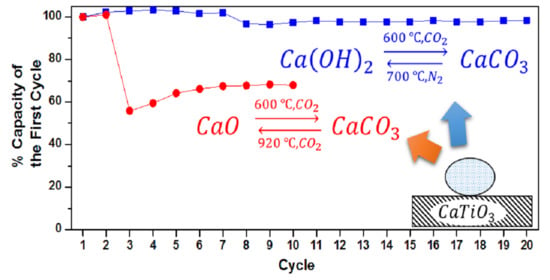
Multicycle Performance of CaTiO3 Decorated CaO-Based CO2 Adsorbent Prepared by a Versatile Aerosol Assisted Self-Assembly Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents Preparation
2.2. Adsorption Characterization
2.3. CO2 Capture Experiments
3. Results and Discussion
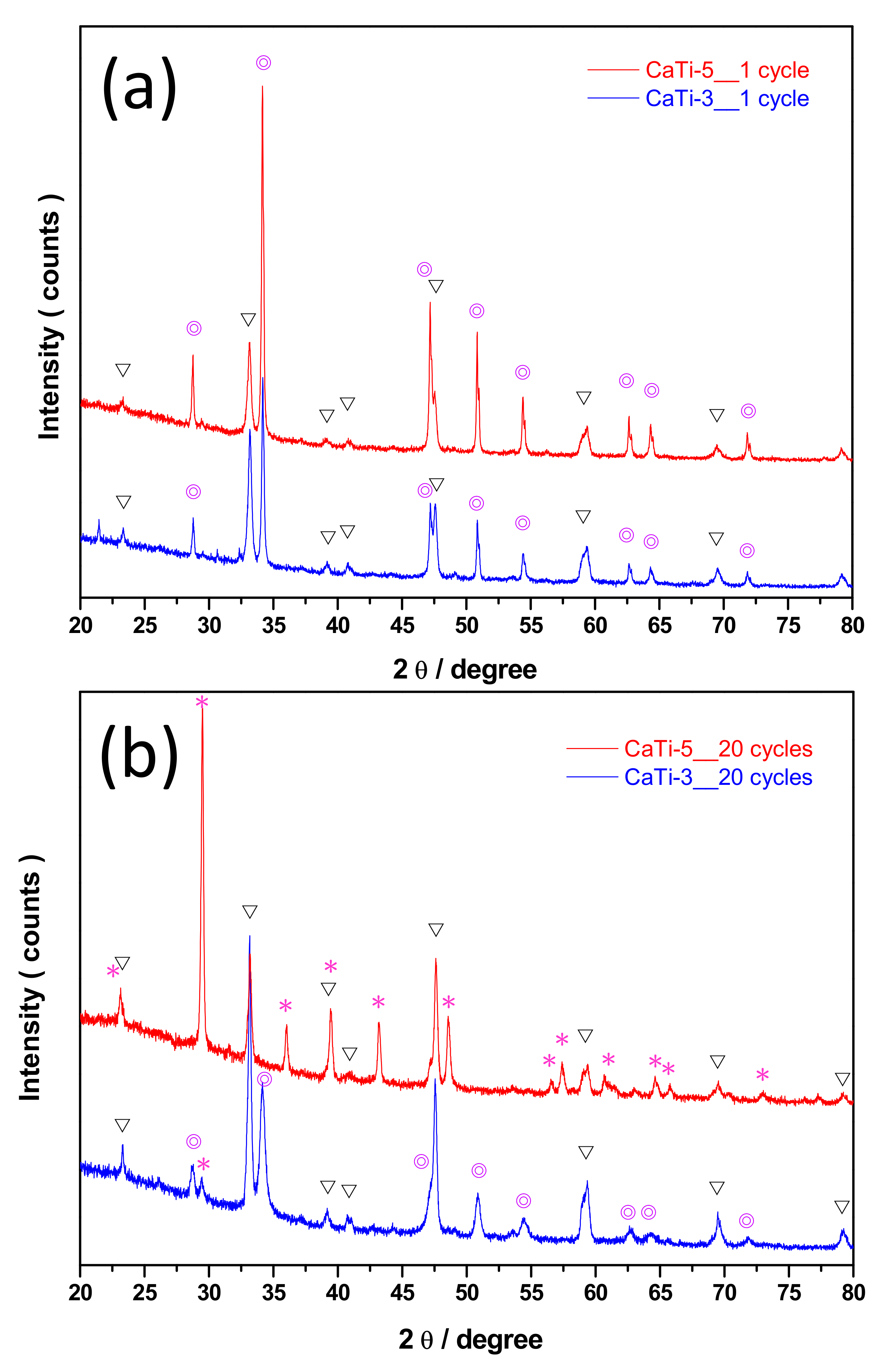
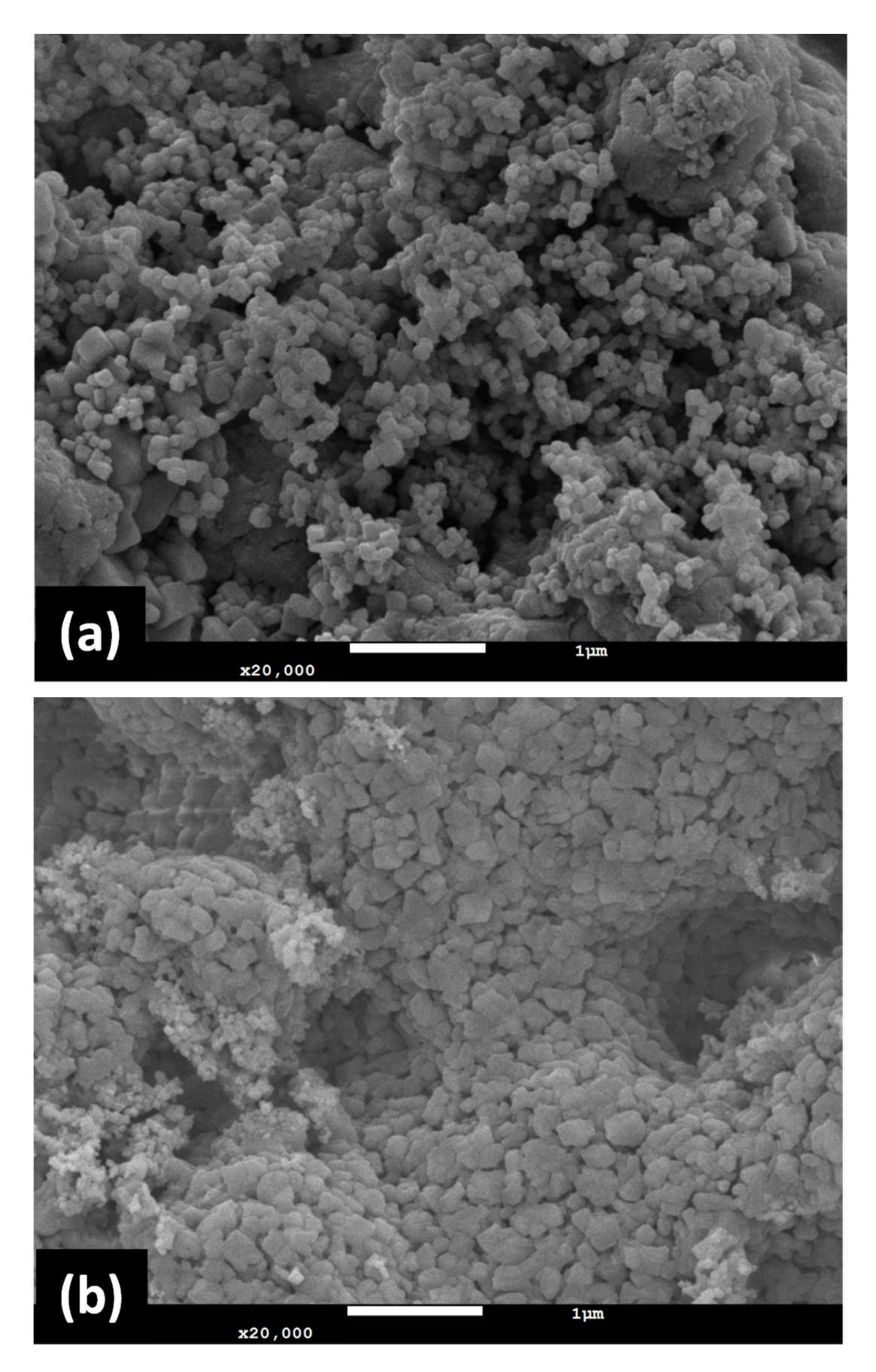
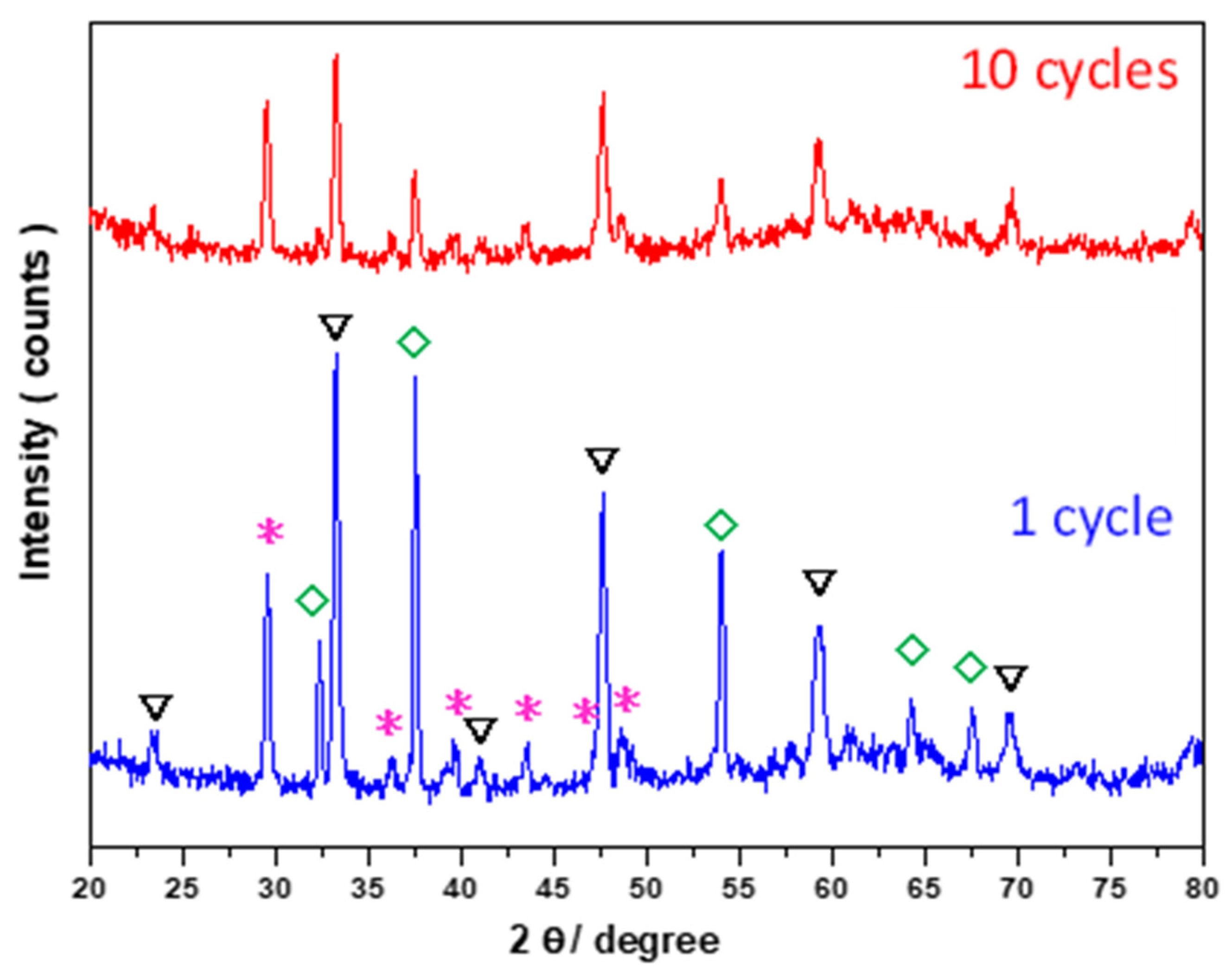
3.1. Properties of Adsorbents
3.2. CO2 Capture Performance of Adsorbents
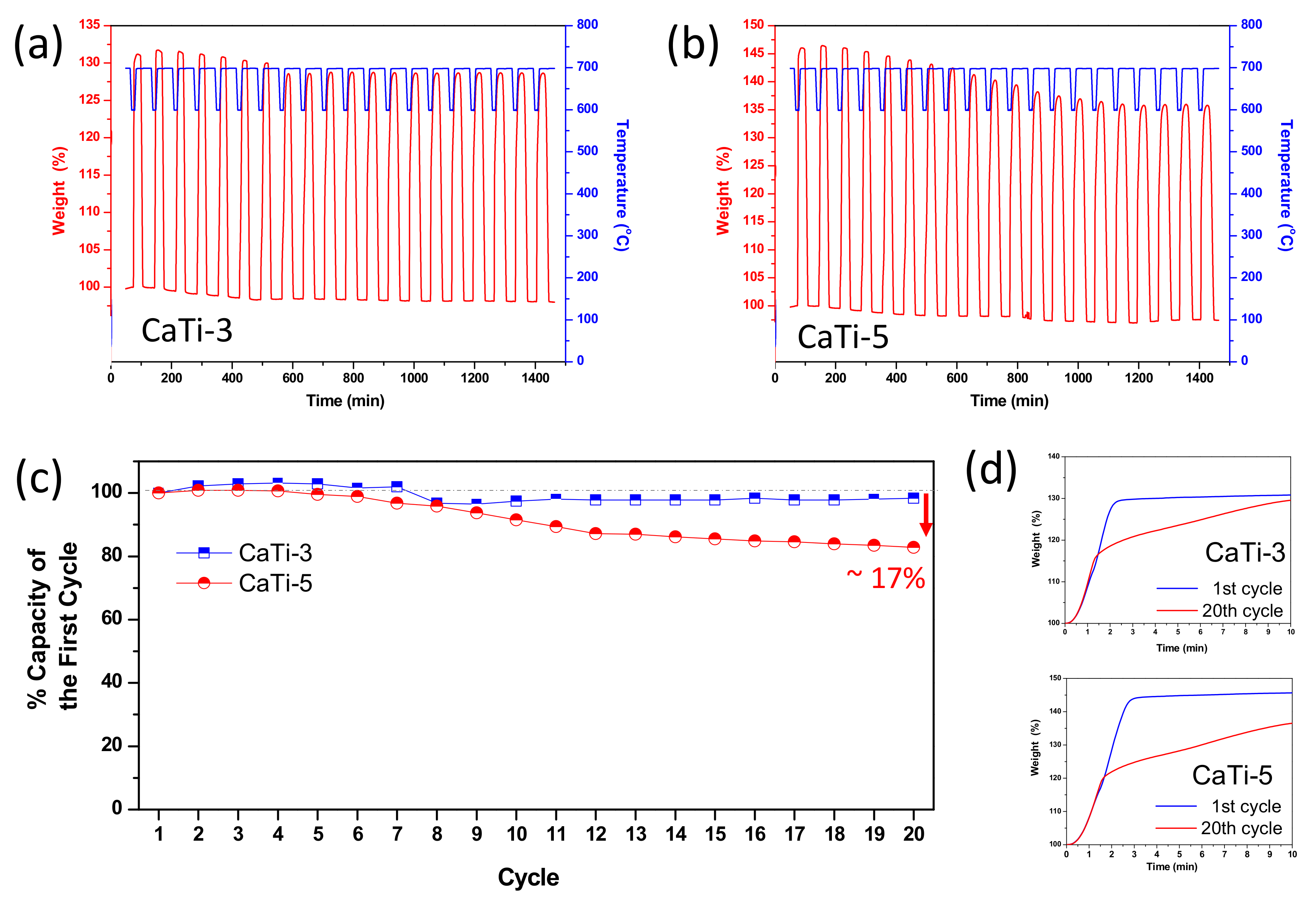
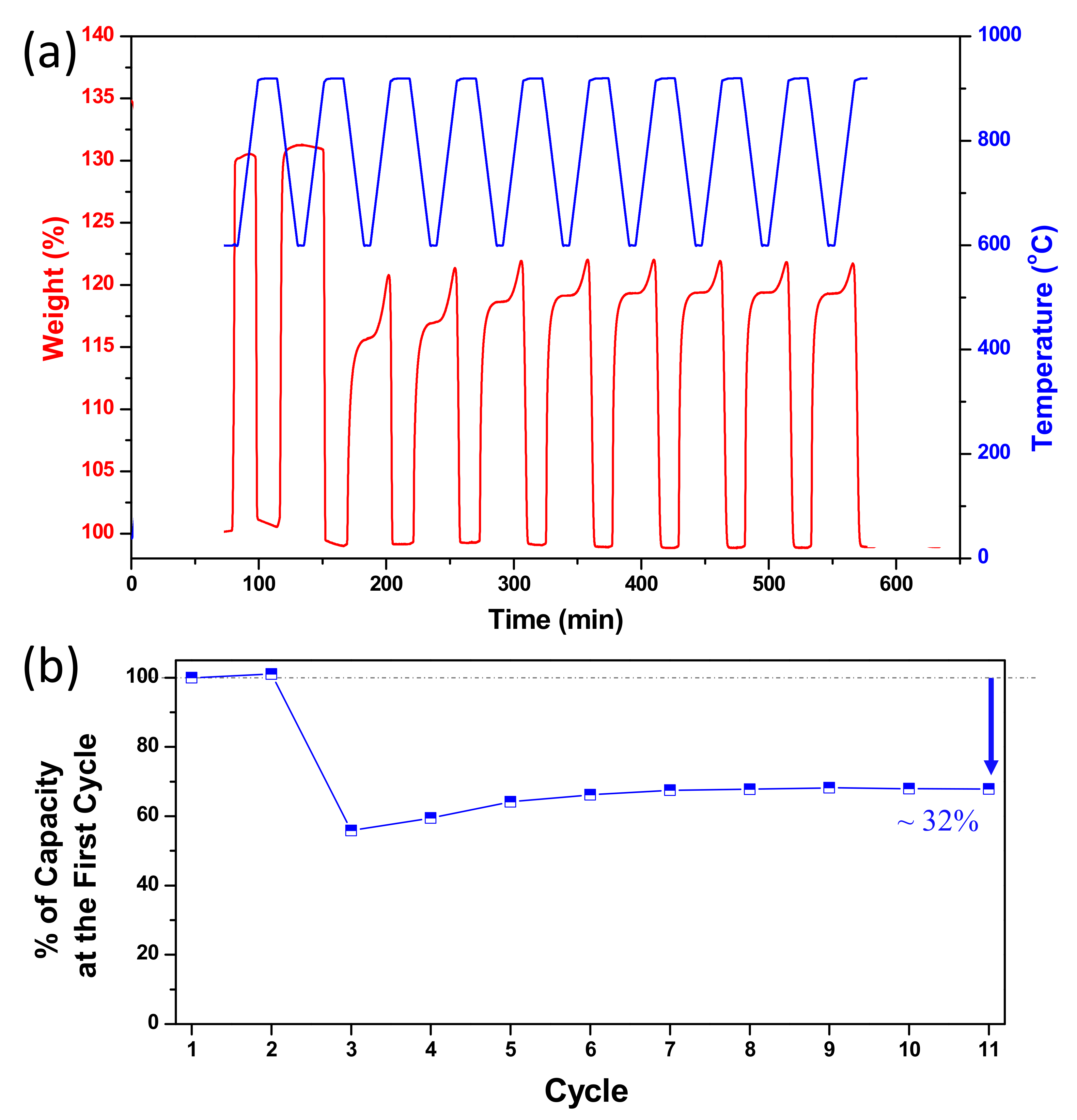
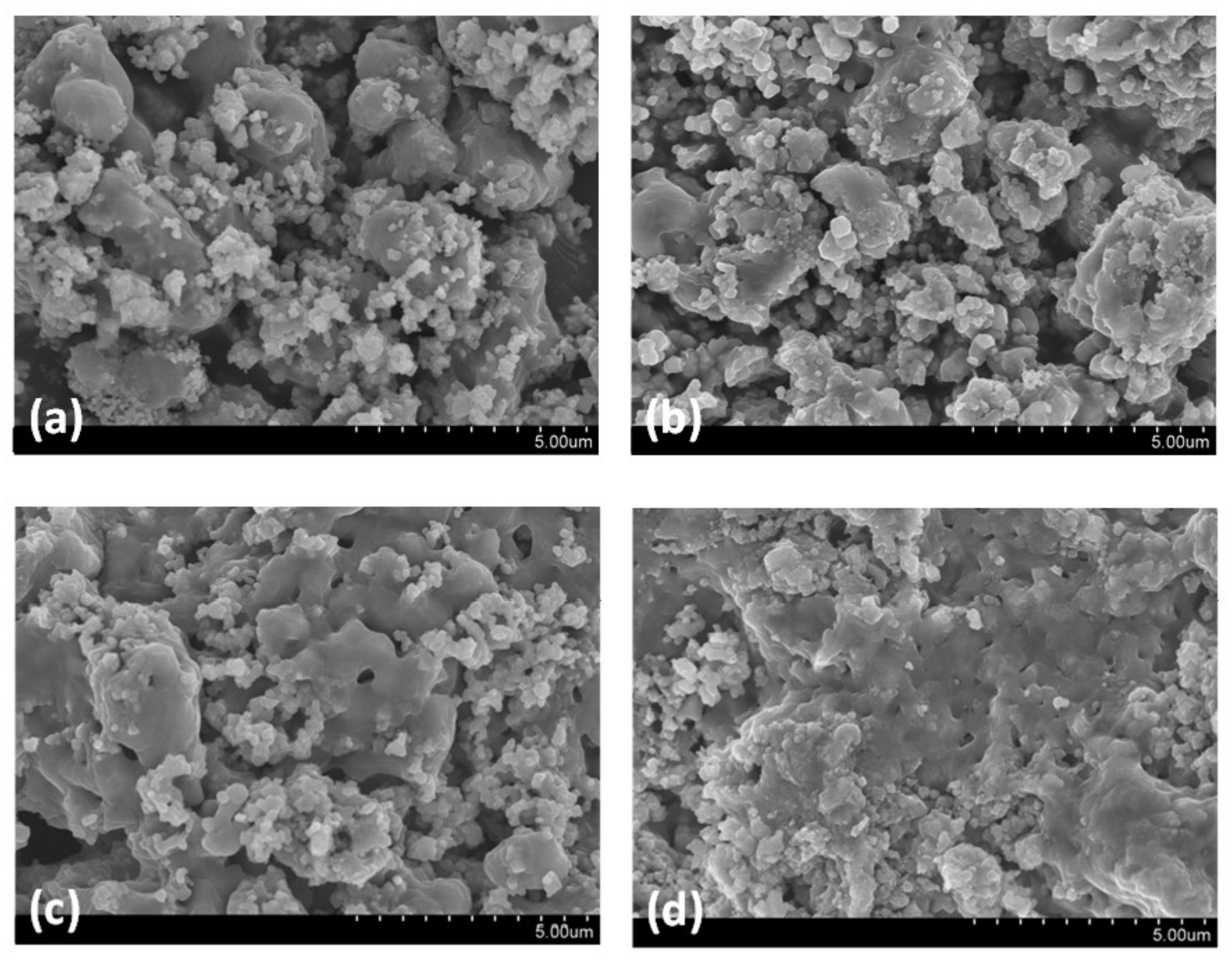
3.3. Multicycle Capture and Regeneration Experiment of Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krödel, M.; Landuyt, A.; Abdala, P.M.; Müller, C.R. Mechanistic understanding of CaO-based sorbents for high-temperature CO2 capture: Advanced characterization and prospects. ChemSusChem 2020, 13, 6259–6272. [Google Scholar] [PubMed]
- Sin, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar]
- Yang, N.; Xue, R.; Huang, G.; Ma, Y.; Wang, J. CO2 adsorption performance and kinetics of ionic liquid-modified calcined magnesite. Nanomaterials 2021, 11, 2614. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Radfarnia, H.R.; Sayari, A. A highly efficient CaO-based CO2 sorbent prepared by a citrate-assisted sol–gel technique. Chem. Eng. J. 2015, 262, 913–920. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, Q.; Shan, S.; Li, S.; Jiang, L.; Wang, Y. Development of CaO-based sorbent doped with mineral rejects–bauxite-tailings in cyclic CO2 capture. J. Taiwan Inst. Chem. Eng. 2015, 46, 155–159. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Liu, L.; Guo, X. Effects of preparation methods on the structure and property of Al-stabilized CaO-based sorbents for CO2 capture. Fuel Process. Technol. 2018, 173, 276–284. [Google Scholar] [CrossRef]
- Liu, F.Q.; Li, W.H.; Liu, B.C.; Li, R.X. Synthesis, characterization, and high temperature CO2 capture of new CaO based hollow sphere sorbents. J. Mater. Chem. A 2013, 1, 8037–8044. [Google Scholar] [CrossRef]
- Sun, H.; Parlett, C.M.A.; Isaacs, M.A.; Liu, X.; Adwek, G.; Wang, J.; Shen, B.; Huang, J.; Wu, C. Development of Ca/KIT-6 adsorbents for high temperature CO2 capture. Fuel 2019, 235, 1070–1076. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Ding, H.; Luo, C.; Zheng, Y.; Zhang, Q.; Li, X.; Sun, J.; Zhang, L. Potential synergy of chlorine and potassium and sodium elements in carbonation enhancement of CaO-based sorbents. ACS Sustain. Chem. Eng. 2018, 6, 11677–11684. [Google Scholar] [CrossRef]
- Sun, P.; Grace, J.R.; Lim, C.J.; Anthony, E.J. The effect of CaO sintering on cyclic CO2 capture in energy systems. AIChE J. 2007, 53, 2432–2442. [Google Scholar] [CrossRef]
- Kierzkowska, A.M.; Pacciani, R.; Muller, C.R. CaO-based CO2 sorbents: From fundamentals to the development of new, highly effective materials. ChemSusChem 2013, 6, 1130–1148. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.A.; Armutlulu, A.; Imtiaz, Q.; Donat, F.; Schäublin, R.; Kierzkowska, A.; Müller, C.R. Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents. Nat. Commun. 2018, 9, 2408–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nityashree, N.; Manohara, G.V.; Maroto-Valer, M.M.; Garcia, S. Advanced high-temperature CO2 sorbents with improved long-term cycling stability. ACS Appl. Mater. Interfaces 2020, 12, 33765–33774. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Y.; Yang, Y.; Li, W.; Zhou, Y.; Zhang, J.; Liu, W.; Zhao, C. Mode investigation of CO2 sorption enhancement for titanium dioxide decorated CaO-based pellets. Fuel 2019, 256, 116009–116017. [Google Scholar] [CrossRef]
- Radfarnia, H.R.; Iliuta, M.C. Metal oxide-stabilized calcium oxide CO2 sorbent for multicycle operation. Chem. Eng. J. 2013, 232, 280–289. [Google Scholar] [CrossRef]
- Guo, H.; Kou, X.; Zhao, Y.; Wang, S.; Sun, Q.; Ma, X. Effect of synergistic interaction between Ce and Mn on the CO2 capture of calcium-based sorbent: Textural properties, electron donation, and oxygen vacancy. Chem. Eng. J. 2018, 334, 237–246. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, K.P.; Yu, C.T.; Chiang, P.C.; Wang, C.F. Development of high-temperature CO2 sorbents made of CaO-based mesoporous silica. Chem. Eng. J. 2010, 161, 129–135. [Google Scholar] [CrossRef]
- Valverde, J.M.; Pontiga, F.; Soria-Hoyo, C.; Quintanilla, M.A.S.; Moreno, H.; Duran, F.J.; Espin, M.J. Improving the gas–solids contact efficiency in a fluidized bed of CO2 adsorbent fine particles. Phys. Chem. Chem. Phys. 2011, 13, 14906–14909. [Google Scholar] [CrossRef]
- Perez-Vaquero, J.; Valverde, J.M.; Quintanilla, M.A.S. Flow properties of CO2 sorbent powders modified with nanosilica. Powder Technol. 2013, 249, 443–455. [Google Scholar] [CrossRef]
- Gunathilake, C.; Jaroniec, M. Mesoporous calcium oxide–silica and magnesium oxide–silica composites for CO2 capture at ambient and elevated temperatures. J. Mater. Chem. A 2016, 4, 10914–10924. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Miccio, F.; Murri, A.N.; Landi, E. Insights into utilization of strontium carbonate for thermochemical energy storage. Renew. Energy 2020, 157, 769–781. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. Calcium-looping for thermochemical energy storage in concentrating solar power applications: Evaluation of the effect of acoustic perturbation on the fluidized bed carbonation. Chem. Eng. J. 2020, 392, 123658–123668. [Google Scholar] [CrossRef]
- Yu, C.; Kuo, H.; Chen, Y. Carbon dioxide removal using calcium aluminate carbonates on titanic oxide under warm-gas conditions. Appl. Energy 2016, 162, 1122–1130. [Google Scholar] [CrossRef]
- Wu, S.F.; Zhu, Y.Q. Behavior of CaTiO3/nano-CaO as a CO2 reactive adsorbent. Ind. Eng. Chem. Res. 2010, 49, 2701–2706. [Google Scholar] [CrossRef]
- Lin, C.J.; Yang, W.T. Ordered mesostructured Cu-doped TiO2 spheres as active visible-light-driven photocatalysts for degradation of paracetamol. Chem. Eng. J. 2014, 237, 131–137. [Google Scholar] [CrossRef]
- Fan, J.; Boettcher, S.W.; Stucky, G.D. Nanoparticle assembly of ordered multicomponent mesostructured metal oxides via a versatile sol-gel process. Chem. Mater. 2006, 18, 6391–6396. [Google Scholar] [CrossRef]
- Wei, S.; Han, R.; Su, Y.; Gao, J.; Zhao, G.; Qin, Y. Pore structure modified CaO-based sorbents with different sized templates for CO2 capture. Energy Fuels 2019, 33, 5398–5407. [Google Scholar] [CrossRef]
- Rodaev, V.V.; Razlivalova, S.S. Performance and durability of the Zr-doped CaO sorbent under cyclic carbonation–decarbonation at different operating parameters. Energies 2021, 14, 4822. [Google Scholar] [CrossRef]



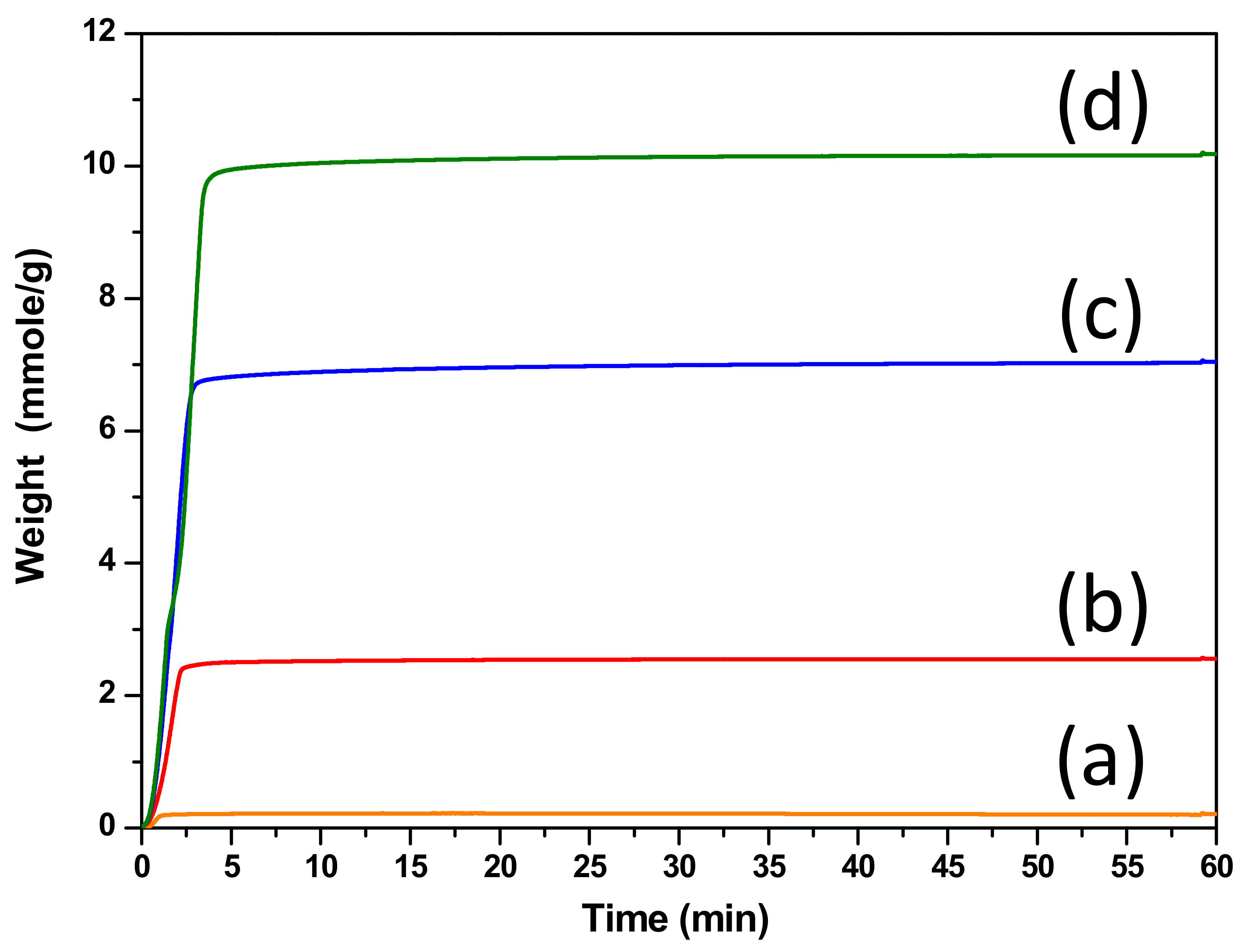

| Sorbent | Surface Area (m2/g) | CO2 Capture Capacity (mmole CO2/g Sorbent) |
|---|---|---|
| CaTi-1.2 | 5 | 0.2 |
| CaTi-2 | 10 | 2.6 |
| CaTi-3 | 17 | 7.0 |
| CaTi-5 | 12 | 10.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, R.-W.; Lin, C.-J.; Liou, Y.-H. Multicycle Performance of CaTiO3 Decorated CaO-Based CO2 Adsorbent Prepared by a Versatile Aerosol Assisted Self-Assembly Method. Nanomaterials 2021, 11, 3188. https://doi.org/10.3390/nano11123188
Chang R-W, Lin C-J, Liou Y-H. Multicycle Performance of CaTiO3 Decorated CaO-Based CO2 Adsorbent Prepared by a Versatile Aerosol Assisted Self-Assembly Method. Nanomaterials. 2021; 11(12):3188. https://doi.org/10.3390/nano11123188
Chicago/Turabian StyleChang, Ren-Wei, Chin-Jung Lin, and Ya-Hsuan Liou. 2021. "Multicycle Performance of CaTiO3 Decorated CaO-Based CO2 Adsorbent Prepared by a Versatile Aerosol Assisted Self-Assembly Method" Nanomaterials 11, no. 12: 3188. https://doi.org/10.3390/nano11123188
APA StyleChang, R.-W., Lin, C.-J., & Liou, Y.-H. (2021). Multicycle Performance of CaTiO3 Decorated CaO-Based CO2 Adsorbent Prepared by a Versatile Aerosol Assisted Self-Assembly Method. Nanomaterials, 11(12), 3188. https://doi.org/10.3390/nano11123188