Nanocellulose Hybrid Lignin Complex Reinforces Cellulose to Form a Strong, Water-Stable Lignin–Cellulose Composite Usable as a Plastic Replacement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Raw Materials
2.2. Preparation of the Lignin–Cellulose Composite
2.3. Characterization
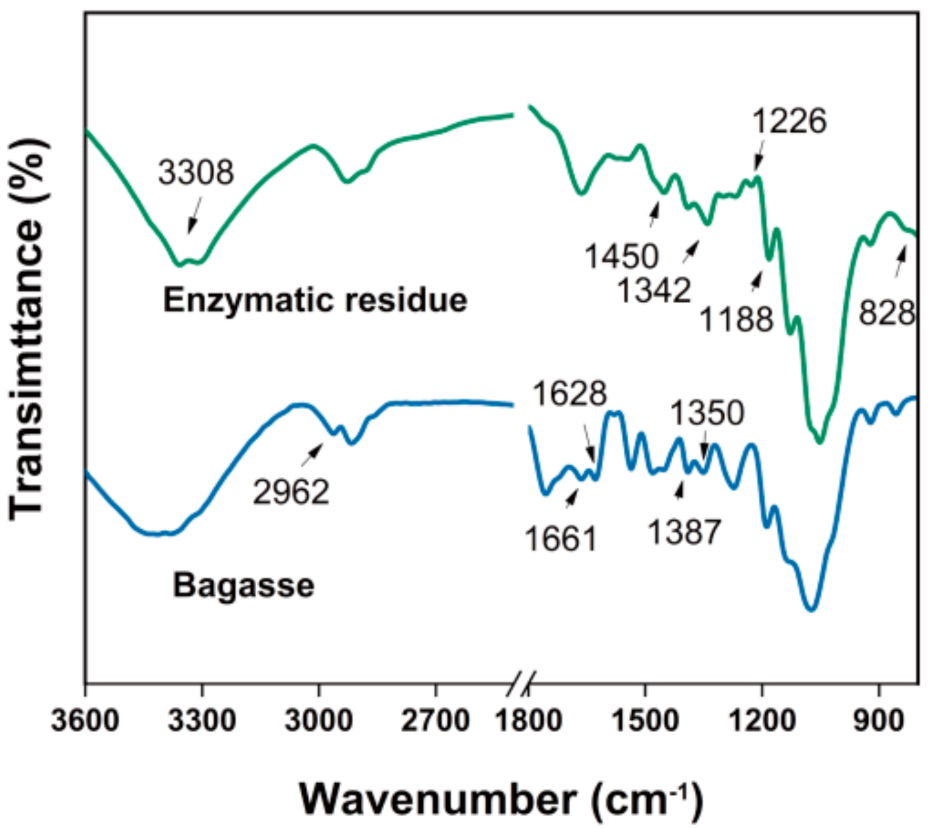
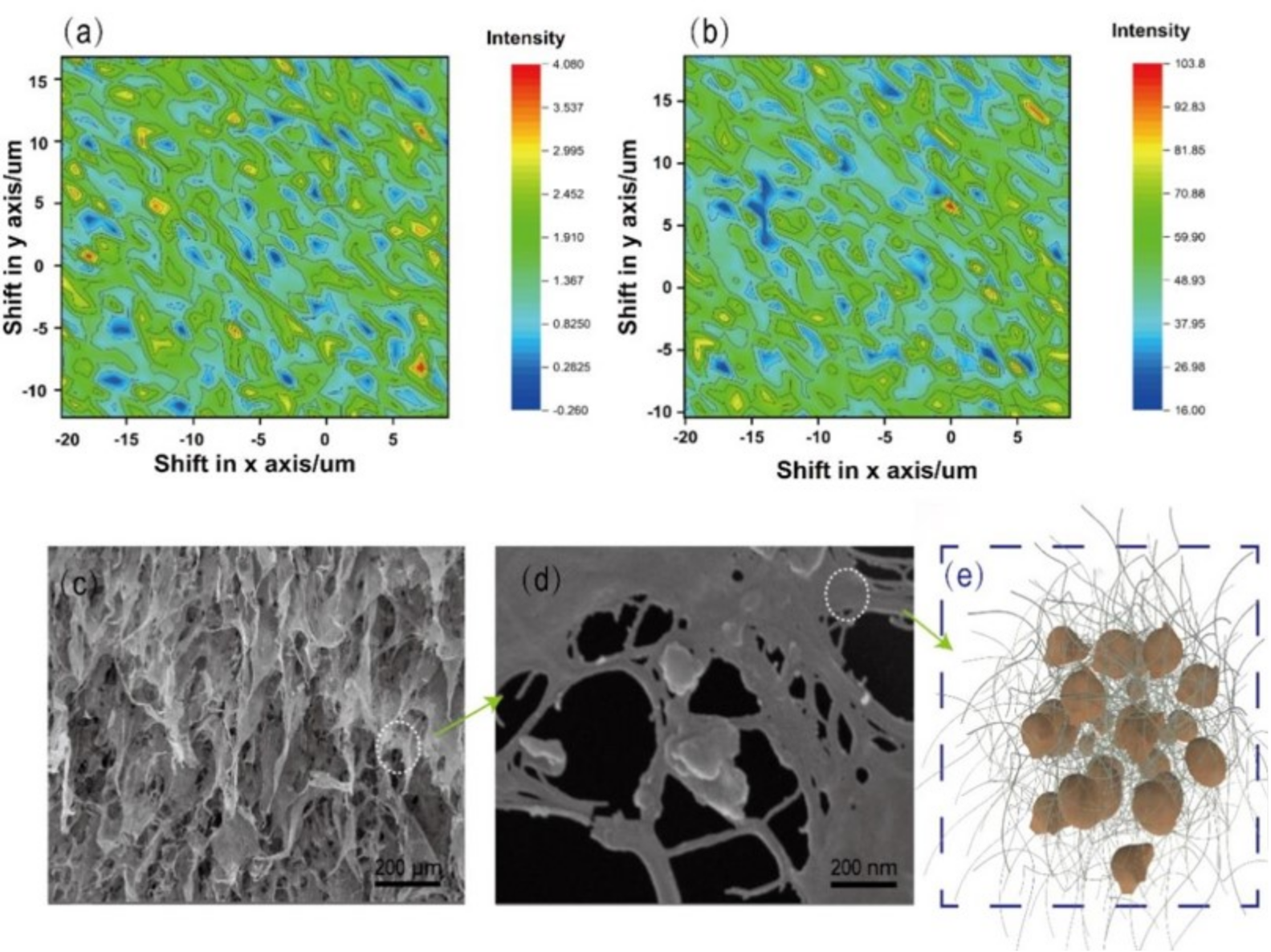
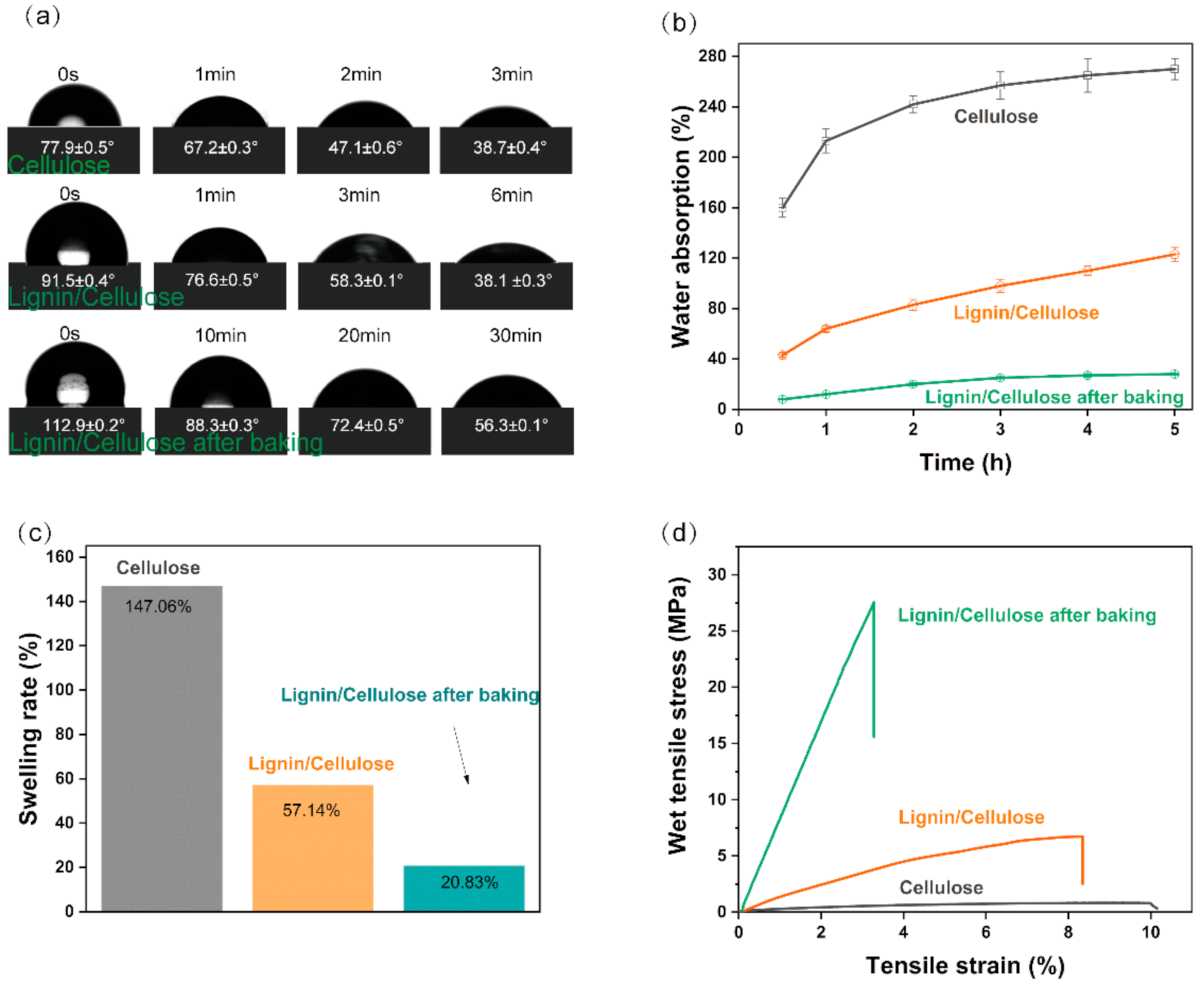
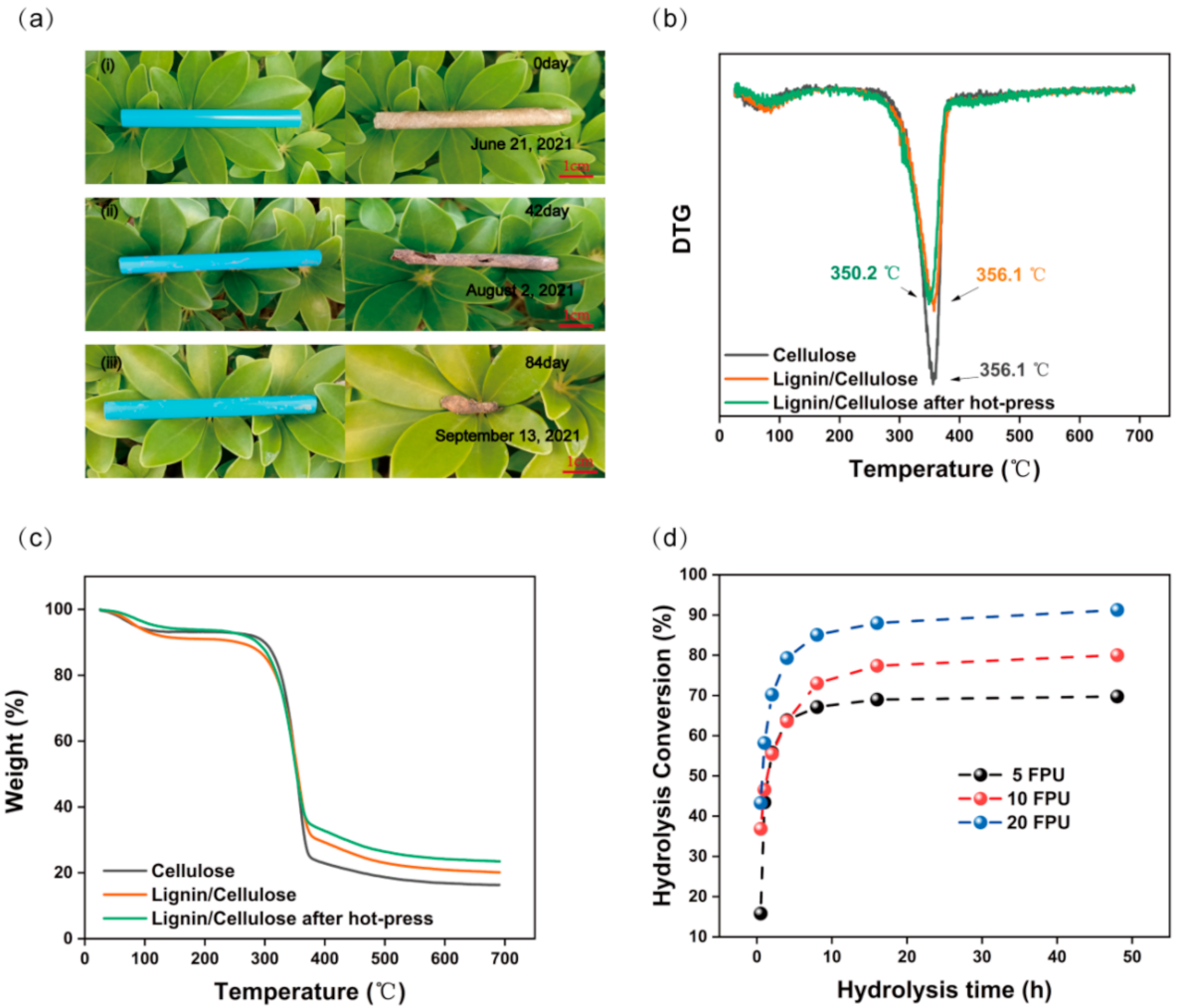
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weinstein, J.E.; Crocker, B.K.; Gray, A.D. From macroplastic to microplastic: Degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ. Toxicol. Chem. 2016, 35, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Vadera, S.; Khan, S. A Critical Analysis of the Rising Global Demand of Plastics and its Adverse Impact on Environmental Sustainability. J. Environ. Pollut. Manag. 2021, 3, 105. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Norton, M. Green chemistry and the plastic pollution challenge: Towards a circular economy. Green Chem. 2020, 22, 6310–6322. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Baniasadi, H.; Polez, R.T.; Kimiaei, E.; Madani, Z.; Rojas, O.J.; Österberg, M.; Seppälä, J. 3D printing and properties of cellulose nanofibrils-reinforced quince seed mucilage bio-inks. Int. J. Biol. Macromol. 2021, 192, 1098–1107. [Google Scholar] [CrossRef]
- Otoni, C.G.; Azeredo, H.M.; Mattos, B.D.; Beaumont, M.; Correa, D.S.; Rojas, O.J. The Food–Materials Nexus: Next Generation Bioplastics and Advanced Materials from Agri-Food Residues. Adv. Mater. 2021, 33, 2102520. [Google Scholar] [CrossRef]
- Muhd Julkapli, N.; Bagheri, S. Nanocellulose as a green and sustainable emerging material in energy applications: A review. Polym. Adv. Technol. 2017, 28, 1583–1594. [Google Scholar] [CrossRef]
- Österberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Chen, C.; Xia, Q.; Liu, Y.; Yao, Y.; Chen, Q.; Hartsfield, M.; Brozena, A.; Tu, K.; Eichhorn, S.J. Lightweight, strong, moldable wood via cell wall engineering as a sustainable structural material. Science 2021, 374, 465–471. [Google Scholar] [CrossRef]
- Thuault, A.; Eve, S.; Blond, D.; Bréard, J.; Gomina, M. Effects of the hygrothermal environment on the mechanical properties of flax fibres. J. Compos. Mater. 2014, 48, 1699–1707. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Bhat, A.; Khan, I.; Usmani, M.A.; Umapathi, R.; Al-Kindy, S.M. Cellulose an ageless renewable green nanomaterial for medical applications: An overview of ionic liquids in extraction, separation and dissolution of cellulose. Int. J. Biol. Macromol. 2019, 129, 750–777. [Google Scholar] [CrossRef]
- Campano, C.; Merayo, N.; Negro, C.; Blanco, Á. Low-fibrillated bacterial cellulose nanofibers as a sustainable additive to enhance recycled paper quality. Int. J. Biol. Macromol. 2018, 114, 1077–1083. [Google Scholar] [CrossRef]
- Tripathi, A.; Ago, M.; Khan, S.A.; Rojas, O.J. Heterogeneous acetylation of plant fibers into micro-and nanocelluloses for the synthesis of highly stretchable, tough, and water-resistant co-continuous filaments via wet-spinning. ACS Appl. Mater. Interfaces 2018, 10, 44776–44786. [Google Scholar] [CrossRef]
- Nypelö, T.; Berke, B.; Spirk, S.; Sirviö, J.A. Periodate oxidation of wood polysaccharides—Modulation of hierarchies. Carbohydr. Polym. 2021, 252, 117105. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, C.; Liang, Z.; He, S.; Kuang, Y.; Song, J.; Mi, R.; Chen, G.; Jiao, M.; Hu, L. Lignin as a wood-inspired binder enabled strong, water stable, and biodegradable paper for plastic replacement. Adv. Funct. Mater. 2020, 30, 1906307. [Google Scholar] [CrossRef]
- Lundahl, M.J.; Klar, V.; Wang, L.; Ago, M.; Rojas, O.J. Spinning of cellulose nanofibrils into filaments: A review. Ind. Eng. Chem. Res. 2017, 56, 8–19. [Google Scholar] [CrossRef]
- Reyes, G.; Lundahl, M.J.; Alejandro-Martín, S.; Arteaga-Pérez, L.E.; Oviedo, C.; King, A.W.; Rojas, O.J. Coaxial spinning of all-cellulose systems for enhanced toughness: Filaments of oxidized nanofibrils sheathed in cellulose II regenerated from a protic ionic liquid. Biomacromolecules 2020, 21, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Ajdary, R.; Trifol, J.; Rojas, O.J.; Seppälä, J. Direct ink writing of aloe vera/cellulose nanofibrils bio-hydrogels. Carbohydr. Polym. 2021, 266, 118114. [Google Scholar] [CrossRef] [PubMed]
- Taajamaa, L.; Laine, J.; Österberg, M.; Rojas, O.; Kontturi, E. In Ultrathin blend films of trimethylsilyl cellulose and cellulose triacetate. In Proceedings of the 2010 TAPPI International Conference on Nanotechnology for the Forest Product Industry, Espoo, Finland, 27–29 September 2010. [Google Scholar]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Nair, S.S.; Yan, N. Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulose 2015, 22, 3137–3150. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Dong, T.; Wang, H.; Si, S. Enabled Cellulose Nanopaper with Outstanding Water Stability and Wet Strength via Activated Residual Lignin as a Reinforcement. Green Chem. 2021, 23, 10062–10070. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Chong, T.Y.; Law, M.C.; San Chan, Y. The potentials of corn waste lignocellulosic fibre as an improved reinforced bioplastic composite. J. Polym. Environ. 2021, 29, 363–381. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Wu, Y.; Bai, F.; Wang, H.; Si, S.; Lu, Y.; Li, X.; Wang, S. Preparation of Cellulose Nanofibers from Bagasse by Phosphoric Acid and Hydrogen Peroxide Enables Fibrillation via a Swelling, Hydrolysis, and Oxidation Cooperative Mechanism. Nanomaterials 2020, 10, 2227. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Si, S.; Wang, Q.; Li, X.; Wang, S. Residual-lignin-endowed molded pulp lunchbox with a sustained wet support strength. Ind. Crop. Prod. 2021, 170, 113756. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In the Protein Protocols Handbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; pp. 17–24. [Google Scholar]
- Zeppa, G.; Conterno, L.; Gerbi, V. Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 2722–2726. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of samples for compositional analysis. Lab. Anal. Proced. 2008, 1617, 65–71. [Google Scholar]
- Zhang, L.; Batchelor, W.; Varanasi, S.; Tsuzuki, T.; Wang, X. Effect of cellulose nanofiber dimensions on sheet forming through filtration. Cellulose 2012, 19, 561–574. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Y.; Zhang, H.; Lv, B.; Qiao, J.; Yu, N.; Zhang, Y.; Di, J.; Li, Q. Mechanical properties of carbon nanotube fibers at extreme temperatures. Nanoscale 2019, 11, 4585–4590. [Google Scholar] [CrossRef] [PubMed]
- Moscou, L.; Lub, S. Practical use of mercury porosimetry in the study of porous solids. Powder Technol. 1981, 29, 45–52. [Google Scholar] [CrossRef]
- Kholodov, V.A.; Milanovskiy, E.Y.; Konstantinov, A.I.; Tyugai, Z.N.; Yaroslavtseva, N.V.; Perminova, I.V. Irreversible sorption of humic substances causes a decrease in wettability of clay surfaces as measured by a sessile drop contact angle method. J. Soils Sediments 2018, 18, 1327–1334. [Google Scholar] [CrossRef]
- Wilson, M.; Carter, M.; Hoff, W. British Standard and RILEM water absorption tests: A critical evaluation. Mater. Struct. 1999, 32, 571–578. [Google Scholar] [CrossRef]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Umeyama, T.; Xu, H.; Ohara, T.; Tsutsui, Y.; Seki, S.; Imahori, H. Photodynamic and Photoelectrochemical Properties of Few-Layered Bismuthene Film on SnO2 Electrode and Its Hybridization with C60. J. Phys. Chem. C 2021, 125, 13954–13962. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. Conversion of liquid hot water, acid and alkali pretreated industrial hemp biomasses to bioethanol. Bioresour. Technol. 2020, 309, 123383. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, L.; Terzopoulou, Z.; Bikiaris, D.N.; Patsiaoura, D.; Chrissafis, K.; Papageorgiou, D.G.; Papageorgiou, G.Z. Synthesis and characterization of in-situ-prepared nanocomposites based on poly (propylene 2,5-furan dicarboxylate) and aluminosilicate clays. Polymers 2018, 10, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloise, A.; Ricchiuti, C.; Giorno, E.; Fuoco, I.; Zumpano, P.; Miriello, D.; Apollaro, C.; Crispini, A.; De Rosa, R.; Punturo, R. Assessment of naturally occurring asbestos in the area of Episcopia (Lucania, Southern Italy). Fibers 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, U.P. An overview of Raman spectroscopy as applied to lignocellulosic materials. Adv. Lignocellul. Charact. 1999, 9, 201–225. [Google Scholar]
- Zhang, C.; Chao, L.; Zhang, Z.; Zhang, L.; Li, Q.; Fan, H.; Zhang, S.; Liu, Q.; Qiao, Y.; Tian, Y. Pyrolysis of cellulose: Evolution of functionalities and structure of bio-char versus temperature. Renew. Sustain. Energy Rev. 2021, 135, 110416. [Google Scholar] [CrossRef]
- Sun, S.-F.; Yang, H.-Y.; Yang, J.; Wang, D.-W.; Shi, Z.-J. Integrated treatment of perennial ryegrass: Structural characterization of hemicelluloses and improvement of enzymatic hydrolysis of cellulose. Carbohydr. Polym. 2021, 254, 117257. [Google Scholar] [CrossRef]
- Jha, S.; Mehta, S.; Chen, Y.; Ma, L.; Renner, P.; Parkinson, D.Y.; Liang, H. Correction to “Design and Synthesis of Lignin-Based Flexible Supercapacitors”. ACS Sustain. Chem. Eng. 2020, 8, 9597–9598. [Google Scholar] [CrossRef]
- Shimizu, F.L.; de Azevedo, G.O.; Coelho, L.F.; Pagnocca, F.C.; Brienzo, M. Minimum Lignin and Xylan Removal to Improve Cellulose Accessibility. BioEnergy Res. 2020, 13, 775–785. [Google Scholar] [CrossRef]
- Merlini, A.; Claumann, C.; Zibetti, A.W.; Coirolo, A.; Rieg, T.; Machado, R.A. Kinetic study of the thermal decomposition of cellulose nanocrystals with different crystal structures and morphologies. Ind. Eng. Chem. Res. 2020, 59, 13428–13439. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, N.; Li, J.; Sui, G. Durable Photoetching Magnetic Biomass-Based Hydrogel with High Performance. Adv. Mater. Interfaces 2021, 8, 2001966. [Google Scholar] [CrossRef]
- Levanic, J.; Šenk, V.P.; Nadrah, P.; Poljanšek, I.; Oven, P.; Haapala, A. Analyzing TEMPO-oxidized cellulose fiber morphology: New insights into optimization of the oxidation process and nanocellulose dispersion quality. ACS Sustain. Chem. Eng. 2020, 8, 17752–17762. [Google Scholar] [CrossRef]
- Irvine, G. The significance of the glass transition of lignin in thermomechanical pulping. Wood Sci. Technol. 1985, 19, 139–149. [Google Scholar] [CrossRef]
- Meshitsuka, G.; Isogai, A. Chemical structures of cellulose, hemicelluloses, and lignin. In Chemical Modification of Lignocellulosic Materials; Routledge: New York, NY, USA, 2017; pp. 11–33. [Google Scholar]
- Xie, Y.; Zhang, K.; Cui, S.; Liu, Y. A Review on the Structure and Biodegradation of Cellulose-Lignin Complexes. Pap. Biomater. 2020, 5, 44–50. [Google Scholar]
- Albornoz-Palma, G.; Ching, D.; Valerio, O.; Mendonça, R.T.; Pereira, M. Effect of lignin and hemicellulose on the properties of lignocellulose nanofibril suspensions. Cellulose 2020, 27, 10631–10647. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Nair, S.S.; Chen, H.; Yan, N.; Farnood, R.; Li, F.-Y. Thermally stable, enhanced water barrier, high strength starch bio-composite reinforced with lignin containing cellulose nanofibrils. Carbohydr. Polym. 2020, 230, 115626. [Google Scholar] [CrossRef]
- Wang, X.; Xia, Q.; Jing, S.; Li, C.; Chen, Q.; Chen, B.; Pang, Z.; Jiang, B.; Gan, W.; Chen, G. Strong, Hydrostable, and Degradable Straws Based on Cellulose-Lignin Reinforced Composites. Small 2021, 17, 2008011. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Samper, M.D.; Bertomeu, D.; Arrieta, M.P.; Ferri, J.M.; López-Martínez, J. Interference of Biodegradable Plastics in the Polypropylene Recycling Process. Materials 2018, 11, 1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, F.; Dong, T.; Chen, W.; Wang, J.; Li, X. Nanocellulose Hybrid Lignin Complex Reinforces Cellulose to Form a Strong, Water-Stable Lignin–Cellulose Composite Usable as a Plastic Replacement. Nanomaterials 2021, 11, 3426. https://doi.org/10.3390/nano11123426
Bai F, Dong T, Chen W, Wang J, Li X. Nanocellulose Hybrid Lignin Complex Reinforces Cellulose to Form a Strong, Water-Stable Lignin–Cellulose Composite Usable as a Plastic Replacement. Nanomaterials. 2021; 11(12):3426. https://doi.org/10.3390/nano11123426
Chicago/Turabian StyleBai, Feitian, Tengteng Dong, Wei Chen, Jinlong Wang, and Xusheng Li. 2021. "Nanocellulose Hybrid Lignin Complex Reinforces Cellulose to Form a Strong, Water-Stable Lignin–Cellulose Composite Usable as a Plastic Replacement" Nanomaterials 11, no. 12: 3426. https://doi.org/10.3390/nano11123426
APA StyleBai, F., Dong, T., Chen, W., Wang, J., & Li, X. (2021). Nanocellulose Hybrid Lignin Complex Reinforces Cellulose to Form a Strong, Water-Stable Lignin–Cellulose Composite Usable as a Plastic Replacement. Nanomaterials, 11(12), 3426. https://doi.org/10.3390/nano11123426






