Co3O4 Nanoneedle Array Grown on Carbon Fiber Paper for Air Cathodes towards Flexible and Rechargeable Zn–Air Batteries
Abstract
:1. Introduction
2. Experimental Section
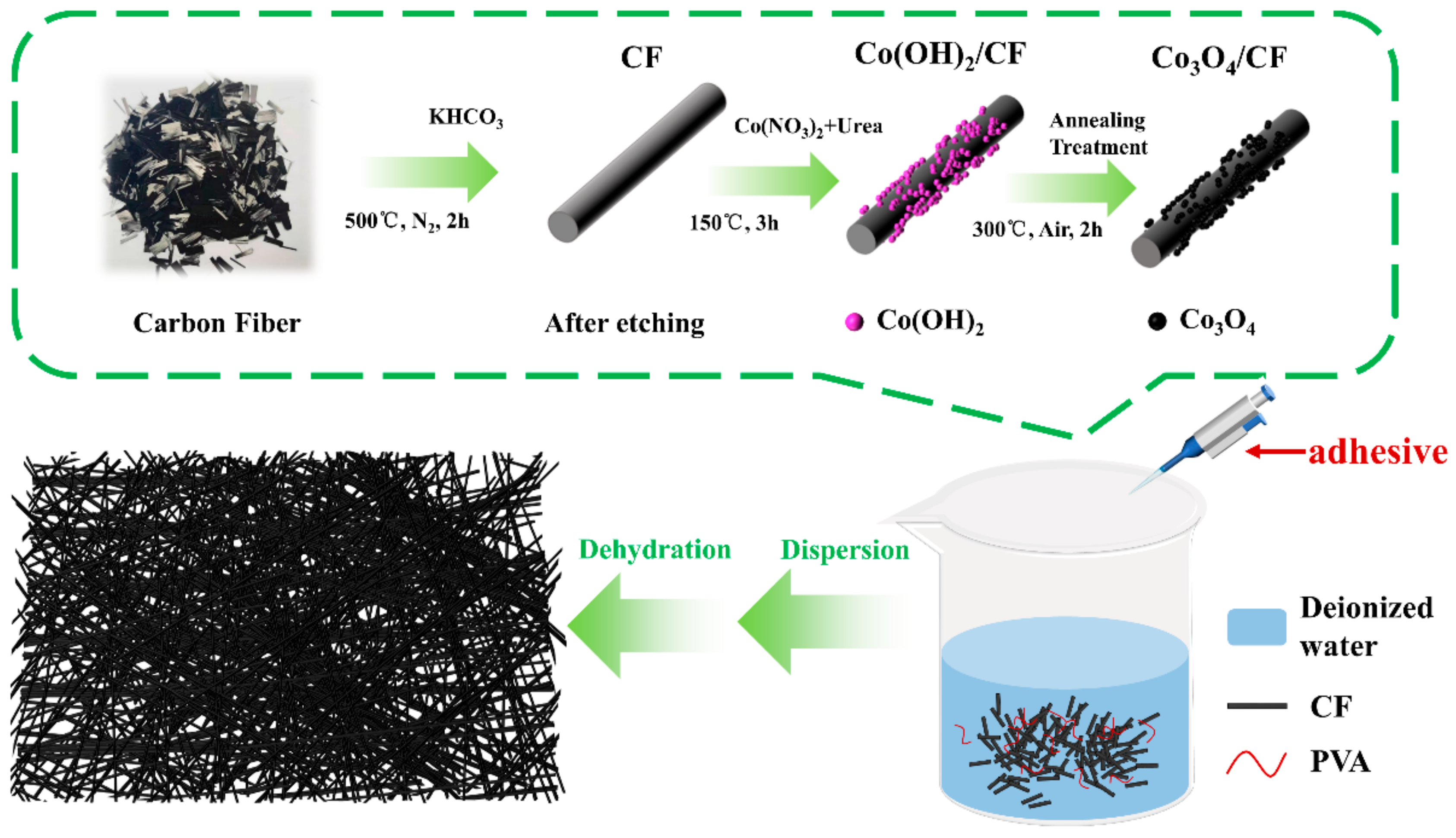
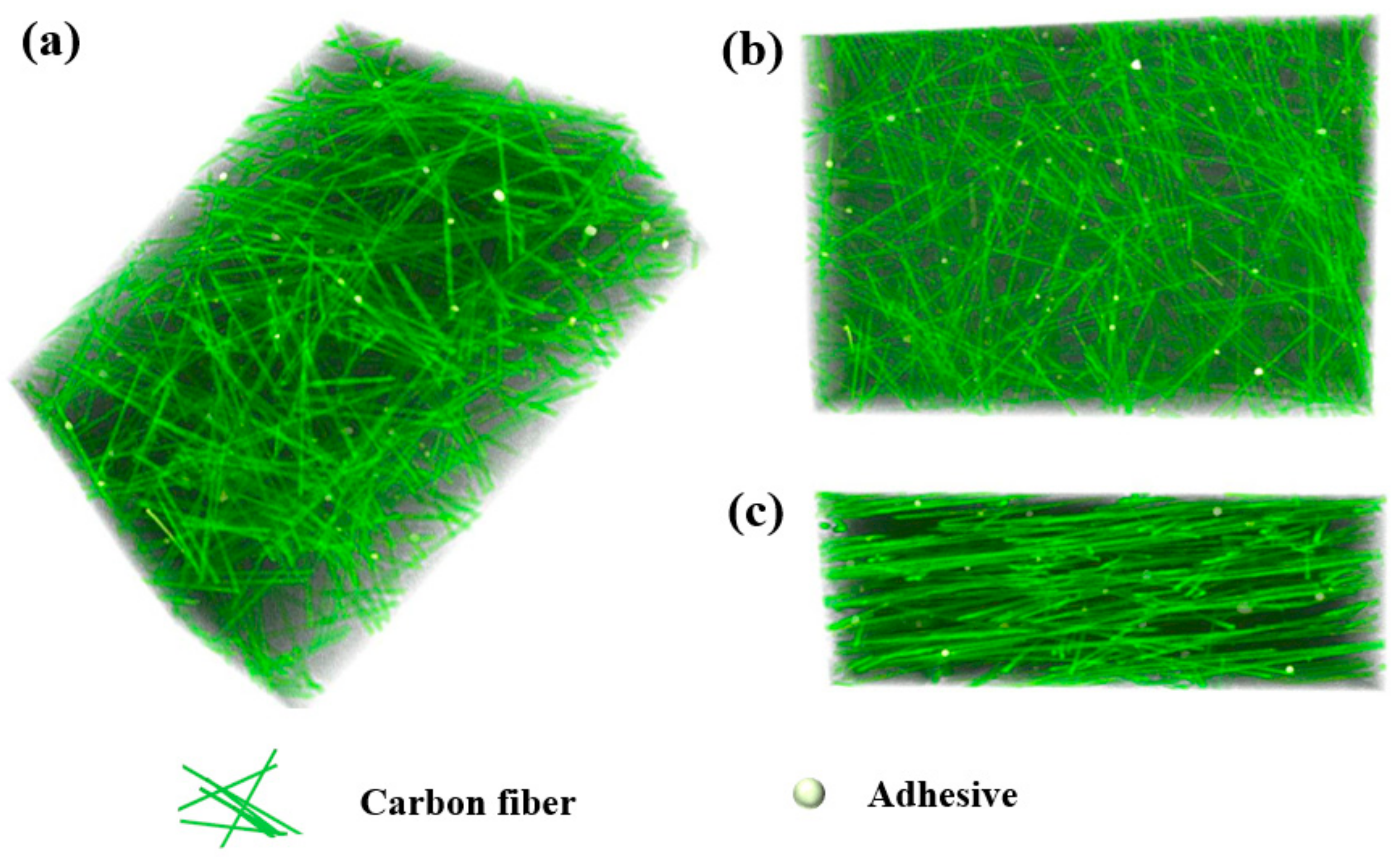
2.1. Preparation of Air Cathodes for ZAB
2.2. Preparation of Hydrogel Electrolyte
2.3. Fabrication of Flexible Zn-Air Battery
2.4. Characterization
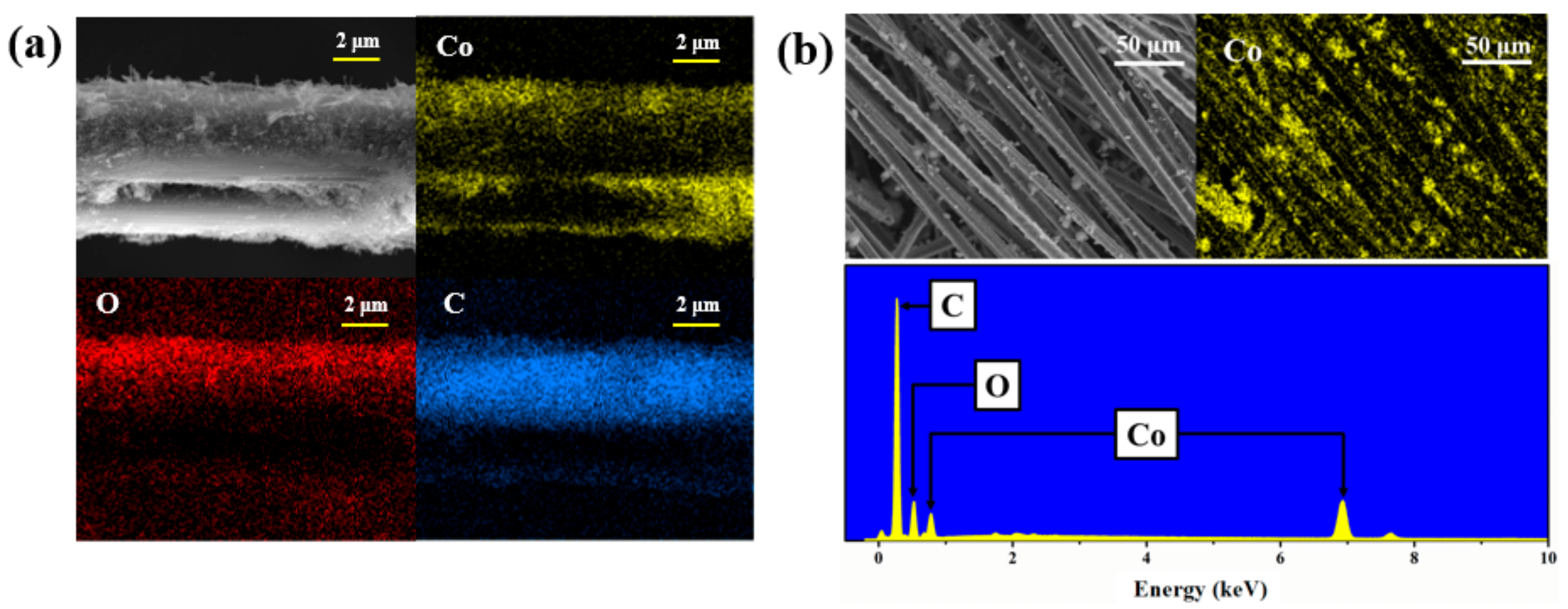
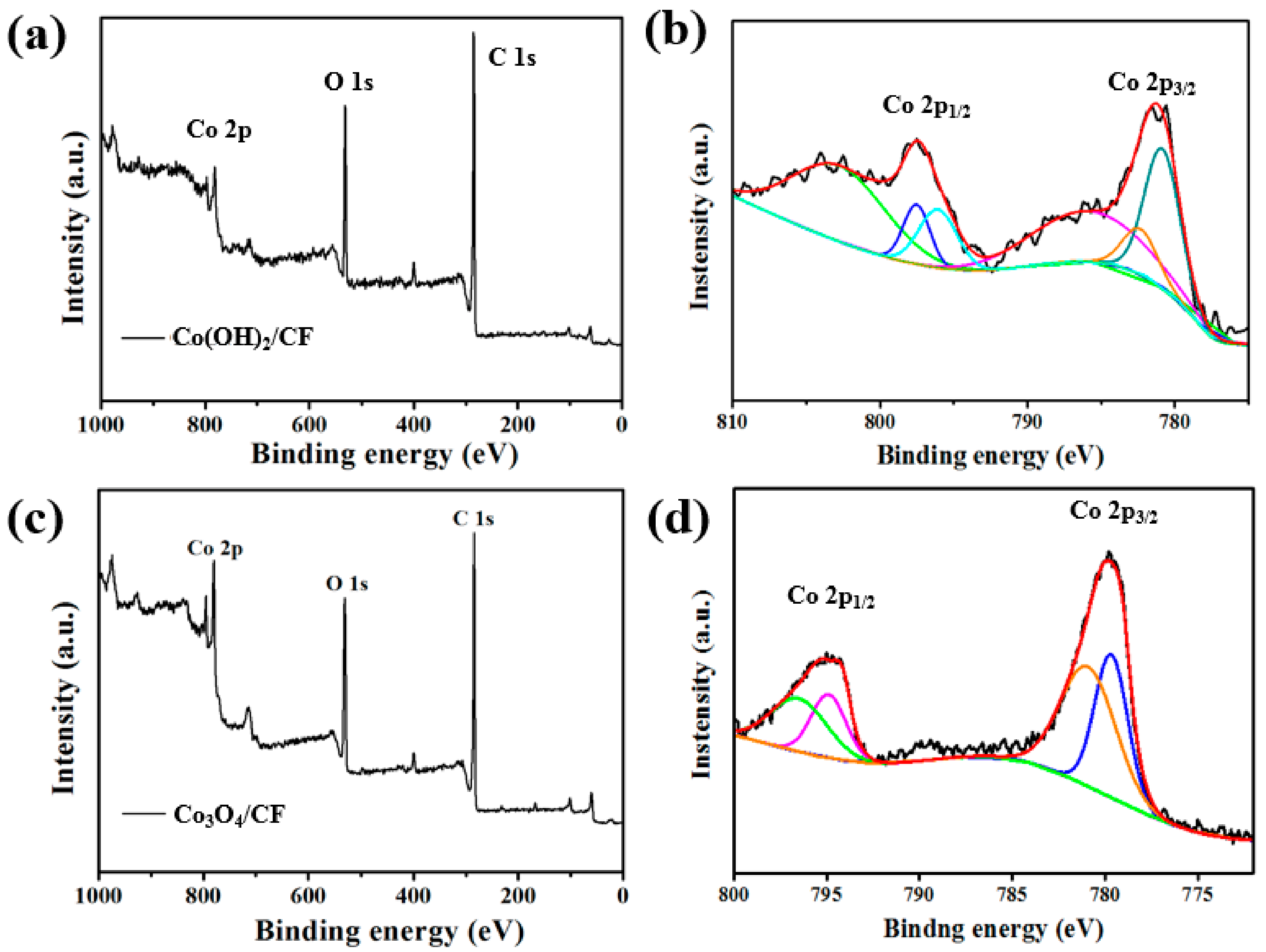
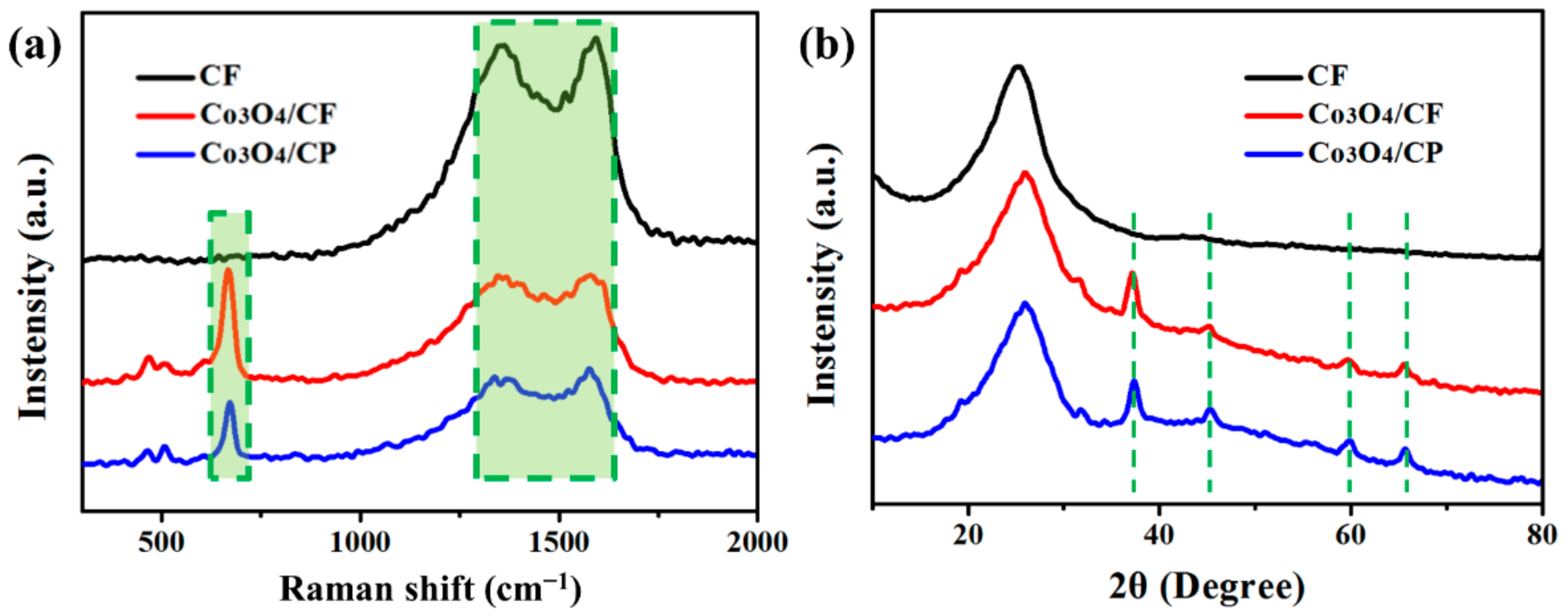
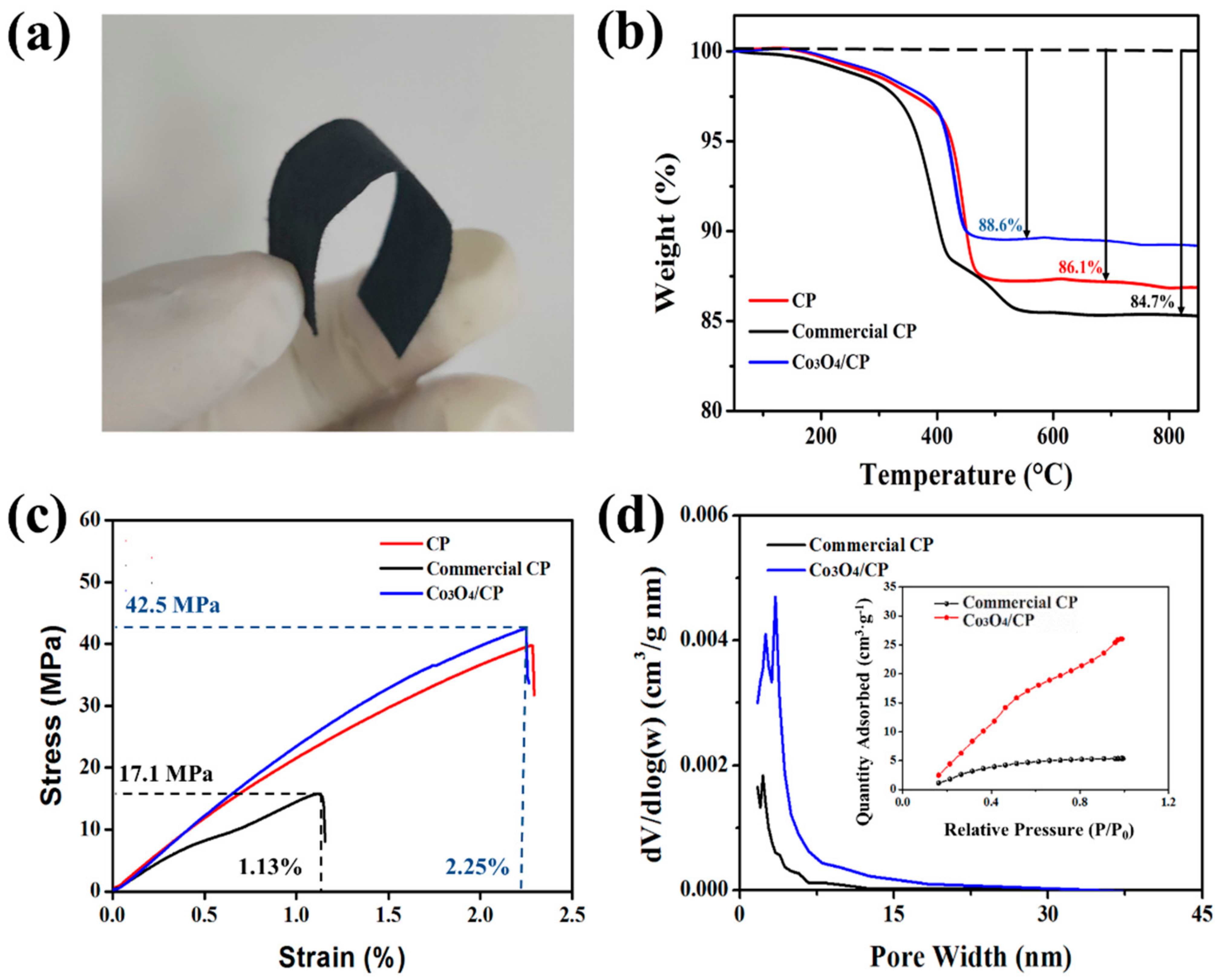
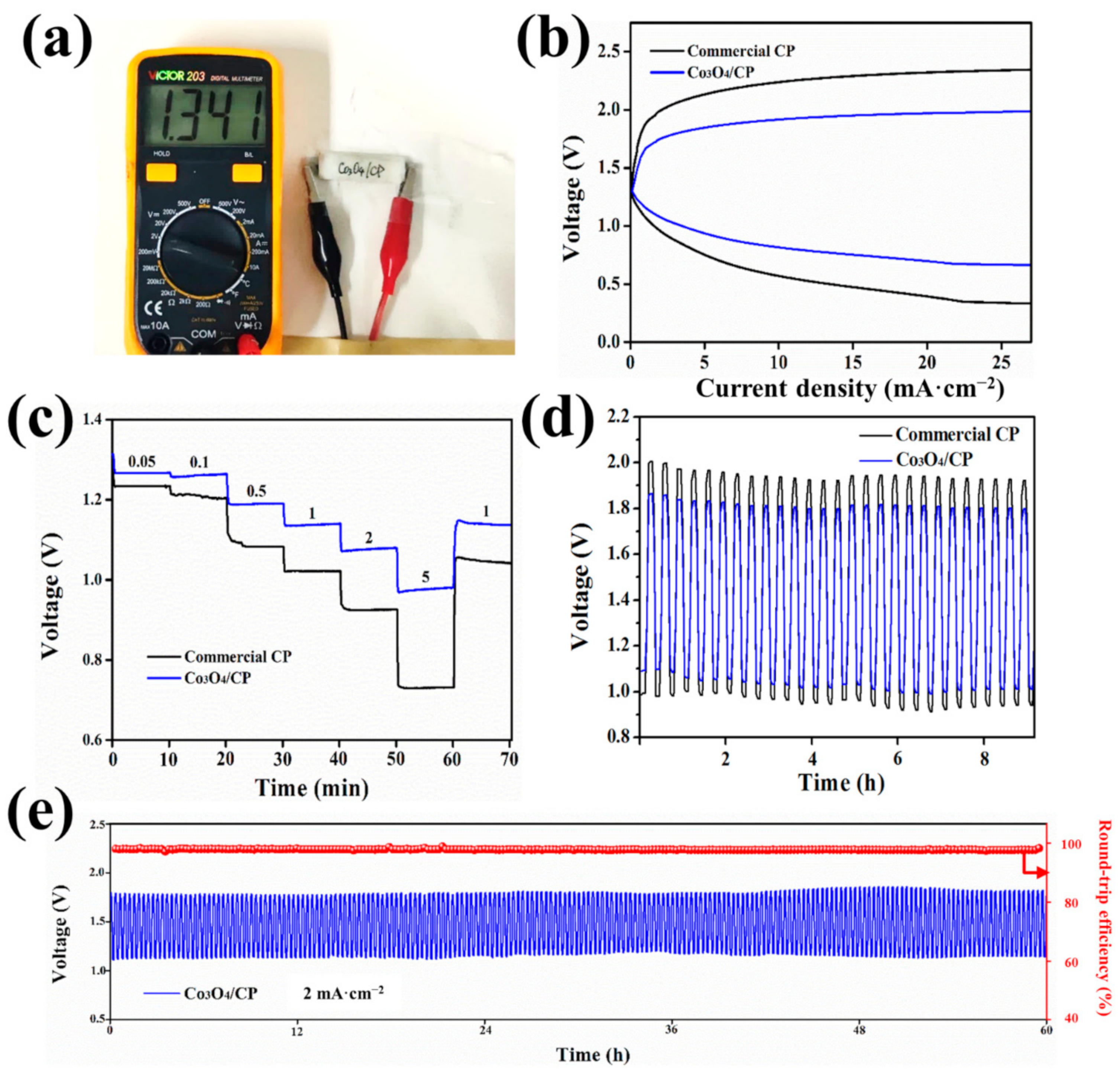
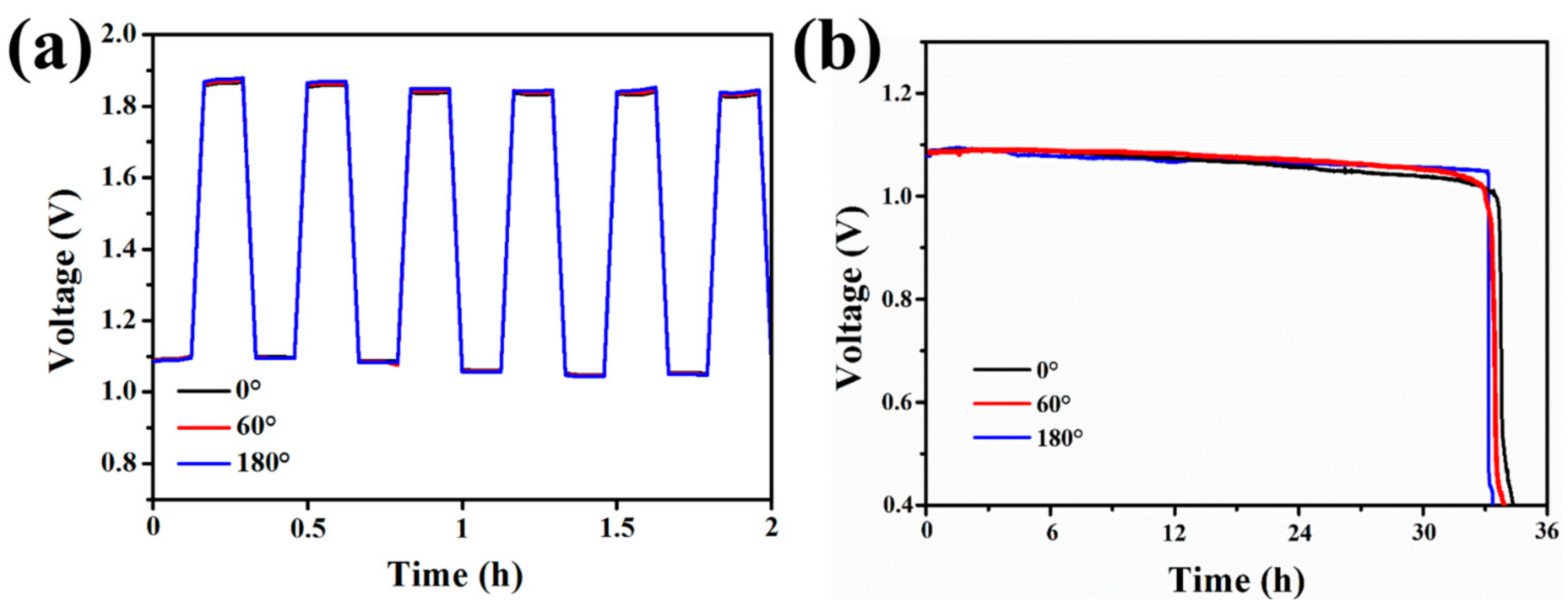
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, J.; Zhang, J.; Song, X.; Zarrin, H.; Tian, X.; Qiao, J.; Rasen, L.; Li, K.; Chen, Z. A flexible solid-state electrolyte for wide-scale integration of rechargeable zinc–air batteries. Energy Environ. Sci. 2015, 9, 663–670. [Google Scholar] [CrossRef]
- Park, J.; Park, M.; Nam, G.; Lee, J.-S.; Cho, J. All-solid-state cable-type flexible zinc-air battery. Adv. Mater. 2015, 27, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc–air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Liang, H.; Li, C.; Bai, J. Enhanced photocatalytic hydrogen production over Co3O4@g-C3N4 p-n junction adhering on one-dimensional carbon fiber. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124200. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Manuel, S.A. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Wang, M.; Ren, G.; Wu, S.; Shen, J. Facilely prepared oxidized carbon Fiber@ Co3O4@ RGO as negative electrode for a novel asymmetric supercapacitor with high areal energy and power density. Appl. Surf. Sci. 2018, 450, 66–76. [Google Scholar] [CrossRef]
- Yang, H.; Leow, W.R.; Chen, X. 3D printing of flexible electronic devices. Small Methods 2018, 450, 1700259. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Zhang, L.; Wan, P. Polymer nanocomposite meshes for flexible electronic devices. Prog. Polym. Sci. 2020, 107, 101279. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, J.; Zang, W.; Kou, Z.; Wang, C.; Ding, X.; Guan, C.; Xiong, T.; Chen, H.; Zhang, Q.; et al. All-solid-state sponge-like squeezable zinc-air battery. Energy Storage Mater. 2019, 23, 375–382. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Blázquez, J.A. High performance secondary zinc-air/silver hybrid battery. J. Energy Storage 2021, 33, 102103. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.-E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Pan, Z.; Wang, X.; Yang, J.; Qiu, Y.; Xu, S.; Lu, Y.; Huang, Q.; Li, W. Hierarchical Co3O4 Nano-micro arrays featuring superior activity as cathode in a flexible and rechargeable zinc–air battery. Adv. Sci. 2019, 6, 1802243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhang, X.; Sun, Y.; Zhang, H.; Wang, C.; Xie, A. Facile synthesis and electrochemical performance of nitrogen-doped porous hollow coaxial carbon fiber/Co3O4 composite. Ceram. Int. 2018, 44, 5848–5854. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, Y.; Xiao, X.; Pang, H. Development and application of carbon fiber in batteries—Science Direct. Chem. Eng. J. 2020, 384, 123294. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Liu, J.; Ma, X.Y.D.; Kong, J.; Zhang, Y.; Yan, T.; Lu, X. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B Environ. 2019, 263, 118344. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Y.; Wang, M.; Fan, X.; Zhang, T.; Peng, L.; Zhou, X.-D.; Qiao, J. High-performing rechargeable/flexible zinc-air batteries by coordinated hierarchical Bi-metallic electrocatalyst and heterostructure anion exchange membrane. Nano Energy 2019, 65, 104021. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Wang, B.; Li, B.-Q.; Cui, X.; Zhang, Q. Defect-rich carbon fiber electrocatalysts with porous graphene skin for flexible solid-state zinc–air batteries. Energy Storage Mater. 2018, 15, 124–130. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, X.F.; Luan, D.; Lou, X.W. NiMn-based bimetal–organic framework nanosheets supported on multi-channel carbon fibers for efficient oxygen electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 18234–18239. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yuan, H.; Huang, J. A bio-inspired nanofibrous Co3O4/TiO2/carbon composite as high-performance anodic material for lithium-ion batteries. J. Alloys Compd. 2020, 819, 153375. [Google Scholar]
- Yin, J.; Li, Z.; Yu, Y.; Lv, Y.; Song, K.; Yang, B.; Yuan, L.; Hu, X. Facile electrodeposition of MFe2O4 (M = Co, Fe) on carbon cloth as air cathodes for Li-O2 batteries. Ceram. Int. 2019, 45, 13401–13408. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Bi, X.; Yang, J.; Zhou, J.; Li, X.; Cheng, J.; Wang, Z.; Wang, B.; Lu, J. Dendrite-free flexible fiber-shaped zn battery with long cycle life in water and air. Adv. Energy Mater. 2019, 9, 1901434. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.H.; Suh, D.H. High micropore number and specific surface of carbon fibers pretreated with a swarm of CO2 micro–nanobubbles. Environ. Chem. Lett. 2021, 19, 3565–3571. [Google Scholar] [CrossRef]
- Choma, J.; Osuchowski, L.; Marszewski, M.; Dziura, A.; Jaroniec, M. Developing microporosity in Kevlar®-derived carbon fibers by CO2 activation for CO2 adsorption. J. CO2 Util. 2016, 16, 17–22. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, K.; Lin, L.; Zhao, W.; Quang, H.T.; Sun, L.; Li, T.; Li, Z.; Liu, X.; Zheng, L.; et al. Large-area synthesis of superclean graphene via selective etching of amorphous carbon with carbon dioxide. Angew. Chem. Int. Ed. 2019, 58, 14446–14451. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Fan, L.; Li, L.; Mao, N.; Qin, X.; Peng, S.; Ramakrishna, S. Hierarchical catalytic electrodes of cobalt-embedded carbon nanotube/carbon flakes arrays for flexible solid-state zinc-air batteries. Carbon 2019, 142, 379–387. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, N.; Zheng, N.; Chen, X.; Mao, J.; Ding, G.; Li, Y.; Sun, F.; Li, Y. MOF-derived porous Co3O4-NC nanoflake arrays on carbon fiber cloth as stable hosts for dendrite-free Li metal anodes. Energy Storage Mater. 2019, 23, 181–189. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B Environ. 2013, 142, 677–683. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Zhong, C.; Liu, Z.; Liu, J.; Ma, L.; Deng, Y.; Han, X.; Wu, T.; Hu, W.; et al. Ultrathin Co3O4 layers with large contact area on carbon fibers as high-performance electrode for flexible zinc–air battery integrated with flexible display. Adv. Energy Mater. 2017, 7, 1700779. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Miao, X.; Yang, W.; Wang, C.; Pan, Q. ZIF-L-Co@carbon fiber paper composite derived Co/Co3O4@C electrocatalyst for ORR in alkali/acidic media and overall seawater splitting. Int. J. Hydrogen Energy 2020, 45, 33028–33036. [Google Scholar] [CrossRef]
- Hu, Z.; Fu, Y.; Hong, Z.; Huang, Y.; Guo, W.; Yang, R.; Xu, J.; Zhou, L.; Yin, S. Composite structural batteries with Co3O4/CNT modified carbon fibers as anode: Computational insights on the interfacial behavior. Compos. Sci. Technol. 2021, 201, 108495. [Google Scholar] [CrossRef]
- Sun, J.; Man, P.; Zhang, Q.; He, B.; Zhou, Z.; Li, C.; Wang, X.; Guo, J.; Zhao, J.; Xie, L.; et al. Hierarchically-structured Co3O4 nanowire arrays grown on carbon nanotube fibers as novel cathodes for high-performance wearable fiber-shaped asymmetric supercapacitors. Appl. Surf. Sci. 2018, 447, 795–801. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, C.; Liu, J.; Zeng, X.; Qu, S.; Han, X.; Deng, Y.; Hu, W.; Lu, J. Atomically thin mesoporous Co3O4 layers strongly coupled with N-rGO nanosheets as high-performance bifunctional catalysts for 1D knittable zinc-air batteries. Adv. Mater. 2018, 30, 1703657. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Zhang, X.; Ma, Y.; Zhou, C.; Yang, J.; Wu, Y.; Li, X.; Luo, Y.; Chen, D. Recent progress in flexible fibrous batteries. ChemElectroChem 2018, 5, 3127–3137. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, Q.; Zhang, Q.; Cui, Y.; Zheng, Z. Fibrous materials for flexible Li–S battery. Adv. Energy Mater. 2021, 11, 2002580. [Google Scholar] [CrossRef]
- Nan, D.; Huang, Z.-H.; Kang, F.-Y.; Shen, W.-C. Research progress on fibrous carbon materials as anode materials for lithium ion batteries. Carbon 2015, 86, 371. [Google Scholar] [CrossRef]
- Deng, L.; Eichhorn, S.J.; Kao, C.-C.; Young, R.J. The effective Young’s modulus of carbon nanotubes in composites. ACS Appl. Mater. Interfaces 2011, 3, 433–440. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Zhang, X.; Zhao, C.; Wang, H.; Li, S.; Liu, Z. Direct conversion of biomass into compact air electrode with atomically dispersed oxygen and nitrogen coordinated copper species for flexible zinc–air batteries. ACS Appl. Energy Mater. 2019, 2, 8659–8666. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, R.; Zang, Y.; Liu, G.; Liu, S.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Shrimp-shell derived carbon nanodots as precursors to fabricate Fe,N-doped porous graphitic carbon electrocatalysts for efficient oxygen reduction in zinc-air batteries. Inorg. Chem. Front. 2016, 3, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Hui, Z.; Zhang, Z. FeCo alloy/N, S dual-doped carbon composite as a high-performance bifunctional catalyst in an advanced rechargeable zinc-air battery. J. Energy Chem. 2021, 56, 64–71. [Google Scholar] [CrossRef]
- Wang, X.; Liao, Z.; Fu, Y.; Neumann, C.; Turchanin, A.; Nam, G.; Feng, X. Confined growth of porous nitrogen-doped cobalt oxide nanoarrays as bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. Energy Storage Mater. 2020, 26, 157–164. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Han, W.; Jia, P.; Li, X.; Jiang, Y.; Ding, Q. Co3O4 Nanoneedle Array Grown on Carbon Fiber Paper for Air Cathodes towards Flexible and Rechargeable Zn–Air Batteries. Nanomaterials 2021, 11, 3321. https://doi.org/10.3390/nano11123321
Li Z, Han W, Jia P, Li X, Jiang Y, Ding Q. Co3O4 Nanoneedle Array Grown on Carbon Fiber Paper for Air Cathodes towards Flexible and Rechargeable Zn–Air Batteries. Nanomaterials. 2021; 11(12):3321. https://doi.org/10.3390/nano11123321
Chicago/Turabian StyleLi, Ziyuan, Wenjia Han, Peng Jia, Xia Li, Yifei Jiang, and Qijun Ding. 2021. "Co3O4 Nanoneedle Array Grown on Carbon Fiber Paper for Air Cathodes towards Flexible and Rechargeable Zn–Air Batteries" Nanomaterials 11, no. 12: 3321. https://doi.org/10.3390/nano11123321
APA StyleLi, Z., Han, W., Jia, P., Li, X., Jiang, Y., & Ding, Q. (2021). Co3O4 Nanoneedle Array Grown on Carbon Fiber Paper for Air Cathodes towards Flexible and Rechargeable Zn–Air Batteries. Nanomaterials, 11(12), 3321. https://doi.org/10.3390/nano11123321






