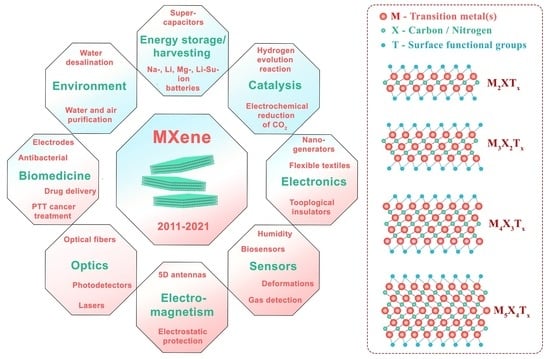
MXenes—A New Class of Two-Dimensional Materials: Structure, Properties and Potential Applications
Abstract
:1. Introduction
2. Methods to Synthesize MXenes
2.1. Materials for Synthesis of MXenes
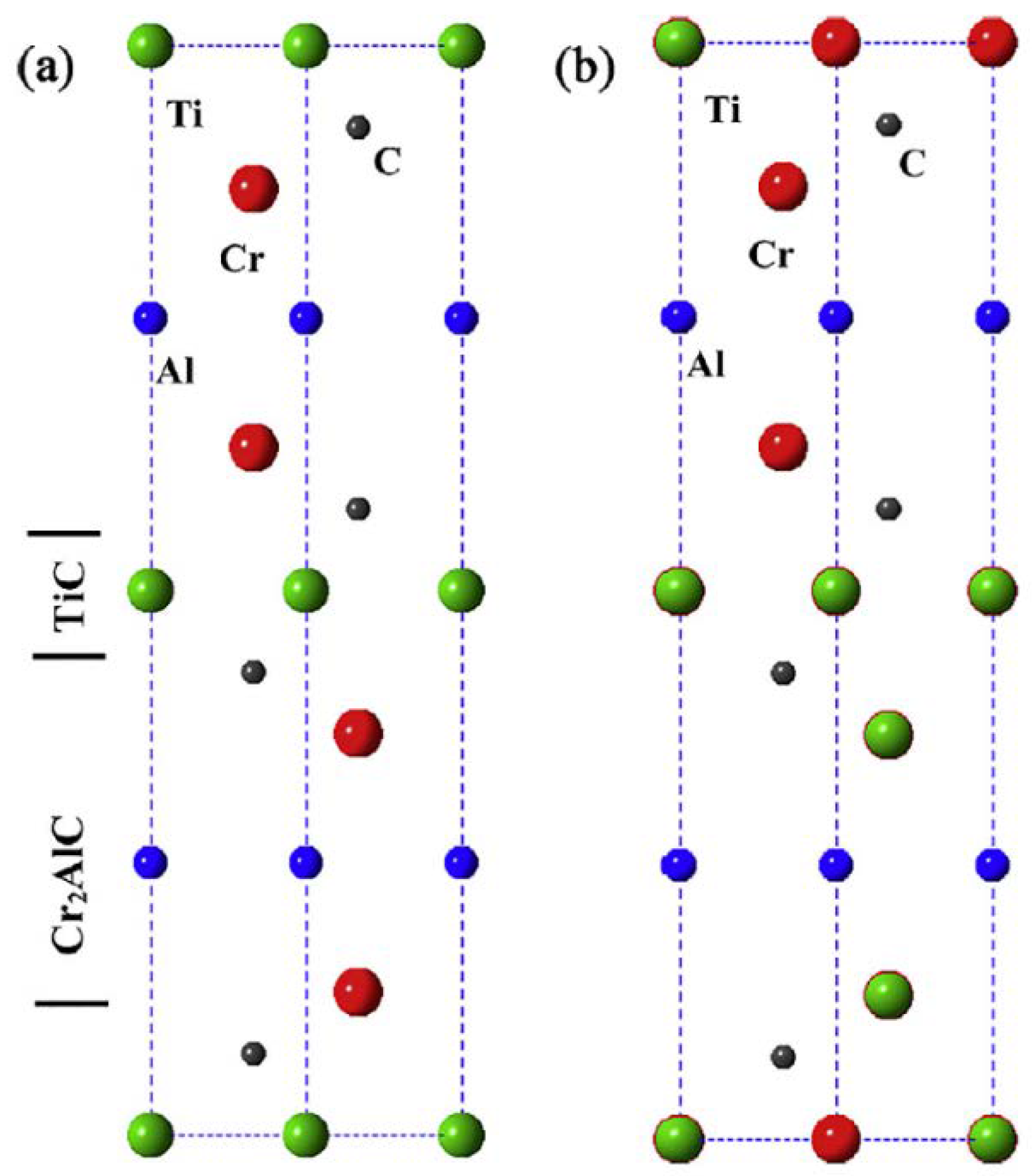
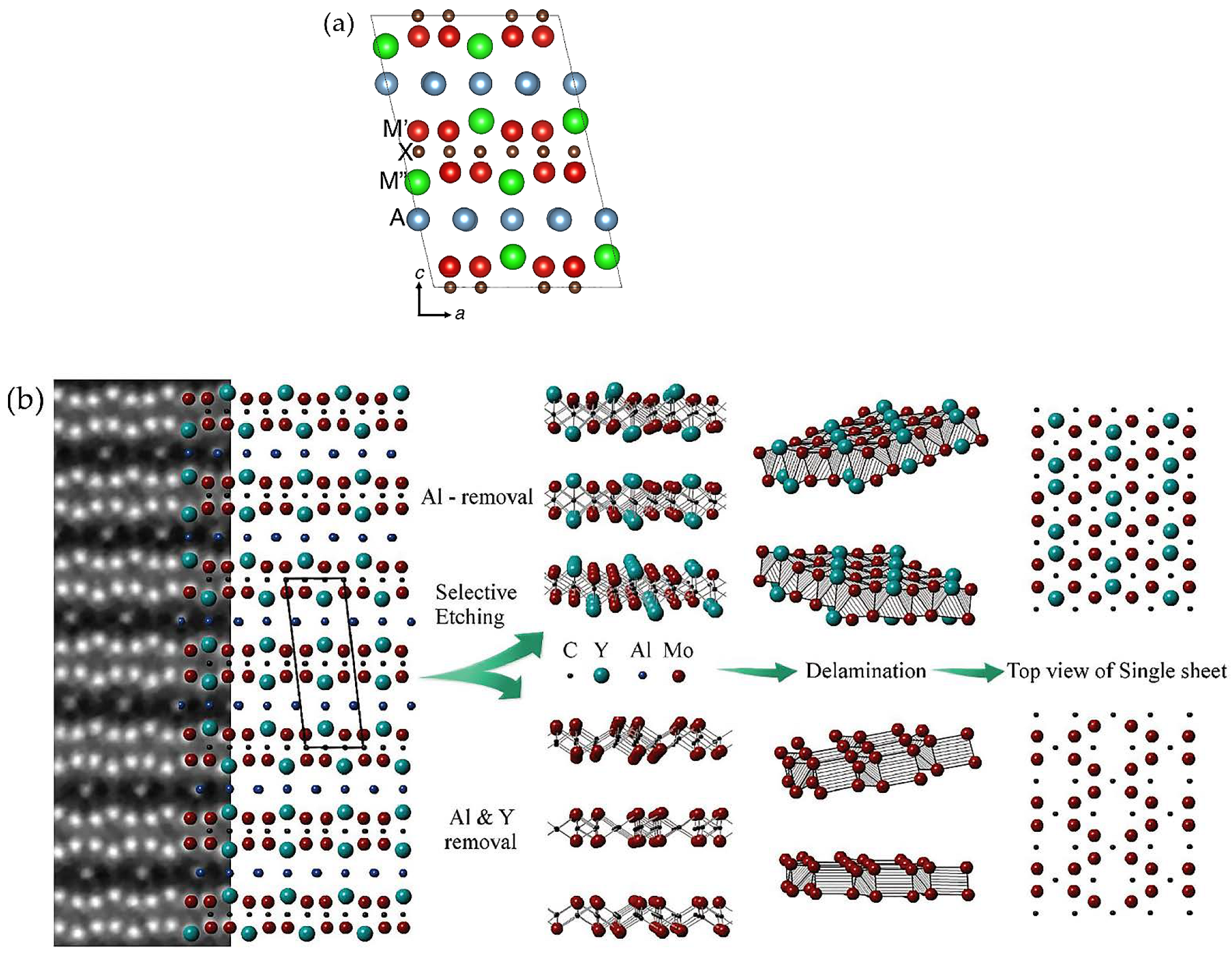
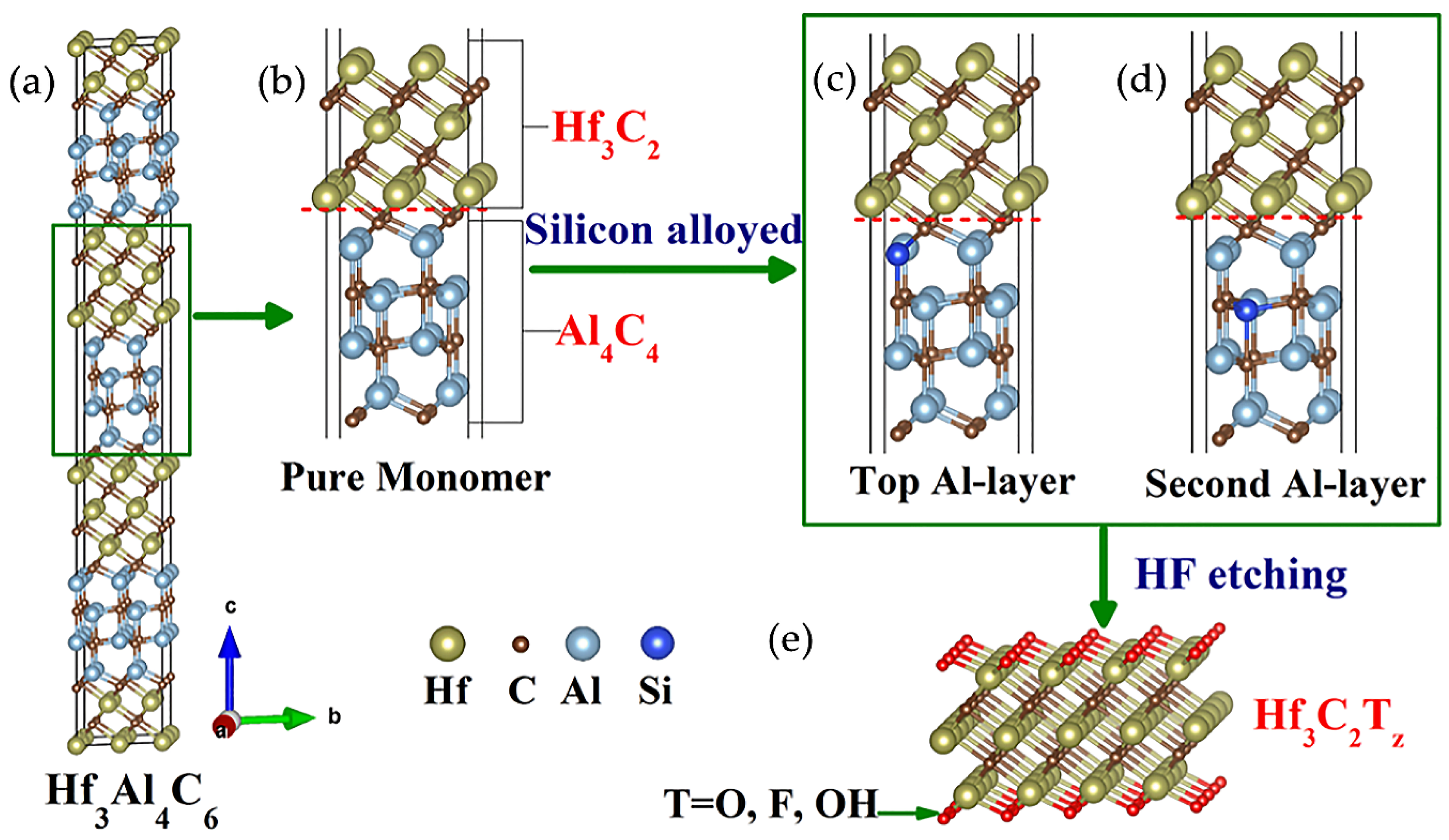
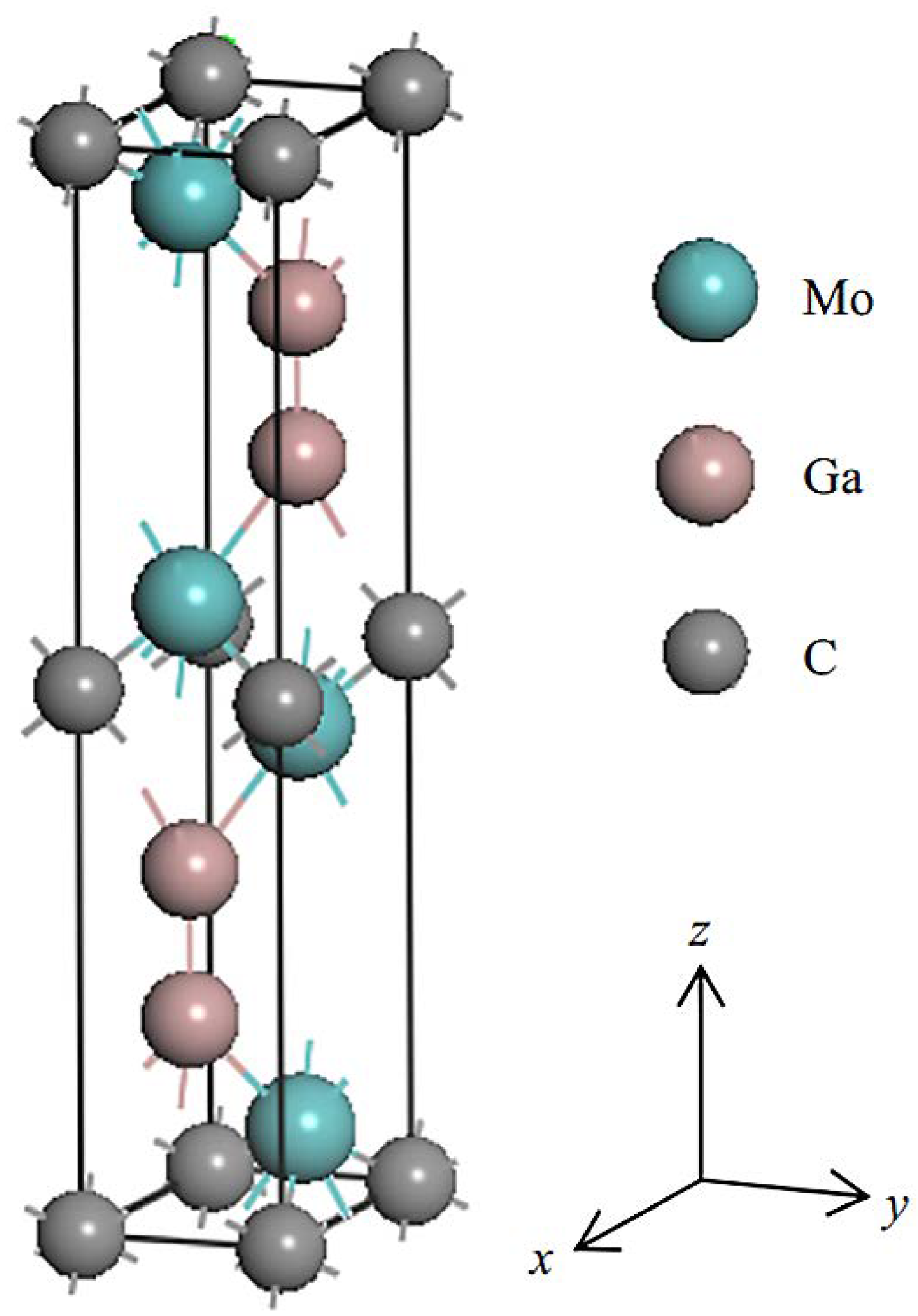
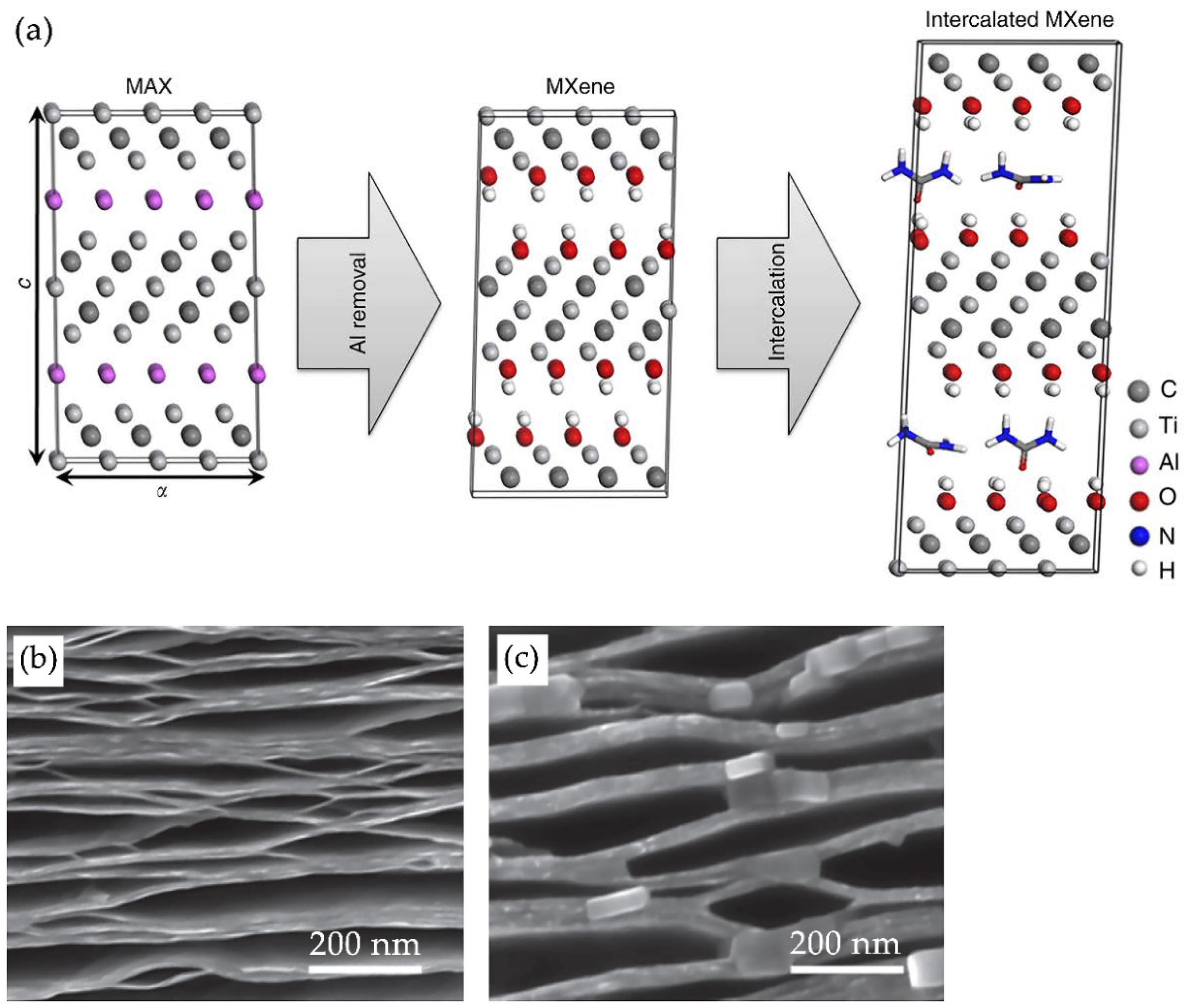
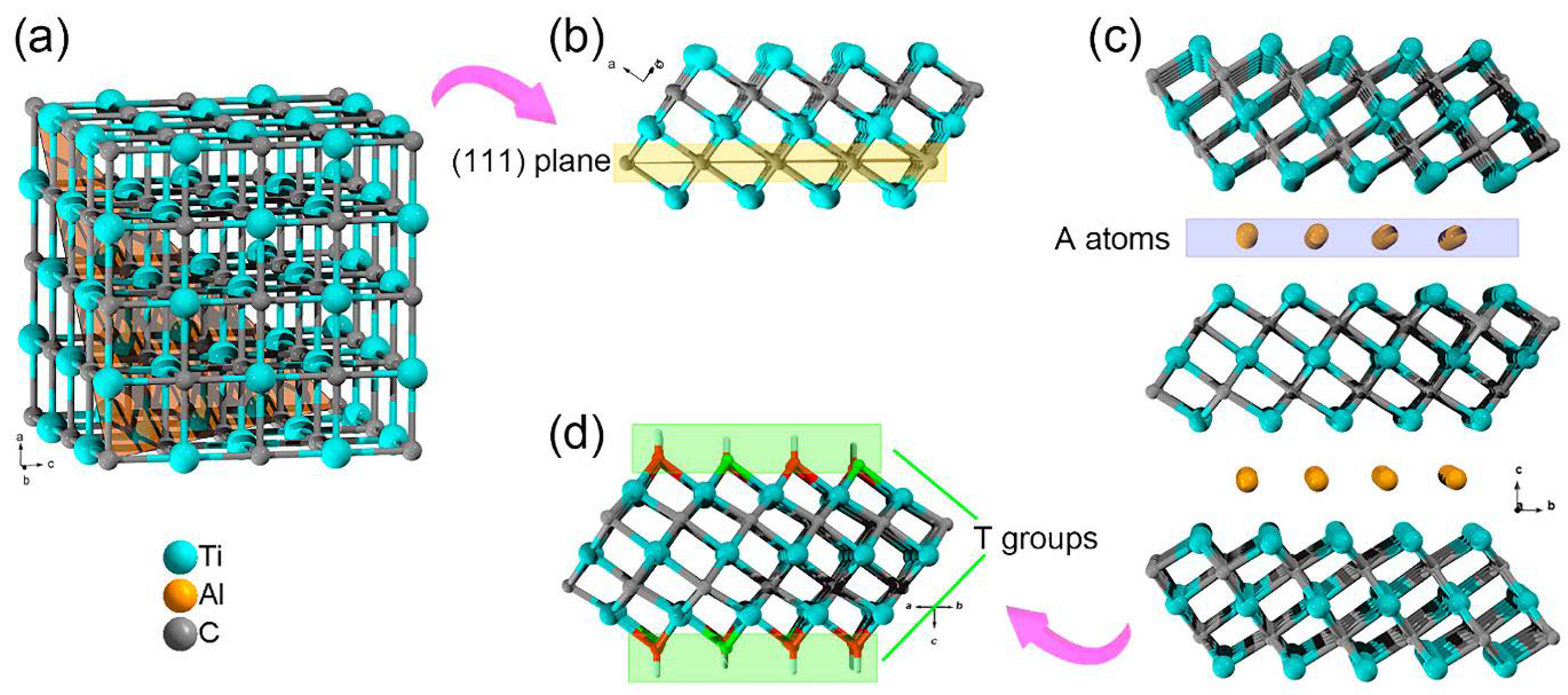
2.1.1. MAX Phases
2.1.2. Other Materials as Precursors of Mxenes
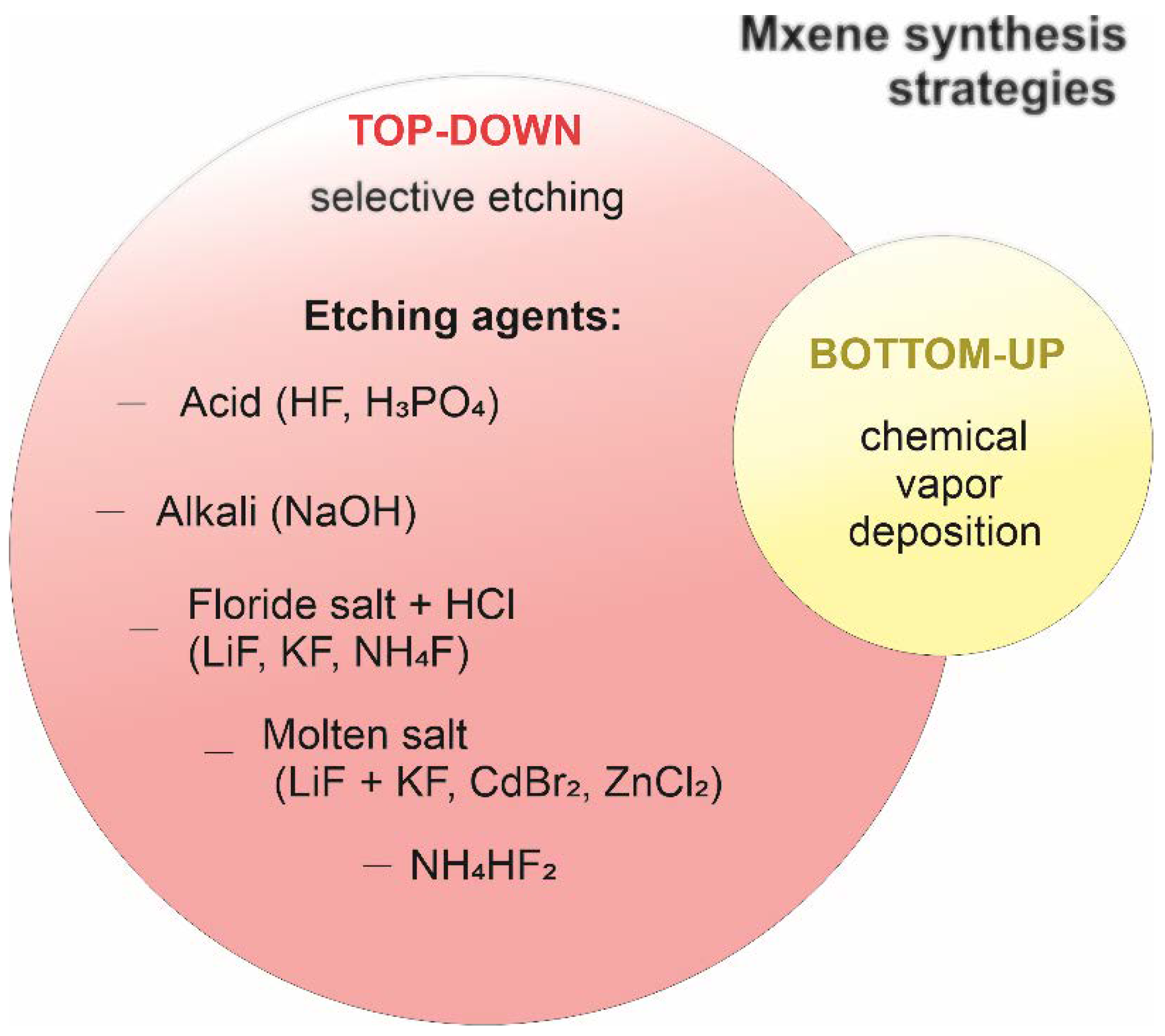
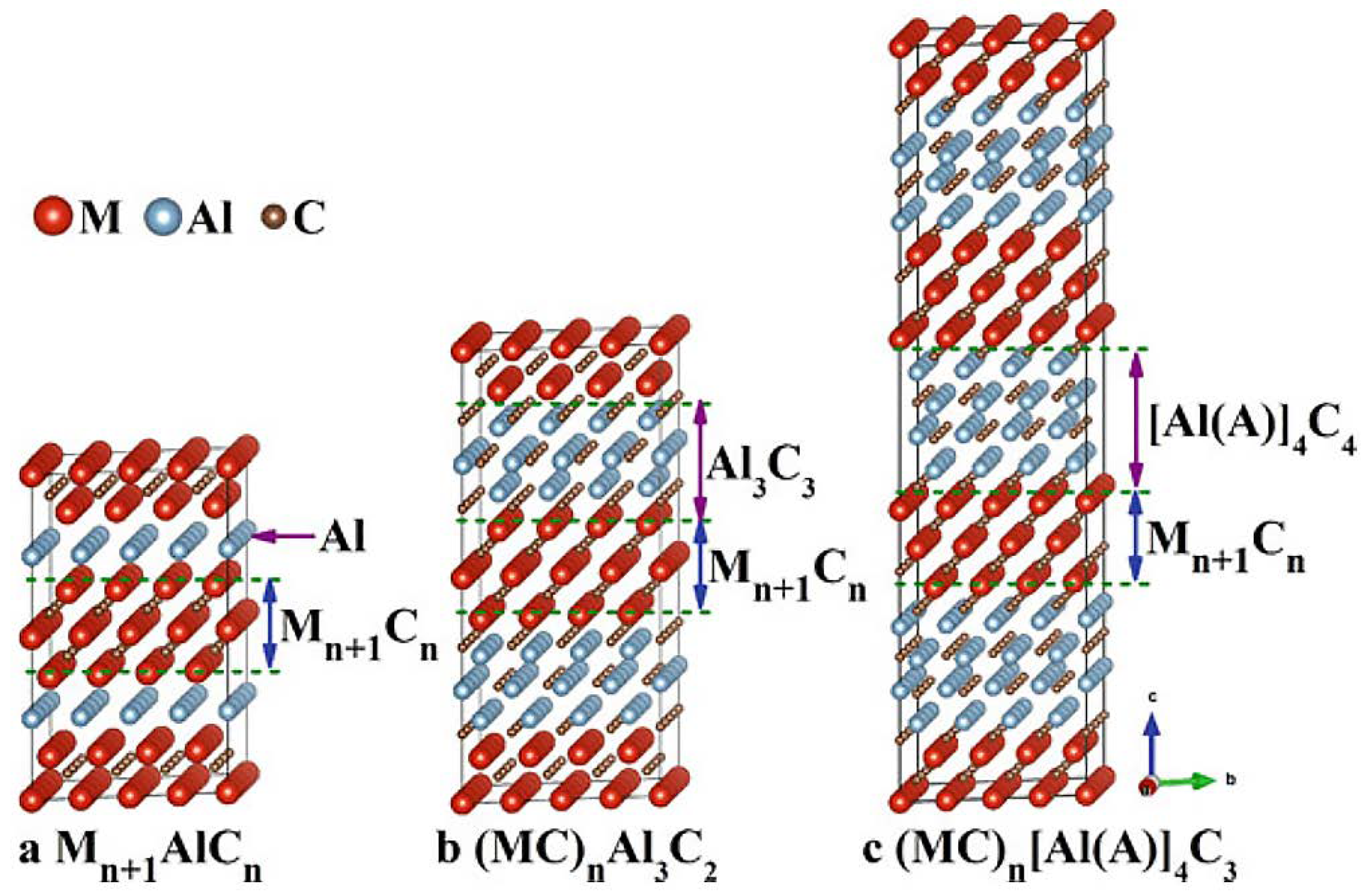
2.2. Strategies of MXene Synthesis
2.2.1. Top-Down Approach
Acid Etching
Etching with Fluoride Salts
Etching with Amonium Hydrofluoride
Reaction with Alkali
Reaction with Molten Fluoride Salts
In-Situ Electrochemical Synthesis
2.2.2. Bottom-Up Approach
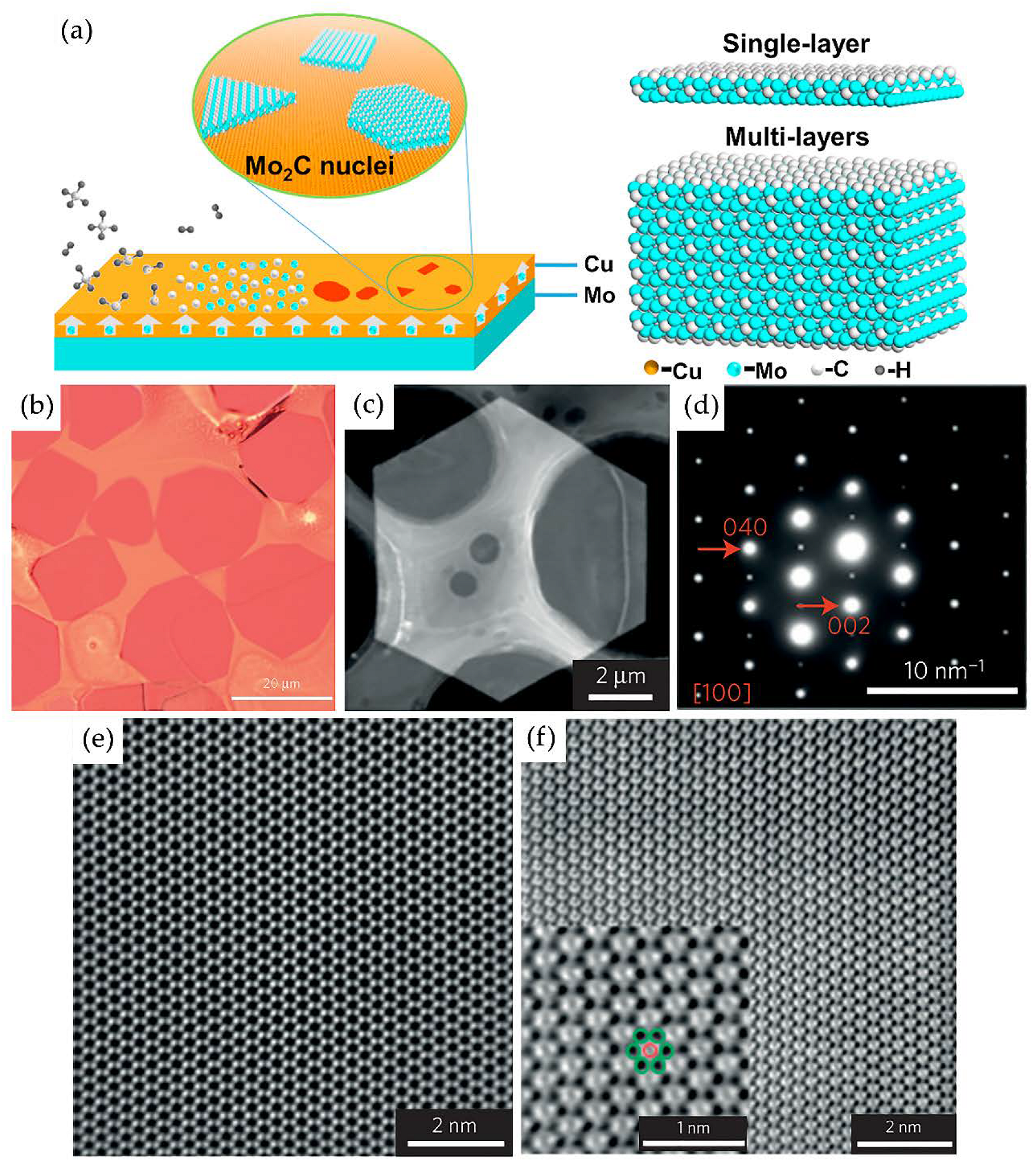
Chemical Vapor Deposition
2.3. Delamination of MXenes Using Intercalating Agents
3. Structure and Properties
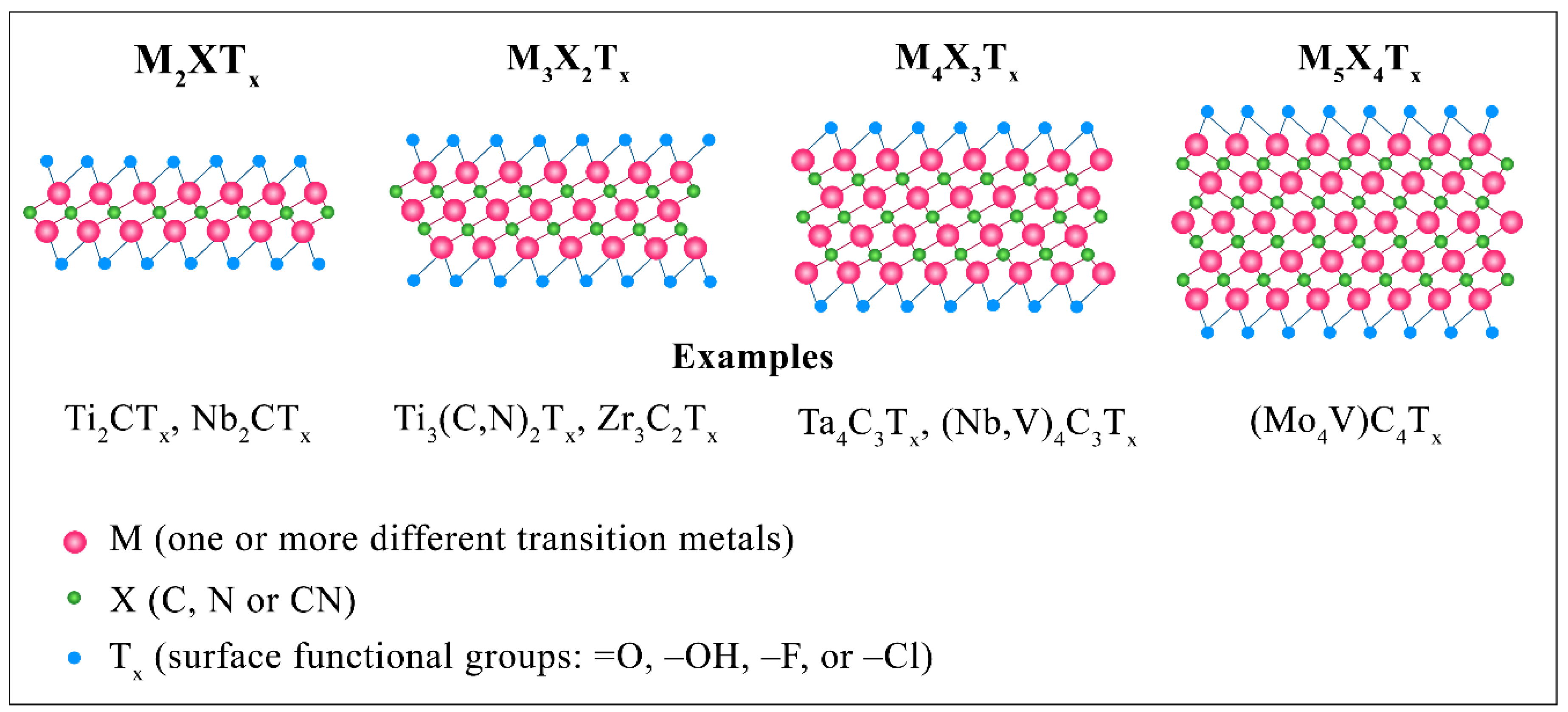
3.1. Structure
3.2. Properties
3.3. Thermal Stability
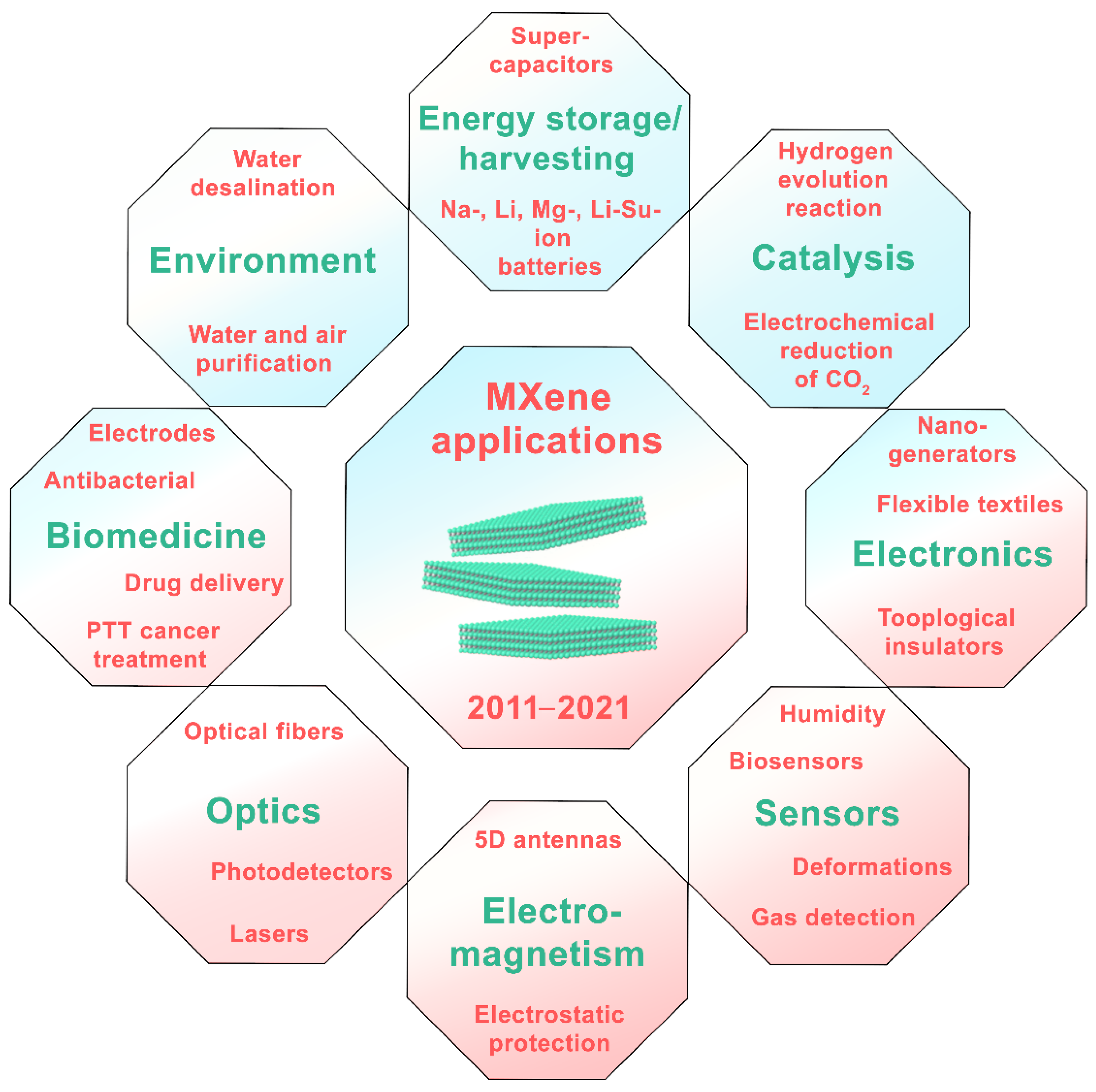
4. MXene Applications
4.1. Biomedicine
4.1.1. Photo-Thermal Therapy
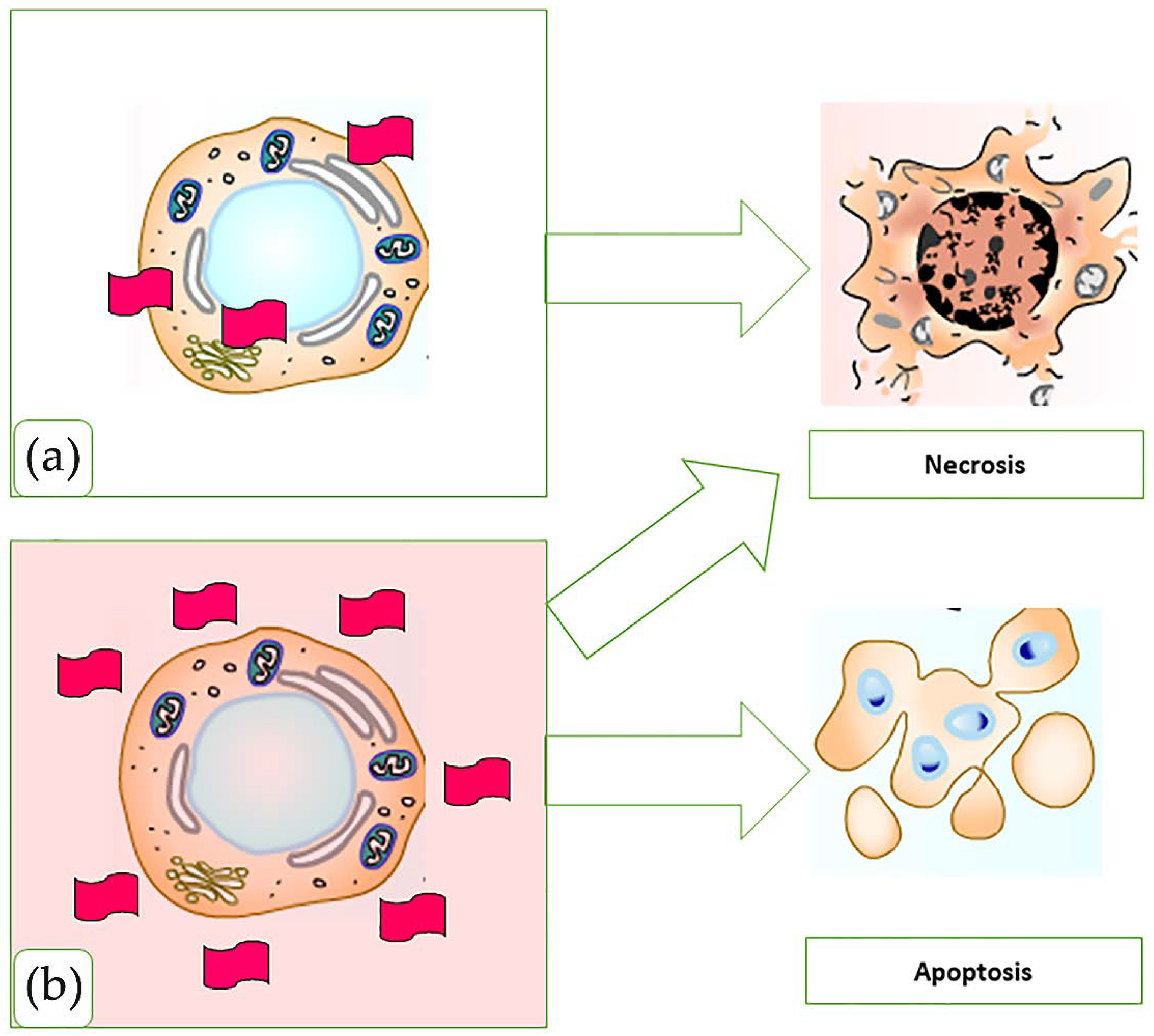
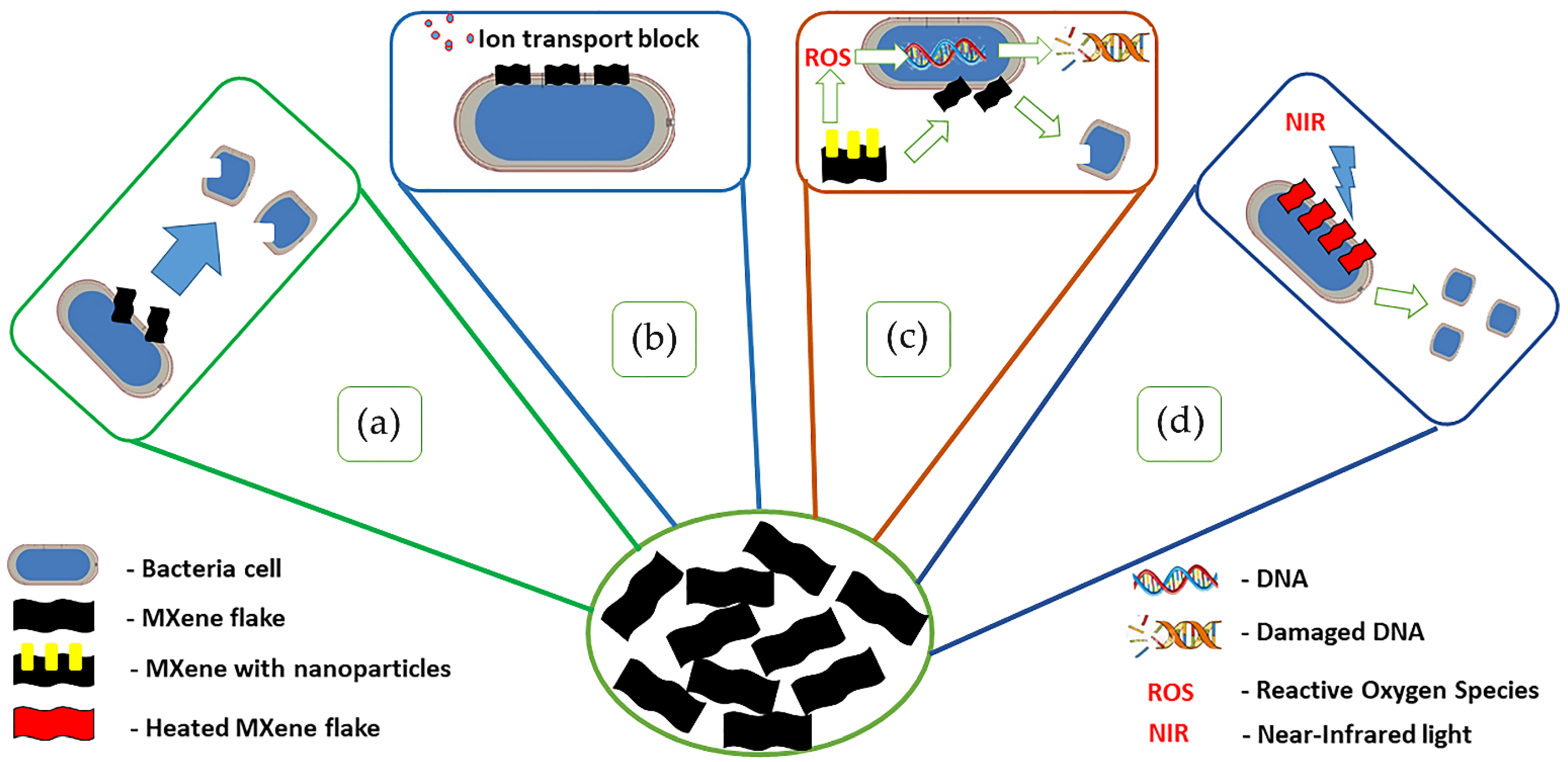
4.1.2. Antibacterial Activity
4.2. Ecological/Environmental Applications
4.2.1. Photothermal Conversion
4.2.2. Adsorption
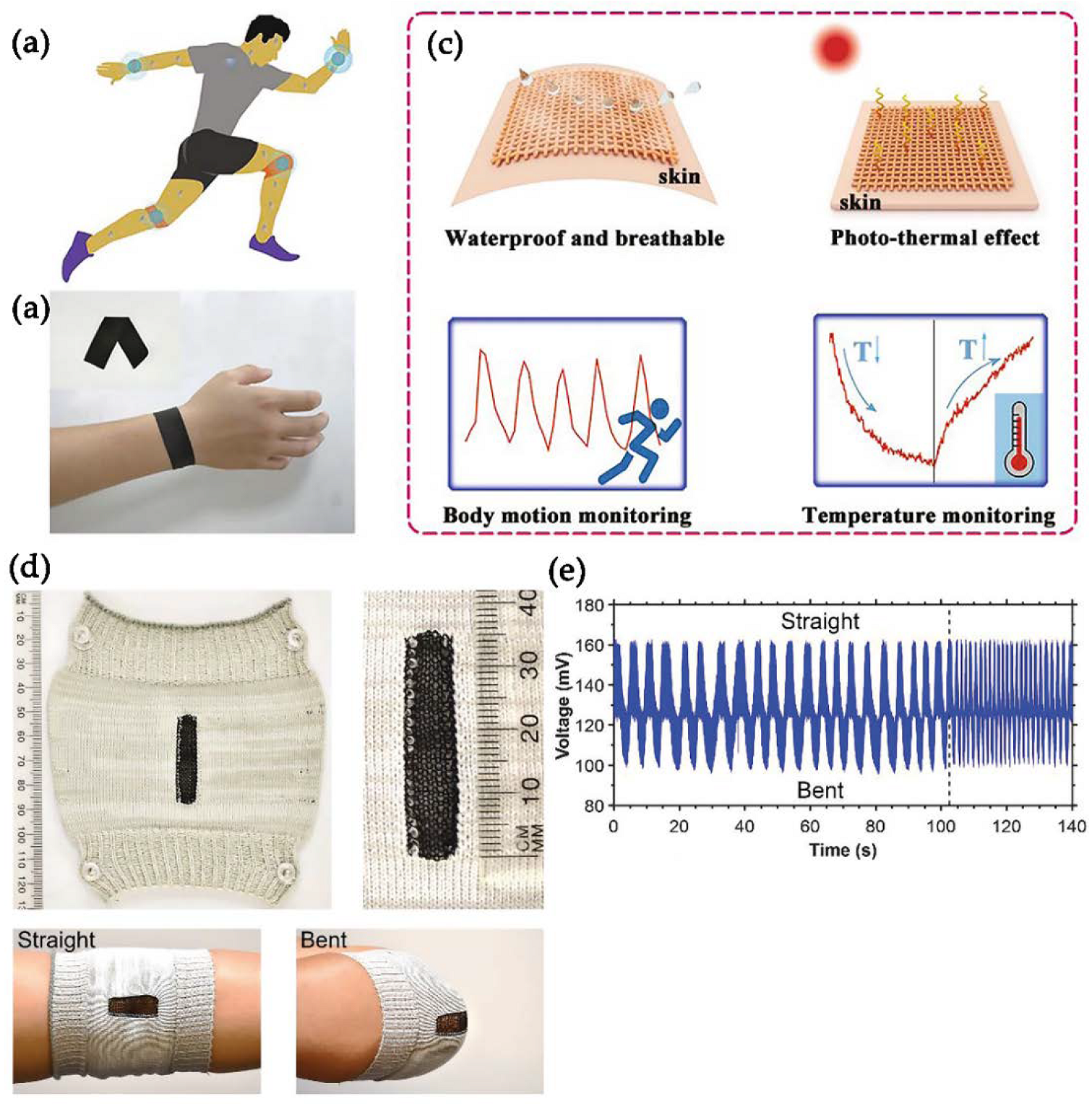
4.3. Multifunctional MXene-Based Smart Textiles
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boehm, H.P.; Clauss, A.; Fischer, G.O.; Hofmann, U. Das adsorptionsverhalten sehr dunner kohlenstoff-folien. Z. Anorg. Allg. Chem. 1962, 316, 119–127. [Google Scholar] [CrossRef]
- Wallace, P.R. The band theory of graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Zhen, Z.; Zhu, H. Structure and Properties of Graphene. In Graphene; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–12. [Google Scholar]
- Xu, Z. Fundamental Properties of Praphene. In Graphene; Elsevier: Amsterdam, The Netherlands, 2018; pp. 73–102. [Google Scholar]
- Spencer, M.J.S.; Morishita, T. (Eds.) Silicene; Springer Series in Materials Science; Springer International Publishing: Cham, Germany, 2016; Volume 235, ISBN 978-3-319-28342-5. [Google Scholar]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.; Anderson, G.; Zhao, R.; Alruqi, A.; Mroczkowska, J.E.; Sumanasekera, G.; Jasinski, J.B. Recent advances in synthesis, properties, and applications of phosphorene. NPJ 2D Mater. Appl. 2017, 1, 5. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Glavin, N.R.; Robinson, J.A. 2D boron nitride: Synthesis and applications. In Semiconductors and Semimetals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 101–147. [Google Scholar]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Ling, Z.; Ren, C.E.; Zhao, M.-Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Gholamirad, F.; Yu, M.; Park, C.M.; Jang, A.; Jang, M.; Taheri-Qazvini, N.; Yoon, Y. Enhanced adsorption performance for selected pharmaceutical compounds by sonicated Ti3C2TX MXene. Chem. Eng. J. 2021, 406, 126789. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.-Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.-L.; Barsoum, M.W.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 2, 17105. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Man Hong, S.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, R.; Feng, Y.; Ouyang, H.; Zhou, N.; Zhang, X.; Wei, Y. Recent development and prospects of surface modification and biomedical applications of MXenes. Nanoscale 2020, 12, 1325–1338. [Google Scholar] [CrossRef]
- Wu, W.; Fang, H.; Ma, H.; Wu, L.; Zhang, W.; Wang, H. Boosting transport kinetics of ions and electrons simultaneously by Ti3C2Tx (MXene) addition for enhanced electrochromic performance. Nano-Micro Lett. 2021, 13, s40820. [Google Scholar]
- Jimmy, J.; Kandasubramanian, B. Mxene functionalized polymer composites: Synthesis and applications. Eur. Polym. J. 2020, 122, 109367. [Google Scholar] [CrossRef]
- Xiao, Z.; Ruan, S.; Kong, L.B.; Que, W.; Zhou, K.; Liu, Y.; Zhang, T. Synthesis and Properties of MXenes. In MXenes and MXenes-Based Composites; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 5–93. [Google Scholar]
- Zhang, Z.; Cai, Z.; Zhang, Y.; Peng, Y.; Wang, Z.; Xia, L.; Ma, S.; Yin, Z.; Wang, R.; Cao, Y.; et al. The recent progress of MXene-Based microwave absorption materials. Carbon N. Y. 2021, 174, 484–499. [Google Scholar] [CrossRef]
- Garg, R.; Agarwal, A.; Agarwal, M. A Review on MXene for energy storage application: Effect of interlayer distance. Mater. Res. Express 2020, 7, 022001. [Google Scholar] [CrossRef]
- Nowotny, H. Structural chemistry of refractory metallic compounds. Angew. Chem. Int. Ed. Eng. 1970, 9, 173–174. [Google Scholar] [CrossRef]
- Jeitschko, W.; Nowotny, H.; Benesovsky, F. Carbides of formula T2MC. J. Less Common Met. 1964, 7, 133–138. [Google Scholar] [CrossRef]
- Barsoum, M.W.; El-Raghy, T. Synthesis and characterization of a remarkable ceramic: Ti3SiC2. J. Am. Ceram. Soc. 1996, 79, 1953–1956. [Google Scholar] [CrossRef]
- Barsoum, M.; El-Raghy, T. The MAX phases: Unique new carbide and nitride materials. Am. Sci. 2001, 89, 334. [Google Scholar] [CrossRef]
- Hu, C.; Lai, C.-C.; Tao, Q.; Lu, J.; Halim, J.; Sun, L.; Zhang, J.; Yang, J.; Anasori, B.; Wang, J.; et al. Mo2Ga2C: A new ternary nanolaminated carbide. Chem. Commun. 2015, 51, 6560–6563. [Google Scholar] [CrossRef] [Green Version]
- Meshkian, R.; Tao, Q.; Dahlqvist, M.; Lu, J.; Hultman, L.; Rosen, J. Theoretical stability and materials synthesis of a chemically ordered MAX phase, Mo2ScAlC2, and its two-dimensional derivate Mo2ScC2 MXene. Acta Mater. 2017, 125, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Persson, I.; El Ghazaly, A.; Tao, Q.; Halim, J.; Kota, S.; Darakchieva, V.; Palisaitis, J.; Barsoum, M.W.; Rosen, J.; Persson, P.O.Å. Tailoring structure, composition, and energy storage properties of MXenes from selective etching of in-plane, chemically ordered MAX phases. Small 2018, 14, 1703676. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, F.; Zhang, J. New MAX-phase compounds in the V–Cr–Al–C system. J. Am. Ceram. Soc. 2008, 91, 1357–1360. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, L.; Sun, L.; Qian, Y.; Wang, J.; Li, M. (Cr2/3Ti1/3)3AlC2 and (Cr5/8Ti3/8)4AlC3: New MAX-phase compounds in Ti-Cr-Al-C system. J. Am. Ceram. Soc. 2014, 97, 67–69. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, E.; Wang, J.; Qian, Y.; Xiang, H.; Li, X.; Jin, Q.; Sun, G.; Chen, X.; Wang, J.; et al. Crystal structure and formation mechanism of (Cr2/3Ti1/3)3AlC2 MAX phase. Acta Mater. 2014, 73, 186–193. [Google Scholar] [CrossRef]
- Wu, J.; Lu, P.; Dai, J.; Zheng, C.; Zhang, T.; Yu, W.W.; Zhang, Y. High performance humidity sensing property of Ti3C2Tx MXene-derived Ti3C2Tx/K2Ti4O9 composites. Sens. Actuators B Chem. 2021, 326. [Google Scholar] [CrossRef]
- Anasori, B.; Dahlqvist, M.; Halim, J.; Moon, E.J.; Lu, J.; Hosler, B.C.; Caspi, E.N.; May, S.J.; Hultman, L.; Eklund, P.; et al. Experimental and theoretical characterization of ordered MAX phases Mo2TiAlC2 and Mo2Ti2AlC3. J. Appl. Phys. 2015, 118, 094304. [Google Scholar] [CrossRef] [Green Version]
- Caspi, E.N.; Chartier, P.; Porcher, F.; Damay, F.; Cabioc’h, T. Ordering of (Cr,V) Layers in nanolamellar (Cr0.5V0.5)n+1AlCn compounds. Mater. Res. Lett. 2015, 3, 100–106. [Google Scholar] [CrossRef]
- Rosen, J.; Dahlqvist, M.; Tao, Q.; Hultman, L. In- and out-of-plane ordered MAX Phases and their mxene derivatives. In 2D Metal Carbides and Nitrides (MXenes); Springer International Publishing: Cham, Germany, 2019; pp. 37–52. [Google Scholar]
- Mei, J.; Ayoko, G.A.; Hu, C.; Bell, J.M.; Sun, Z. Two-dimensional fluorine-free mesoporous Mo2C MXene via UV-induced selective etching of Mo2Ga2C for energy storage. Sustain. Mater. Technol. 2020, 25, e00156. [Google Scholar] [CrossRef]
- Mendes, R.G.; Ta, H.Q.; Yang, X.; Li, W.; Bachmatiuk, A.; Choi, J.; Gemming, T.; Anasori, B.; Lijun, L.; Fu, L.; et al. In Situ N-doped graphene and Mo nanoribbon formation from Mo2Ti2C3 MXene monolayers. Small 2020, 16, 1907115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anasori, B.; Shi, C.; Moon, E.J.; Xie, Y.; Voigt, C.A.; Kent, P.R.C.; May, S.J.; Billinge, S.J.L.; Barsoum, M.W.; Gogotsi, Y. Control of electronic properties of 2D carbides (MXenes) by manipulating their transition metal layers. Nanoscale Horiz. 2016, 1, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O.Å.; et al. Two-dimensional Mo1.33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering. Nat. Commun. 2017, 8, 14949. [Google Scholar] [CrossRef]
- Dahlqvist, M.; Lu, J.; Meshkian, R.; Tao, Q.; Hultman, L.; Rosen, J. Prediction and synthesis of a family of atomic laminate phases with Kagomé-like and in-plane chemical ordering. Sci. Adv. 2017, 3, e1700642. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, M.; Wang, V.; Sevik, C.; Ranjbar, A.; Arai, M.; Yunoki, S. Electronic structures of iMAX phases and their two-dimensional derivatives: A family of piezoelectric materials. Phys. Rev. Mater. 2018, 2, 074002. [Google Scholar] [CrossRef] [Green Version]
- Meshkian, R.; Dahlqvist, M.; Lu, J.; Wickman, B.; Halim, J.; Thörnberg, J.; Tao, Q.; Li, S.; Intikhab, S.; Snyder, J.; et al. W-based atomic laminates and their 2D derivative W1.33C MXene with vacancy ordering. Adv. Mater. 2018, 30, 1706409. [Google Scholar] [CrossRef]
- Arróyave, R.; Talapatra, A.; Duong, T.; Son, W.; Radovic, M. Out-of-plane ordering in quaternary MAX alloys: An alloy theoretic perspective. Mater. Res. Lett. 2018, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Petruhins, A.; Lu, J.; Hultman, L.; Rosen, J. Synthesis of atomically layered and chemically ordered rare-earth (RE) i -MAX phases; (Mo2/3RE1/3)2GaC with RE = Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu. Mater. Res. Lett. 2019, 7, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Dahlqvist, M.; Rosen, J. Predictive theoretical screening of phase stability for chemical order and disorder in quaternary 312 and 413 MAX phases. Nanoscale 2020, 12, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zha, X.; Chen, F.Y.; Ye, Q.; Eklund, P.; Du, S.; Huang, Q. A Two-dimensional zirconium carbide by selective etching of Al3C3 from nanolaminated Zr3Al3C5. Angew. Chem. Int. Ed. 2016, 55, 5008–5013. [Google Scholar] [CrossRef] [Green Version]
- Zha, X.-H.; Zhou, J.; Eklund, P.; Bai, X.; Du, S.; Huang, Q. Non-MAX Phase Precursors for MXenes. In 2D Metal Carbides and Nitrides (MXenes); Springer International Publishing: Cham, Germnay, 2019; pp. 53–68. [Google Scholar]
- Zhou, Y.-C.; He, L.-F.; Lin, Z.-J.; Wang, J.-Y. Synthesis and structure–property relationships of a new family of layered carbides in Zr-Al(Si)-C and Hf-Al(Si)-C systems. J. Eur. Ceram. Soc. 2013, 33, 2831–2865. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Zhou, X.; Chen, F.; Gao, G.; Wang, S.; Shen, C.; Chen, T.; Zhi, C.; Eklund, P.; et al. Synthesis and electrochemical properties of two-dimensional hafnium carbide. ACS Nano 2017, 11, 3841–3850. [Google Scholar] [CrossRef] [Green Version]
- Hadi, M.A. New ternary nanolaminated carbide Mo2Ga2C: A first-principles comparison with the MAX phase counterpart Mo2GaC. Comput. Mater. Sci. 2016, 117, 422–427. [Google Scholar] [CrossRef]
- Meshkian, R.; Näslund, L.-Å.; Halim, J.; Lu, J.; Barsoum, M.W.; Rosen, J. Synthesis of two-dimensional molybdenum carbide, Mo2C, from the gallium based atomic laminate Mo2Ga2C. Scr. Mater. 2015, 108, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Malaki, M.; Maleki, A.; Varma, R.S. MXenes and ultrasonication. J. Mater. Chem. A 2019, 7, 10843–10857. [Google Scholar] [CrossRef]
- Telkhozhayeva, M.; Teblum, E.; Konar, R.; Girshevitz, O.; Perelshtein, I.; Aviv, H.; Tischler, Y.R.; Nessim, G.D. Higher Ultrasonic Frequency Liquid Phase Exfoliation Leads to Larger and Monolayer to Few-Layer Flakes of 2D Layered Materials. Langmuir 2021, 37, 4504–4514. [Google Scholar] [CrossRef]
- Mohammed Al-antaki, A.H.; Kellici, S.; Power, N.P.; Lawrance, W.D.; Raston, C.L. Continuous flow vortex fluidic-mediated exfoliation and fragmentation of two-dimensional MXene. R. Soc. Open Sci. 2020, 7, 192255. [Google Scholar] [CrossRef]
- Kumar, S.; Rehman, M.A.; Lee, S.; Kim, M.; Hong, H.; Park, J.-Y.; Seo, Y. Supercapacitors based on Ti3C2Tx MXene extracted from supernatant and current collectors passivated by CVD-graphene. Sci. Rep. 2021, 11, 649. [Google Scholar] [CrossRef]
- Zhou, Y.; Maleski, K.; Anasori, B.; Thostenson, J.O.; Pang, Y.; Feng, Y.; Zeng, K.; Parker, C.B.; Zauscher, S.; Gogotsi, Y.; et al. Ti 3 C 2 T x MXene-Reduced Graphene Oxide Composite Electrodes for Stretchable Supercapacitors. ACS Nano 2020, 14, 3576–3586. [Google Scholar] [CrossRef]
- Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Poźniak, S.; Wojciechowski, T.; Birowska, M.; Popielski, M.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Moszczyńska, D.; et al. Multilayered stable 2D nano-sheets of Ti2NTx MXene: Synthesis, characterization, and anticancer activity. J. Nanobiotechnol. 2019, 17, 114. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, X.; Li, S.; Wei, J.; Li, P.; Wang, Y.; Lee, C.-S. Two-dimensional MXene-based materials for photothermal therapy. Nanophotonics 2020, 9, 2233–2249. [Google Scholar] [CrossRef]
- Verger, L.; Xu, C.; Natu, V.; Cheng, H.-M.; Ren, W.; Barsoum, M.W. Overview of the synthesis of MXenes and other ultrathin 2D transition metal carbides and nitrides. Curr. Opin. Solid State Mater. Sci. 2019, 23, 149–163. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.-Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Syamsai, R.; Rodriguez, J.R.; Pol, V.G.; Van Le, Q.; Batoo, K.M.; Farooq, S.A.; Pandiaraj, S.; Muthumareeswaran, M.R.; Raslan, E.H.; Grace, A.N. Double transition metal MXene (TixTa4−xC3) 2D materials as anodes for Li-ion batteries. Sci. Rep. 2021, 11, 688. [Google Scholar] [CrossRef]
- Zhang, C.J.; Ma, Y.; Zhang, X.; Abdolhosseinzadeh, S.; Sheng, H.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-dimensional transition metal carbides and nitrides (MXenes): Synthesis, properties, and electrochemical energy storage applications. Energy Environ. Mater. 2020, 3, 29–55. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Wang, B.; Shen, C.; Zhang, C.; Hu, Q.; Zhou, A.; Liu, B. Synthesis and electrochemical performance of Ti3C2Tx with hydrothermal process. Electron. Mater. Lett. 2016, 12, 702–710. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Dyatkin, B.; Gogotsi, Y.; Barsoum, M.W. Kinetics of aluminum extraction from Ti3AlC2 in hydrofluoric acid. Mater. Chem. Phys. 2013, 139, 147–152. [Google Scholar] [CrossRef]
- Soundiraraju, B.; George, B.K. Two-dimensional titanium nitride (Ti2N) MXene: Synthesis, characterization, and potential application as surface-enhanced raman scattering substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef]
- Venkateshalu, S.; Cherusseri, J.; Karnan, M.; Kumar, K.S.; Kollu, P.; Sathish, M.; Thomas, J.; Jeong, S.K.; Grace, A.N. New method for the synthesis of 2D vanadium nitride (MXene) and its application as a supercapacitor electrode. ACS Omega 2020, 5, 17983–17992. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Jiang, F.; Wang, Y.; Mi, L.; Yu, Y.; Song, L. Fabrication and thermal stability of NH4HF2-etched Ti3C2 MXene. Ceram. Int. 2017, 43, 6322–6328. [Google Scholar] [CrossRef]
- Natu, V.; Pai, R.; Sokol, M.; Carey, M.; Kalra, V.; Barsoum, M.W. 2D Ti3C2Tz MXene synthesized by water-free etching of Ti3AlC2 in polar organic solvents. Chem 2020, 6. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-free synthesis of high-purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chemie 2018, 130, 6223–6227. [Google Scholar] [CrossRef]
- Ye, Q.; Xiao, P.; Liu, W.; Chen, K.; Chen, T.; Xue, J.; Du, S.; Huang, Q. Exploring the potential of exfoliated ternary ultrathin Ti4AlN3 nanosheets for fabricating hybrid patterned polymer brushes. RSC Adv. 2015, 5, 70339–70344. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale 2016, 8, 11385–11391. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element replacement approach by reaction with lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef] [Green Version]
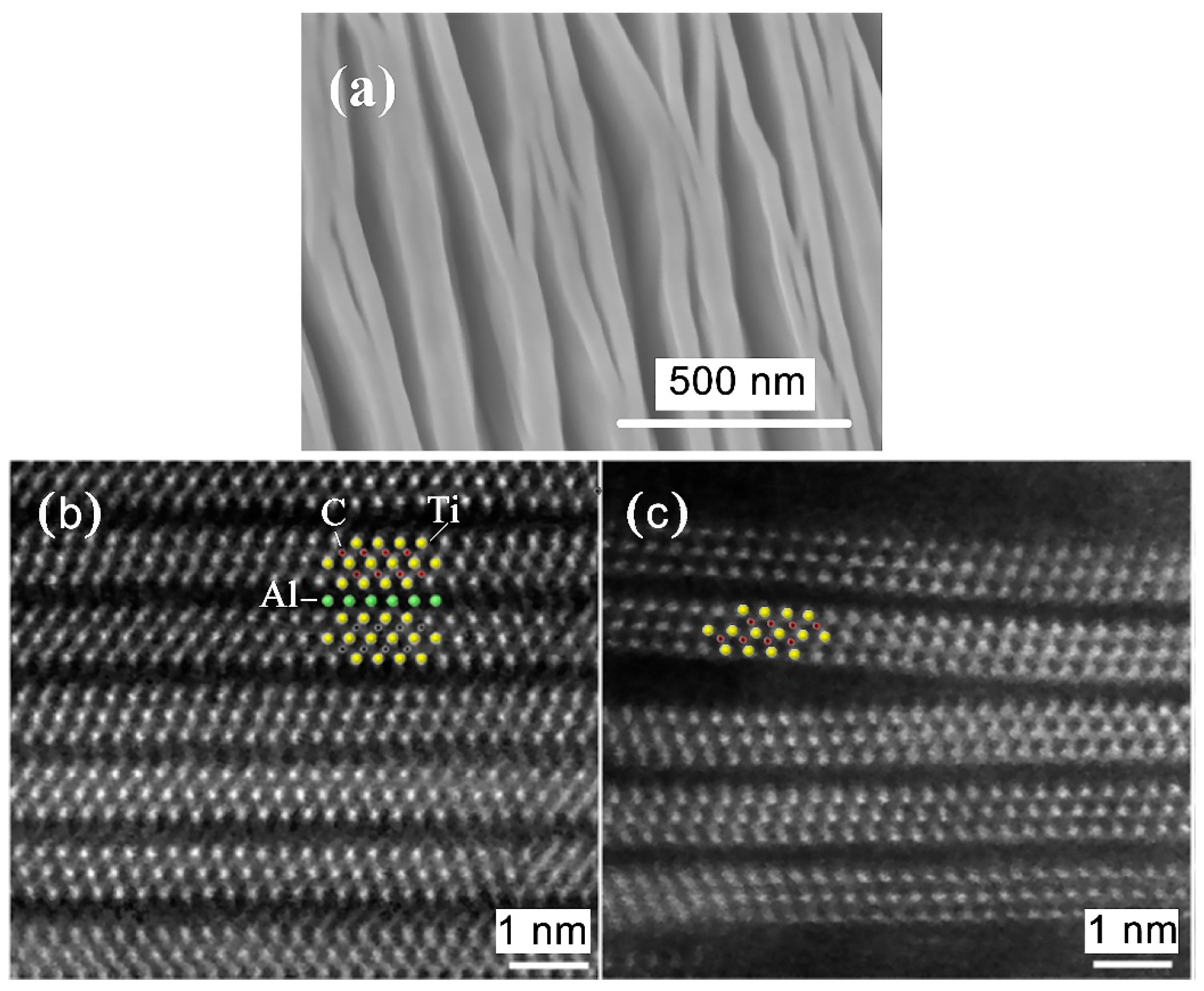
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.-L.; Cheng, H.-M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef]
- Xu, C.; Chen, L.; Liu, Z.; Cheng, H.-M.; Ren, W. Bottom-Up Synthesis of 2D Transition Metal Carbides and Nitrides. In 2D Metal Carbides and Nitrides (MXenes); Springer International Publishing: Cham, Germany, 2019; pp. 89–109. [Google Scholar]
- Geng, D.; Zhao, X.; Li, L.; Song, P.; Tian, B.; Liu, W.; Chen, J.; Shi, D.; Lin, M.; Zhou, W.; et al. Controlled growth of ultrathin Mo2C superconducting crystals on liquid Cu surface. 2D Mater. 2016, 4, 011012. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Wang, Y.; Wang, T.; Shang, S.; Ren, W. Magnetron-sputtering deposited molybdenum carbide MXene thin films as a saturable absorber for passively Q-switched lasers. J. Mater. Chem. C 2020, 8, 1608–1613. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”. Dalt. Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Hart, J.L.; Hantanasirisakul, K.; Lang, A.C.; Anasori, B.; Pinto, D.; Pivak, Y.; van Omme, J.T.; May, S.J.; Gogotsi, Y.; Taheri, M.L. Control of MXenes’ electronic properties through termination and intercalation. Nat. Commun. 2019, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D tantalum carbide (MXene). Adv. Mater. 2018, 30, 1703284. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, Y.; Xu, F.; Hu, J.; Chen, N.; Du, G.; Jiang, C. K+ intercalation of NH4HF2 -Exfoliated Ti3C2 MXene as binder-free electrodes with high electrochemical capacitance. Phys. Status Solidi. 2020, 217, 1900806. [Google Scholar] [CrossRef]
- Kim, H.; Alshareef, H.N. MXetronics: MXene-enabled electronic and photonic devices. ACS Mater. Lett. 2020, 2, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Attanayake, N.H.; Banjade, H.R.; Thenuwara, A.C.; Anasori, B.; Yan, Q.; Strongin, D.R. Electrocatalytic CO2 reduction on earth abundant 2D Mo2C and Ti3C2 MXenes. Chem. Commun. 2021, 57, 1675–1678. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Sa, B.; Fang, Y.; Lin, J.; Huang, Y.; Tang, C.; Zhou, J.; Miao, N.; Sun, Z. M2C-type MXenes: Promising catalysts for CO2 capture and reduction. Appl. Surf. Sci. 2020, 521, 146436. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Dong, L.M.; Ye, C.; Zheng, L.L.; Gao, Z.F.; Xia, F. Two-dimensional metal carbides and nitrides (MXenes): Preparation, property, and applications in cancer therapy. Nanophotonics 2020, 9, 2125–2145. [Google Scholar] [CrossRef] [Green Version]
- Reding, B.; Carter, P.; Qi, Y.; Li, Z.; Wu, Y.; Wannemuehler, M.; Bratlie, K.M.; Wang, Q. Manipulate intestinal organoids with niobium carbide nanosheets. J. Biomed. Mater. Res. Part A 2021, 109, 479–487. [Google Scholar] [CrossRef]
- Gao, L.; Ma, C.; Wei, S.; Kuklin, A.V.; Zhang, H.; Ågren, H. Applications of few-layer Nb2C MXene: Narrow-band photodetectors and femtosecond mode-locked fiber lasers. ACS Nano 2021, 15, 954–965. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Peng, S.; He, B.; Wang, J.; Du, Y.; Zhang, W.; Han, K.; Dang, F. Highly efficient Nb2C MXene cathode catalyst with uniform O-terminated surface for lithium–oxygen batteries. Adv. Energy Mater. 2021, 11, 2002721. [Google Scholar] [CrossRef]
- Wang, T.; Sun, X.; Guo, X.; Zhang, J.; Yang, J.; Tao, S.; Guan, J.; Zhou, L.; Han, J.; Wang, C.; et al. Ultraefficiently calming cytokine storm using Ti3C2Tx MXene. Small Methods 2021. [Google Scholar] [CrossRef]
- Deysher, G.; Shuck, C.E.; Hantanasirisakul, K.; Frey, N.C.; Foucher, A.C.; Maleski, K.; Sarycheva, A.; Shenoy, V.B.; Stach, E.A.; Anasori, B.; et al. Synthesis of Mo4VAlC4 MAX phase and two-dimensional Mo4VC4 MXene with five atomic layers of transition metals. ACS Nano 2020, 14, 204–217. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H.; Vlcek, L.; Peng, J.; Brady, A.B.; Osti, N.C.; Mamontov, E.; Tyagi, M.; Nanda, J.; Greenbaum, S.G.; et al. Multiscale and multimodal characterization of 2D titanium carbonitride MXene. Adv. Mater. Interfaces 2020, 7, 1902207. [Google Scholar] [CrossRef]
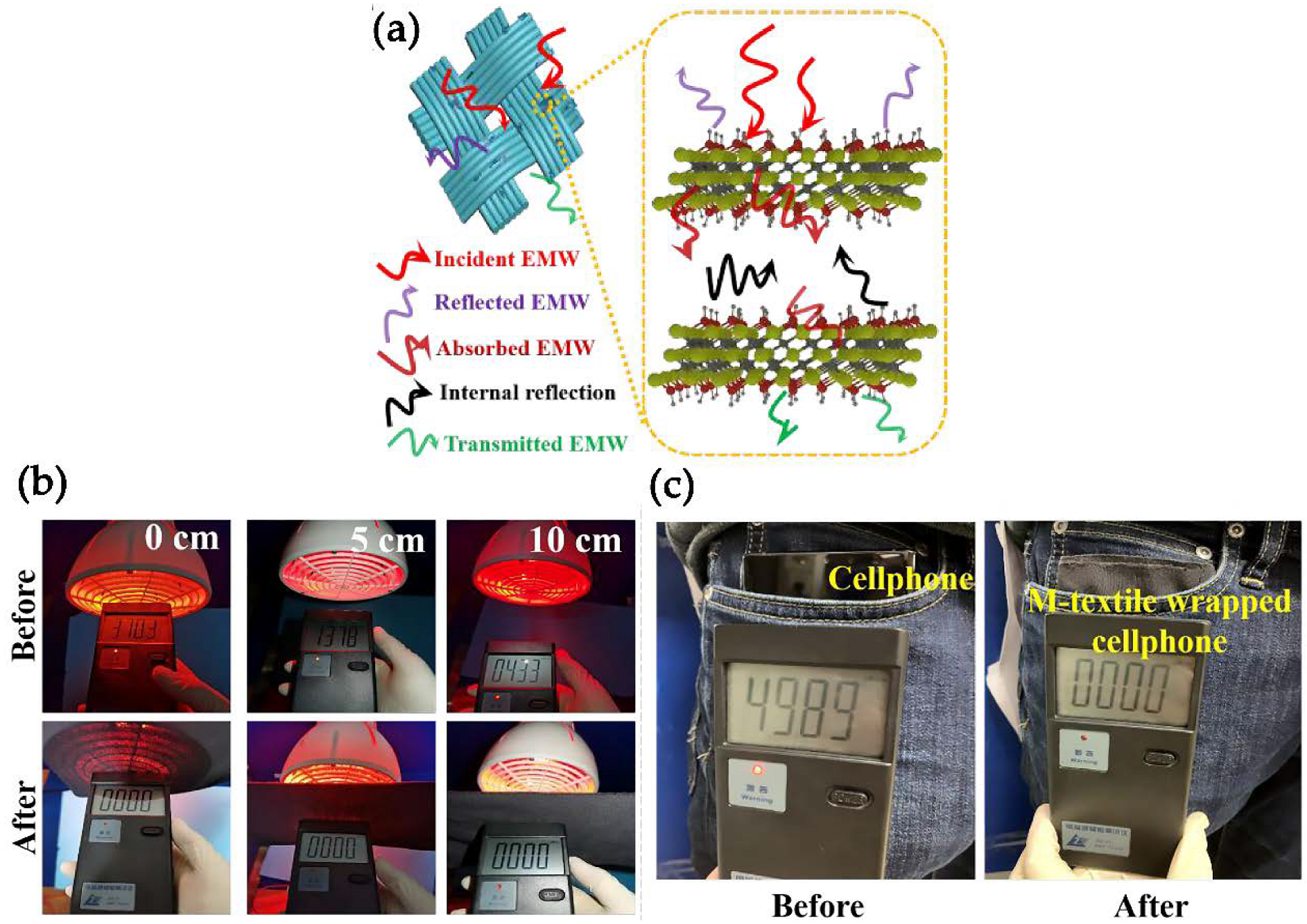
- Iqbal, A.; Shahzad, F.; Hantanasirisakul, K.; Kim, M.K.; Kwon, J.; Hong, J.; Kim, H.; Kim, D.; Gogotsi, Y.; Koo, C.M. Anomalous absorption of electromagnetic waves by 2D transition metal carbonitride Ti3CNTx (MXene). Science 2020, 369, 446–450. [Google Scholar] [CrossRef]
- Du, C.-F.; Zhao, X.; Wang, Z.; Yu, H.; Ye, Q. Recent advanced on the MXene–organic hybrids: Design, synthesis, and their applications. Nanomaterials 2021, 11, 166. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Wang, D.; Xu, Q. Nano friction and adhesion properties on Ti3C2 and Nb2C MXene studied by AFM. Tribol. Int. 2021, 153. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Yang, J.; Yao, G.; Sun, S.; Chen, Z.; Yuan, S.; Wu, K.; Fu, X.; Wang, Q.; Cui, W. Structural, magnetic properties of in-plane chemically ordered (Mo2/3R)2AlC (R = Gd, Tb, Dy, Ho, Er and Y) MAX phase and enhanced capacitance of Mo1.33C MXene derivatives. Carbon N. Y. 2021, 179, 104–110. [Google Scholar] [CrossRef]
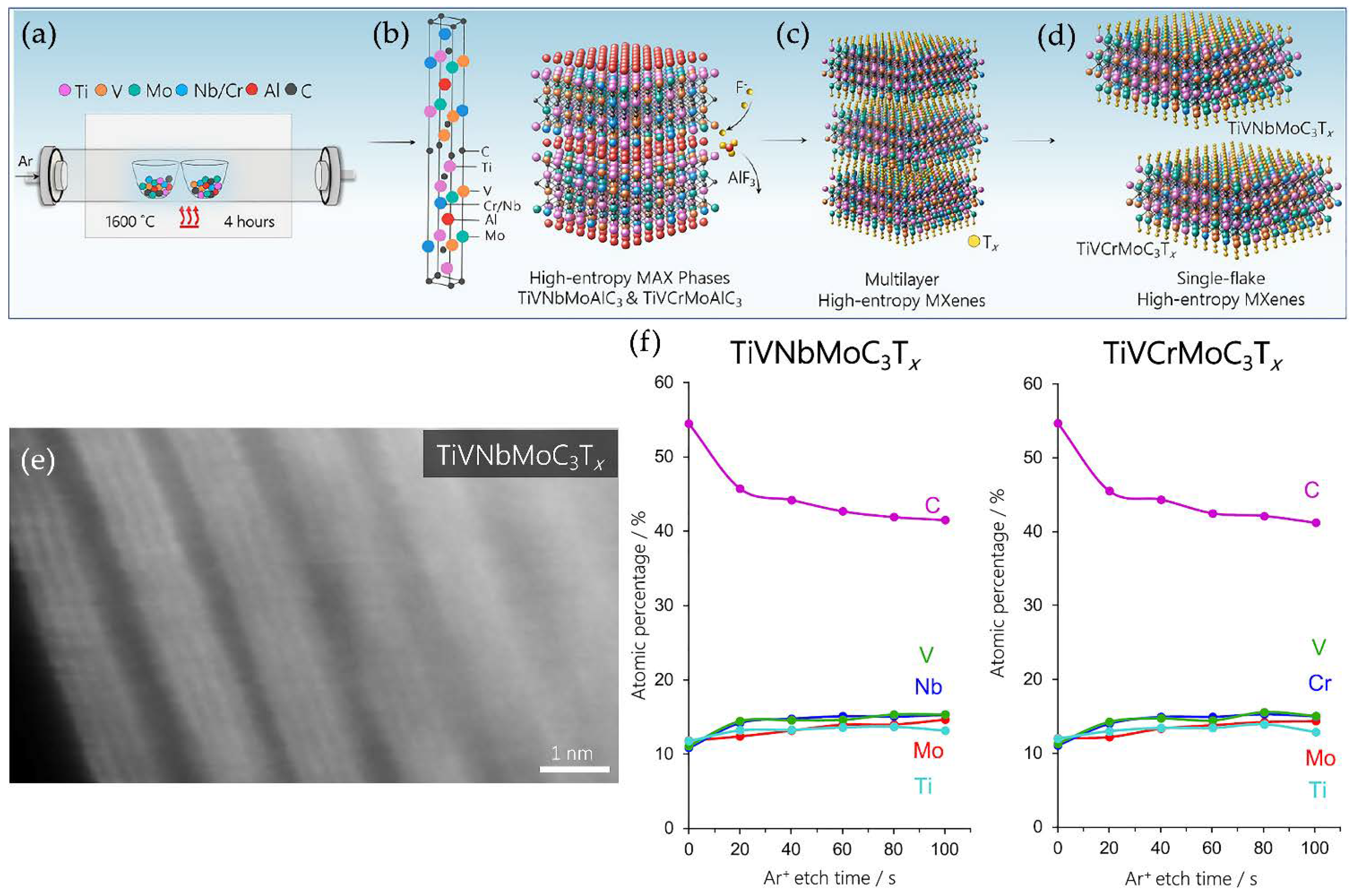
- Nemani, S.K.; Zhang, B.; Wyatt, B.C.; Hood, Z.D.; Manna, S.; Khaledialidusti, R.; Hong, W.; Sternberg, M.G.; Sankaranarayanan, S.K.R.S.; Anasori, B. High-entropy 2D carbide MXenes: TiVNbMoC3 and TiVCrMoC3. ACS Nano 2021, acsnano.1c02775. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, P.; Xue, T.; Ge, Y.; Ai, S.; Sheng, Y.; Wu, R.; Xu, L.; Tang, K.; Wen, Y. A novel graphene-like titanium carbide MXene/Au–Ag nanoshuttles bifunctional nanosensor for electrochemical and SERS intelligent analysis of ultra-trace carbendazim coupled with machine learning. Ceram. Int. 2021, 47. [Google Scholar] [CrossRef]
- Mockute, A.; Persson, P.O.Å.; Magnus, F.; Ingason, A.S.; Olafsson, S.; Hultman, L.; Rosen, J. Synthesis and characterization of arc deposited magnetic (Cr,Mn)2AlC MAX phase films. Phys. Status Solidi—Rapid Res. Lett. 2014, 8, 420–423. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Q.; Li, X.; Meng, C.; Ye, B.; Gou, B. Low-temperature synthesis of Ti3Al(Sn)C2 solid solution using replacement reaction. J. Mater. Sci. Mater. Electron. 2020, 31, 20601–20610. [Google Scholar] [CrossRef]
- Tunca, B.; Greaves, G.; Hinks, J.A.; Persson, P.O.Å.; Vleugels, J.; Lambrinou, K. In situ He+ irradiation of the double solid solution (Ti0.5,Zr0.5)2(Al0.5,Sn0.5)C MAX phase: Defect evolution in the 350–800 °C temperature range. Acta Mater. 2021, 206, 116606. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Enyashin, A.N.; Ivanovskii, A.L. Two-dimensional titanium carbonitrides and their hydroxylated derivatives: Structural, electronic properties and stability of MXenes Ti3C2−xNx(OH)2 from DFTB calculations. J. Solid State Chem. 2013, 207, 42–48. [Google Scholar] [CrossRef] [Green Version]
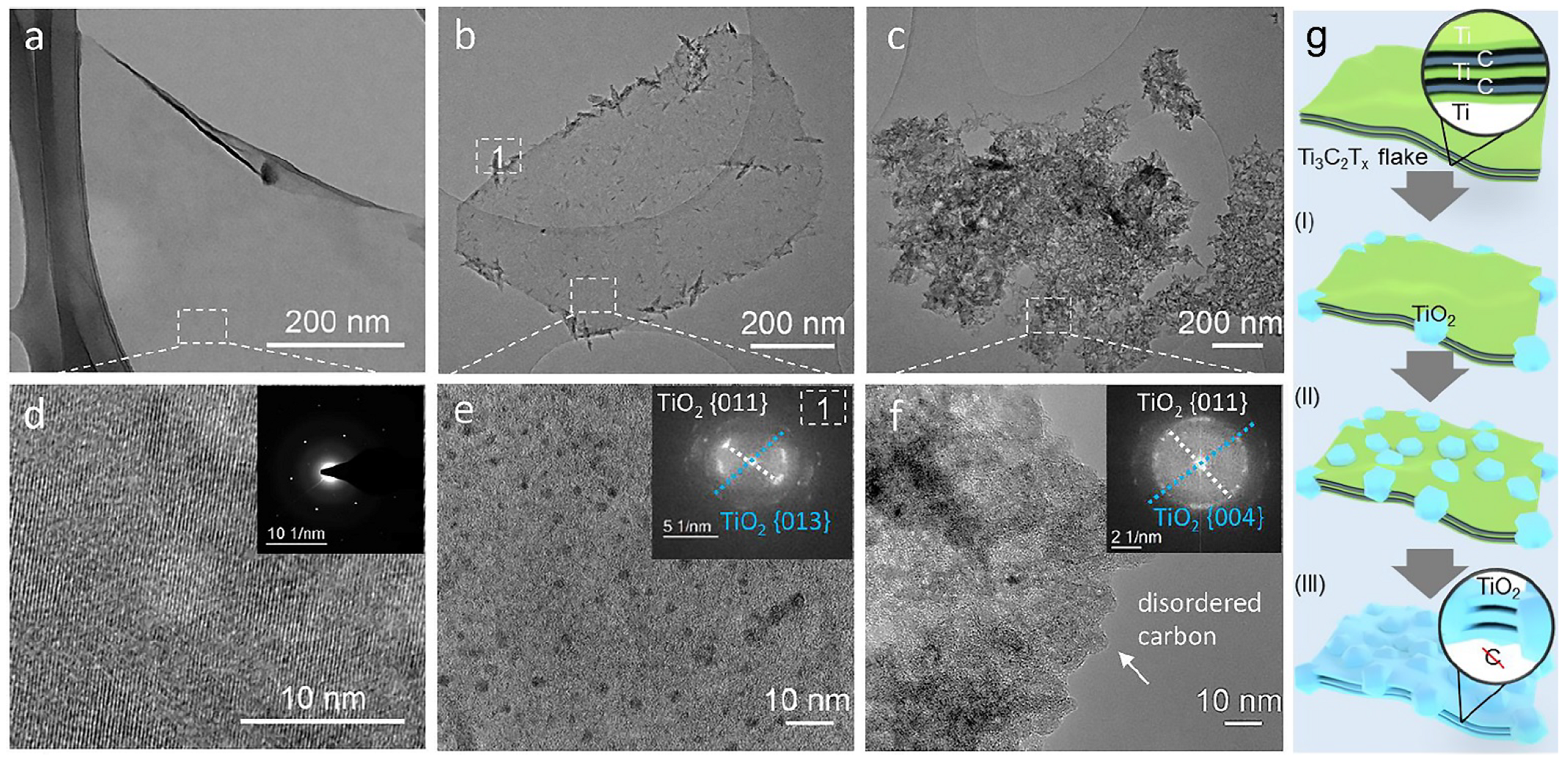
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation stability of colloidal two-dimensional titanium carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
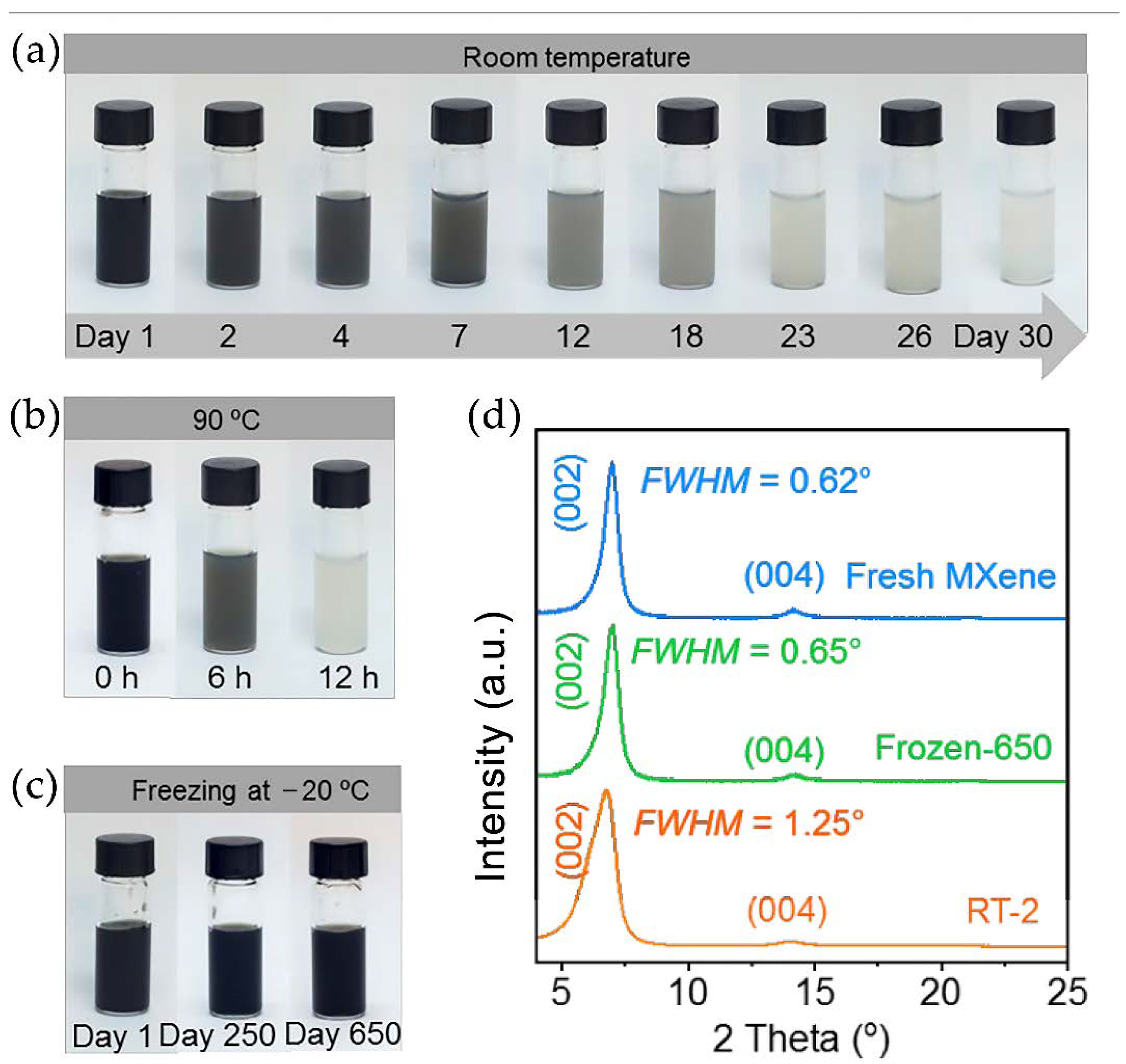
- Zhang, J.; Kong, N.; Hegh, D.; Usman, K.A.S.; Guan, G.; Qin, S.; Jurewicz, I.; Yang, W.; Razal, J.M. Freezing titanium carbide aqueous dispersions for ultra-long-term storage. ACS Appl. Mater. Interfaces 2020, 12, 34032–34040. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Seyedin, S.; Zhang, J.; Usman, K.A.S.; Qin, S.; Glushenkov, A.M.; Yanza, E.R.S.; Jones, R.T.; Razal, J.M. Facile solution processing of stable mxene dispersions towards conductive composite fibers. Glob. Chall. 2019, 3, 1900037. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Wang, B.; Hu, Q.; Wang, L.; Zhou, A. The Synthesis process and thermal stability of V2C MXene. Materials 2018, 11, 2112. [Google Scholar] [CrossRef] [Green Version]
- Kołtunowicz, T.; Gałaszkiewicz, P.; Kierczyński, K.; Rogalski, P.; Okal, P.; Pogrebnjak, A.D.; Buranich, V.; Pogorielov, M.; Diedkova, K.; Zahorodna, V.; et al. Investigation of AC electrical properties of MXene-PCL nanocomposites for application in small and medium power generation. Energies 2021, 14, 7123. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpińska, D.; Dybko, A.; Chudy, M.; Jastrzębska, A.M.; Olszyna, A.; Brzózka, Z. Future applications of MXenes in biotechnology, nanomedicine, and sensors. Trends Biotechnol. 2020, 38, 264–279. [Google Scholar] [CrossRef]
- Li, W.; Song, Z.; Zhong, J.; Qian, J.; Tan, Z.; Wu, X.; Chu, H.; Nie, W.; Ran, X. Multilayer-structured transparent MXene/PVDF film with excellent dielectric and energy storage performance. J. Mater. Chem. C 2019, 7, 10371–10378. [Google Scholar] [CrossRef]
- Tu, S.; Jiang, Q.; Zhang, X.; Alshareef, H.N. Large dielectric constant enhancement in mxene percolative polymer composites. ACS Nano 2018, 12, 3369–3377. [Google Scholar] [CrossRef]
- Fei, M.; Lin, R.; Lu, Y.; Zhang, X.; Bian, R.; Cheng, J.; Luo, P.; Xu, C.; Cai, D. MXene-reinforced alumina ceramic composites. Ceram. Int. 2017, 43, 17206–17210. [Google Scholar] [CrossRef]
- Guo, J.; Legum, B.; Anasori, B.; Wang, K.; Lelyukh, P.; Gogotsi, Y.; Randall, C.A. Cold sintered ceramic nanocomposites of 2D MXene and zinc oxide. Adv. Mater. 2018, 30, 1801846. [Google Scholar] [CrossRef]
- Petrus, M.; Woźniak, J.; Cygan, T.; Lachowski, A.; Moszczyńska, D.; Adamczyk-Cieślak, B.; Rozmysłowska-Wojciechowska, A.; Wojciechowski, T.; Ziemkowska, W.; Jastrzębska, A.; et al. Influence of Ti3C2Tx MXene and surface-modified Ti3C2Tx MXene addition on microstructure and mechanical properties of silicon carbide composites sintered via spark plasma sintering method. Materials 2021, 14, 3558. [Google Scholar] [CrossRef]
- Iqbal, A.; Sambyal, P.; Kwon, J.; Han, M.; Hong, J.; Kim, S.J.; Kim, M.-K.; Gogotsi, Y.; Koo, C.M. Enhanced absorption of electromagnetic waves in Ti3C2T MXene films with segregated polymer inclusions. Compos. Sci. Technol. 2021, 213, 108878. [Google Scholar] [CrossRef]
- Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Yu, B. MXene as emerging nanofillers for high-performance polymer composites: A review. Compos. Part B Eng. 2021, 217, 108867. [Google Scholar] [CrossRef]
- Wang, N.-N.; Wang, H.; Wang, Y.-Y.; Wei, Y.-H.; Si, J.-Y.; Yuen, A.C.Y.; Xie, J.-S.; Yu, B.; Zhu, S.-E.; Lu, H.-D.; et al. Robust, lightweight, hydrophobic, and fire-retarded polyimide/MXene aerogels for effective oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 40512–40523. [Google Scholar] [CrossRef]
- Hai, Y.; Jiang, S.; Zhou, C.; Sun, P.; Huang, Y.; Niu, S. Fire-safe unsaturated polyester resin nanocomposites based on MAX and MXene: A comparative investigation of their properties and mechanism of fire retardancy. Dalt. Trans. 2020, 49, 5803–5814. [Google Scholar] [CrossRef]
- Yu, B.; Yuen, A.C.Y.; Xu, X.; Zhang, Z.-C.; Yang, W.; Lu, H.; Fei, B.; Yeoh, G.H.; Song, P.; Wang, H. Engineering MXene surface with POSS for reducing fire hazards of polystyrene with enhanced thermal stability. J. Hazard. Mater. 2021, 401, 123342. [Google Scholar] [CrossRef]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Tang, S.; Guo, Z.; Wang, X.; Mo, S.; Huang, X.; Liu, G.; Zheng, N. Core-shell Pd@Au nanoplates as theranostic agents for in vivo photoacoustic imaging, CT imaging, and photothermal therapy. Adv. Mater. 2014, 26. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, L.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef]
- Han, X.; Jing, X.; Yang, D.; Lin, H.; Wang, Z.; Ran, H.; Li, P.; Chen, Y. Therapeutic mesopore construction on 2D Nb2C MXenes for targeted and enhanced chemo-photothermal cancer therapy in NIR-II biowindow. Theranostics 2018, 8. [Google Scholar] [CrossRef]
- Yin, H.; Guan, X.; Lin, H.; Pu, Y.; Fang, Y.; Yue, W.; Zhou, B.; Wang, Q.; Chen, Y.; Xu, H. Nanomedicine-enabled photonic thermogaseous cancer therapy. Adv. Sci. 2020, 7, 1901954. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-dimensional ultrathin MXene ceramic nanosheets for photothermal conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef]
- Liu, G.; Zou, J.; Tang, Q.; Yang, X.; Zhang, Y.; Zhang, Q.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy. ACS Appl. Mater. Interfaces 2017, 9, 40077–40086. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D ultrathin MXene-based drug-delivery nanoplatform for synergistic photothermal ablation and chemotherapy of cancer. Adv. Healthc. Mater. 2018, 7, 1701394. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Liang, X.; Liu, Q.; Qu, M.; Zou, Q.; Li, J.; Tan, H.; Liu, L.; Fan, D.; et al. Two-dimensional MXene (Ti3C2)-Integrated cellulose hydrogels: Toward smart three-dimensional network nanoplatforms exhibiting light-induced swelling and bimodal photothermal/chemotherapy anticancer activity. ACS Appl. Mater. Interfaces 2018, 10, 27631–27643. [Google Scholar] [CrossRef]
- Hussein, E.A.; Zagho, M.M.; Rizeq, B.R.; Younes, N.N.; Pintus, G.; Mahmoud, K.A.; Nasrallah, G.K.; Elzatahry, A.A. Plasmonic MXene-based nanocomposites exhibiting photothermal therapeutic effects with lower acute toxicity than pure MXene. Int. J. Nanomedicine 2019, 14, 4529–4539. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Han, Q.; Yang, W.; Gan, X.; Yang, Y.; Xie, K.; Xie, L.; Deng, Y. Two-dimensional MXene/cobalt nanowire heterojunction for controlled drug delivery and chemo-photothermal therapy. Mater. Sci. Eng. C 2020, 116, 111212. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yi, W.; Sun, T.; Tian, Y.; Zhang, P.; Si, J.; Hou, X.; Hou, J. Surface modification engineering of two-dimensional titanium carbide for efficient synergistic multitherapy of breast cancer. J. Mater. Chem. B 2020, 8, 6402–6417. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, M.; Lin, H.; Dai, C.; Ren, C.; Zhang, S.; Peng, W.; Chen, Y. 2D magnetic titanium carbide MXene for cancer theranostics. J. Mater. Chem. B 2018, 6, 3541–3548. [Google Scholar] [CrossRef]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A Two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. [Google Scholar] [CrossRef]
- Xiang, H.; Lin, H.; Yu, L.; Chen, Y. Hypoxia-irrelevant photonic thermodynamic cancer nanomedicine. ACS Nano 2019, 13, 2223–2235. [Google Scholar] [CrossRef]
- Zada, S.; Dai, W.; Kai, Z.; Lu, H.; Meng, X.; Zhang, Y.; Cheng, Y.; Yan, F.; Fu, P.; Zhang, X.; et al. Algae extraction controllable delamination of vanadium carbide nanosheets with enhanced near-infrared photothermal performance. Angew. Chem. Int. Ed. 2020, 59, 6601–6606. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [Green Version]
- Rasool, K.; Mahmoud, K.A.; Johnson, D.J.; Helal, M.; Berdiyorov, G.R.; Gogotsi, Y. Efficient antibacterial membrane based on two-dimensional Ti3C2Tx (MXene) nanosheets. Sci. Rep. 2017, 7, 1598. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Street, R.M.; McDaniel, R.M.; Barsoum, M.W.; Schauer, C.L. Antibacterial properties of electrospun Ti3C2Tz (MXene)/chitosan nanofibers. RSC Adv. 2018, 8, 35386–35394. [Google Scholar] [CrossRef] [Green Version]
- Jastrzębska, A.M.; Karwowska, E.; Wojciechowski, T.; Ziemkowska, W.; Rozmysłowska, A.; Chlubny, L.; Olszyna, A. The atomic structure of Ti2C and Ti3C2 MXenes is responsible for their antibacterial activity toward E. coli bacteria. J. Mater. Eng. Perform. 2019, 28, 1272–1277. [Google Scholar] [CrossRef]
- Dwivedi, N.; Dhand, C.; Kumar, P.; Srivastava, A.K. Emergent 2D materials for combating infectious diseases: The potential of MXenes and MXene–graphene composites to fight against pandemics. Mater. Adv. 2021, 2, 2892–2905. [Google Scholar] [CrossRef]
- Arabi Shamsabadi, A.; Sharifian Gh., M.; Anasori, B.; Soroush, M. Antimicrobial mode-of-action of colloidal Ti3C2Tx MXene nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 16586–16596. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Mitrzak, J.; Szuplewska, A.; Chudy, M.; Woźniak, J.; Petrus, M.; Wojciechowski, T.; Vasilchenko, A.S.; Jastrzębska, A.M. Engineering of 2D Ti3C2 MXene surface charge and its influence on biological properties. Materials 2020, 13, 2347. [Google Scholar] [CrossRef]
- Zheng, K.; Li, S.; Jing, L.; Chen, P.; Xie, J. Synergistic antimicrobial titanium carbide (MXene) conjugated with gold nanoclusters. Adv. Healthc. Mater. 2020, 9, 2001007. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasool, K.; Madhavan, V.E.; Aïssa, B.; Gogotsi, Y.; Mahmoud, K.A. Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 2018, 6, 3522–3533. [Google Scholar] [CrossRef]
- Wu, F.; Zheng, H.; Wang, W.; Wu, Q.; Zhang, Q.; Guo, J.; Pu, B.; Shi, X.; Li, J.; Chen, X.; et al. Rapid eradication of antibiotic-resistant bacteria and biofilms by MXene and near-infrared light through photothermal ablation. Sci. China Mater. 2021, 64, 748–758. [Google Scholar] [CrossRef]
- Unal, M.A.; Bayrakdar, F.; Fusco, L.; Besbinar, O.; Shuck, C.E.; Yalcin, S.; Erken, M.T.; Ozkul, A.; Gurcan, C.; Panatli, O.; et al. 2D MXenes with antiviral and immunomodulatory properties: A pilot study against SARS-CoV-2. Nano Today 2021, 38, 101136. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Huang, J.; Cheng, G.; He, Y. Volumetric solar steam generation enhanced by reduced graphene oxide nanofluid. Appl. Energy 2018, 220, 302–312. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, J.; Wang, J.; Wang, X. Design and optimization of solar steam generation system for water purification and energy utilization: A review. Rev. Adv. Mater. Sci. 2019, 58, 226–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, N.; Zhou, Z. Adsorptive environmental applications of MXene nanomaterials: A review. RSC Adv. 2018, 8, 19895–19905. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, T.; Yang, Z.; Chen, C.; Luo, W.; Song, J.; Hitz, E.; Jia, C.; Zhou, Y.; Liu, B.; et al. 3D-Printed, All-in-one evaporator for high-efficiency solar steam generation under 1 sun illumination. Adv. Mater. 2017, 29, 1700981. [Google Scholar] [CrossRef]
- Han, R.; Xie, Y.; Ma, X. Crosslinked P84 copolyimide/MXene mixed matrix membrane with excellent solvent resistance and permselectivity. Chinese J. Chem. Eng. 2019, 27, 877–883. [Google Scholar] [CrossRef]
- Ju, M.; Yang, Y.; Zhao, J.; Yin, X.; Wu, Y.; Que, W. Macroporous 3D MXene architecture for solar-driven interfacial water evaporation. J. Adv. Dielectr. 2019, 9, 1950047. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Natu, G.; Ahmad, K.; Chattopadhyay, A. Phosphorus induced crystallinity in carbon dots for solar light assisted seawater desalination. J. Mater. Chem. A 2018, 6, 4111–4118. [Google Scholar] [CrossRef]
- Weinstein, L.A.; Loomis, J.; Bhatia, B.; Bierman, D.M.; Wang, E.N.; Chen, G. Concentrating solar power. Chem. Rev. 2015, 115, 12797–12838. [Google Scholar] [CrossRef]
- Zhao, X.; Zha, X.-J.; Pu, J.-H.; Bai, L.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Macroporous three-dimensional MXene architectures for highly efficient solar steam generation. J. Mater. Chem. A 2019, 7, 10446–10455. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An effective 2D light-to-heat conversion material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef] [Green Version]
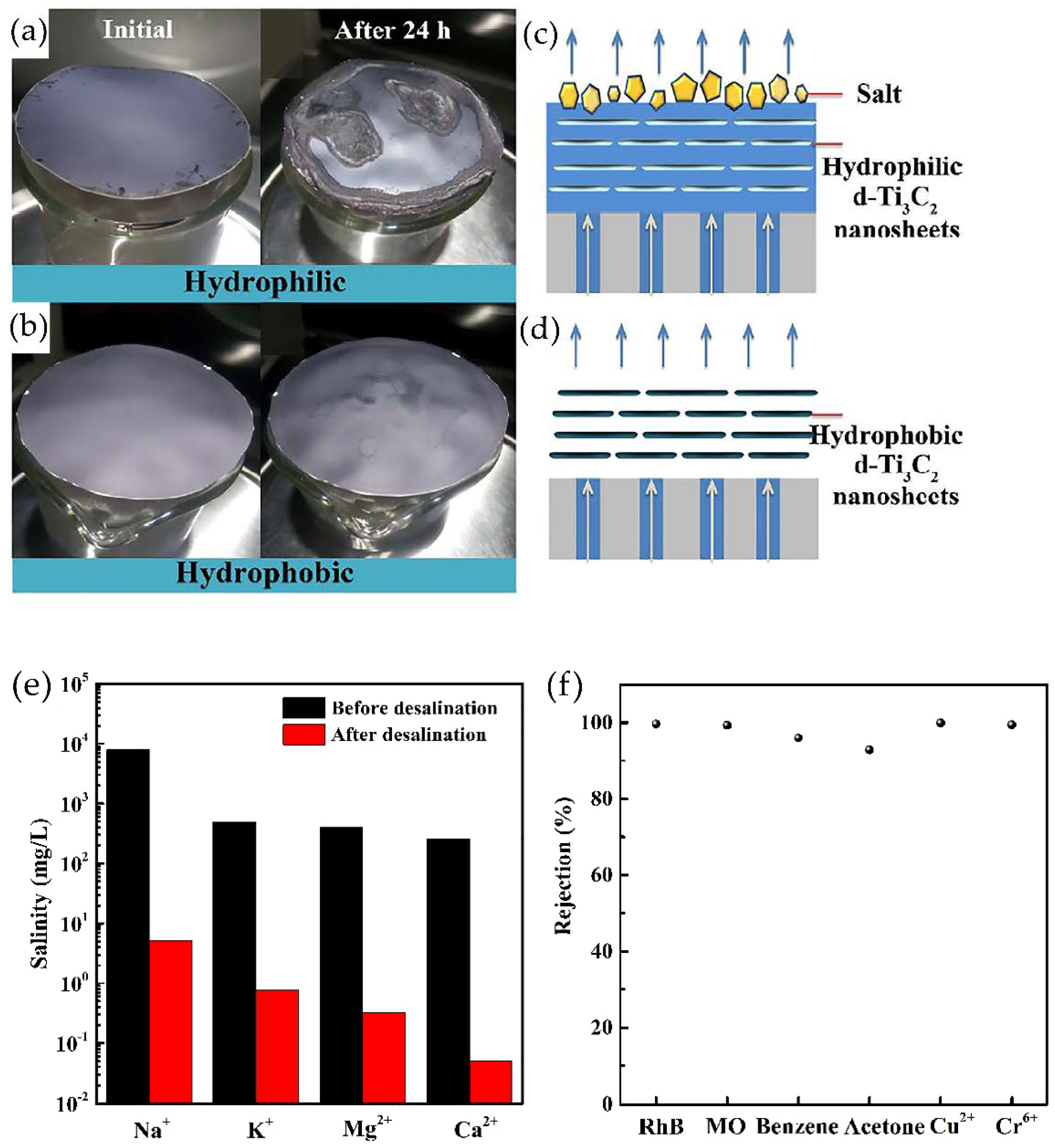
- Zhao, J.; Yang, Y.; Yang, C.; Tian, Y.; Han, Y.; Liu, J.; Yin, X.; Que, W. A hydrophobic surface enabled salt-blocking 2D Ti3C2 MXene membrane for efficient and stable solar desalination. J. Mater. Chem. A 2018, 6, 16196–16204. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Jun, B.-M.; Yoon, M.; Taheri-Qazvini, N.; Snyder, S.A.; Jang, M.; Heo, J.; Yoon, Y. Applications of MXene-based membranes in water purification: A review. Chemosphere 2020, 254, 126821. [Google Scholar] [CrossRef]
- Li, K.; Chang, T.; Li, Z.; Yang, H.; Fu, F.; Li, T.; Ho, J.S.; Chen, P. Biomimetic MXene textures with enhanced light-to-heat conversion for solar steam generation and wearable thermal management. Adv. Energy Mater. 2019, 9, 1901687. [Google Scholar] [CrossRef]
- Zhao, X.; Zha, X.-J.; Tang, L.-S.; Pu, J.-H.; Ke, K.; Bao, R.-Y.; Liu, Z.; Yang, M.-B.; Yang, W. Self-assembled core-shell polydopamine@MXene with synergistic solar absorption capability for highly efficient solar-to-vapor generation. Nano Res. 2020, 13, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Zhang, W.; Xu, L.; Cheng, M.; Su, Y.; Xue, J.; Liu, J.; Hou, S. A facile synthesis of porous MXene-based freestanding film and its spectacular electrosorption performance for organic dyes. Sep. Purif. Technol. 2021, 263, 118365. [Google Scholar] [CrossRef]
- My Tran, N.; Thanh Hoai Ta, Q.; Sreedhar, A.; Noh, J.S. Ti3C2Tx MXene playing as a strong methylene blue adsorbent in wastewater. Appl. Surf. Sci. 2021, 537. [Google Scholar] [CrossRef]
- Choi, J.; Ide, A.; Truong, Y.B.; Kyratzis, I.L.; Caruso, R.A. High surface area mesoporous titanium–zirconium oxide nanofibrous web: A heavy metal ion adsorbent. J. Mater. Chem. A 2013, 1, 5847. [Google Scholar] [CrossRef]
- Kang, K.M.; Kim, D.W.; Ren, C.E.; Cho, K.M.; Kim, S.J.; Choi, J.H.; Nam, Y.T.; Gogotsi, Y.; Jung, H.-T. Selective molecular separation on Ti3C2Tx—Graphene oxide membranes during pressure-driven filtration: Comparison with graphene oxide and MXenes. ACS Appl. Mater. Interfaces 2017, 9, 44687–44694. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Q.; Fu, H.; Zou, G.; Zhang, Q. Heavy-metal adsorption behavior of two-dimensional alkalization-intercalated MXene by First-principles calculations. J. Phys. Chem. C 2015, 119, 20923–20930. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, Y.; Wang, X.; Mao, Y.; Cao, W.; Hu, P.; Peng, X. Two-dimensional titanium carbide for efficiently reductive removal of highly toxic chromium(VI) from water. ACS Appl. Mater. Interfaces 2015, 7, 1795–1803. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, L.; Chen, K.; Zhang, Y.; Deng, Q.; Du, S.; Huang, Q.; Zheng, L.; Zhang, J.; Chai, Z.; et al. Loading actinides in multilayered structures for nuclear waste treatment: The first case study of uranium capture with vanadium carbide MXene. ACS Appl. Mater. Interfaces 2016, 8, 16396–16403. [Google Scholar] [CrossRef]
- Fu, K.; Liu, X.; Yu, D.; Luo, J.; Wang, Z.; Crittenden, J.C. Highly efficient and selective Hg(II) removal from water using multilayered Ti3C2Ox MXene via adsorption coupled with catalytic reduction mechanism. Environ. Sci. Technol. 2020, 54, 16212–16220. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Qu, W.; Zhang, M.; Feng, Y.; Zhao, J.; Yang, J.; Shih, K. Role of sulfur trioxide (SO3) in gas-phase elemental mercury immobilization by mineral sulfide. Environ. Sci. Technol. 2019, 53, 3250–3257. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, selective heavy metal removal from water by a metal–organic framework/polydopamine composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- Lei, H.; Hao, Z.; Chen, K.; Chen, Y.; Zhang, J.; Hu, Z.; Song, Y.; Rao, P.; Huang, Q. Insight into adsorption performance and mechanism on efficient removal of methylene blue by accordion-like V2CTx MXene. J. Phys. Chem. Lett. 2020, 11, 4253–4260. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Huang, G.; Xie, W.; Wang, W.; Liu, Y.; Gao, J. Preparation of graphene oxide aerogel and its adsorption for Cu2+ ions. Carbon N. Y. 2012, 50, 4856–4864. [Google Scholar] [CrossRef]
- Kim, S.; Yu, M.; Yoon, Y. Fouling and retention mechanisms of selected cationic and anionic dyes in a Ti3C2Tx MXene-ultrafiltration hybrid system. ACS Appl. Mater. Interfaces 2020, 12, 16557–16565. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, J.; Choe, A.; Cho, S.; Kim, J.; Ko, H. Mimicking human and biological skins for multifunctional skin electronics. Adv. Funct. Mater. 2020, 30, 1904523. [Google Scholar] [CrossRef]
- Lou, M.; Abdalla, I.; Zhu, M.; Wei, X.; Yu, J.; Li, Z.; Ding, B. Highly wearable, breathable, and washable sensing textile for human motion and pulse monitoring. ACS Appl. Mater. Interfaces 2020, 12, 19965–19973. [Google Scholar] [CrossRef]
- Liu, X.; Jin, X.; Li, L.; Wang, J.; Yang, Y.; Cao, Y.; Wang, W. Air-permeable, multifunctional, dual-energy-driven MXene-decorated polymeric textile-based wearable heaters with exceptional electrothermal and photothermal conversion performance. J. Mater. Chem. A 2020, 8, 12526–12537. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Kornienko, V.; Gogotsi, O.; Oleshko, O.; Kolesnyk, M.; Mishchenko, O.; Zahorodna, V.; Buranich, V.; Pogrebnjak, A.; Zozulia, Y.; et al. Bio-functionalization of electrospun polymeric nanofibers by Ti3C2Tx MXene. In Proceedings of the 2020 IEEE 10th International Conference Nanomaterials: Applications & Properties (NAP), Sumy, Ukraine, 9–13 November 2020; pp. 02BA10-1–02BA10-5. [Google Scholar]
- Li, T.; Chen, L.; Yang, X.; Chen, X.; Zhang, Z.; Zhao, T.; Li, X.; Zhang, J. A flexible pressure sensor based on an MXene–textile network structure. J. Mater. Chem. C 2019, 7, 1022–1027. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W.; Zhang, H.; Wang, Q.; Guan, F.; Yu, Z. Flexible and multifunctional silk textiles with biomimetic leaf-like MXene/silver nanowire nanostructures for electromagnetic interference shielding, humidity monitoring, and self-derived hydrophobicity. Adv. Funct. Mater. 2019, 29, 1905197. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, J.; Hu, Q.; Nie, W.; Wang, Z.; Zou, L.; Li, C. Vapor phase polymerized conducting polymer/MXene textiles for wearable electronics. Nanoscale 2021, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Lei, Z.; Wang, L.; Tian, M.; Zhu, S.; Xiao, H.; Tang, X.; Qu, L. Flexible MXene-decorated fabric with interwoven conductive networks for integrated joule heating, electromagnetic interference shielding, and strain sensing performances. ACS Appl. Mater. Interfaces 2020, 12, 14459–14467. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, K.; Lu, M.; Jiao, E.; Zhang, H.; Shi, J.; Lu, M. Highly thermal conductivity and flame retardant flexible graphene/MXene paper based on an optimized interface and nacre laminated structure. Compos. Part A Appl. Sci. Manuf. 2021, 141. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, M.; Wu, K.; Yao, S.; Du, X.; Chen, G.; Zhang, Q.; Liang, L.; Lu, M. Anisotropic thermal conductivity and electromagnetic interference shielding of epoxy nanocomposites based on magnetic driving reduced graphene oxide@Fe3O4. Compos. Sci. Technol. 2019, 174, 1–10. [Google Scholar] [CrossRef]
- Jia, X.; Shen, B.; Zhang, L.; Zheng, W. Waterproof MXene-decorated wood-pulp fabrics for high-efficiency electromagnetic interference shielding and Joule heating. Compos. Part B Eng. 2020, 198, 108250. [Google Scholar] [CrossRef]
- Luo, J.; Gao, S.; Luo, H.; Wang, L.; Huang, X.; Guo, Z.; Lai, X.; Lin, L.; Li, R.K.Y.; Gao, J. Superhydrophobic and breathable smart MXene-based textile for multifunctional wearable sensing electronics. Chem. Eng. J. 2021, 406. [Google Scholar] [CrossRef]
- Cao, W.; Ma, C.; Mao, D.; Zhang, J.; Ma, M.; Chen, F. MXene-reinforced cellulose nanofibril inks for 3D-printed smart fibres and textiles. Adv. Funct. Mater. 2019, 29, 1905898. [Google Scholar] [CrossRef]
- Seyedin, S.; Uzun, S.; Levitt, A.; Anasori, B.; Dion, G.; Gogotsi, Y.; Razal, J.M. MXene composite and coaxial fibers with high stretchability and conductivity for wearable strain sensing textiles. Adv. Funct. Mater. 2020, 30, 1910504. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Ji, H.; Wang, N.; Feng, S.; Xiao, H. Electromagnetic interference shielding with absorption-dominant performance of Ti3C2TX MXene/non-woven laminated fabrics. Text. Res. J. 2021, 004051752110062. [Google Scholar] [CrossRef]
- Rubežienė, V.; Varnaitė-Žuravliova, S. EMI shielding textile materials. In Materials for Potential EMI Shielding Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–378. [Google Scholar]
- Roh, J.-S.; Chi, Y.-S.; Kang, T.J.; Nam, S. Electromagnetic shielding effectiveness of multifunctional metal composite fabrics. Text. Res. J. 2008, 78, 825–835. [Google Scholar] [CrossRef]
- Lee, P.-C.; Kim, B.-R.; Jeoung, S.K.; Kim, Y.K. Electromagnetic Interference Shielding Effectiveness of Polypropylene/Conducting Fiber Composites. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; p. 120015. [Google Scholar]
- Alameri, B.M. Electromagnetic interference (EMI) produced by high voltage transmission lines. EUREKA Phys. Eng. 2020, 5, 43–50. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, Q.; Huang, B.; Feng, S.; Lu, C. Facile fabrication of densely packed Ti3C2 MXene/nanocellulose composite films for enhancing electromagnetic interference shielding and electro-/photothermal performance. ACS Nano 2021, 15, 12405–12417. [Google Scholar] [CrossRef]
- Ma, C.; Cao, W.T.; Zhang, W.; Ma, M.G.; Sun, W.M.; Zhang, J.; Chen, F. Wearable, ultrathin and transparent bacterial celluloses/MXene film with Janus structure and excellent mechanical property for electromagnetic interference shielding. Chem. Eng. J. 2021, 403. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Z.; Ma, X.; He, G.; Xu, T.; Tan, J.; Wang, L.; Zhang, X.; Qu, L.; Zhang, X. A lightweight MXene-coated nonwoven fabric with excellent flame retardancy, EMI shielding, and electrothermal/photothermal conversion for wearable heater. Chem. Eng. J. 2021, 430, 132605. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, F.; Yue, Y.; Han, D.; Lin, L.; Zhao, S.; Zhao, Y.; Pan, Z.; Li, C.; Nyström, G.; et al. Flexible and ultrathin waterproof cellular membranes based on high-conjunction metal-wrapped polymer nanofibers for electromagnetic interference shielding. Adv. Mater. 2020, 32, 1908496. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Luo, Y.; Lin, S.; Zhou, M.; Du, Z.; Cheng, X.; Wang, H. Hierarchical design of waterproof, highly sensitive, and wearable sensing electronics based on MXene-reinforced durable cotton fabrics. Chem. Eng. J. 2021, 408, 127363. [Google Scholar] [CrossRef]


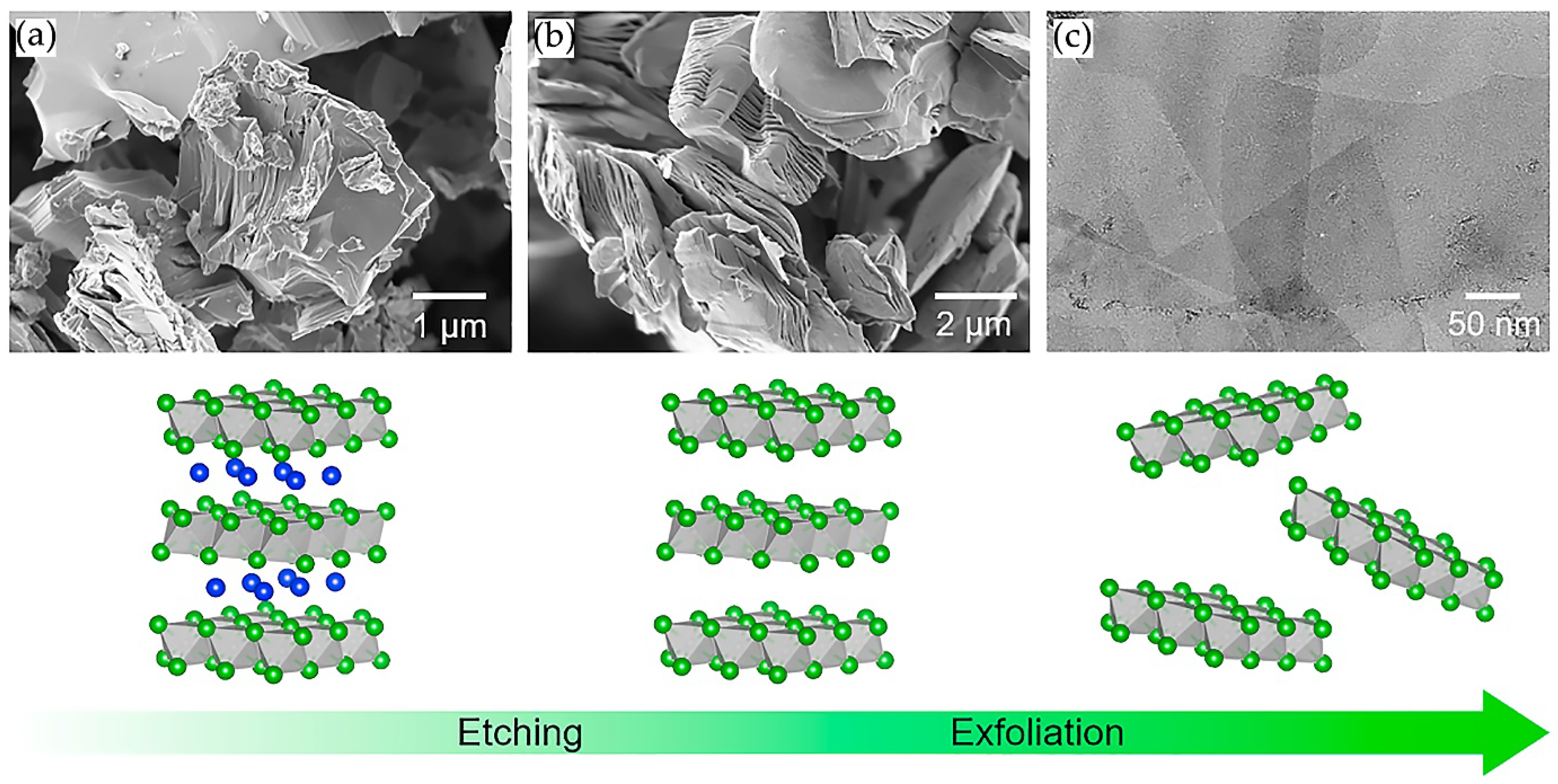
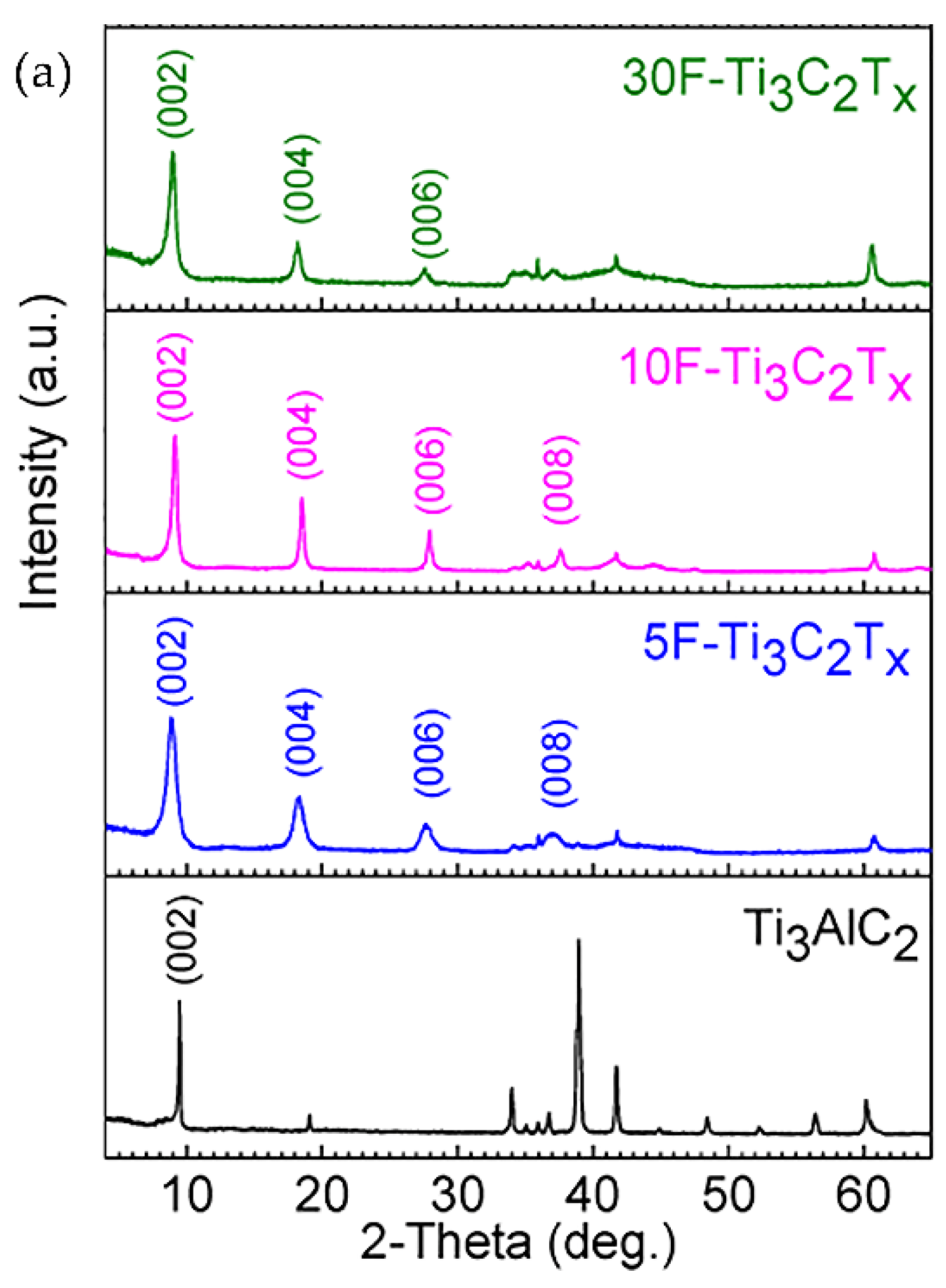

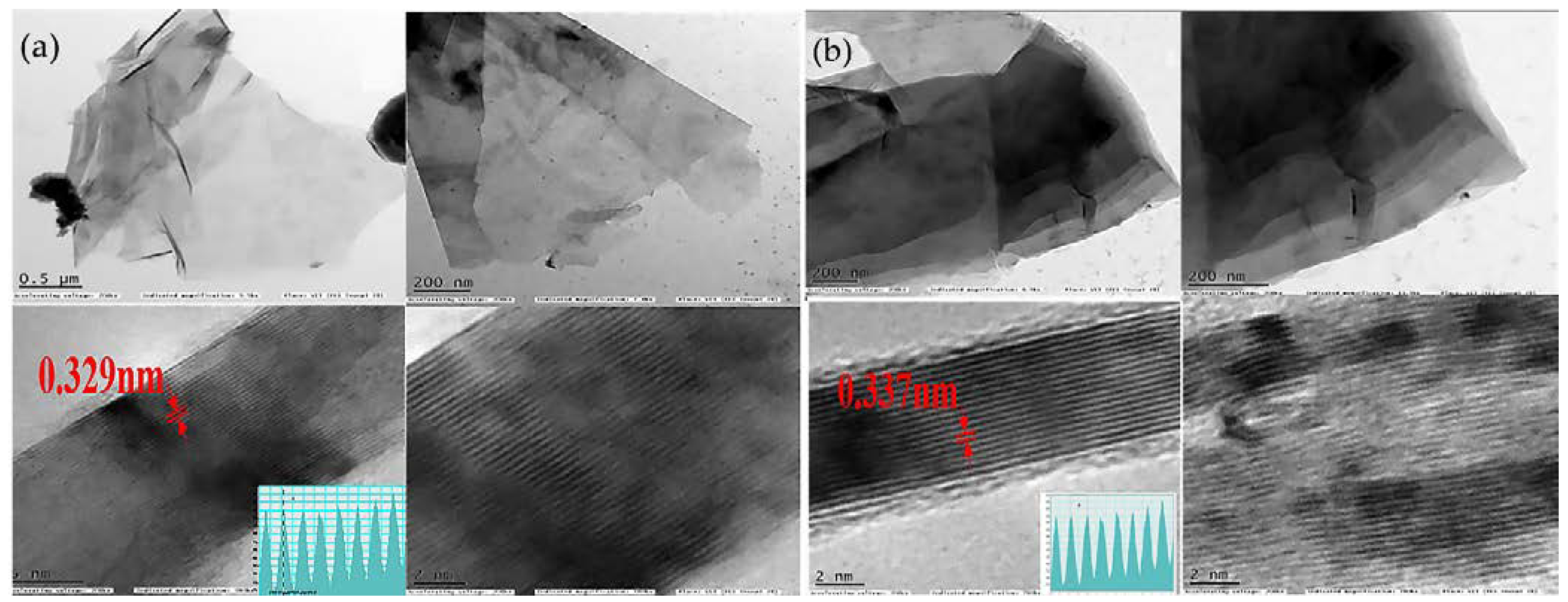
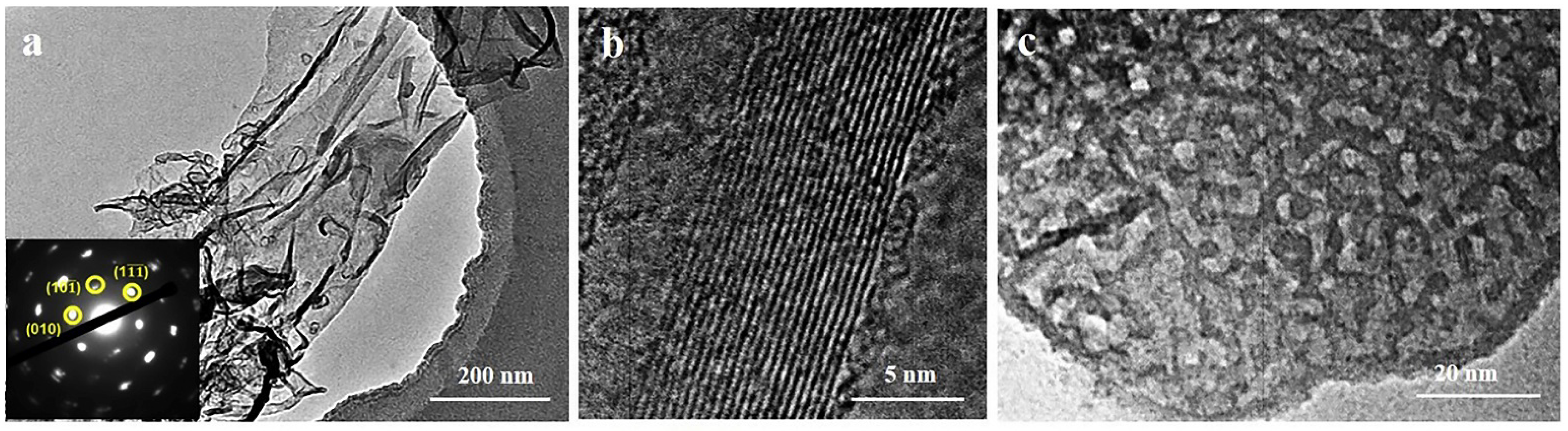
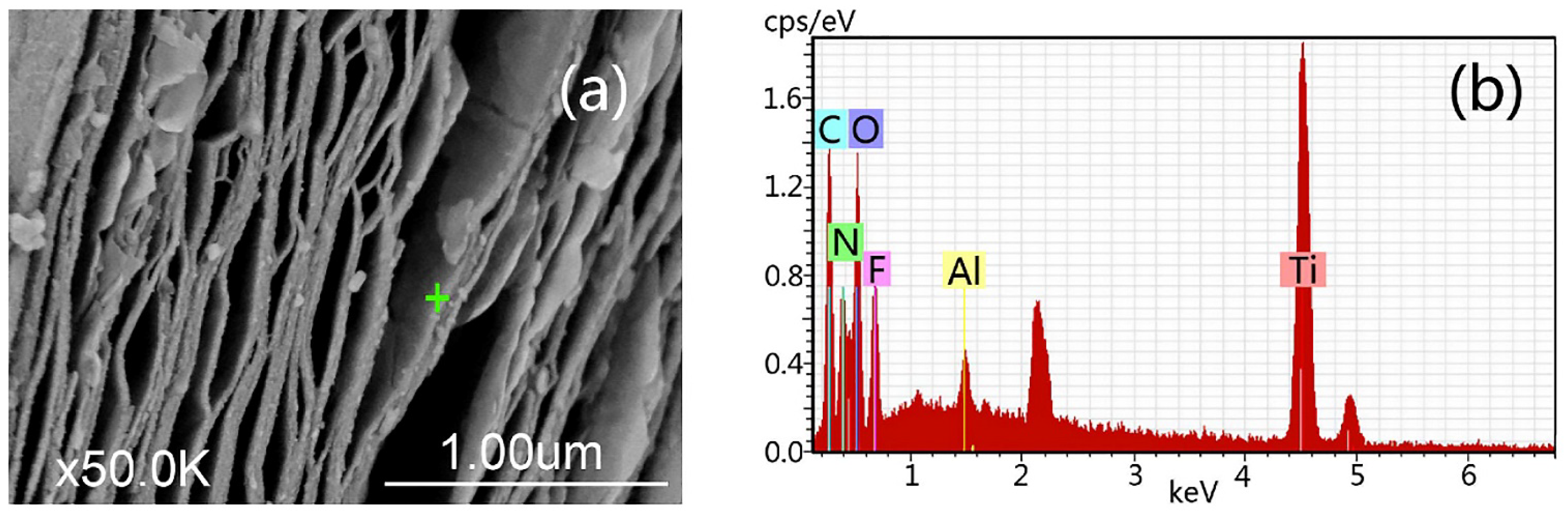
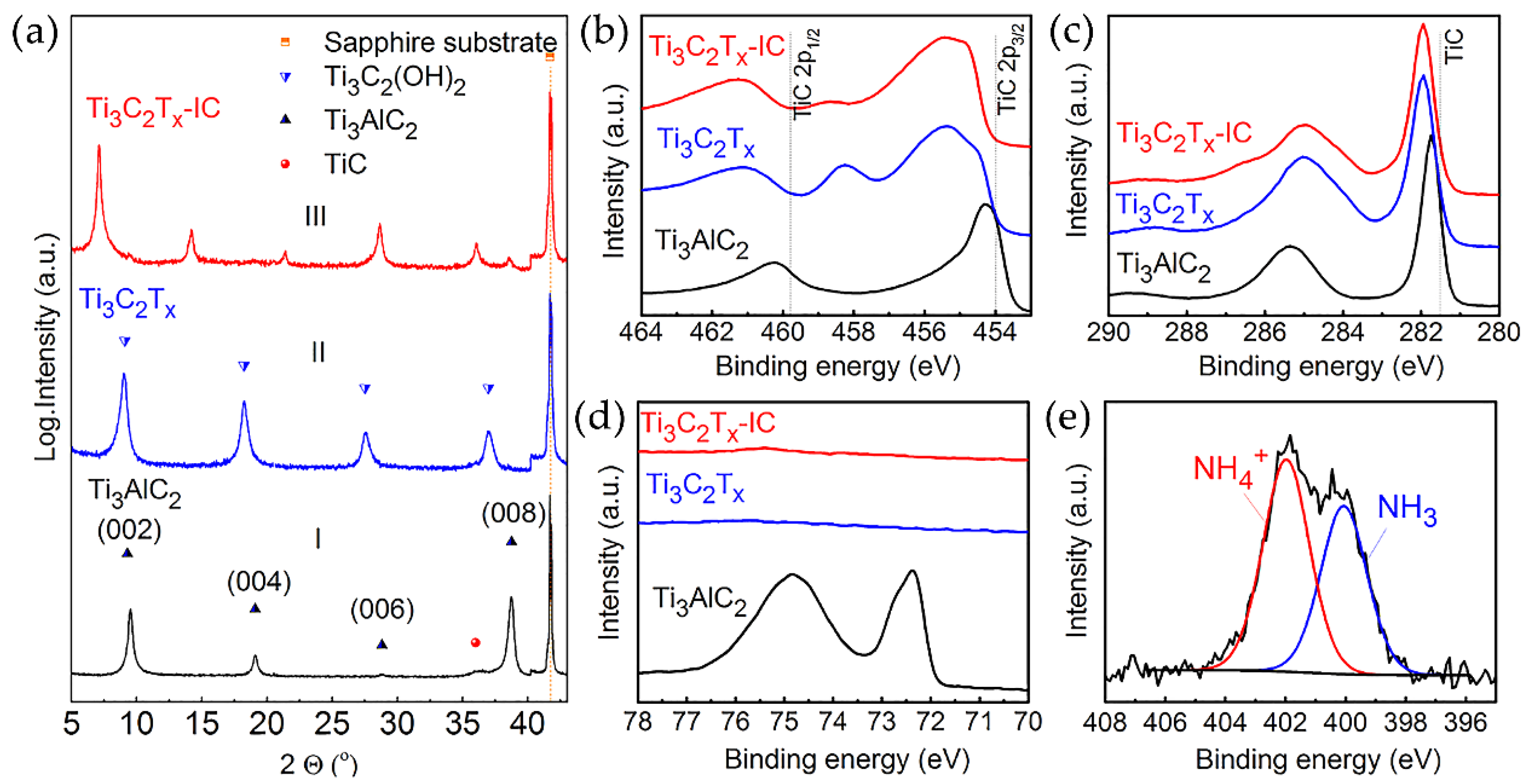
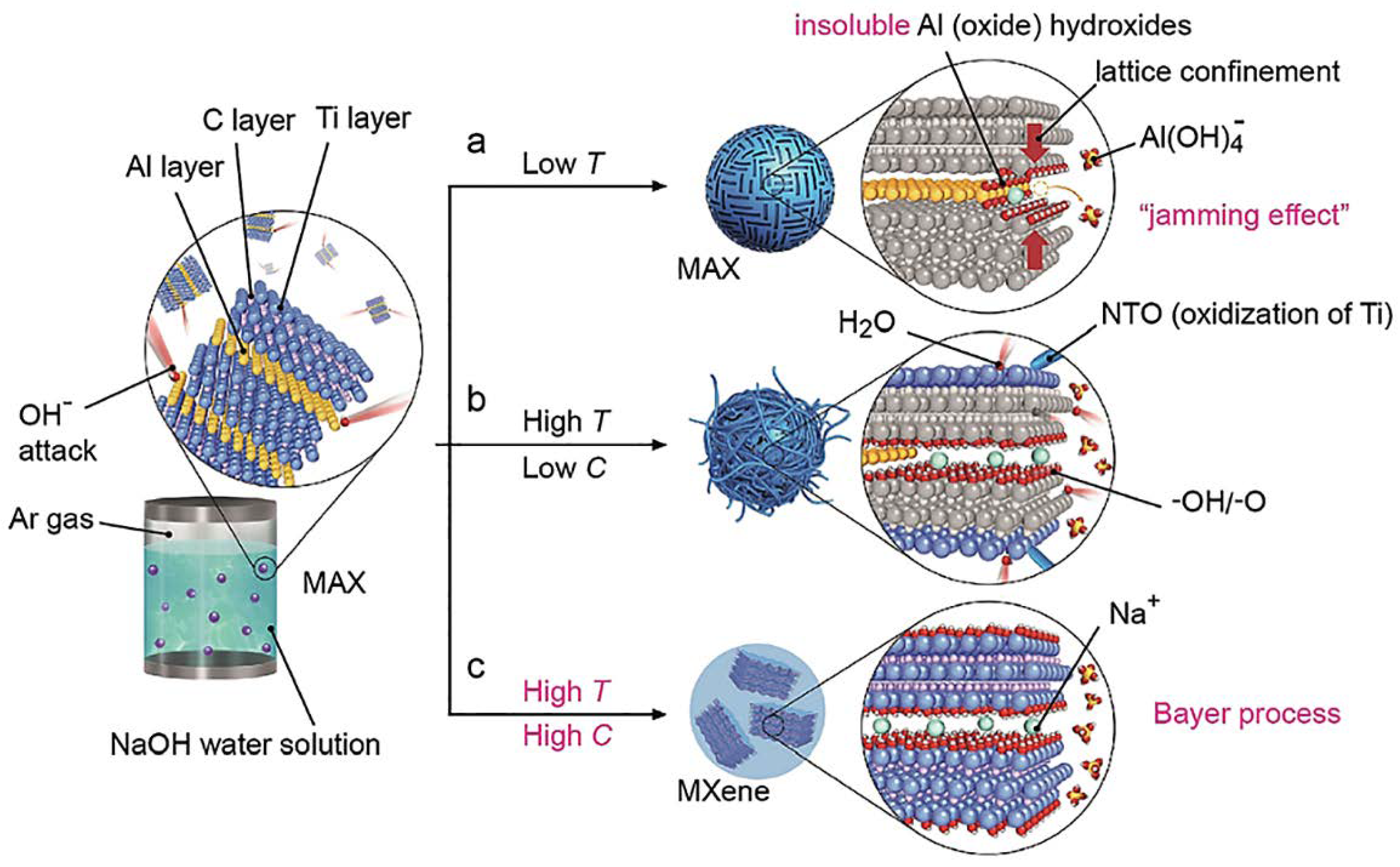
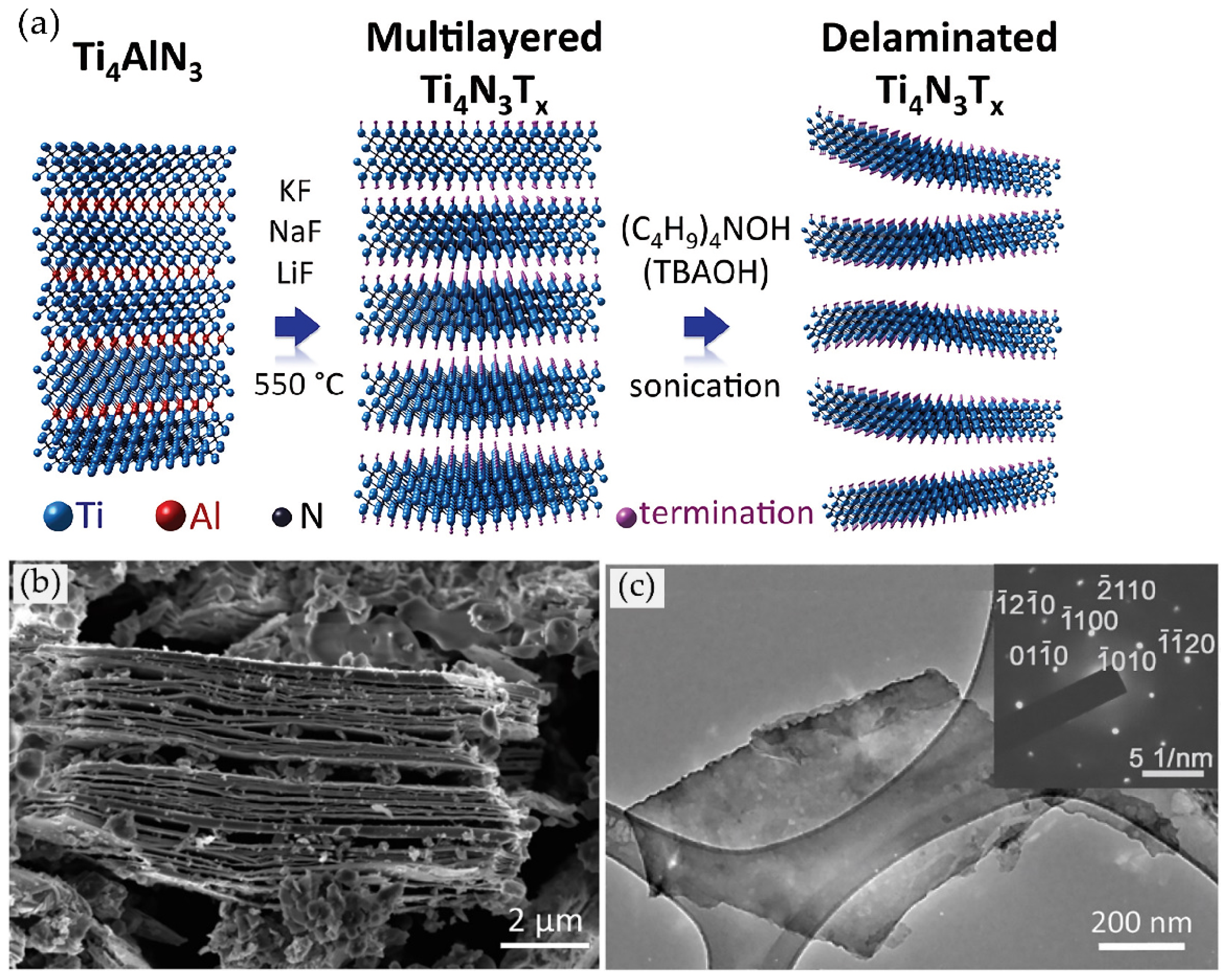

| № | MXene | Synthesis | Specific Features | Applications | Refs |
|---|---|---|---|---|---|
| 1 | Mo2C | Selective etching of Ga atoms from Mo2Ga2C using:
magnetron sputtering | High activity of the electrochemical reaction of hydrogen evolution, since the basal planes of Mo2CTx are catalytically active with respect to the reaction of hydrogen evolution; In the air, Mo2C is stable at 200 °C, and at 590 °C, it is completely oxidized to MoO3; Adsorbed COOH groups can spontaneously dissociate into a water molecule and CO, adsorbed on Mo2C; Flexible batteries (bending angle of about 110 °) based on Mo2C have excellent capacity retention of ~89% and ~74% for lithium-ion and sodium-ion batteries, respectively | Electrocatalysts for the evolution of hydrogen; high-performance flexible energy storage devices; electrocatalyst for the reduction of CO2; saturable absorber for passively Q-switched lasers | [37,80,89,90,91] |
| 2 | Nb2C | Selective etching of Al atoms from Nb2AlC powder using 50% aqueous solution of hydrofluoric acid | High biocompatibility; High efficiency of photothermal conversion while maintaining the required photothermal stability; Capable of penetrating organelles through Matrigel; Demonstrates ultra-stable pulses in the telecommunications and mid-infrared regions; At a center wavelength of 1882 nm, the 69th order harmonic can be achieved from 411 MHz; Excellent electrocatalytic characteristics when terminating the surface with -O functional groups. Nb2C cathodes are stable for 130 cycles at an ultra-high current density of 3 A/g | Photothermal therapy of cancer; Protecting and stimulating the survival of intestinal cells during various procedures; Building block for narrow-band photoelectrochemical photodetectors and mode synchronizers; cathode material for lithium–oxygen batteries | [92,93,94,95] |
| 3 | Ti3C2 | Selective etching of Al atoms from Ti3AlC2 using:
NH4HF2- containing polar organic solvents (without water) | High biocompatibility; High electron affinity between thrombin and Ti3C2; High efficiency of fluorescence quenching; Ultra-high ability to remove the typical cytokine IL-6 through a mechanism of chemisorption | Photothermal therapy of cancer; Aptasensor based on Förster resonance energy transfer for the quantitative determination of thrombin; Hemoperfusion absorbent for blocking cytokine storm for treatment of severe COVID-19 infection; Anode material of Na-ion batteries | [72,76,92,96] |
| 4 | Ta4C3 | Etching Al atoms from Ta4AlC3 with 40% aqueous solution of HF + solution of N-methylpyrrolidone | High biocompatibility; The efficiency of photothermal conversion is about 44.7%; Excellent photothermal stability; High photothermal performance in the near-infrared range | Photoacoustic computed tomography of tumors with contrast enhancement; In vivo photothermal ablation of tumor xenografts; Theranostics | [82] |
| 5 | Mo2TiC2 | Etching of Al atoms from Mo2TiAlC2 with a 48–51% aqueous solution of HF | Shows properties of a semiconductor. Resistivity increases with decreasing temperature (dρ/dT < 0) in the measuring range 10–250 K | Applications in electronics and optics | [39] |
| 6 | TixTa4−xC3 | Hydrofluoric acid etching of Al atoms from TixTa4−xAlC3 | Stable electrochemical characteristics, high capacity, and good performance: reversible specific capacity of 459 mAh/g at 0.5 °C for 200 cycles with a capacity retention of about 97%; Bimetallic MXene accumulates ions on the surface of its layers | Anode material for lithium-ion batteries | [64] |
| 7 | (Mo4V)C4 | Hydrofluoric acid etching of Al atoms from (MoV)5AlC4 (the ratio of Mo:V precursor powders was 4:1) | The presence of disordered M-positions; The structure is stable up to 900 °C with subsequent transformation into the orthorhombic (Mo, V) 2C phase, and at 1500 °C into cubic c (Mo, V) C; The specific electrical resistance of MXene is about 1.20 mΩ cm, and the conductivity was 833 S/cm). However, the resistance is worse compared to Mo2C and Mo2TiC2 (0.80 and 0.67 mΩ cm) | - | [97] |
| 8 | Ti4N3 | Etching of Ti4AlN3 in a mixture of 59 wt.% KF + 29 wt.% LiF + 12 wt.% NaF at 550 °C with TBAOH | Ti4N3 with functional surface groups O, F, or OH has higher states at the Fermi level compared to similar two-dimensional carbides; Magnetic moment about 7.0 μB per unit cell | Electrodes in electrochemical capacitors; Plasmonic material for conversion optics | [75] |
| 9 | V2N | Etching of V2AlN with a LiF-HCl mixture, followed by treatment with TMAOH or DMSO separating agents | Outstanding electrochemical stability; Higher electronic conductivity than carbide MXene | Electrodes for supercapacitor | [70] |
| 10 | Ti3CN | Etching of Al atoms from Ti3AlCN powder in 30% HF solution | Abnormally high absorption of electromagnetic waves in the layered structure of carbonitride MXene after thermal annealing at 350 °C; Electrical conductivity at 2475 S/cm after annealing at 250 °C | Anode material for Na- and Li-ion batteries;Spectral filter that induces mode-locked laser pulses for photonic applications; Lightweight, ultra-thin, and flexible EMI shielding materials | [98,99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogorielov, M.; Smyrnova, K.; Kyrylenko, S.; Gogotsi, O.; Zahorodna, V.; Pogrebnjak, A. MXenes—A New Class of Two-Dimensional Materials: Structure, Properties and Potential Applications. Nanomaterials 2021, 11, 3412. https://doi.org/10.3390/nano11123412
Pogorielov M, Smyrnova K, Kyrylenko S, Gogotsi O, Zahorodna V, Pogrebnjak A. MXenes—A New Class of Two-Dimensional Materials: Structure, Properties and Potential Applications. Nanomaterials. 2021; 11(12):3412. https://doi.org/10.3390/nano11123412
Chicago/Turabian StylePogorielov, Maksym, Kateryna Smyrnova, Sergiy Kyrylenko, Oleksiy Gogotsi, Veronika Zahorodna, and Alexander Pogrebnjak. 2021. "MXenes—A New Class of Two-Dimensional Materials: Structure, Properties and Potential Applications" Nanomaterials 11, no. 12: 3412. https://doi.org/10.3390/nano11123412
APA StylePogorielov, M., Smyrnova, K., Kyrylenko, S., Gogotsi, O., Zahorodna, V., & Pogrebnjak, A. (2021). MXenes—A New Class of Two-Dimensional Materials: Structure, Properties and Potential Applications. Nanomaterials, 11(12), 3412. https://doi.org/10.3390/nano11123412