Combined Layer-by-Layer/Hydrothermal Synthesis of Fe3O4@MIL-100(Fe) for Ofloxacin Adsorption from Environmental Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. MIL-100(Fe)
2.2.2. Fe3O4@MIL-100(Fe)
2.3. Characterization Techniques
2.4. Analytical Measurements
2.5. Adsorption Experiments
2.5.1. Adsorption Equilibrium Study
2.5.2. Kinetic Study
3. Results and Discussion
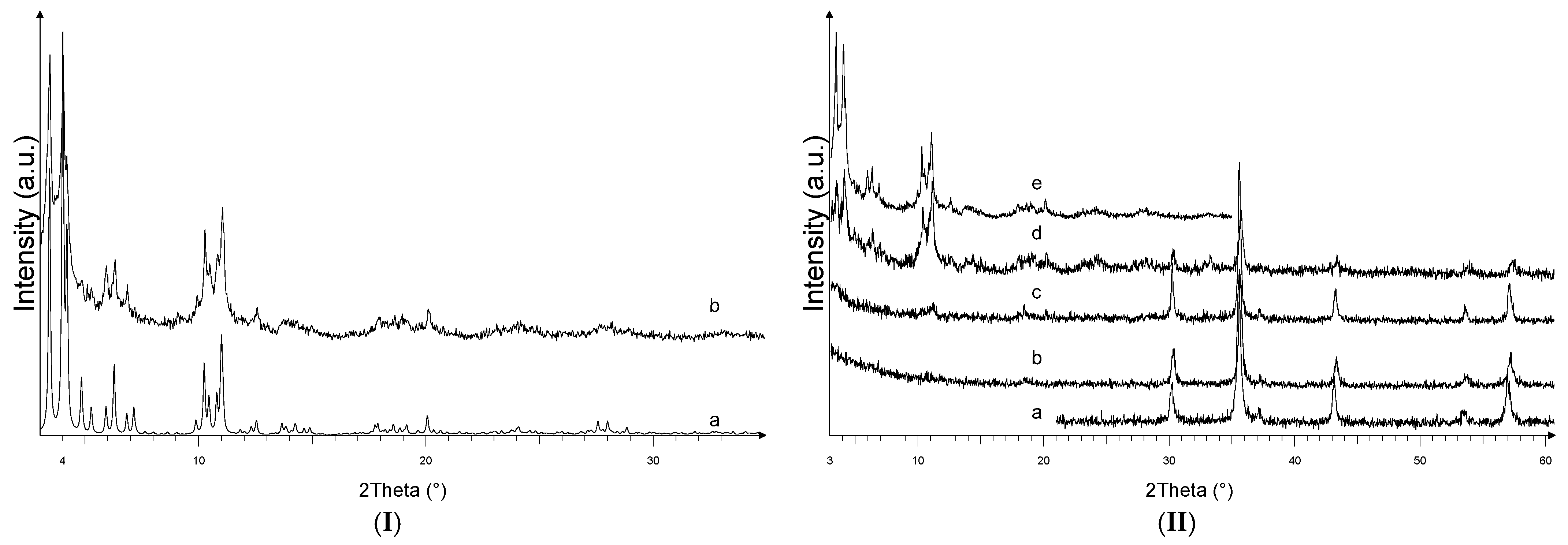
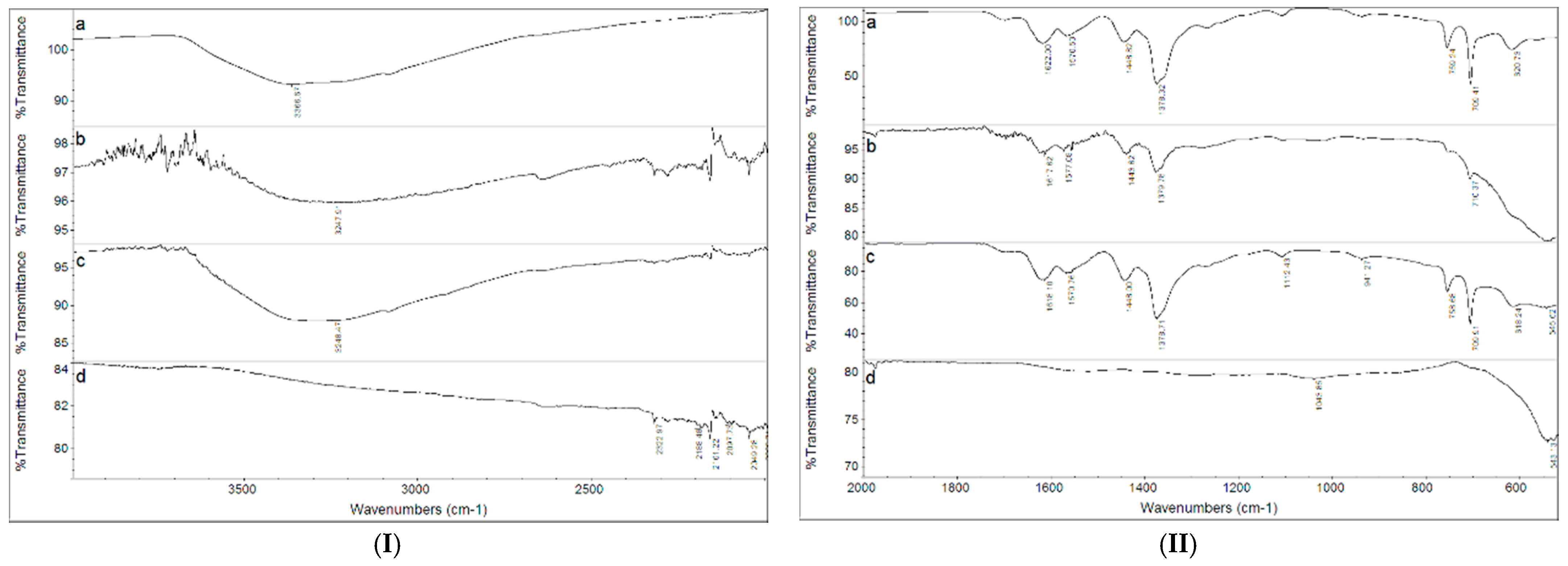
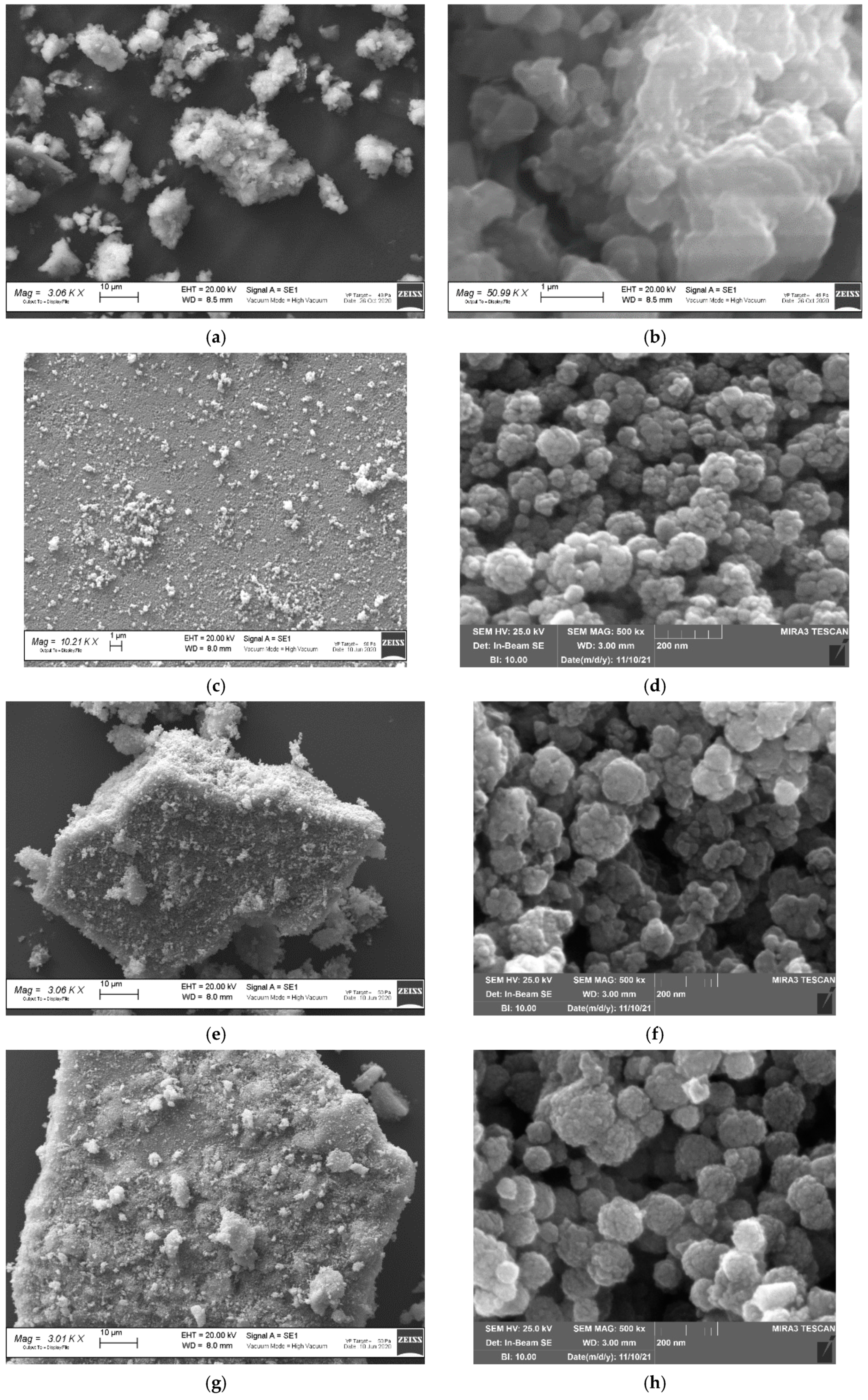
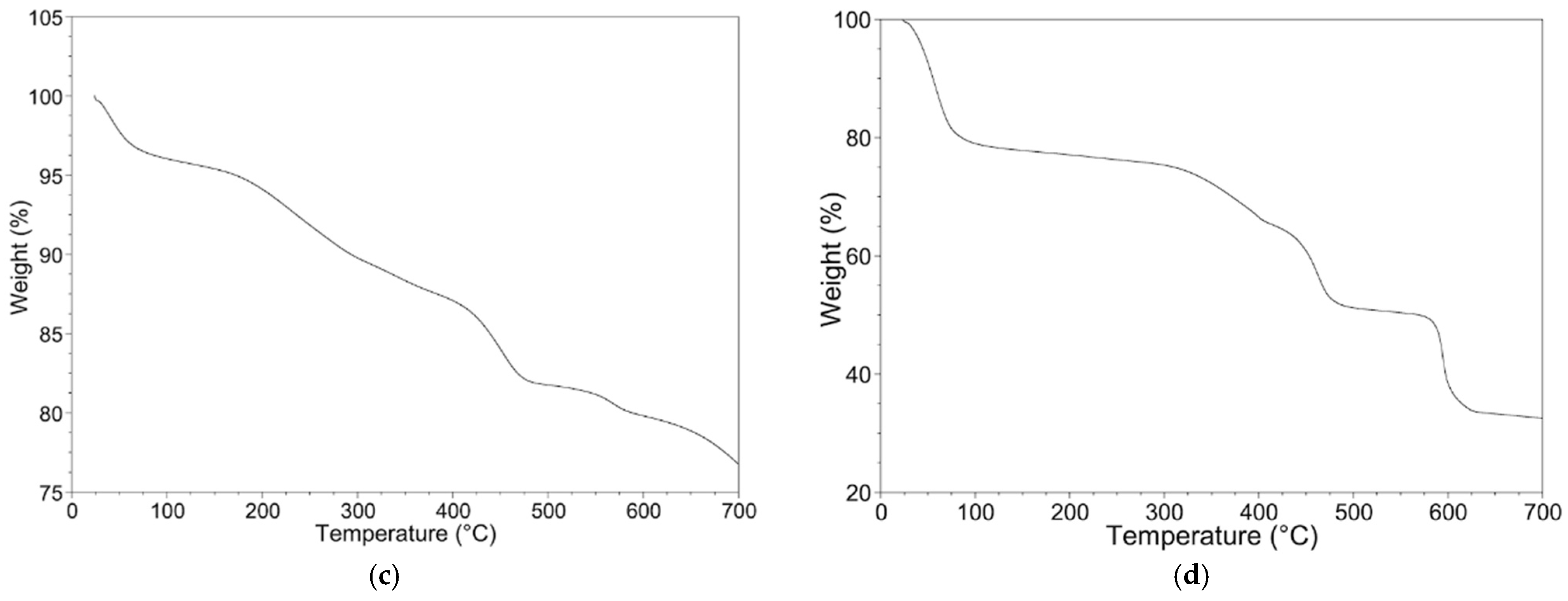
3.1. Fe3O4, MIL-100(Fe) and Fe3O4@MIL-100(Fe) Characterization
3.2. Preliminary Adsorption Experiments
3.3. Isotherm and Kinetic Studies
3.3.1. Isotherm Study
3.3.2. Kinetic Study
3.3.3. Ofloxacin Removal under Relevant Environmental Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters/Ions | Tap Water | River Water | |
|---|---|---|---|
| pH | 7.7 | 7.9 | |
| Conductivity at 20 °C | µS cm−1 | 278 | 297 |
| TOC | mg L−1 | <2 | 4.9 |
| Cl− | mg L−1 | 4.5 | 3.8 |
| NO3− | mg L−1 | 0.6 | 1.5 |
| SO42− | mg L−1 | 5.0 | 12.5 |
| HCO3− | mg L−1 | 195 | 200 |
| Ca2+ | mg L−1 | 38 | 56 |
| Mg2+ | mg L−1 | 12 | 7.0 |
| Na+ | mg L−1 | 11 | 5.0 |
References
- Yaghi, O.M.; Li, H.; Davis, C.; Richardson, D.; Groy, T.L. Synthetic Strategies, Structure Patterns, and Emerging Properties in the Chemistry of Modular Porous Solids. Acc. Chem. Res. 1998, 31, 474–484. [Google Scholar] [CrossRef]
- Wang, P.-L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal–Organic Frameworks for Food Safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Hu, Y.H.; Zhang, L. Hydrogen Storage in Metal-Organic Frameworks. Adv. Mater. 2010, 22, E117–E130. [Google Scholar] [CrossRef]
- Li, Y.; Wen, G. Advances in Metal-Organic Frameworks for Acetylene Storage. Eur. J. Inorg. Chem. 2020, 2020, 2303–2311. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Lin, W. Nanoscale metal–organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef]
- Anderson, S.L.; Boyd, P.G.; Gładysiak, A.; Nguyen, T.N.; Palgrave, R.G.; Kubicki, D.; Emsley, L.; Bradshaw, D.; Rosseinsky, M.J.; Smit, B.; et al. Nucleobase pairing and photodimerization in a biologically derived metal-organic framework nanoreactor. Nat. Commun. 2019, 10, 1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Chai, Y.; Li, P.; Wang, B. Metal–Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Acc. Chem. Res. 2019, 52, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-Organic Frameworks in Green Analytical Chemistry. Separations 2019, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Healy, C.; Patil, K.M.; Wilson, B.H.; Hermanspahn, L.; Harvey-Reid, N.C.; Howard, B.I.; Kleinjan, C.; Kolien, J.; Payet, F.; Telfer, S.G.; et al. The thermal stability of metal-organic frameworks. Coord. Chem. Rev. 2020, 419, 213388. [Google Scholar] [CrossRef]
- Wu, H.; Yildirim, T.; Zhou, W. Exceptional Mechanical Stability of Highly Porous Zirconium Metal–Organic Framework UiO-66 and Its Important Implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Boyd, P.G.; Sarkisov, L.; Smit, B. Improving the Mechanical Stability of Metal–Organic Frameworks Using Chemical Caryatids. ACS Cent. Sci. 2018, 4, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Canioni, R.; Roch-Marchal, C.; Sécheresse, F.; Horcajada, P.; Serre, C.; Hardi-Dan, M.; Férey, G.; Grenèche, J.-M.; Lefebvre, F.; Chang, J.-S.; et al. Stable polyoxometalate insertion within the mesoporous metal organic framework MIL-100(Fe). J. Mater. Chem. 2011, 21, 1226–1233. [Google Scholar] [CrossRef]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.-Y.; Seo, Y.-K.; Chang, J.-S.; Grenèche, J.-M.; Margiolaki, I.; Férey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large pores. Chem. Commun. 2007, 2820–2822. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Liu, D.; Zhang, J. Applications of Porous Metal–Organic Framework MIL-100(M) (M = Cr, Fe, Sc, Al, V). Cryst. Growth Des. 2018, 18, 7730–7744. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef]
- Yoon, J.W.; Seo, Y.-K.; Hwang, Y.K.; Chang, J.-S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E.; et al. Controlled Reducibility of a Metal-Organic Framework with Coordinatively Unsaturated Sites for Preferential Gas Sorption. Angew. Chem. Int. Ed. 2010, 49, 5949–5952. [Google Scholar] [CrossRef]
- Yoon, J.W.; Lee, J.S.; Lee, S.; Cho, K.H.; Hwang, Y.K.; Daturi, M.; Jun, C.-H.; Krishna, R.; Chang, J.-S. Adsorptive Separation of Acetylene from Light Hydrocarbons by Mesoporous Iron Trimesate MIL-100(Fe). Chem. Eur. J. 2015, 21, 18431–18438. [Google Scholar] [CrossRef]
- Rapeyko, A.; Arias, K.S.; Climent, M.J.; Corma, A.; Iborra, S. Polymers from biomass: One pot two-step synthesis of furilydenepropanenitrile derivatives with MIL-100(Fe) catalyst. Catal. Sci. Technol. 2017, 7, 3008–3016. [Google Scholar] [CrossRef] [Green Version]
- Taherzade, S.; Soleimannejad, J.; Tarlani, A. Application of Metal-Organic Framework Nano-MIL-100(Fe) for Sustainable Release of Doxycycline and Tetracycline. Nanomaterials 2017, 7, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singco, B.; Liu, L.-H.; Chen, Y.-T.; Shih, Y.-H.; Huang, H.-Y.; Lin, C.-H. Approaches to drug delivery: Confinement of aspirin in MIL-100(Fe) and aspirin in the de novo synthesis of metal–organic frameworks. Microporous Mesoporous Mater. 2016, 223, 254–260. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Wu, S.-H.; Jia, S.-Y.; Qin, F.-X.; Zhou, S.-M.; Ren, H.-T.; Na, P.; Liu, Y. Novel hematite nanorods and magnetite nanoparticles prepared from MIL-100(Fe) template for the removal of As(V). Mater. Lett. 2014, 132, 8–10. [Google Scholar] [CrossRef]
- Jia, Y.; Jin, Q.; Li, Y.; Sun, Y.; Huo, J.; Zhao, X. Investigation of the adsorption behaviour of different types of dyes on MIL-100(Fe) and their removal from natural water. Anal. Methods 2015, 7, 1463–1470. [Google Scholar] [CrossRef]
- Berardozzi, E.; Tuninetti, J.S.; Einschlag, F.S.G.; Azzaroni, O.; Ceolín, M.; Rafti, M. Comparison of Arsenate Adsorption from Neutral pH Aqueous Solutions Using Two Different Iron-Trimesate Porous Solids: Kinetics, Equilibrium Isotherms, and Synchrotron X-ray Absorption Experiments. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1185–1194. [Google Scholar] [CrossRef]
- Jang, H.-Y.; Kang, J.-K.; Park, J.-A.; Lee, S.-C.; Kim, S.-B. Metal-organic framework MIL-100(Fe) for dye removal in aqueous solutions: Prediction by artificial neural network and response surface methodology modeling. Environ. Pollut. 2020, 267, 115583. [Google Scholar] [CrossRef]
- Tan, K.L.; Foo, K.Y. Preparation of MIL-100 via a novel water-based heatless synthesis technique for the effective remediation of phenoxyacetic acid-based pesticide. J. Environ. Chem. Eng. 2021, 9, 104923. [Google Scholar] [CrossRef]
- Zhuang, S.; Liu, Y.; Wang, J. Mechanistic insight into the adsorption of diclofenac by MIL-100: Experiments and theoretical calculations. Environ. Pollut. 2019, 253, 616–624. [Google Scholar] [CrossRef]
- Chen, S.; Zang, Z.; Zhang, S.; Ouyang, G.; Han, R. Preparation of MIL-100(Fe) and multi-walled carbon nanotubes nanocomposite with high adsorption capacity towards Oxytetracycline from solution. J. Environ. Chem. Eng. 2021, 9, 104780. [Google Scholar] [CrossRef]
- Li, W.; Cao, J.; Xiong, W.; Yang, Z.; Sun, S.; Jia, M.; Xu, Z. In-situ growing of metal-organic frameworks on three-dimensional iron network as an efficient adsorbent for antibiotics removal. Chem. Eng. J. 2020, 392, 124844. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Umar, A.; Khan, M.A.; Algarni, H.; Kansal, S.K. Removal of fluoroquinolone drug, levofloxacin, from aqueous phase over iron based MOFs, MIL-100(Fe). J. Solid State Chem. 2020, 281, 121029. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, and the European Economic and Social Committee: European Union Strategic Approach to Pharmaceuticals in the Environment; European Commision: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX:52019DC0128 (accessed on 18 November 2021).
- Merlin, C. Reducing the Consumption of Antibiotics: Would That Be Enough to Slow Down the Dissemination of Resistances in the Downstream Environment? Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophia, A.C.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Helbling, D.E.; Dichtel, W.R. Best Practices for Evaluating New Materials as Adsorbents for Water Treatment. ACS Mater. Lett. 2020, 2, 1532–1544. [Google Scholar] [CrossRef]
- Doherty, C.M.; Knystautas, E.; Buso, D.; Villanova, L.; Konstas, K.; Hill, A.J.; Takahashi, M.; Falcaro, P. Magnetic framework composites for polycyclic aromatic hydrocarbon sequestration. J. Mater. Chem. 2012, 22, 11470. [Google Scholar] [CrossRef]
- Huo, S.-H.; Yan, X.-P. Facile magnetization of metal–organic framework MIL-101 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples. Analyst 2012, 137, 3445. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Yan, X.; Kong, L.; Zhang, G.; Liu, H.; Qiu, J.; Yeung, K.L. Synthesis of Fe3O4@ZIF-8 magnetic core–shell microspheres and their potential application in a capillary microreactor. Chem. Eng. J. 2013, 228, 398–404. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, L.; Bao, C.; Ma, J.; Liu, M.; Wang, F. Magnetic responsive metal–organic frameworks nanosphere with core–shell structure for highly efficient removal of methylene blue. Chem. Eng. J. 2016, 283, 1127–1136. [Google Scholar] [CrossRef]
- Yu, S.; Wan, J.; Chen, K. A facile synthesis of superparamagnetic Fe3O4 supraparticles@MIL-100(Fe) core–shell nanostructures: Preparation, characterization and biocompatibility. J. Colloid Interface Sci. 2016, 461, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, J.; Jin, Y.; Fu, Y.; Zhong, Y.; Zhu, W. Facile synthesis of MIL-100(Fe) under HF-free conditions and its application in the acetalization of aldehydes with diols. Chem. Eng. J. 2015, 259, 183–190. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, Y.-X.J.; Yu, J.C.; Cham-Fai Leung, K. Tuning the Grain Size and Particle Size of Superparamagnetic Fe3O4 Microparticles. Chem. Mater. 2009, 21, 5079–5087. [Google Scholar] [CrossRef]
- Capsoni, D.; Guerra, G.; Puscalau, C.; Maraschi, F.; Bruni, G.; Monteforte, F.; Profumo, A.; Sturini, M. Zinc Based Metal-Organic Frameworks as Ofloxacin Adsorbents in Polluted Waters: ZIF-8 vs. Zn3(BTC)2. Int. J. Environ. Res. Public Health 2021, 18, 1433. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Alinia Asli, M.; Vossoughi, M. Metal-organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Huang, S.; Yang, K.-L.; Liu, X.-F.; Pan, H.; Zhang, H.; Yang, S. MIL-100(Fe)-catalyzed efficient conversion of hexoses to lactic acid. RSC Adv. 2017, 7, 5621–5627. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Wu, Z. High adsorption for ofloxacin and reusability by the use of ZIF-8 for wastewater treatment. Microporous Mesoporous Mater. 2020, 308, 110494. [Google Scholar] [CrossRef]
- Kida, K.; Okita, M.; Fujita, K.; Tanaka, S.; Miyake, Y. Formation of high crystalline ZIF-8 in an aqueous solution. CrystEngComm 2013, 15, 1794. [Google Scholar] [CrossRef]
- Moradi, S.E.; Haji Shabani, A.M.; Dadfarnia, S.; Emami, S. Effective removal of ciprofloxacin from aqueous solutions using magnetic metal–organic framework sorbents: Mechanisms, isotherms and kinetics. J. Iran. Chem. Soc. 2016, 13, 1617–1627. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Shi, L.; Xue, J.; Liang, A.; Zhang, D. Opposite impacts of chemical oxidation for ofloxacin adsorption on activated carbon and carbon nanotubes. Sci. Total Environ. 2021, 771, 145455. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4583, Ofloxacin. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ofloxacin#section=Dissociation-Constants (accessed on 5 August 2021).
- Aslam, S.; Zeng, J.; Subhan, F.; Li, M.; Lyu, F.; Li, Y.; Yan, Z. In situ one-step synthesis of Fe3O4@MIL-100(Fe) core-shells for adsorption of methylene blue from water. J. Colloid Interface Sci. 2017, 505, 186–195. [Google Scholar] [CrossRef]
- Li, Z.; Ma, M.; Zhang, S.; Zhang, Z.; Zhou, L.; Yun, J.; Liu, R. Efficiently removal of ciprofloxacin from aqueous solution by MIL-101(Cr)-HSO3: The enhanced electrostatic interaction. J. Porous Mater. 2020, 27, 189–204. [Google Scholar] [CrossRef]
- Zhang, H.; Jieying, W.; Zhengji, L.; Fan, R.; Chen, Q.; Shan, X.; Jiang, C.; Sun, G. Extraction of phenylurea herbicides from rice and environmental water utilizing MIL-100(Fe)-functionalized magnetic adsorbents. New J. Chem. 2020, 44, 1548–1555. [Google Scholar] [CrossRef]


 ), Freundlich (
), Freundlich (  ), BET (
), BET (  ) for Ofloxacin (OFL) on (a) MIL-100 and (b) Fe3O4@MIL-100_H. (Experimental conditions: MOF 10 mg, 20 mL OFL solution from 10 to 293 mg L−1; RDS ≤ 10%).
) for Ofloxacin (OFL) on (a) MIL-100 and (b) Fe3O4@MIL-100_H. (Experimental conditions: MOF 10 mg, 20 mL OFL solution from 10 to 293 mg L−1; RDS ≤ 10%).
 ), Freundlich (
), Freundlich (  ), BET (
), BET (  ) for Ofloxacin (OFL) on (a) MIL-100 and (b) Fe3O4@MIL-100_H. (Experimental conditions: MOF 10 mg, 20 mL OFL solution from 10 to 293 mg L−1; RDS ≤ 10%).
) for Ofloxacin (OFL) on (a) MIL-100 and (b) Fe3O4@MIL-100_H. (Experimental conditions: MOF 10 mg, 20 mL OFL solution from 10 to 293 mg L−1; RDS ≤ 10%).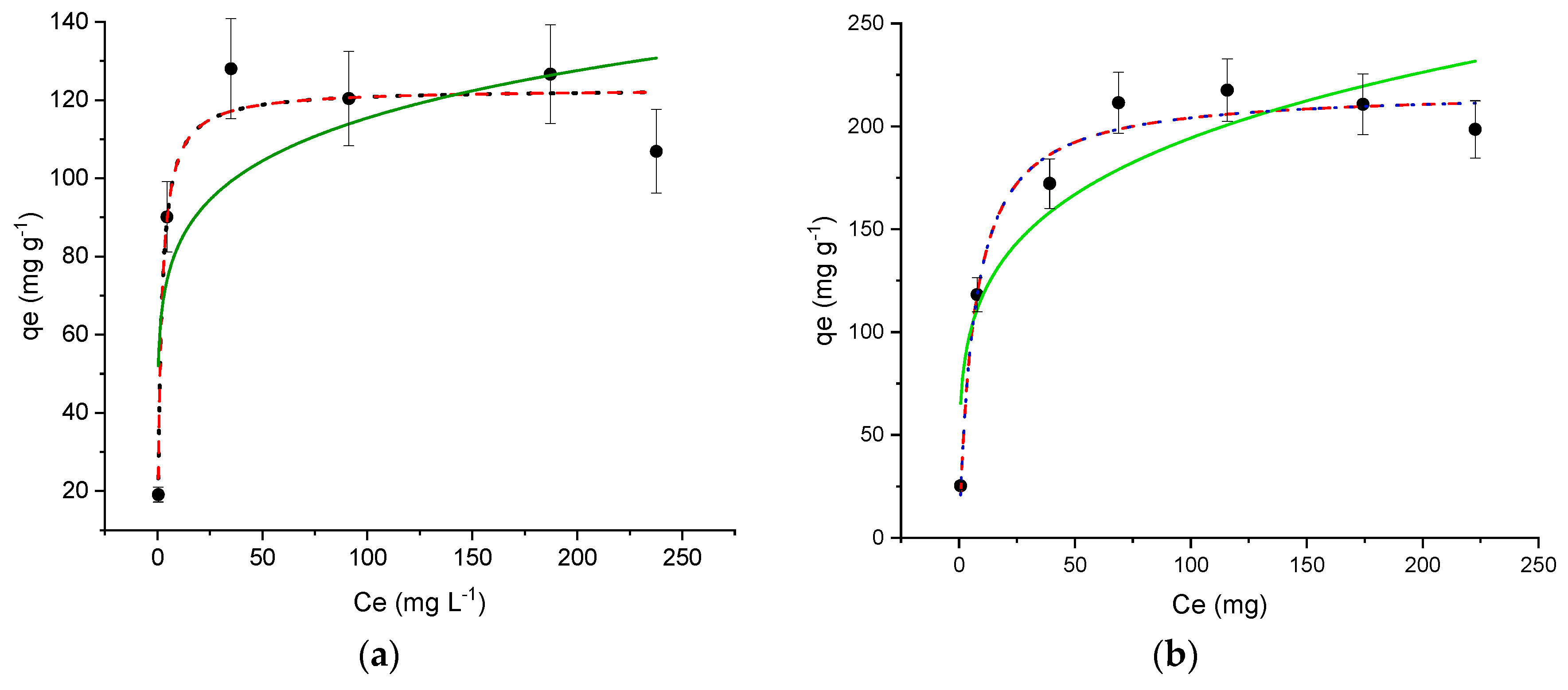
 ), pseudo-second order (
), pseudo-second order (  ) for OFL on (a) MIL-100 and (b) Fe3O4@MIL-100_H (Experimental conditions: MOF 20 mg, 40 mL tap water, OFL initial concentration 20 mg L−1; RDS ≤ 10%).
) for OFL on (a) MIL-100 and (b) Fe3O4@MIL-100_H (Experimental conditions: MOF 20 mg, 40 mL tap water, OFL initial concentration 20 mg L−1; RDS ≤ 10%).
 ), pseudo-second order (
), pseudo-second order (  ) for OFL on (a) MIL-100 and (b) Fe3O4@MIL-100_H (Experimental conditions: MOF 20 mg, 40 mL tap water, OFL initial concentration 20 mg L−1; RDS ≤ 10%).
) for OFL on (a) MIL-100 and (b) Fe3O4@MIL-100_H (Experimental conditions: MOF 20 mg, 40 mL tap water, OFL initial concentration 20 mg L−1; RDS ≤ 10%).
| Synthesis Procedure | Layer by Layer 5 Shell | Layer by Layer 20 Shell | Layer by Layer 5 Shell + Reflux | Layer by Layer 5 Shell + Hydrothermal |
|---|---|---|---|---|
| Sample name | Fe3O4@MIL-100_5 | Fe3O4@MIL-100_20 | Fe3O4@MIL-100_R | Fe3O4@MIL-100_H |
| Sample | m%s | m%* | m%c | Fe3O4 Mass% | MIL-100 Mass% |
|---|---|---|---|---|---|
| Fe3O4 | 92.8 | 93.2 | 93.1 | - | - |
| MIL-100 | 21.7 | 24.0 | 23.6 | - | - |
| Fe3O4@MIL-100_20 | 76.9 | 80.2 | - | 81.5 | 18.5 |
| Fe3O4@MIL-100_H | 33.6 | 42.9 | - | 27.3 | 72.7 |
| Adsorption Model | Isotherm Parameters | MIL-100 | Fe3O4@MIL-100_H |
|---|---|---|---|
| qm exp (mg g−1) | 127 | 199 | |
| Freundlich | KF (mg(1-n) Ln g−1) | 59 ± 18 | 71 ± 19 |
| 1/n | 0.14 ± 0.07 | 0.22 ± 0.07 | |
| R2 | 0.594 | 0.824 | |
| Langmuir | KL (L mg−1) | 0.6 ± 0.2 | 0.15 ± 0.03 |
| qm (mg g−1) | 123 ± 5 | 218 ± 7 | |
| R2 | 0.944 | 0.972 | |
| BET | Ks (L mg−1) | 0.6 ± 0.2 | 0.15 ± 0.04 |
| KL (L mg−1) | 0 | 6.7E-18 ± 0 | |
| qm (mg g−1) | 123 ± 6 | 218 ± 7 | |
| R2 | 0.925 | 0.965 |
| Kinetic Model | Kinetic Parameter | MIL-100 | Fe3O4@MIL-100_H |
|---|---|---|---|
| qe exp (mg g−1) | 18.3 | 45.3 | |
| Pseudo-first order | k1 (min−1) | 0.6 ± 0.2 | 0.30 ± 0.06 |
| R2 | 0.929 | 0.928 | |
| qe (mg g−1) | 15.3 ± 0.6 | 38 ± 1 | |
| Pseudo-second order | k2 (g mg−1 min−1) | 0.05 ± 0.02 | 0.015 ± 0.003 |
| R2 | 0.927 | 0.974 | |
| qe (mg g−1) | 16.5 ± 0.6 | 41 ± 1 |
| R% | R% | |
|---|---|---|
| MOF | Tap Water | River Water |
| MIL-100 | 88 | 80 |
| Fe3O4@MIL-100_H | 95 | 87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturini, M.; Puscalau, C.; Guerra, G.; Maraschi, F.; Bruni, G.; Monteforte, F.; Profumo, A.; Capsoni, D. Combined Layer-by-Layer/Hydrothermal Synthesis of Fe3O4@MIL-100(Fe) for Ofloxacin Adsorption from Environmental Waters. Nanomaterials 2021, 11, 3275. https://doi.org/10.3390/nano11123275
Sturini M, Puscalau C, Guerra G, Maraschi F, Bruni G, Monteforte F, Profumo A, Capsoni D. Combined Layer-by-Layer/Hydrothermal Synthesis of Fe3O4@MIL-100(Fe) for Ofloxacin Adsorption from Environmental Waters. Nanomaterials. 2021; 11(12):3275. https://doi.org/10.3390/nano11123275
Chicago/Turabian StyleSturini, Michela, Constantin Puscalau, Giulia Guerra, Federica Maraschi, Giovanna Bruni, Francesco Monteforte, Antonella Profumo, and Doretta Capsoni. 2021. "Combined Layer-by-Layer/Hydrothermal Synthesis of Fe3O4@MIL-100(Fe) for Ofloxacin Adsorption from Environmental Waters" Nanomaterials 11, no. 12: 3275. https://doi.org/10.3390/nano11123275
APA StyleSturini, M., Puscalau, C., Guerra, G., Maraschi, F., Bruni, G., Monteforte, F., Profumo, A., & Capsoni, D. (2021). Combined Layer-by-Layer/Hydrothermal Synthesis of Fe3O4@MIL-100(Fe) for Ofloxacin Adsorption from Environmental Waters. Nanomaterials, 11(12), 3275. https://doi.org/10.3390/nano11123275









