Mussel-Inspired Fabrication of PDA@PAN Electrospun Nanofibrous Membrane for Oil-in-Water Emulsion Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments and Characterization
2.3. Preparation of PAN Nanofibrous Membrane
2.4. Fabrication of PDA@PAN Nanofibrous Membrane
2.5. Oil-In-Water Emulsion Separation Test
3. Results and Discussion
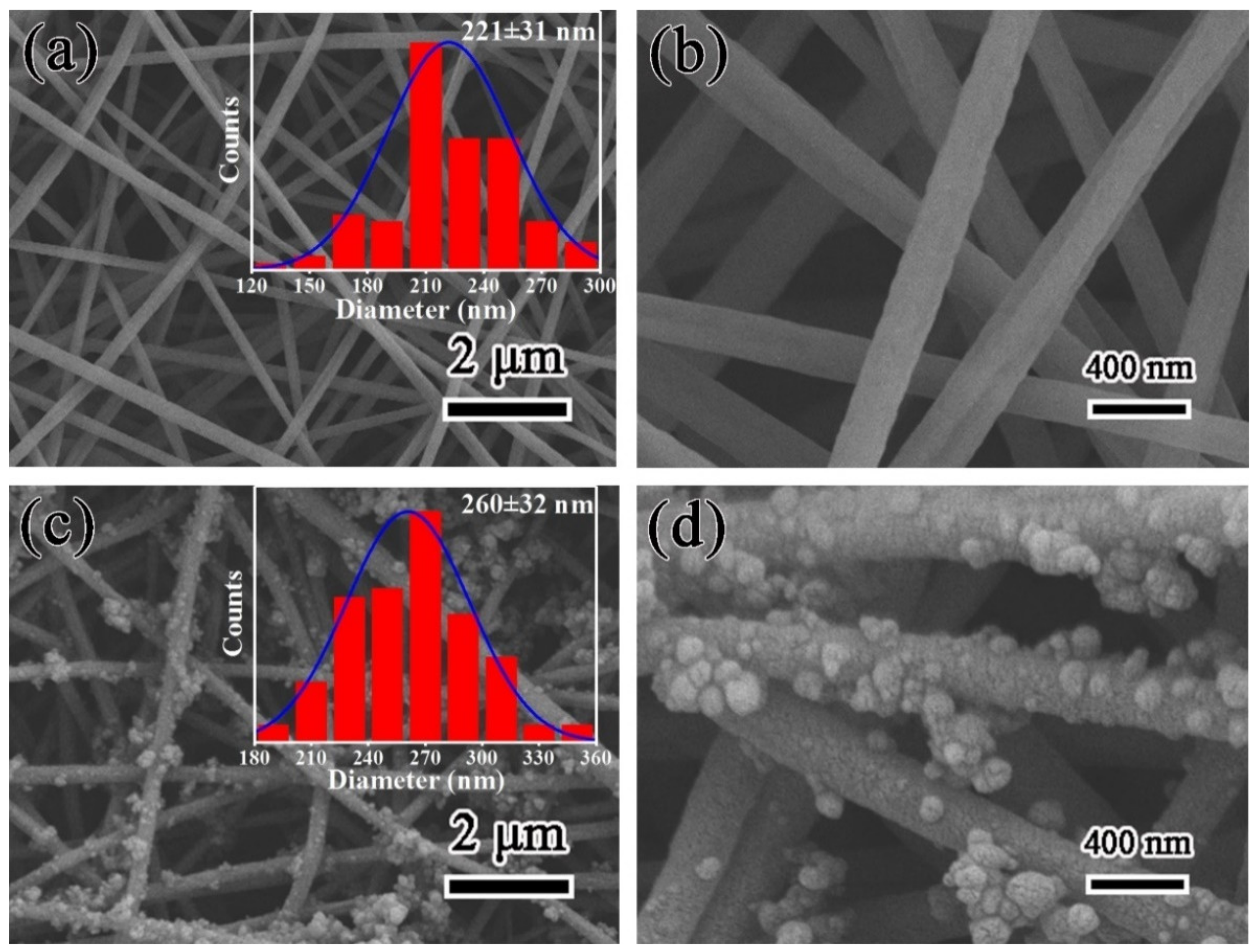
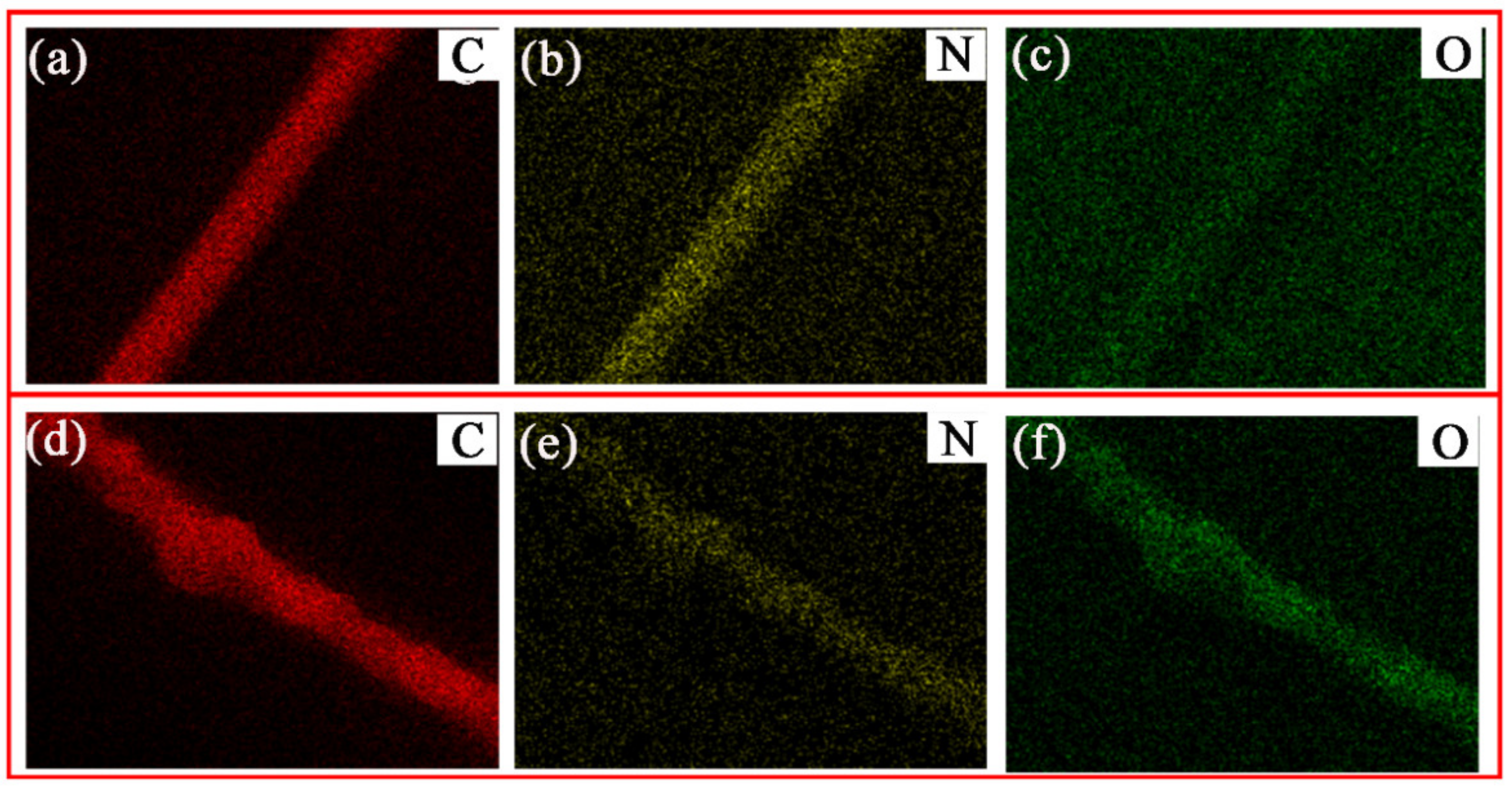
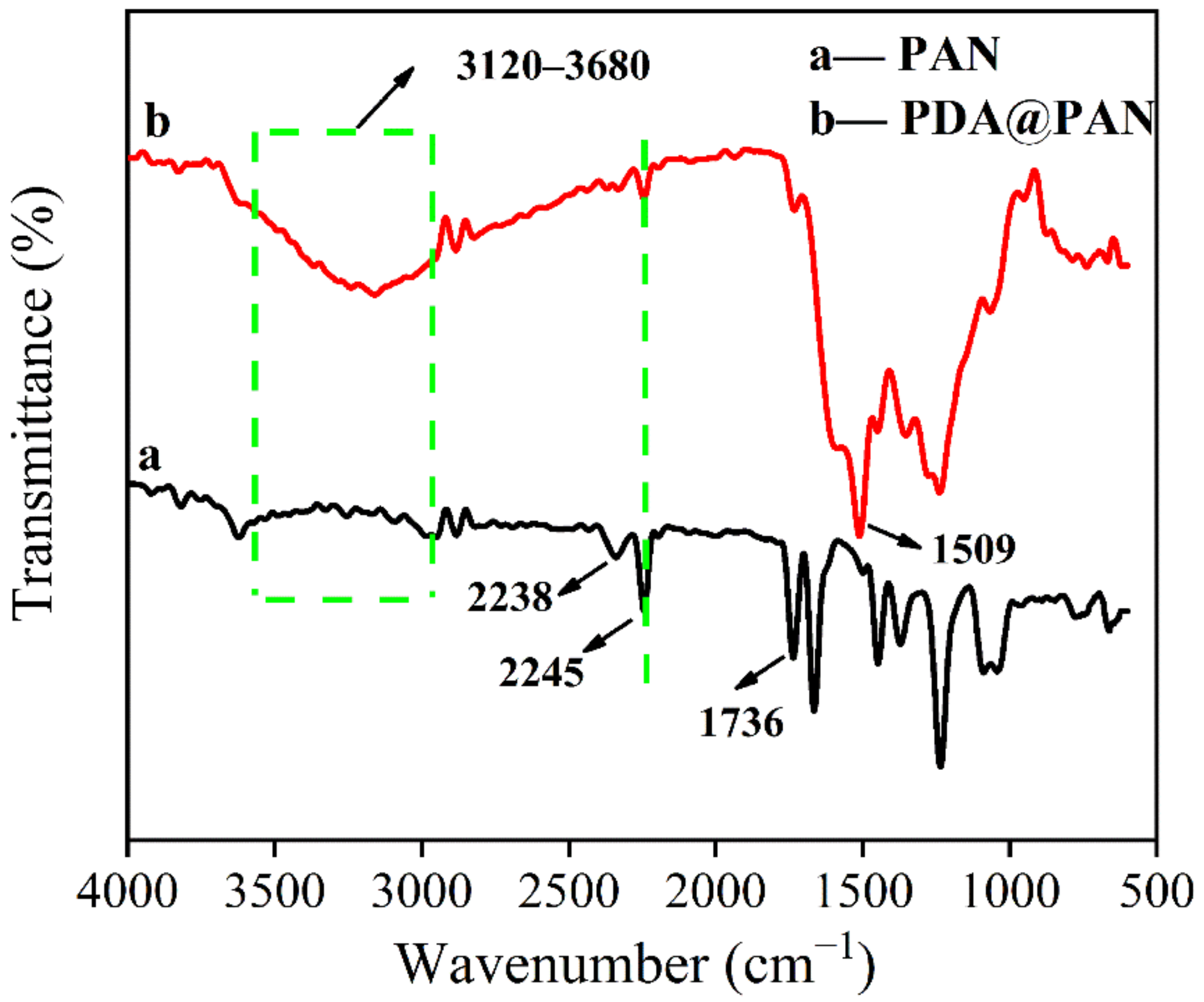
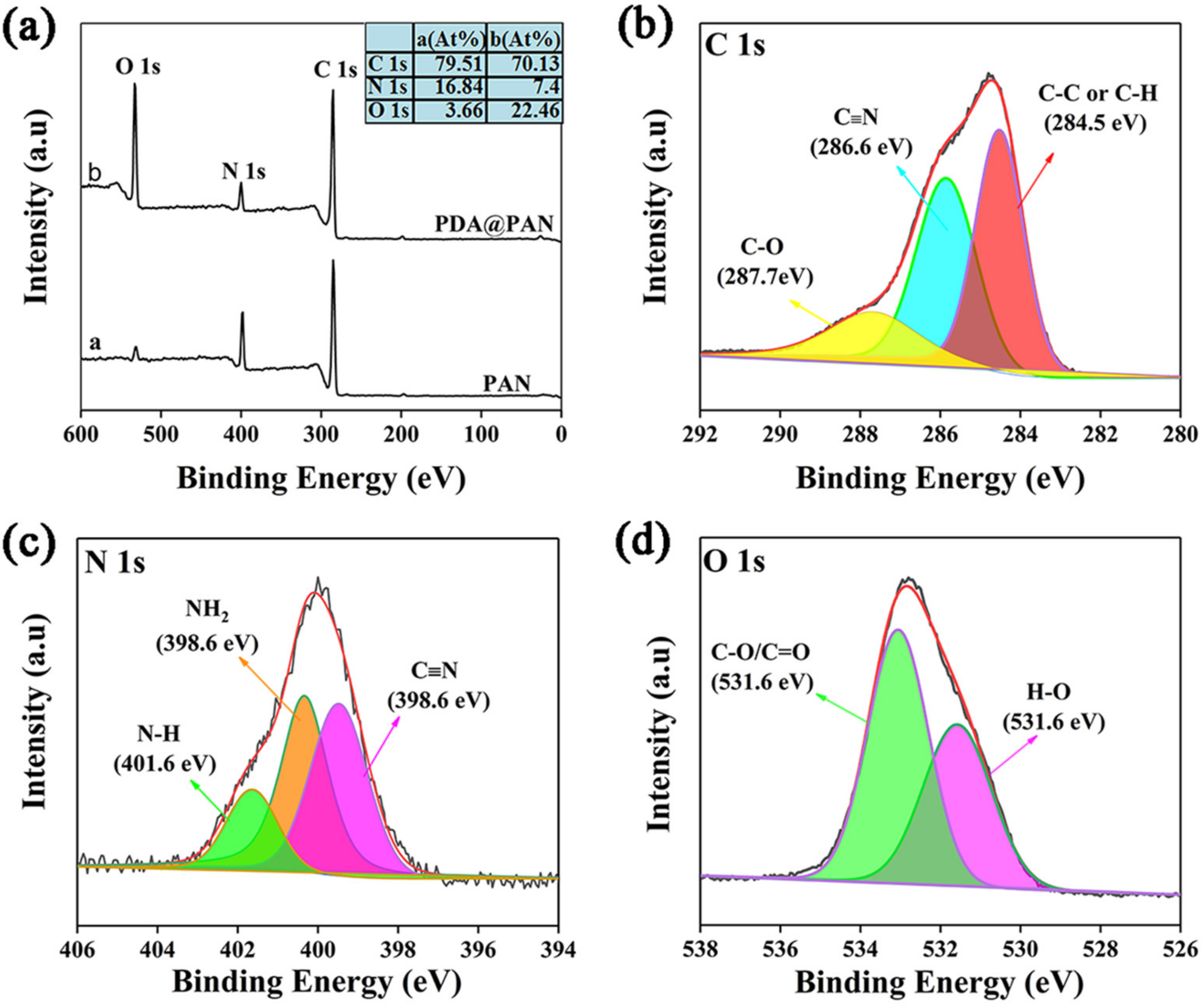
3.1. Morphology and Structure of PDA@PAN Nanofiber Membrane
3.2. Hydrophilicity of PDA@PAN Nanofibrous Membrane in Air
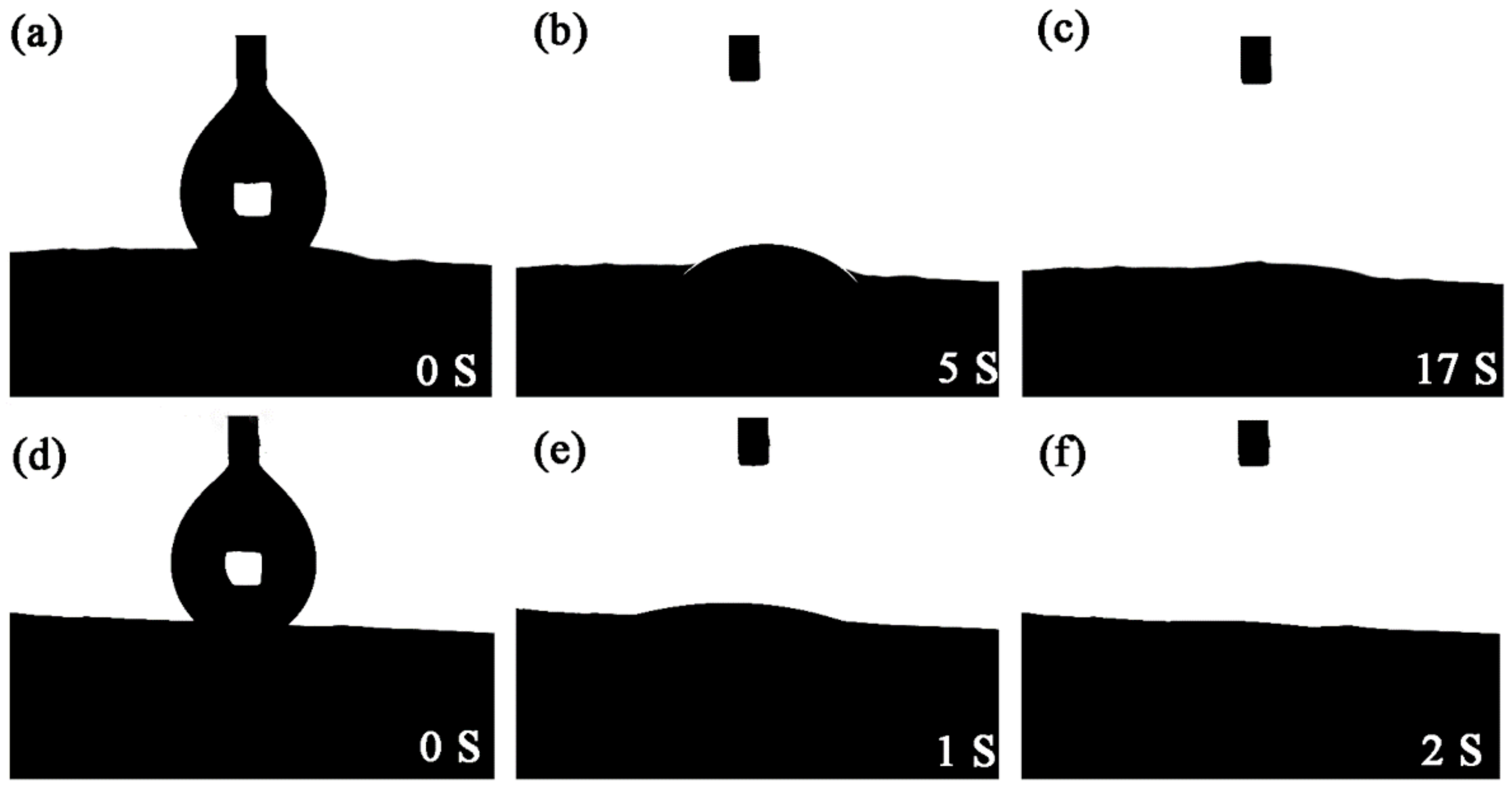
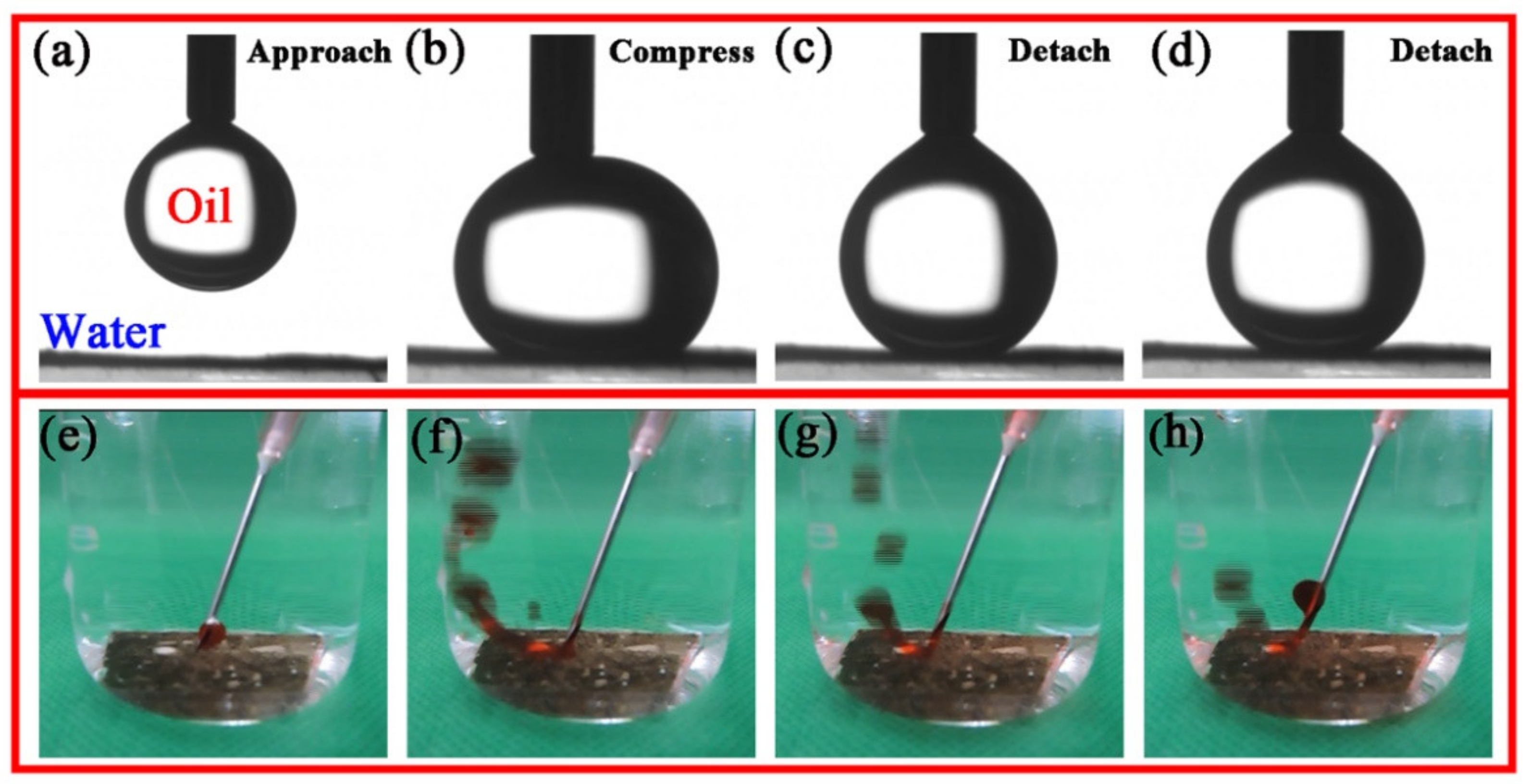
3.3. Underwater Oleophobicity of PDA@PAN Nanofiber Membrane
3.4. Separation Performance of PDA@PAN Nanofibrous Membrane for Oil-In-Water Emulsion
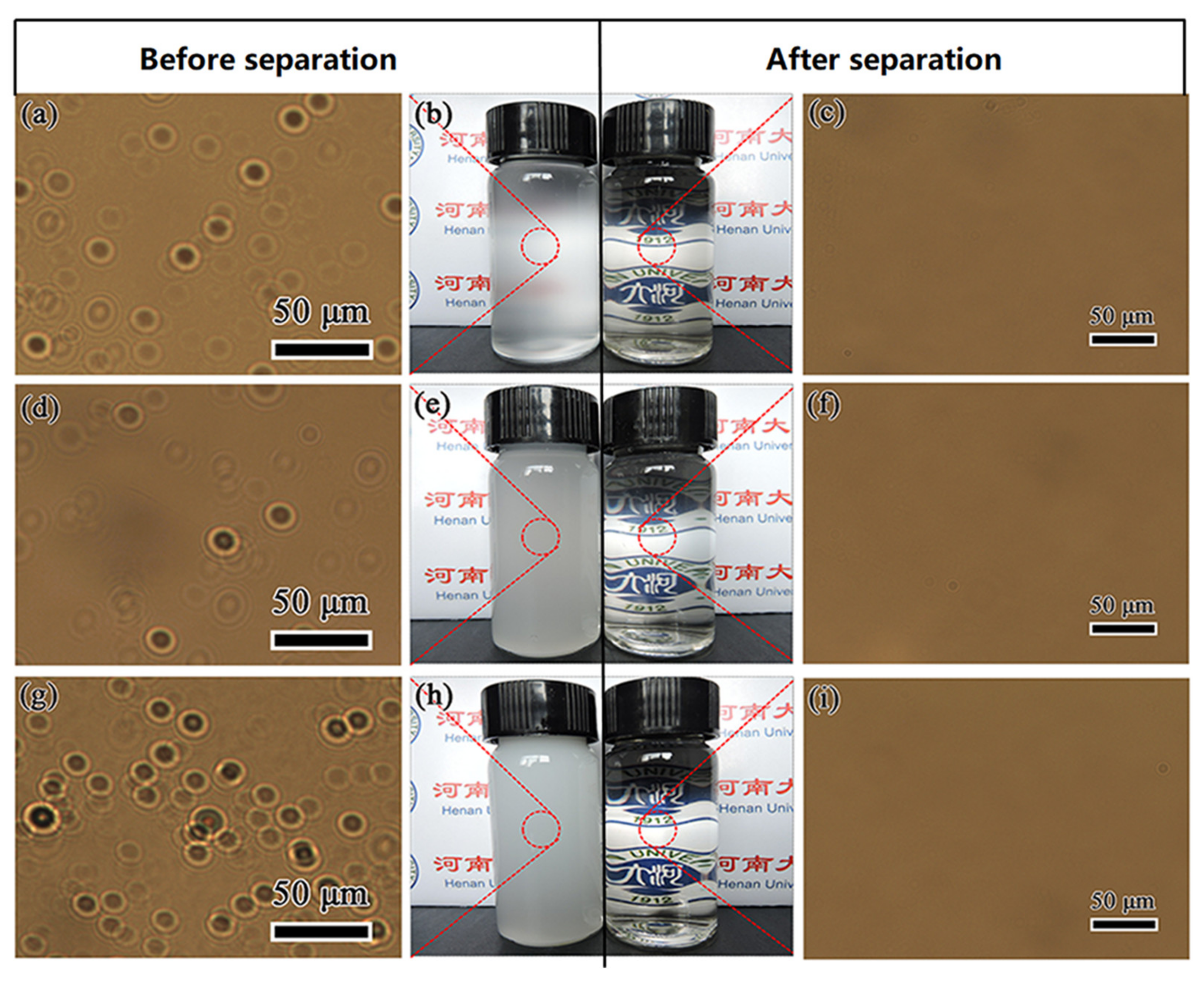
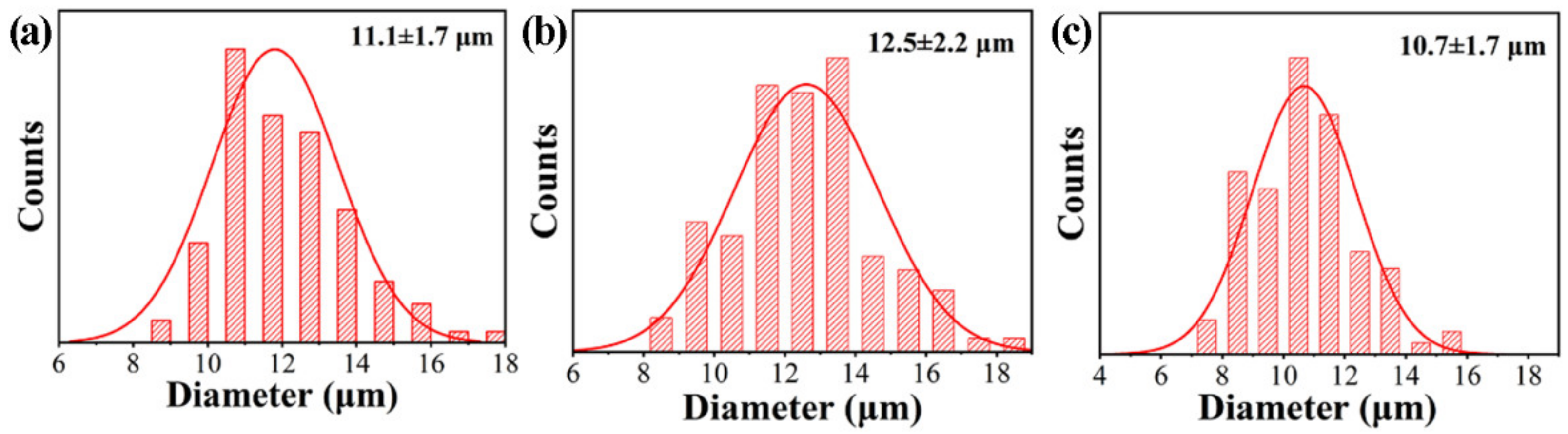
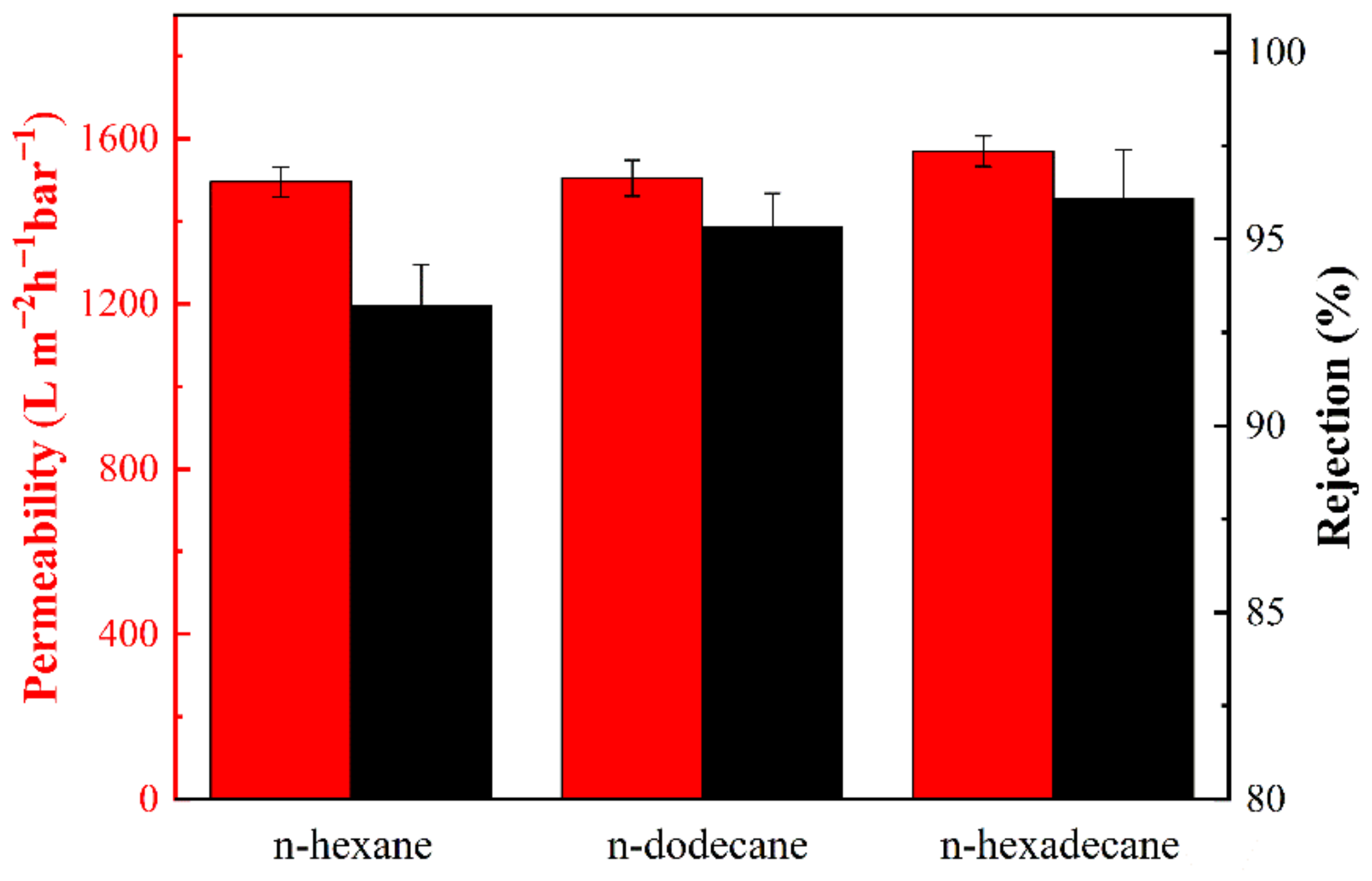
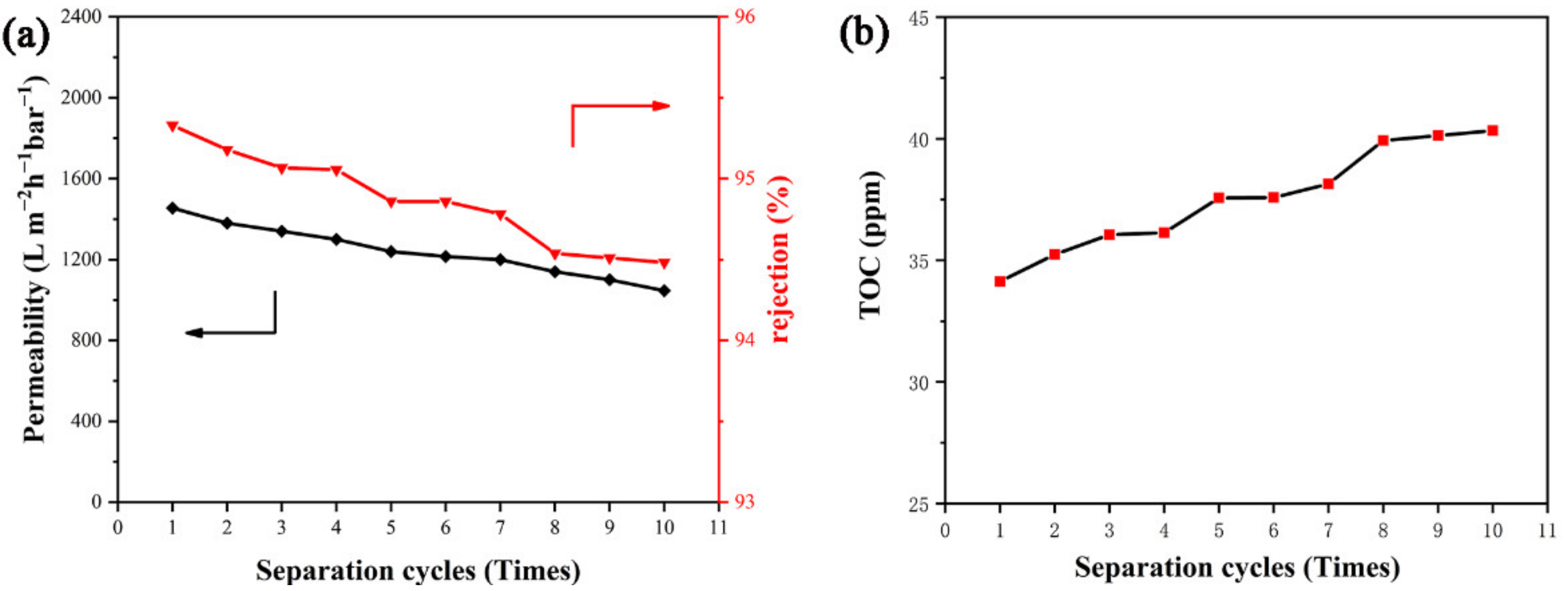
3.5. Separation Efficiency of PDA@PAN Nanofibrous Membrane for Emulsified Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Kim, S.; Kallem, P.; Choi, H. Capillary effect in Janus electrospun nanofiber membrane for oil/water emulsion separation. Chemosphere 2019, 221, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhao, H.Y.; Zhu, H.W.; Huang, J.; Shi, L.A.; Yu, S.H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef]
- Wu, J.; Wei, W.; Li, S.; Zhong, Q.; Liu, F.; Zheng, J.; Wang, J. The effect of membrane surface charges on demulsification and fouling resistance during emulsion separation. J. Membr. Sci. 2018, 563, 126–133. [Google Scholar] [CrossRef]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M. Preparation, characterization and fouling analysis of in-air hydrophilic/underwater oleophobic bio-inspired polydopamine coated PES membranes for oily wastewater treatment. J. Membr. Sci. 2019, 582, 402–413. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Q.; Pan, X.; Jin, Y.; Lu, W.; Ding, D.; Guo, Q. Graphene oxide/polyacrylonitrile fiber hierarchical-structured membrane for ultra-fast microfiltration of oil-water emulsion. Chem. Eng. J. 2017, 307, 643–649. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Zhu, P.; Bai, Y. One-step plant-inspired reaction that transform membrane hydrophobicity into high hydrophilicity and underwater super oleophobicity for oil-in-water emulsion separation. Appl. Surf. Sci. 2019, 479, 423–429. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Wang, A.; Lu, Y.; Li, J.; Zhu, Y.; Jin, J. Zwitterionic Nanofibrous Membranes with a Superior Antifouling Property for Gravity-Driven Crude Oil-in-Water Emulsion Separation. Langmuir 2019, 35, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Cherukupally, P.; Sun, W.; Wong, A.P.Y.; Williams, D.R.; Ozin, G.A.; Bilton, A.M.; Park, C.B. Surface-engineered sponges for recovery of crude oil microdroplets from wastewater. Nat. Sustain. 2020, 3, 136–143. [Google Scholar] [CrossRef]
- Xu, H.; Jia, W.; Ren, S.; Wang, J. Novel and recyclable demulsifier of expanded perlite grafted by magnetic nanoparticles for oil separation from emulsified oil wastewaters. Chem. Eng. J. 2018, 337, 10–18. [Google Scholar] [CrossRef]
- Jiang, Y.; Hou, J.; Xu, J.; Shan, B. Switchable oil/water separation with efficient and robust Janus nanofiber membranes. Carbon 2017, 115, 477–485. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y. Oil/water mixtures and emulsions separation of stearic acid-functionalized sponge fabricated via a facile one-step coating method. Sep. Purif. Technol. 2017, 181, 183–191. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Yu, H. Dynamic Membrane Filtration: Formation, Filtration, Cleaning, and Applications. Chem. Eng. Technol. 2018, 41, 7–18. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, J.; Wang, F.; Li, Z.; Yu, J.; Ding, B. Superhydrophilic and underwater superoleophobic nanofibrous membrane with hierarchical structured skin for effective oil-in-water emulsion separation. J. Mater. Chem. A 2017, 5, 497–502. [Google Scholar] [CrossRef]
- Sun, J.; Bi, H.; Su, S.; Jia, H.; Xie, X.; Sun, L. One-step preparation of GO/SiO2 membrane for highly efficient separation of oil-in-water emulsion. J. Membr. Sci. 2018, 553, 131–138. [Google Scholar] [CrossRef]
- Lin, Y.M.; Rutledge, G.C. Separation of oil-in-water emulsions stabilized by different types of surfactants using electrospun fiber membranes. J. Membr. Sci. 2018, 563, 247–258. [Google Scholar] [CrossRef]
- Zhan, H.; Peng, N.; Lei, X.; Huang, Y.; Li, D.; Tao, R.; Chang, C. UV-induced self-cleanable TiO2/nanocellulose membrane for selective separation of oil/water emulsion. Carbohydr. Polym. 2018, 201, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, L.; Zhang, J.; Guo, T.; Li, X.; Xing, W.; Xue, Q. High-efficiency separation performance of oil-water emulsions of polyacrylonitrile nanofibrous membrane decorated with metal-organic frameworks. Appl. Surf. Sci. 2019, 476, 61–69. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Wu, G.; Chen, S.-C.; Wang, Y.Z. Highly-efficient, Rapid and continuous separation of surfactant-stabilized Oil/Water emulsions by selective under-liquid adhering emulsified droplets. J. Hazard. Mater. 2020, 400, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Zhang, J.; Kang, Y.L.; Wu, G.; Chen, S.C.; Wang, Y.Z. Reusable and Recyclable Superhydrophilic Electrospun Nanofibrous Membranes with In Situ Co-cross-linked Polymer-Chitin Nanowhisker Network for Robust Oil-in-Water Emulsion Separation. ACS Sustain. Chem. Eng. 2018, 6, 1753–1762. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2016, 19, 403–414. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Hua, D.; Xiong, R.; Zhao, J.; Rao, W.; Huang, S.; Zhan, X.; Chen, F.; Huang, C. Electrospun fibers for oil-water separation. Rsc. Adv. 2016, 6, 12868–12884. [Google Scholar] [CrossRef]
- Hai, A.; Durrani, A.A.; Selvaraj, M.; Banat, F.; Abu Haija, M. Oil-water emulsion separation using intrinsically superoleophilic and superhydrophobic PVDF membrane. Sep. Purif. Technol. 2019, 212, 388–395. [Google Scholar] [CrossRef]
- Sun, H.; Tang, B.; Wu, P. Hydrophilic hollow zeolitic imidazolate framework-8 modified ultrafiltration membranes with significantly enhanced water separation properties. J. Membr. Sci. 2018, 551, 283–293. [Google Scholar] [CrossRef]
- Wei, W.; Sun, M.; Zhang, L.; Zhao, S.; Wu, J.; Wang, J. Underwater oleophobic PTFE membrane for efficient and reusable emulsion separation and the influence of surface wettability and pore size. Sep. Purif. Technol. 2017, 189, 32–39. [Google Scholar] [CrossRef]
- Hao, J.; Fan, Z.; Xiao, C.; Zhao, J.; Liu, H.; Chen, L. Effect of stretching on continuous oil/water separation performance of polypropylene hollow fiber membrane. Iran. Polym. J 2017, 26, 941–948. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, G.; Bai, R.; Shen, S.; Zhou, X.; Wyman, I. Fabrication of superhydrophilic and underwater superoleophobic membranes via an in situ crosslinking blend strategy for highly efficient oil/water emulsion separation. J. Membr. Sci. 2019, 569, 60–70. [Google Scholar] [CrossRef]
- Yan, L.; Li, P.; Zhou, W.; Wang, Z.; Fan, X.; Chen, M.; Fang, Y.; Liu, H. Shrimp Shell-Inspired Antifouling Chitin Nanofibrous Membrane for Efficient Oil/Water Emulsion Separation with In Situ Removal of Heavy Metal Ions. ACS Sustain. Chem. Eng. 2019, 7, 2064–2072. [Google Scholar] [CrossRef]
- Qing, W.; Li, X.; Wu, Y.; Shao, S.; Guo, H.; Yao, Z.; Chen, Y.; Zhang, W.; Tang, C.Y. In situ silica growth for superhydrophilic-underwater superoleophobic Silica/PVA nanofibrous membrane for gravity-driven oil-in-water emulsion separation. J. Membr. Sci. 2020, 612, 118476. [Google Scholar] [CrossRef]
- Kong, W.; Li, F.; Pan, Y.; Zhao, X. Hygro-responsive, Photo-decomposed Superoleophobic/Superhydrophilic Coating for On-Demand Oil-Water Separation. ACS Appl. Mater. Interfaces 2021, 13, 35142–35152. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, J.; Yang, N.; Sha, S.; Yang, C.; Zhao, J.; Duoerkun, A.; Hong, Y.; Wu, C. Underwater superoleophobic graphene oxide-connected cotton fibers membrane for antifouling oil/water separation. J. Water Process Eng. 2021, 44, 102334. [Google Scholar] [CrossRef]
- Yuan, M.; Teng, Z.; Wang, S.; Xu, Y.; Wu, P.; Zhu, Y.; Wang, C.; Wang, G. Polymeric carbon nitride modified polyacrylonitrile fabrics with efficient self-cleaning and water disinfection under visible light. Chem. Eng. J. 2020, 391, 123506. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, S.; Zhu, Y.; Jin, J. Alkaline-induced superhydrophilic/underwater superoleophobic polyacrylonitrile membranes with ultralow oil-adhesion for high-efficient oil/water separation. J. Membr. Sci. 2016, 513, 67–73. [Google Scholar] [CrossRef]
- Almasian, A.; Jalali, M.L.; Fard, G.C.; Maleknia, L. Surfactant grafted PDA-PAN nanofiber: Optimization of synthesis, characterization and oil absorption property. Chem. Eng. J. 2017, 326, 1232–1241. [Google Scholar] [CrossRef]
- Lee, H.A.; Ma, Y.; Zhou, F.; Hong, S.; Lee, H. Material-Independent Surface Chemistry beyond Polydopamine Coating. Acc. Chem. Res. 2019, 52, 704–713. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, Y.; Qin, W.Z.; He, A.; Xu, Z.K. Polydopamine Coatings with Nanopores for Versatile Molecular Separation. ACS Appl. Mater. Interfaces 2017, 9, 14437–14444. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; He, S.; Wan, X.; Zhao, S.; Bai, Y. Thermally and chemically stable poly(arylene ether nitrile)/halloysite nanotubes intercalated graphene oxide nanofibrous composite membranes for highly efficient oil/water emulsion separation in harsh environment. J. Membr. Sci. 2018, 567, 76–88. [Google Scholar] [CrossRef]
- Luo, C.; Liu, Q. Oxidant-Induced High-Efficient Mussel-Inspired Modification on PVDF Membrane with Superhydrophilicity and Underwater Superoleophobicity Characteristics for Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 8297–8307. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Ni, X.X.; Zhang, D.B.; Zheng, H.; Wang, J.B.; Zhang, Q.Q. Engineering a self-driven PVDF/PDA hybrid membranes based on membrane micro-reactor effect to achieve super-hydrophilicity, excellent antifouling properties and hemocompatibility. Appl. Surf. Sci. 2018, 444, 672–690. [Google Scholar] [CrossRef]
- Kumar, P.S.; Venkatesh, K.; Gui, E.L.; Jayaraman, S.; Singh, G.; Arthanareeswaran, G. Electrospun carbon nanofibers/TiO2-PAN hybrid membranes for effective removal of metal ions and cationic dye. Environ. Nanotechnol. Monit. Manag. 2018, 10, 366–376. [Google Scholar] [CrossRef]
- Zhan, H.; Zuo, T.; Tao, R.; Chang, C. Robust Tunicate Cellulose Nanocrystal/Palygorskite Nanorod Membranes for Multifunctional Oil/Water Emulsion Separation. ACS Sustain. Chem. Eng. 2018, 6, 10833–10840. [Google Scholar] [CrossRef]
- Yuan, T.; Meng, J.; Hao, T.; Wang, Z.; Zhang, Y. A Scalable Method toward Superhydrophilic and Underwater Superoleophobic PVDF Membranes for Effective Oil/Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 14896–14904. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, X.; Li, X.; Shao, C.; Han, C.; Li, X.; Liu, Y. Bismuth oxychloride (BiOCl)/copper phthalocyanine (CuTNPc) heterostructures immobilized on electrospun polyacrylonitrile nanofibers with enhanced activity for floating photocatalysis. J. Colloid Interface Sci. 2018, 525, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, Y.; Li, X.; Li, Y.; Sun, B.; Chao, S.; Wang, C. Facile hydrothermal synthesis of branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane as a highly efficient and reusable bilirubin adsorbent in hemoperfusion. J. Colloid Interface Sci. 2018, 514, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, Z.; Xie, A.; Meng, M.; Cui, Y.; Liu, S.; Lu, J.; Zhou, S.; Yan, Y.; Dong, H. Bio-inspired fabrication of superhydrophilic nanocomposite membrane based on surface modification of SiO2 anchored by polydopamine towards effective oil-water emulsions separation. Sep. Purif. Technol. 2019, 209, 434–442. [Google Scholar] [CrossRef]
- Park, J.H.; Joo, Y.L. A facile precursor route to highly loaded metal/ceramic nanofibers as a robust surface-enhanced Raman template. Appl. Surf. Sci. 2017, 416, 742–750. [Google Scholar] [CrossRef]
- Jin, X.; Sun, X.; Chen, G.; Ding, L.; Li, Y.; Liu, Z.; Wang, Z.; Pan, W.; Hu, C.; Wang, J. pH-sensitive carbon dots for the visualization of regulation of intracellular pH inside living pathogenic fungal cells. Carbon 2015, 81, 388–395. [Google Scholar] [CrossRef]
- Liu, P.; Wang, X.; Ma, J.; Liu, H.; Ning, P. Highly efficient immobilization of NZVI onto bio-inspired reagents functionalized polyacrylonitrile membrane for Cr(VI) reduction. Chemosphere 2019, 220, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pan, X.; Xue, Q.; He, D.; Zhu, L.; Guo, Q. Antifouling hydrolyzed polyacrylonitrile/graphene oxide membrane with spindle-knotted structure for highly effective separation of oil-water emulsion. J. Membr. Sci. 2017, 532, 38–46. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, X.; Lv, Y.; Wu, Z.; Chen, F.; Chen, Z. Excellent Surface Enhanced Raman Scattering of SiO2 Fiber Membrane Embedded with Ag Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2018, 28, 251–257. [Google Scholar] [CrossRef]
- Venkatesh, K.; Arthanareeswaran, G.; Bose, A.C. PVDF mixed matrix nano-filtration membranes integrated with 1D-PANI/TiO2 NFs for oil–water emulsion separation. Rsc. Adv. 2016, 6, 18899–18908. [Google Scholar] [CrossRef]
- He, Y.; Wan, M.; Wang, Z.; Zhang, X.; Zhao, Y.; Sun, L. Fabrication and characterization of degradable and durable fluoride-free super-hydrophobic cotton fabrics for oil/water separation. Surf. Coat. Technol. 2019, 378, 125079. [Google Scholar] [CrossRef]
- Venkatesh, K.; Arthanareeswaran, G.; Bose, A.C.; Kumar, P.S. Hydrophilic hierarchical carbon with TiO2 nanofiber membrane for high separation efficiency of dye and oil-water emulsion. Sep. Purif. Technol. 2020, 241, 116709. [Google Scholar] [CrossRef]
- Venkatesh, K.; Arthanareeswaran, G.; Chandra Bose, A.; Suresh Kumar, P.; Kweon, J. Diethylenetriaminepentaacetic acid-functionalized multi-walled carbon nanotubes/titanium oxide-PVDF nanofiber membrane for effective separation of oil/water emulsion. Sep. Purif. Technol. 2021, 257, 117926. [Google Scholar] [CrossRef]
- Pagidi, A.; Saranya, R.; Arthanareeswaran, G.; Ismail, A.F.; Matsuura, T. Enhanced oil–water separation using polysulfone membranes modified with polymeric additives. Desalination 2014, 344, 280–288. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; He, Y.; Wang, Z.; Zhao, Y.; Sun, L. Mussel-Inspired Fabrication of PDA@PAN Electrospun Nanofibrous Membrane for Oil-in-Water Emulsion Separation. Nanomaterials 2021, 11, 3434. https://doi.org/10.3390/nano11123434
Zhao H, He Y, Wang Z, Zhao Y, Sun L. Mussel-Inspired Fabrication of PDA@PAN Electrospun Nanofibrous Membrane for Oil-in-Water Emulsion Separation. Nanomaterials. 2021; 11(12):3434. https://doi.org/10.3390/nano11123434
Chicago/Turabian StyleZhao, Haodong, Yali He, Zhihua Wang, Yanbao Zhao, and Lei Sun. 2021. "Mussel-Inspired Fabrication of PDA@PAN Electrospun Nanofibrous Membrane for Oil-in-Water Emulsion Separation" Nanomaterials 11, no. 12: 3434. https://doi.org/10.3390/nano11123434
APA StyleZhao, H., He, Y., Wang, Z., Zhao, Y., & Sun, L. (2021). Mussel-Inspired Fabrication of PDA@PAN Electrospun Nanofibrous Membrane for Oil-in-Water Emulsion Separation. Nanomaterials, 11(12), 3434. https://doi.org/10.3390/nano11123434






