Detailed Calorimetric Analysis of Mixed Micelle Formation from Aqueous Binary Surfactants for Design of Nanoscale Drug Carriers
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
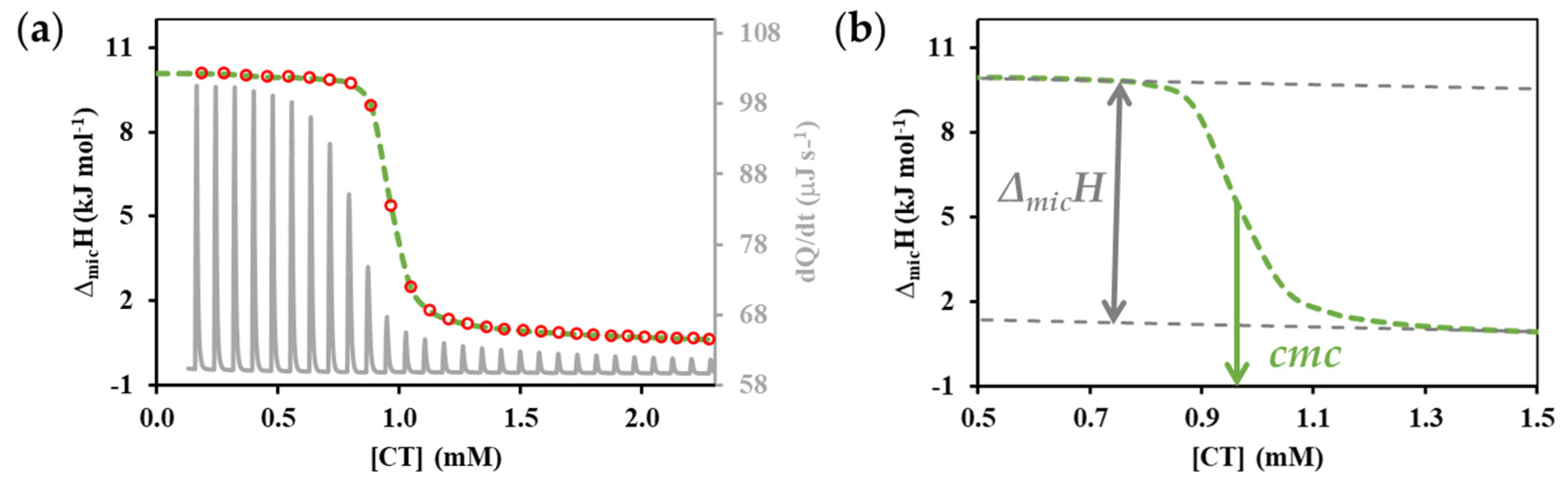
3.1. Micelle Formation of Individual Surfactants
3.2. Thermodynamics (cmc, ΔmicG, ΔmicH and ΔmicS) of Mixed Micelle Formation
3.3. Composition Dependence of Solubilization Capability of Mixed Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Sagir, M.; Mushtaq, M.; Tahir, M.S.; Tahir, M.B.; Shaik, A.R. Surfactants. In Surfactants for Enhanced Oil Recovery Applications; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–11. [Google Scholar]
- Malik, N.A. Drug Solubilization by Surfactants: Experimental Methods and Theoretical Perspectives. Mini Rev. Med. Chem. 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Pisárčik, M.; Lukáč, M.; Jampílek, J.; Bilka, F.; Bilková, A.; Pašková, L.; Devínsky, F.; Horáková, R.; Březina, M.; Opravil, T. Silver nanoparticles stabilized with phosphorus-containing heterocyclic surfactants: Synthesis, physico-chemical properties, and biological activity determination. Nanomaterials 2021, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Durval, I.J.B.; Ribeiro, B.G.; Aguiar, J.S.; Rufino, R.D.; Converti, A.; Sarubbo, L.A. Application of a Biosurfactant Produced by Bacillus cereus UCP 1615 from Waste Frying Oil as an Emulsifier in a Cookie Formulation. Fermentation 2021, 7, 189. [Google Scholar] [CrossRef]
- Cirelli, A.F.; Ojeda, C.; Castro, M.J.L.; Salgot, M. Surfactants in Sludge-Amended Agricultural Soils: A Review. Org. Farming Pest Control. Remediat. Soil Pollut. 2009, 227–251. [Google Scholar] [CrossRef]
- Ivanković, T.; Hrenović, J. Surfactants in the environment. Arh. Hig. Rada Toksikol. 2010, 61, 95–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, remediation and green surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Lourenço, M.J.; Alexandre, J.; Huisman, C.; Paredes, X.; de Castro, C.N. The balance between energy, environmental security, and technical performance: The regulatory challenge of nanofluids. Nanomaterials 2021, 11, 1871. [Google Scholar] [CrossRef]
- Al Sabagh, A.M.; Abdel-Hamid, T.M.; Abdel-Salam, F.H.; El-Din, M.R.N.; Mohamed, A. Surface activity and thermodynamic properties of some green surfactants from wastes in formation water at reservoir conditions. J. Dispers. Sci. Technol. 2020, 41, 1–14. [Google Scholar] [CrossRef]
- Al-Soufi, W.; Novo, M. A Surfactant Concentration Model for the Systematic Determination of the Critical Micellar Concentration and the Transition Width. Molecules 2021, 26, 5339. [Google Scholar] [CrossRef]
- Vitková, Z.; Tárník, M.; Pavlovičová, J.; Vitko, A.; Oremusová, J.; Murgaš, J.; Babinec, A. From Static to Dynamic Modeling of Surfactants Micellization. Tenside Surfactants Deterg. 2021, 58, 4–12. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant Self-Assembling and Critical Micelle Concentration: One Approach Fits All? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef]
- Noor, S.; Taj, M.B.; Rehman, A.; Naz, I. Comparative solubilization of reactive dyes in single and mixed surfactants. J. Dispers. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Staples, E.; Penfold, J.; Tucker, I. Adsorption of Mixed Surfactants at the Oil-Water Interface. J. Phys. Chem. B 2000, 104, 606–614. [Google Scholar] [CrossRef]
- Loh, W.; Brinatti, C.; Tam, K.C. Use of isothermal titration calorimetry to study surfactant aggregation in colloidal systems. Biochim. Biophys. Acta -Gen. Subj. 2016, 1860, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Wyrzykowski, D.; Szutkowski, K.; Greber, K.; Lubecka, E.A.; Zhukov, I. Thermodynamics, size, and dynamics of zwitterionic dodecylphosphocholine and anionic sodium dodecyl sulfate mixed micelles. J. Therm. Anal. Calorim. 2016, 123, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Juhász, Á.; Tabajdi, R.; Dékány, I.; Csapó, E. Thermodynamic Characterization of Temperature- and Composition-Dependent Mixed Micelle Formation in Aqueous Medium. J. Surfactants Deterg. 2017, 20, 1291–1299. [Google Scholar] [CrossRef] [Green Version]
- Storm, S.; Jakobtorweihen, S.; Smirnova, I. Solubilization in mixed micelles studied by molecular dynamics simulations and COSMOmic. J. Phys. Chem. B 2014, 118, 3593–3604. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Lemon oil solubilization in mixed surfactant solutions: Rationalizing microemulsion & nanoemulsion formation. Food Hydrocoll. 2012, 26, 268–276. [Google Scholar] [CrossRef]
- Kumar, A.; Kansal, S.K.; Ibhadon, A.O.; Mehta, S.K. Mixed surfactant (altering chain length and head group) aggregates as an effective carrier for tuberculosis drug. Chem. Phys. Lipids 2018, 215, 11–17. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Zhao, Y.; Liu, H.; Zhao, Y.; Li, X.; Lin, Q. Biodegradable Micelles for NIR/GSH-Triggered Chemophototherapy of Cancer. Nanomaterials 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.X.; Zhang, S.L.; Yang, Y.Q.; Yu, Z.J.; Li, L.; Wang, Y.S.; Zhang, L.S. Temperature-resistant and salt-tolerant mixed surfactant system for EOR in the Tahe Oilfield. Pet. Sci. 2021, 18, 667–678. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Aleshina, A.L.; Arkharova, N.A.; Orekhov, A.S.; Kuklin, A.I.; Philippova, O.E. Disruption of cationic/anionic viscoelastic surfactant micellar networks by hydrocarbon as a basis of enhanced fracturing fluids clean-up. Nanomaterials 2020, 10, 2353. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Marques, E.F. Synergism and polymorphism in mixed surfactant systems. Curr. Opin. Colloid Interface Sci. 1999, 4, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Páhi, A.B.; Király, Z.; Mastalir, Á.; Dudás, J.; Puskás, S.; Vágó, Á. Thermodynamics of micelle formation of the counterion coupled gemini surfactant bis(4- (2-dodecyl) benzenesuifonate)-jeffamine salt and its dynamic adsorption on sandstone. J. Phys. Chem. B 2008, 112, 15320–15326. [Google Scholar] [CrossRef] [PubMed]
- Páhi, A.B.; Király, Z.; Puskás, S. Mass spectrometric characterization of the non-ionic gemini surfactant Surfynol 465 and a microcalorimetric study of its micelle formation in water. Colloids Surf. A Physicochem. Eng. Asp. 2009, 345, 13–17. [Google Scholar] [CrossRef]
- Heintz, A.; Lehmann, J.K.; Kozlova, S.A.; Balantseva, E.V.; Bazyleva, A.B.; Ondo, D. Micelle formation of alkylimidazolium ionic liquids in water and in ethylammonium nitrate ionic liquid: A calorimetric study. Fluid Phase Equilib. 2010, 294, 187–196. [Google Scholar] [CrossRef]
- Prasad, M.; Chakraborty, I.; Rakshit, A.K.; Moulik, S.P. Critical evaluation of micellization behavior of nonionic surfactant MEGA 10 in comparison with ionic surfactant tetradecyltriphenylphosphonium bromide studied by microcalorimetric method in aqueous medium. J. Phys. Chem. B 2006, 110, 9815–9821. [Google Scholar] [CrossRef]
- Clint, J.H. Micellization of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 1327–1334. [Google Scholar] [CrossRef]
- Holland, P.M.; Rubingh, D.N. Nonideal multicomponent mixed micelle model. J. Phys. Chem. 1983, 87, 1984–1990. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Minardi, R.M.; Schulz, E.P.; Pieroni, O.; Schulz, P.C. The Composition of Mixed Micelles Formed by Dodecyl Trimethyl Ammonium Bromide and Benzethonium Chloride in Water. J. Surfactants Deterg. 2012, 15, 147–155. [Google Scholar] [CrossRef]


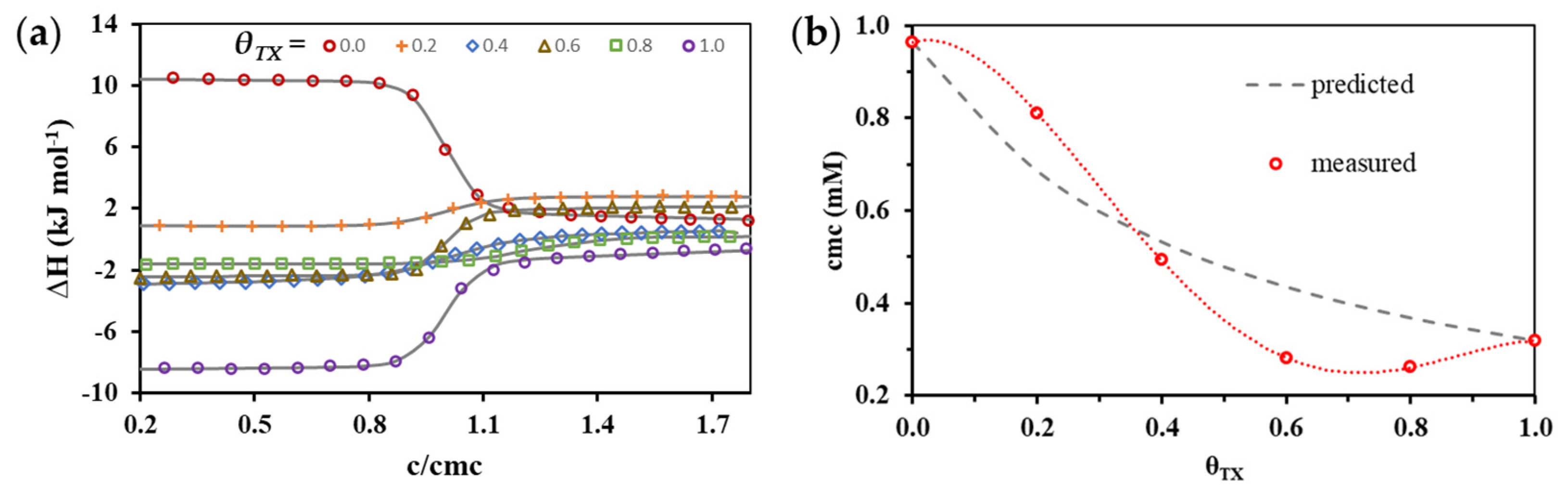
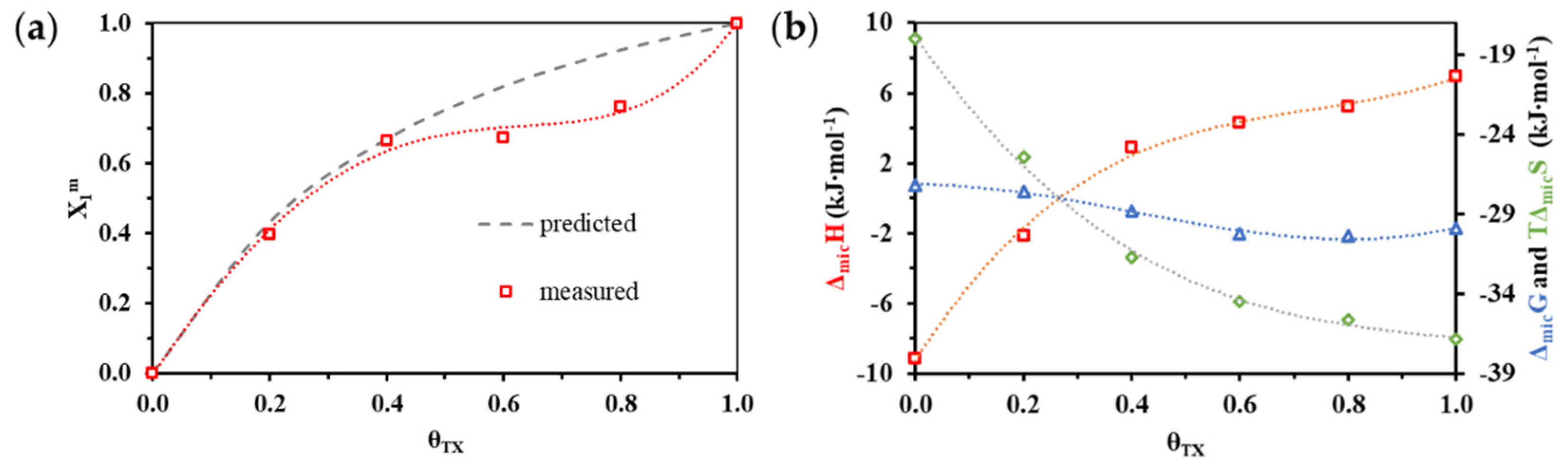
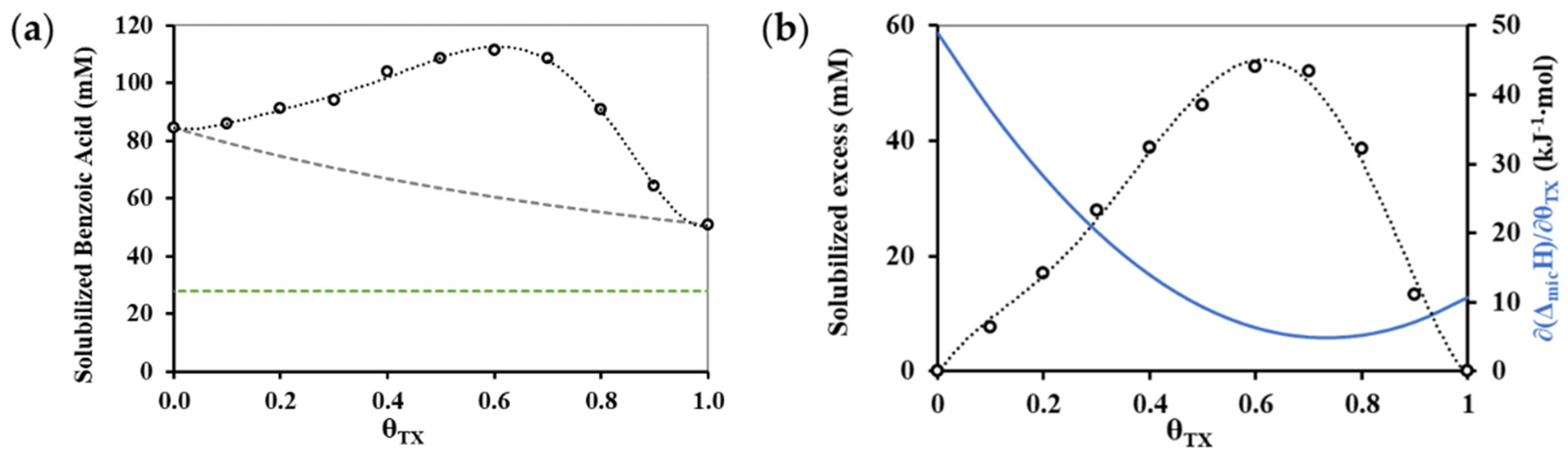
| θTX | cmc (mM) | ΔmicH (kJ mol−1) |
|---|---|---|
| 0.0 1 | 0.964 ± 0.005 | −9.16 ± 0.77 |
| 0.2 | 0.810 ± 0.009 | −2.14 ± 0.07 |
| 0.4 | 0.493 ± 0.003 | 2.92 ± 0.06 |
| 0.6 | 0.281 ± 0.002 | 4.28 ± 0.28 |
| 0.8 | 0.261 ± 0.002 | 5.22 ± 0.36 |
| 1.0 2 | 0.319 ± 0.003 | 6.96 ± 0.72 |
| θTX | VNaOH (mL) | Solubilized Amount (mM) |
|---|---|---|
| 0.0 1 | 4.10 ± 0.10 | 50.8 ± 1.9 |
| 0.1 | 4.77 ± 0.06 | 63.6 ± 1.1 |
| 0.2 | 6.20 ± 0.10 | 91.0 ± 1.9 |
| 0.3 | 7.10 ± 0.10 | 108.3 ± 1.9 |
| 0.4 | 7.27 ± 0.06 | 111.5 ± 1.1 |
| 0.5 | 7.10 ± 0.10 | 108.3 ± 1.9 |
| 0.6 | 6.87 ± 0.06 | 103.8 ± 1.1 |
| 0.7 | 6.33 ± 0.06 | 93.6 ± 1.1 |
| 0.8 | 6.20 ± 0.10 | 91.0 ± 1.9 |
| 0.9 | 5.90 ± 0.10 | 85.3 ± 1.9 |
| 1.0 2 | 5.83 ± 0.06 | 84.0 ± 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, Á.; Seres, L.; Varga, N.; Ungor, D.; Wojnicki, M.; Csapó, E. Detailed Calorimetric Analysis of Mixed Micelle Formation from Aqueous Binary Surfactants for Design of Nanoscale Drug Carriers. Nanomaterials 2021, 11, 3288. https://doi.org/10.3390/nano11123288
Juhász Á, Seres L, Varga N, Ungor D, Wojnicki M, Csapó E. Detailed Calorimetric Analysis of Mixed Micelle Formation from Aqueous Binary Surfactants for Design of Nanoscale Drug Carriers. Nanomaterials. 2021; 11(12):3288. https://doi.org/10.3390/nano11123288
Chicago/Turabian StyleJuhász, Ádám, László Seres, Norbert Varga, Ditta Ungor, Marek Wojnicki, and Edit Csapó. 2021. "Detailed Calorimetric Analysis of Mixed Micelle Formation from Aqueous Binary Surfactants for Design of Nanoscale Drug Carriers" Nanomaterials 11, no. 12: 3288. https://doi.org/10.3390/nano11123288
APA StyleJuhász, Á., Seres, L., Varga, N., Ungor, D., Wojnicki, M., & Csapó, E. (2021). Detailed Calorimetric Analysis of Mixed Micelle Formation from Aqueous Binary Surfactants for Design of Nanoscale Drug Carriers. Nanomaterials, 11(12), 3288. https://doi.org/10.3390/nano11123288











