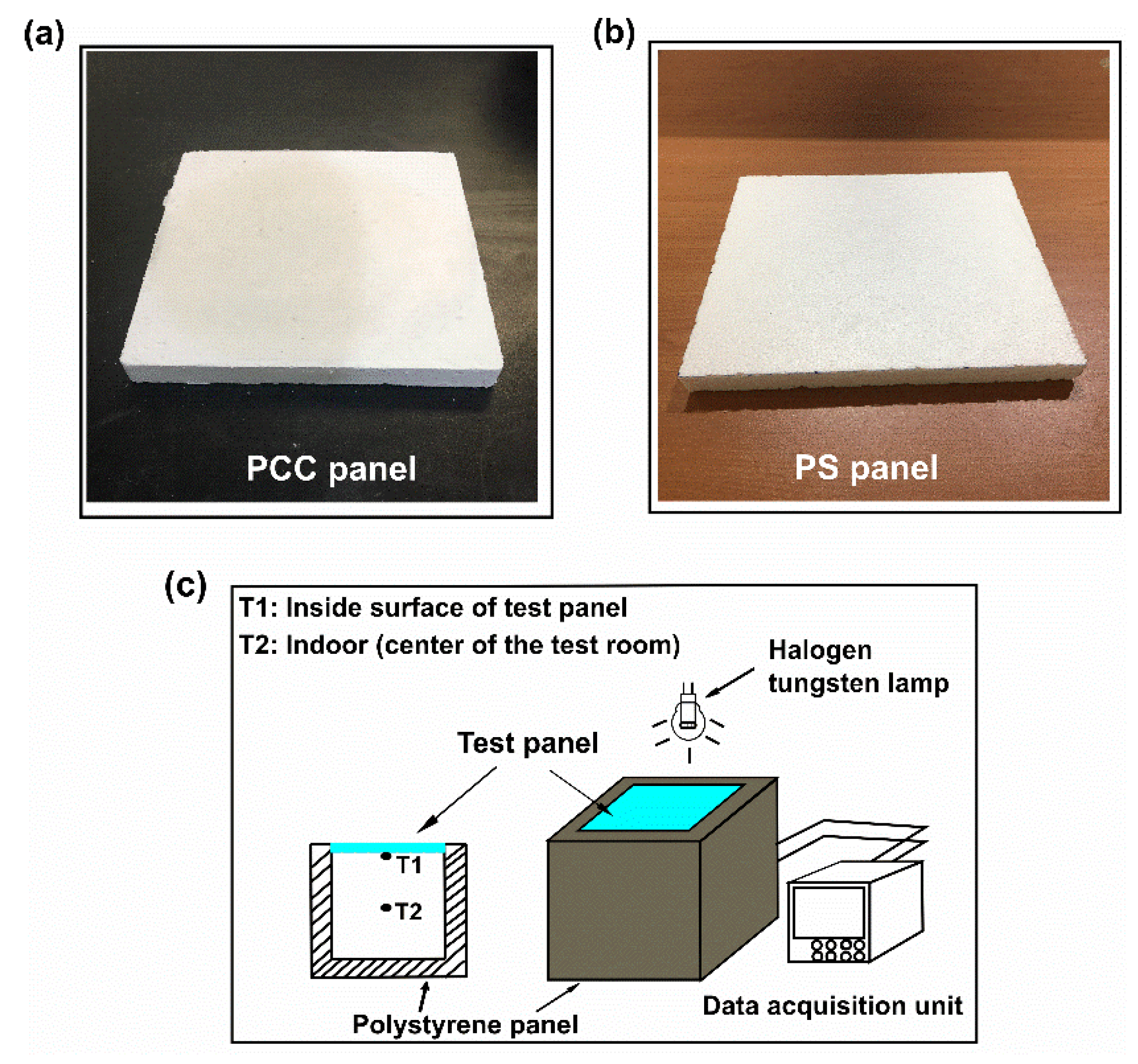
2.4.1. Fabrication of PCC Panel and Polystyrene Panel
2.4.2. Thermal Performance Evaluation
3. Results and Discussion
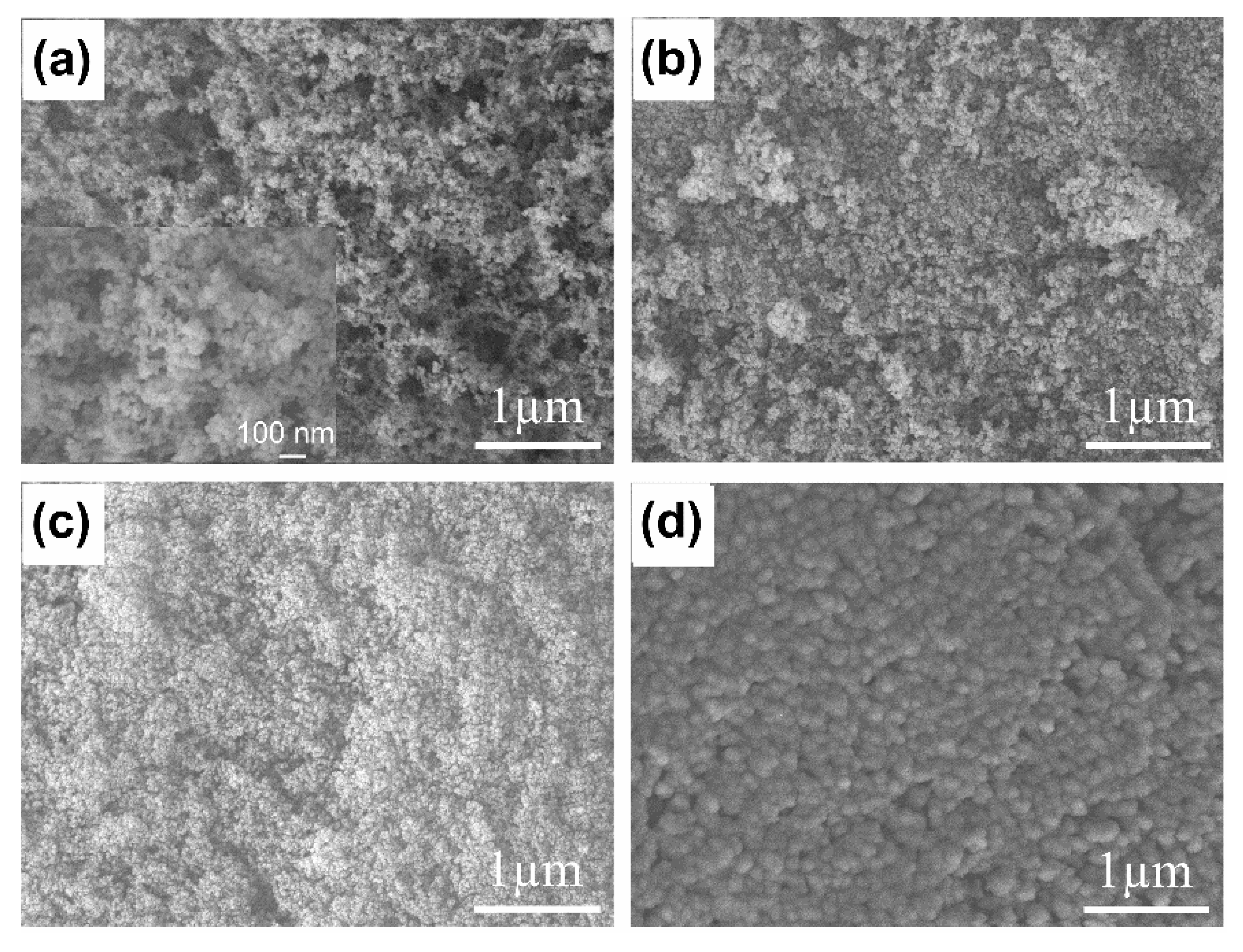
3.1. Characterization of n-Octadecane/FS PCCs
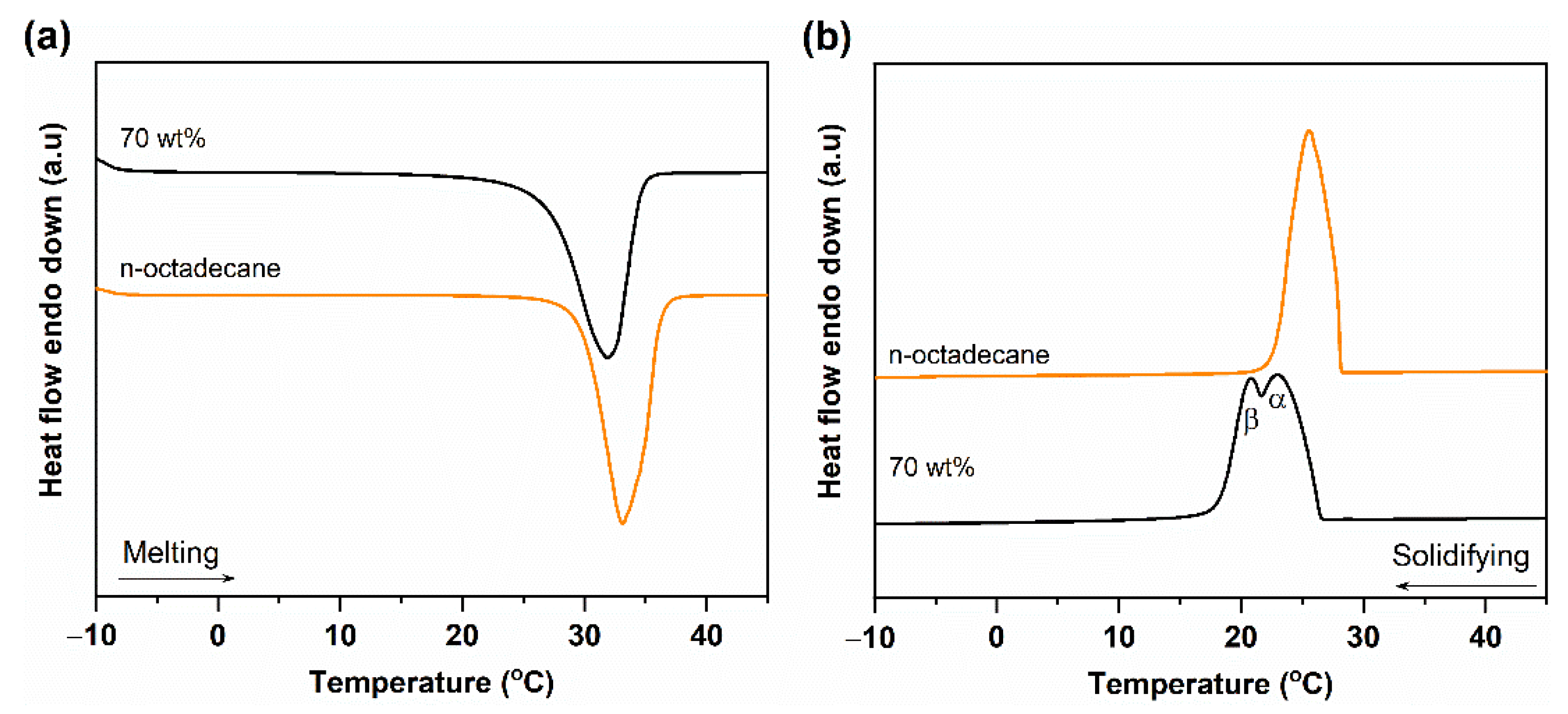
3.2. Phase Change Properties of n-Octadecane/FS PCC
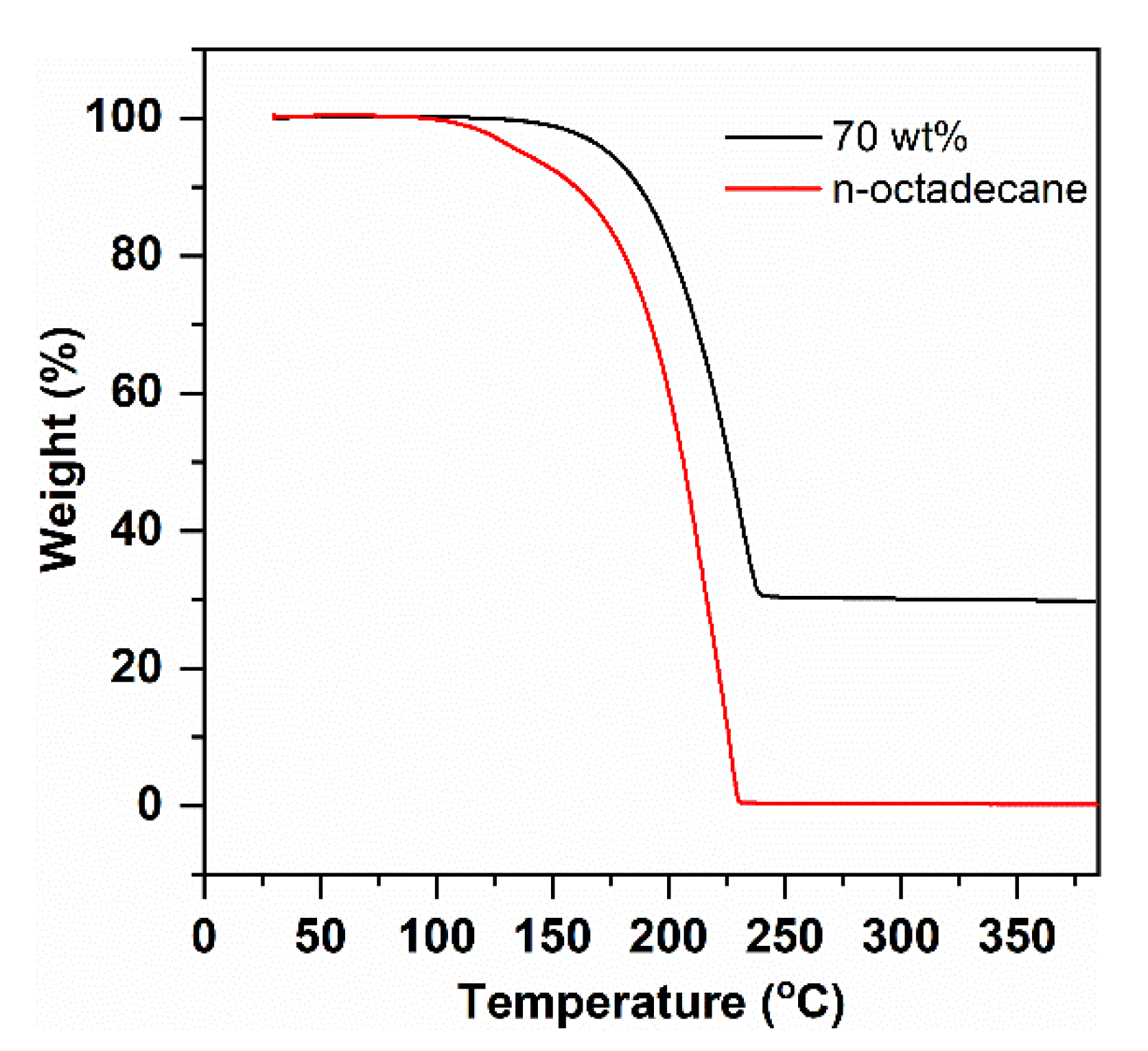
3.3. Thermal Stability of n-Octadecane/FS PCC
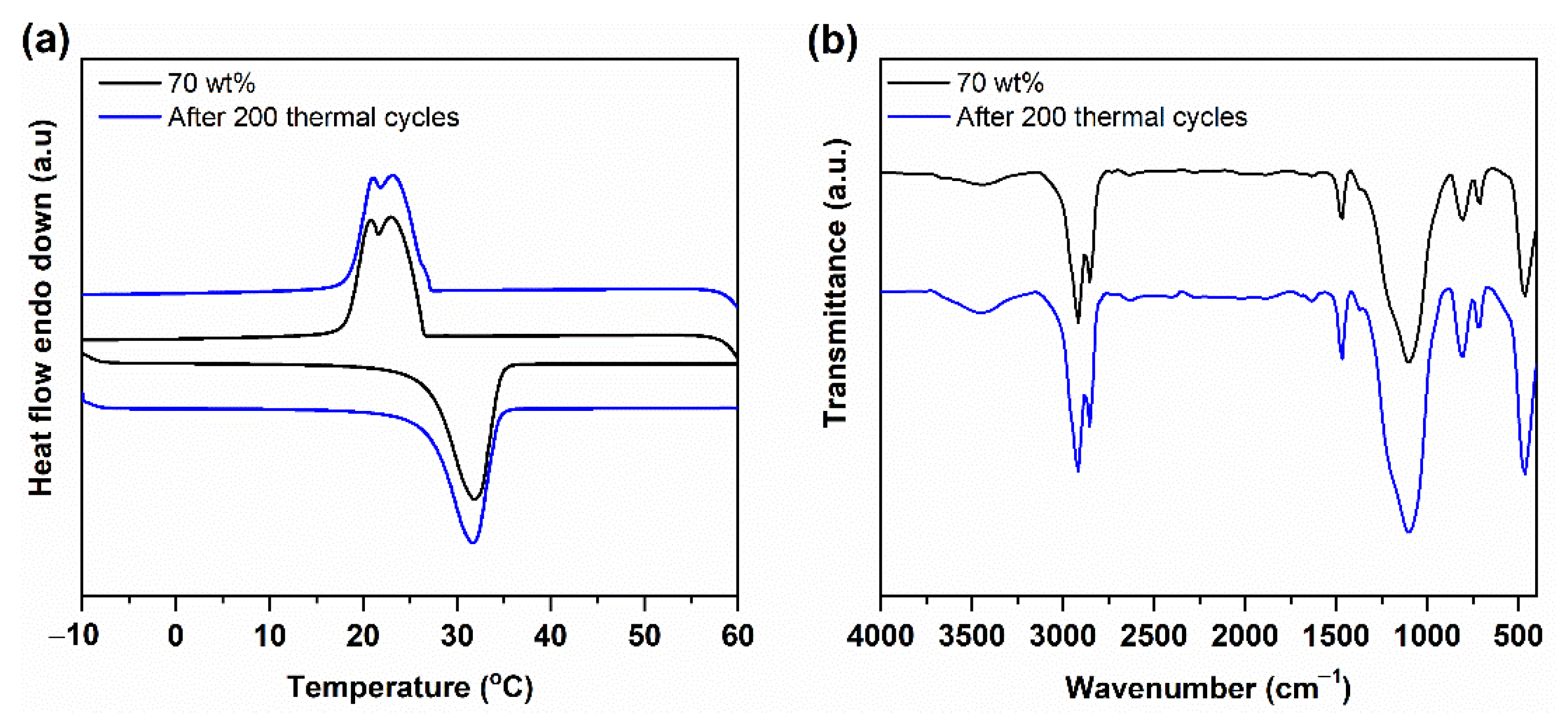
3.4. Thermal Reliability and Chemical Stability of n-Octadecane/FS PCC
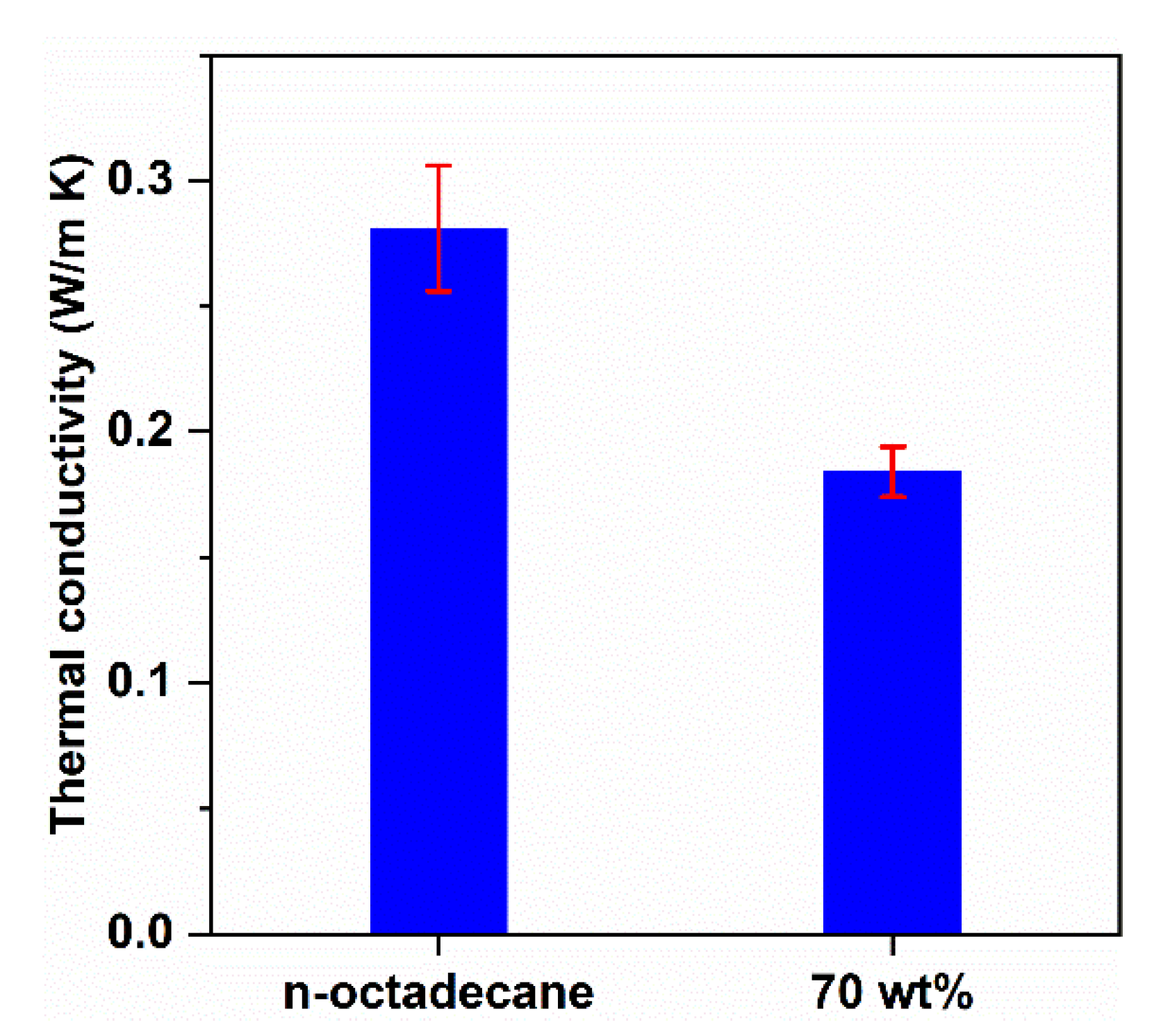
3.5. Thermal Conductivity of n-Octadecane/FS PCC
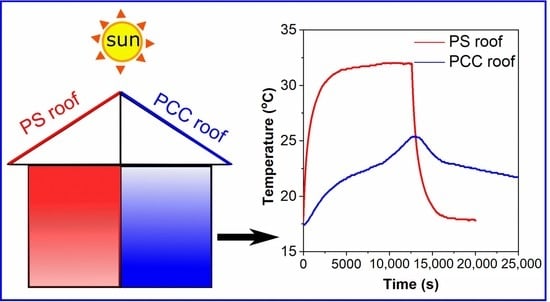
3.6. Thermal Performance of PCC Panel
3.6.1. Properties of PCC Panel
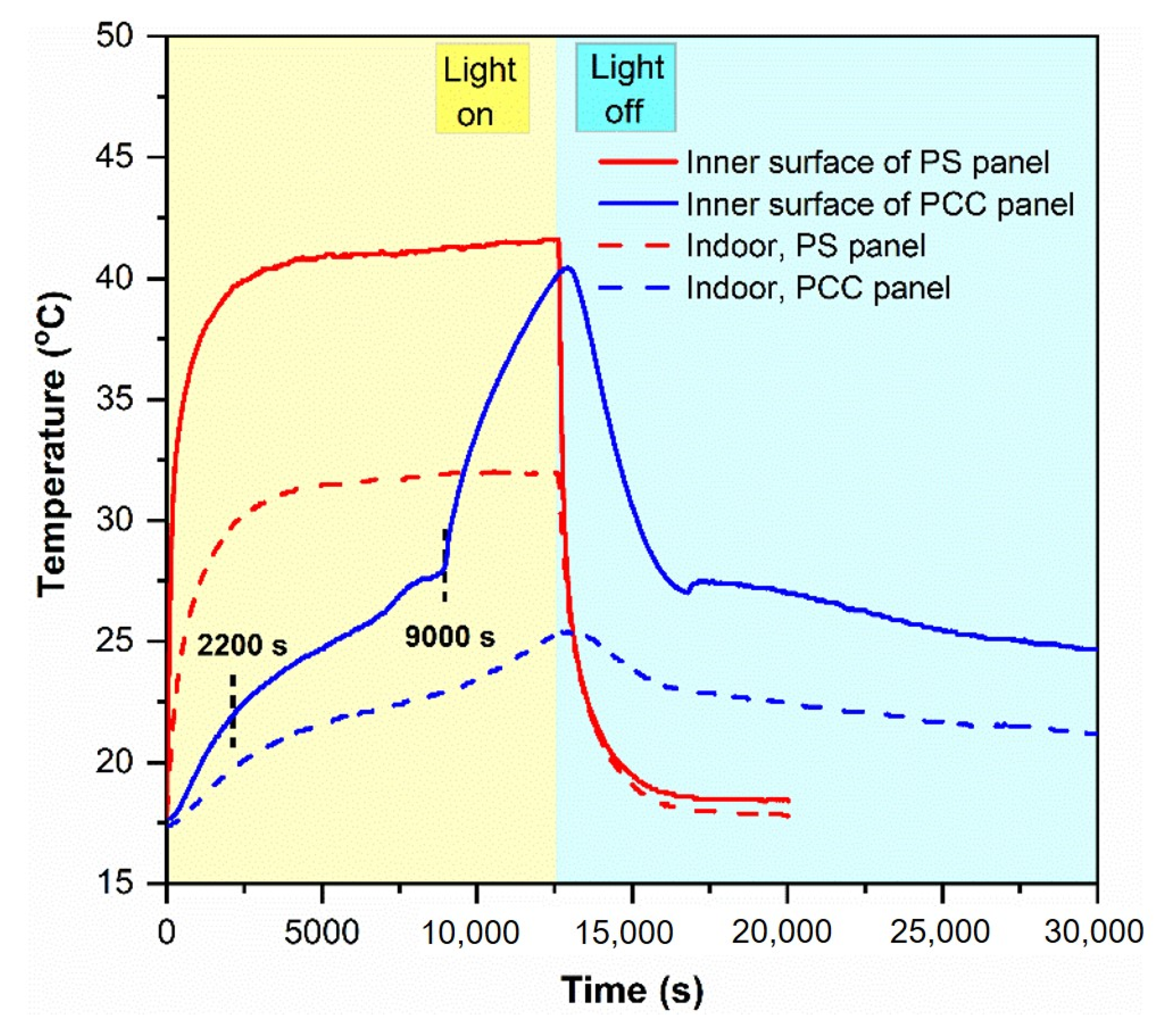
3.6.2. Thermal Performance of the PCC Panel in a Test Room
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| PCM | Phase change material |
| PCC | Phase change composite |
| FS | Fumed silica |
| PS | Polystyrene |
| TM | Melting temperature (°C) |
| TS | Solidifying temperature (°C) |
| TS,α | Solidifying temperature of α crystallization phase (°C) |
| TS,β | Solidifying temperature of β crystallization phase (°C) |
| ΔHM | Melting phase change enthalpy (J/g) |
| ΔHS | Solidifying phase change enthalpy (J/g) |
| F | Crystallization fraction (%) |
| ΔHM,PCC | Melting phase change enthalpy of phase change composite (J/g) |
| ΔHS,PCC | Solidifying phase change enthalpy of phase change composite (J/g) |
| ΔHM,PCM | Melting phase change enthalpy of pure phase change material (J/g) |
| ΔHS,PCM | Solidifying phase change enthalpy of pure phase change material (J/g) |
| Relative mass proportion of the PCM in the composite | |
| SEM | Scanning electron microscopy |
| FT-IR | Fourier transform infrared spectroscopy |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
References
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Drissi, S.; Ling, T.-C.; Mo, K.H.; Eddhahak, A. A review of microencapsulated and composite phase change materials: Alteration of strength and thermal properties of cement-based materials. Renew. Sustain. Energy Rev. 2019, 110, 467–484. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, Y.; Feng, J.; Feng, J. Synthesis of white cement bonded porous fumed silica-based composite for thermal insulation with low thermal conductivity via a facile cast-in-place approach. Constr. Build. Mater. 2019, 206, 620–629. [Google Scholar] [CrossRef]
- Zalba, B.; Marı́n, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Shilei, L.; Neng, Z.; Guohui, F. Eutectic mixtures of capric acid and lauric acid applied in building wallboards for heat energy storage. Energy Build. 2006, 38, 708–711. [Google Scholar] [CrossRef]
- Weinläder, H.; Beck, A.; Fricke, J. PCM-facade-panel for daylighting and room heating. Sol. Energy 2005, 78, 177–186. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, J.; Liu, L.; Xu, T.; Wu, H.; Zhou, X. Study on properties of phase change foam concrete block mixed with paraffin/fumed silica composite phase change material. Renew. Energy 2020, 150, 1127–1135. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; Ye, R.; Fang, X.; Zhang, Z. A calcium chloride hexahydrate/expanded perlite composite with good heat storage and insulation properties for building energy conservation. Renew. Energy 2017, 114, 733–743. [Google Scholar] [CrossRef]
- Camuffo, D. Chapter 2—Temperature: A Key Variable in Conservation and Thermal Comfort. In Microclimate for Cultural Heritage, 3rd ed.; Camuffo, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 15–42. [Google Scholar]
- Tyagi, V.V.; Pandey, A.K.; Buddhi, D.; Kothari, R. Thermal performance assessment of encapsulated PCM based thermal management system to reduce peak energy demand in buildings. Energy Build. 2016, 117, 44–52. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Kim, S.; Chang, S.J.; Chung, O.; Jeong, S.-G.; Kim, S. Thermal characteristics of mortar containing hexadecane/xGnP SSPCM and energy storage behaviors of envelopes integrated with enhanced heat storage composites for energy efficient buildings. Energy Build. 2014, 70, 472–479. [Google Scholar] [CrossRef]
- Shuja, S.Z.; Yilbas, B.S.; Javani, N.; Ahmed, S. Thermal characteristics of n-octadecane and carbon nanotubes mixture. Appl. Therm. Eng. 2016, 98, 646–655. [Google Scholar] [CrossRef]
- Wi, S.; Seo, J.; Jeong, S.-G.; Chang, S.J.; Kang, Y.; Kim, S. Thermal properties of shape-stabilized phase change materials using fatty acid ester and exfoliated graphite nanoplatelets for saving energy in buildings. Sol. Energy Mater. Sol. Cells 2015, 143, 168–173. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; Barbanera, M.; Cabeza, L.F. Palm oil-based bio-PCM for energy efficient building applications: Multipurpose thermal investigation and life cycle assessment. J. Energy Storage 2020, 28, 101129. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Luo, L. A review of potential materials for thermal energy storage in building applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Tang, J.; Yang, M.; Yu, F.; Chen, X.; Tan, L.; Wang, G. 1-Octadecanol@hierarchical porous polymer composite as a novel shape-stability phase change material for latent heat thermal energy storage. Appl. Energy 2017, 187, 514–522. [Google Scholar] [CrossRef]
- Nomura, T.; Zhu, C.; Sheng, N.; Tabuchi, K.; Sagara, A.; Akiyama, T. Shape-stabilized phase change composite by impregnation of octadecane into mesoporous SiO2. Sol. Energy Mater. Sol. Cells 2015, 143, 424–429. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Chen, X.; Wang, G.; Huang, X.; Li, A.; Dong, W. Nanoconfinement effects on thermal properties of nanoporous shape-stabilized composite PCMs: A review. Nano Energy 2018, 53, 769–797. [Google Scholar] [CrossRef]
- Hoseini, A.; Malekian, A.; Bahrami, M. Deformation and thermal resistance study of aerogel blanket insulation material under uniaxial compression. Energy Build. 2016, 130, 228–237. [Google Scholar] [CrossRef]
- Alam, M.; Singh, H.; Brunner, S.; Naziris, C. Experimental characterisation and evaluation of the thermo-physical properties of expanded perlite—Fumed silica composite for effective vacuum insulation panel (VIP) core. Energy Build. 2014, 69, 442–450. [Google Scholar] [CrossRef]
- Tang, X.; Yu, Y. Preparation of fumed silica compacts for thermal insulation using wet processing method. Int. J. Appl. Ceram. Technol. 2018, 15, 232–236. [Google Scholar] [CrossRef]
- Chen, J.; Ling, Z.; Fang, X.; Zhang, Z. Experimental and numerical investigation of form-stable dodecane/hydrophobic fumed silica composite phase change materials for cold energy storage. Energy Convers. Manag. 2015, 105, 817–825. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Wang, T.; Zhu, P. Effect of fumed silica additive on supercooling, thermal reliability and thermal stability of Na2HPO4·12H2O as inorganic PCM. Thermochim. Acta 2019, 675, 1–8. [Google Scholar] [CrossRef]
- Fu, W.; Zou, T.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Preparation and properties of phase change temperature-tuned composite phase change material based on sodium acetate trihydrate–urea/fumed silica for radiant floor heating system. Appl. Therm. Eng. 2019, 162, 114253. [Google Scholar] [CrossRef]
- Mitran, R.A.; Berger, D.; Munteanu, C.; Matei, C. Evaluation of Different Mesoporous Silica Supports for Energy Storage in Shape-Stabilized Phase Change Materials with Dual Thermal Responses. J. Phys. Chem. C 2015, 119, 15177–15184. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Shape-stabilised n-octadecane/activated carbon nanocomposite phase change material for thermal energy storage. J. Taiwan Inst. Chem. Eng. 2015, 55, 189–197. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, S.; Chen, K.; Gao, X.; Chang, P.; Tian, C.; Wang, J.; Huang, Y. Preparation and properties of nanoencapsulated n-octadecane phase change material with organosilica shell for thermal energy storage. Energy Convers. Manag. 2015, 105, 908–917. [Google Scholar] [CrossRef]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Du, X.; Wang, S.; Du, Z.; Cheng, X.; Wang, H. Preparation and characterization of flame-retardant nanoencapsulated phase change materials with poly(methylmethacrylate) shells for thermal energy storage. J. Mater. Chem. A 2018, 6, 17519–17529. [Google Scholar] [CrossRef]
- Geng, L.; Wang, S.; Wang, T.; Luo, R. Facile Synthesis and Thermal Properties of Nanoencapsulated n-Dodecanol with SiO2 Shell as Shape-Formed Thermal Energy Storage Material. Energy Fuels 2016, 30, 6153–6160. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Jeon, J.; Lee, J.-H.; Kim, S. Optimal preparation of PCM/diatomite composites for enhancing thermal properties. Int. J. Heat Mass Transf. 2013, 62, 711–717. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Wang, M.; Liu, Z. A n-octadecane/hierarchically porous TiO2 form-stable PCM for thermal energy storage. Renew. Energy 2020, 145, 1465–1473. [Google Scholar] [CrossRef]
- Sami, S.; Etesami, N. Improving thermal characteristics and stability of phase change material containing TiO2 nanoparticles after thermal cycles for energy storage. Appl. Therm. Eng. 2017, 124, 346–352. [Google Scholar] [CrossRef]
- Park, I.; Peng, H.-g.; Gidley, D.W.; Xue, S.; Pinnavaia, T.J. Epoxy−Silica Mesocomposites with Enhanced Tensile Properties and Oxygen Permeability. Chem. Mater. 2006, 18, 650–656. [Google Scholar] [CrossRef]
- Motahar, S.; Nikkam, N.; Alemrajabi, A.A.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M. A novel phase change material containing mesoporous silica nanoparticles for thermal storage: A study on thermal conductivity and viscosity. Int. Commun. Heat Mass Transf. 2014, 56, 114–120. [Google Scholar] [CrossRef]


| Sample | TM (°C) | ΔHM (J/g) | TS,α (°C) | Ts,β (°C) | ΔHS (J/g) | F (%) |
|---|---|---|---|---|---|---|
| n-octadecane | 28.81 ± 0.12 | 230.5 ± 6.0 | 28.21 ± 0.14 | 229.4 ± 3.7 | 100 | |
| 70 wt% | 26.76 ± 0.10 | 155.8 ± 2.5 | 26.69 ± 0.37 | 22.09 ± 0.34 | 154.7 ± 1.2 | 96.5 ± 3.4 |
| 70 wt% after 200 thermal cycles | 26.61 ± 0.24 | 154.2 ± 4.1 | 27.07 ± 0.17 | 22.23 ± 0.22 | 153.2 ± 4.0 | 95.5 ± 4.6 |
| Phase-Change Composite | PCM Content (wt%) | TM (°C) | ΔHM (J/g) | Ref |
|---|---|---|---|---|
| Fatty acid ester/exfoliated graphite | 80 | 26.9 | 77.6 | [15] |
| CaCl2‧6H2O/expanded perlite | 55 | 27.4 | 87.4 | [9] |
| n-Dodecanol/SiO2 nano-shell | 55.5 | 21.0 | 116.7 | [35] |
| n-Hexadecane/xGnP | 48.8 | 16–25 | 96.4 | [13] |
| n-Hexadecane/diatomite | 47 | 23.7 | 120.1 | [36] |
| n-Octadecane/porous TiO2 | 50 | 27.9 | 85.8 | [37] |
| n-Octadecane/activated carbon | 43.4 | - | 101.8 | [29] |
| n-Octadecane/PMMA | 60 | 32.6 | 125.4 | [34] |
| n-Octadecane /SiO2 micro-shell | 66.2 | 23.3 | 142.2 | [33] |
| n-Octadecane/FS | 70 | 26.7 | 155.8 | This work |
| Material | Latent Heat (J/g) | Density (kg/m3) | Thermal Conductivity (W/m K) |
|---|---|---|---|
| PS panel | 0 | 20 ± 0.5 | 0.045 ± 0.007 |
| PCC panel | 155.8 ± 2.5 | 700 ± 18 | 0.184 ± 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, G.T.; Hwang, H.S.; Lee, J.; Cha, D.A.; Park, I. n-Octadecane/Fumed Silica Phase Change Composite as Building Envelope for High Energy Efficiency. Nanomaterials 2021, 11, 566. https://doi.org/10.3390/nano11030566
Nguyen GT, Hwang HS, Lee J, Cha DA, Park I. n-Octadecane/Fumed Silica Phase Change Composite as Building Envelope for High Energy Efficiency. Nanomaterials. 2021; 11(3):566. https://doi.org/10.3390/nano11030566
Chicago/Turabian StyleNguyen, Giang Tien, Ha Soo Hwang, Jiyoung Lee, Dong An Cha, and In Park. 2021. "n-Octadecane/Fumed Silica Phase Change Composite as Building Envelope for High Energy Efficiency" Nanomaterials 11, no. 3: 566. https://doi.org/10.3390/nano11030566
APA StyleNguyen, G. T., Hwang, H. S., Lee, J., Cha, D. A., & Park, I. (2021). n-Octadecane/Fumed Silica Phase Change Composite as Building Envelope for High Energy Efficiency. Nanomaterials, 11(3), 566. https://doi.org/10.3390/nano11030566