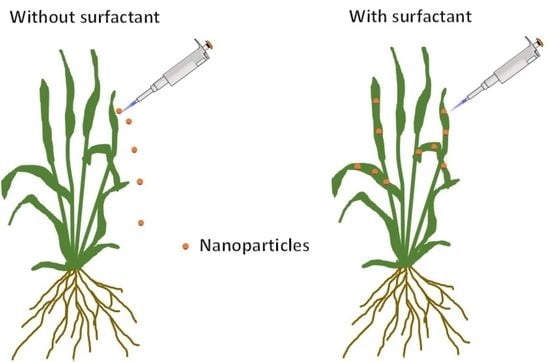
Metabolomic Response of Early-Stage Wheat (Triticum aestivum) to Surfactant-Aided Foliar Application of Copper Hydroxide and Molybdenum Trioxide Nanoparticles
Abstract
:1. Synopsis
2. Introduction
3. Materials and Methods
3.1. Characterization and Stability of Cu(OH)2 and MoO3 NMs
3.2. Wheat Growth and Leaf Exposure Assay
3.3. Plant Tissue Digestions and Nutrient Measurements
3.4. Plant Tissue Extractions and Targeted Metabolites Measurements
4. Results and Discussion
4.1. NM Characterization and Dissolution
4.2. Plant Responses to the Surfactant Solution
4.3. Wheat Leaf Responses to Metal-Surfactant Suspensions
4.4. Wheat Root Responses to Metal-Surfactant Suspensions
4.5. Comparison of Wheat Responses to Cu(OH)2 NMs and MoO3 NMs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Alshaal, T.; El-Ramady, H. Foliar application: From plant nutrition to biofortification. Environ. Biodivers. Soil Secur. 2017, 1, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.; Gao, Q.; Deng, C.; Wang, Y.; Lee, W.-Y.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Foliar exposure of Cu(OH)2 nanopesticide to basil (Ocimum basilicum): Variety-dependent copper translocation and biochemical responses. J. Agric. Food Chem. 2018, 66, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Chen, J.; Han, H.; Yuan, Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806. [Google Scholar] [CrossRef]
- Shallan, M.A.; Hassan, H.M.; Namich, A.A.; Ibrahim, A.A. Biochemical and physiological effects of TiO2 and SiO2 nanoparticles on cotton plant under drought stress. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1540–1551. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhao, L.; Keller, A.A. Interactions, transformations, and bioavailability of nano-copper exposed to root exudates. Environ. Sci. Technol. 2017, 51, 9774–9783. [Google Scholar] [CrossRef] [Green Version]
- Cervantes-Avilés, P.; Huang, X.; Keller, A.A. Dissolution and Aggregation of Metal Oxide Nanoparticles in Root Exudates and Soil Leachate: Implications for Nanoagrochemical Application. Environ. Sci. Technol. 2021, 55, 13443–13451. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharm. 2017, 329, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Cécillon, L.; Bureau, S.; Barthès, V.; Ouerdane, L.; Carrière, M.; Sarret, G. Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation. J. Hazard. Mater. 2014, 264, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Peralta-Videa, J.R.; Rico, C.; Sahi, S.; Viveros, M.N.; Bartonjo, J.; Zhao, L.; Gardea-Torresdey, J.L. Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2014, 48, 4376–4385. [Google Scholar] [CrossRef]
- Peirce, C.A.; McBeath, T.M.; Priest, C.; McLaughlin, M.J. The timing of application and inclusion of a surfactant are important for absorption and translocation of foliar phosphoric acid by wheat leaves. Front. Plant Sci. 2019, 10, 1532. [Google Scholar] [CrossRef] [Green Version]
- Green, J.M.; Beestman, G.B. Recently patented and commercialized formulation and adjuvant technology. Crop Prot. 2007, 26, 320–327. [Google Scholar] [CrossRef]
- Castro, M.J.; Ojeda, C.; Cirelli, A.F. Advances in surfactants for agrochemicals. Environ. Chem. Lett. 2014, 12, 85–95. [Google Scholar] [CrossRef]
- Wang, P.; Keller, A.A. Partitioning of hydrophobic organic compounds within soil–water–surfactant systems. Water Res. 2008, 42, 2093–2101. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, Y.; Zhang, C.; Zhu, Y.; Lei, J.; Ma, Y.; Du, F. Wettability of ionic surfactants SDS and DTAB on wheat (Triticum aestivum) leaf surfaces. J. Disper. Sci. Technol. 2018, 39, 1820–1828. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Lei, J.; Ma, Y.; Du, F. The wetting behavior of aqueous surfactant solutions on wheat (Triticum aestivum) leaf surfaces. Soft Matter. 2017, 13, 503–513. [Google Scholar] [CrossRef]
- Peirce, C.A.; Priest, C.; McBeath, T.M.; McLaughlin, M.J. Uptake of phosphorus from surfactant solutions by wheat leaves: Spreading kinetics, wetted area, and drying time. Soft Matter. 2016, 12, 209–218. [Google Scholar] [CrossRef]
- Castro, M.J.; Ojeda, C.; Cirelli, A.F. Surfactants in Agriculture. In Green Materials for Energy, Products and Depollution; Springer: Berlin/Heidelberg, Germany, 2013; pp. 287–334. [Google Scholar]
- Januszkiewicz, K.; Mrozek-Niećko, A.; Różański, J. Effect of surfactants and leaf surface morphology on the evaporation time and coverage area of ZnIDHA droplets. Plant Soil 2019, 434, 93–105. [Google Scholar] [CrossRef]
- Banks, M.-L.L.; Kremer, R.J.; Eivazi, F.; Motavalli, P.P.; Nelson, K.A. Effects of selected surfactants on nutrient uptake in corn (Zea mays L.). J. Plant Nutr. 2015, 38, 1036–1049. [Google Scholar] [CrossRef]
- Peirce, C.; McBeath, T.; Fernández, V.; McLaughlin, M. Wheat leaf properties affecting the absorption and subsequent translocation of foliar-applied phosphoric acid fertiliser. Plant Soil 2014, 384, 37–51. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef]
- Zimbovskaya, M.M.; Polyakov, A.Y.; Volkov, D.S.; Kulikova, N.A.; Lebedev, V.A.; Pankratov, D.A.; Konstantinov, A.I.; Parfenova, A.M.; Zhilkibaev, O.; Perminova, I.V. Foliar Application of Humic-Stabilized Nanoferrihydrite Resulted in an Increase in the Content of Iron in Wheat Leaves. Agronomy 2020, 10, 1891. [Google Scholar] [CrossRef]
- Larue, C.; Veronesi, G.; Flank, A.-M.; Surble, S.; Herlin-Boime, N.; Carrière, M. Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J. Toxicol. Environ. Health A 2012, 75, 722–734. [Google Scholar] [CrossRef]
- Read, T.L.; Doolette, C.L.; Li, C.; Schjoerring, J.K.; Kopittke, P.M.; Donner, E.; Lombi, E. Optimising the foliar uptake of zinc oxide nanoparticles: Do leaf surface properties and particle coating affect absorption? Physiol. Plantarum 2020, 170, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Luque, A.; Cifuentes, Z.; Beckstead, J.A.; Sillero, J.C.; Ávila, C.; Rubio, J.; Ryan, R.O. Effect of amphotericin B nanodisks on plant fungal diseases. Pest Manag. Sci. 2012, 68, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, I.; Tarafdar, J. Perspectives of biosynthesized magnesium nanoparticles in foliar application of wheat plant. J. Bionanosci. 2015, 9, 209–214. [Google Scholar] [CrossRef]
- Ghafari, H.; Razmjoo, J. Effect of foliar application of nano-iron oxidase, iron chelate and iron sulphate rates on yield and quality of wheat. Int. J. Agron. Plant Prod. 2013, 4, 2997–3003. [Google Scholar]
- Avellan, A.; Yun, J.; Morais, B.P.; Clement, E.T.; Rodrigues, S.M.; Lowry, G.V. Critical Review: Role of Inorganic Nanoparticle Properties on Their Foliar Uptake and in Planta Translocation. Environ. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanism of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Deshpande, P.; Dapkekar, A.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017, 165, 394–401. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Adisa, I.O.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Effects of manganese nanoparticle exposure on nutrient acquisition in wheat (Triticum aestivum L.). Agronomy 2018, 8, 158. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Tian, L.; Shen, J.; Sun, G.; Wang, B.; Ji, R.; Zhao, L. Foliar Application of SiO2 Nanoparticles Alters Soil Metabolite Profiles and Microbial Community Composition in the Pakchoi (Brassica chinensis L.) Rhizosphere Grown in Contaminated Mine Soil. Environ. Sci. Technol. 2020, 54, 13137–13146. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Wang, J.; Tian, L.; Li, F.; Liu, S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; White, J.C.; Huang, Y. C60 fullerols enhance copper toxicity and alter the leaf metabolite and protein profile in Cucumber. Environ. Sci. Technol. 2019, 53, 2171–2180. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Paglia, K.; Vaniya, A.; Wancewicz, B.; Keller, A.A. Metabolomics reveals the molecular mechanisms of copper induced cucumber leaf (Cucumis sativus) senescence. Environ. Sci. Technol. 2018, 52, 7092–7100. [Google Scholar] [CrossRef]
- Zhang, H.; Du, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; White, J.C.; Keller, A.; Guo, H.; Ji, R.; Zhao, L. Metabolomics reveals how cucumber (Cucumis sativus) reprograms metabolites to cope with silver ions and silver nanoparticle-induced oxidative stress. Environ. Sci. Technol. 2018, 52, 8016–8026. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Chehregani, A.; Lucini, L.; Majd, A.; Gholami, M. Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci. Total Environ. 2018, 616, 1540–1551. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Keller, A.A. Comparative metabolic response between cucumber (Cucumis sativus) and corn (Zea mays) to a Cu(OH)2 nanopesticide. J. Agric. Food Chem. 2017, 66, 6628–6636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Hu, Q.; Huang, Y.; Keller, A.A. Response at genetic, metabolic, and physiological levels of maize (Zea mays) exposed to a Cu(OH)2 nanopesticide. ACS Sustain. Chem. Eng. 2017, 5, 8294–8301. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Hu, Q.; Huang, Y.; Fulton, A.N.; Hannah-Bick, C.; Adeleye, A.S.; Keller, A.A. Activation of antioxidant and detoxification gene expression in cucumber plants exposed to a Cu(OH)2 nanopesticide. Environ. Sci. Nano 2017, 4, 1750–1760. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Huang, Y.; Hannah-Bick, C.; Fulton, A.N.; Keller, A.A. Application of metabolomics to assess the impact of Cu(OH)2 nanopesticide on the nutritional value of lettuce (Lactuca sativa): Enhanced Cu intake and reduced antioxidants. NanoImpact 2016, 3, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, S.; Keller, A.A. Omics to address the opportunities and challenges of nanotechnology in agriculture. Crit. Rev. Environ. Sci Technol. 2021, 51, 2595–2636. [Google Scholar] [CrossRef]
- Huang, X.; Cervantes-Avilés, P.; Li, W.; Keller, A.A. Drilling into the Metabolomics to Enhance Insight on Corn and Wheat Responses to Molybdenum Trioxide Nanoparticles. Environ. Sci. Technol. 2021, 55, 13452–13464. [Google Scholar] [CrossRef]
- Majumdar, S.; Long, R.W.; Kirkwood, J.S.; Minakova, A.S.; Keller, A.A. Unraveling Metabolic and Proteomic Features in Soybean Plants in Response to Copper Hydroxide Nanowires Compared to a Commercial Fertilizer. Environ. Sci. Technol. 2021, 55, 13477–13489. [Google Scholar] [CrossRef]
- Hewitt, E.; Bolle-Jones, E. Molybdenum as a Plant Nutrient: II. The Effects of Molybdenum Deficiency on Some Horticultural and Agricultural Crop Plants in Sand Culture. Int. J. Hortic. Sci. 1952, 27, 257–265. [Google Scholar] [CrossRef]
- Gupta, U.C.; Srivastava, P.C.; Gupta, S.C. Role of micronutrients: Boron and molybdenum in crops and in human health and nutrition. Curr. Nutr. Food Sci. 2011, 7, 126–136. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Rao, A.S. Impact of SiO2 and Mo nano particles on seed germination of rice (Oryza sativa L.). Int. J. Agric. Food Sci. Technol. 2013, 4, 809–816. [Google Scholar]
- Kanneganti, A.; Talasila, M. MoO3 nanoparticles: Synthesis, characterization and its hindering effect on germination of Vigna Unguiculata seeds. J. Eng. Res. Appl. 2014, 4, 116–120. [Google Scholar]
- Mushinskiy, A.; Aminova, E. Effect of Iron, Copper and Molybdenum Nanoparticles on Morphometric Parameters of Solanum tuberosum L. Plants. In IOP Conference Series: Earth and Environmental Science; IOP Pub.: Bristol, UK, 2019; Volume 341, p. 12195. [Google Scholar]
- Abbasifar, A.; ValizadehKaji, B.; Iravani, M.A. Effect of green synthesized molybdenum nanoparticles on nitrate accumulation and nitrate reductase activity in spinach. J. Plant Nutr. 2020, 43, 13–27. [Google Scholar] [CrossRef]
- Sharma, P.K.; Raghubanshi, A.S.; Shah, K. Examining the uptake and bioaccumulation of molybdenum nanoparticles and their effect on antioxidant activities in growing rice seedlings. Environ. Sci. Pollut. Res. 2021, 28, 13439–13453. [Google Scholar] [CrossRef]
- Majumdar, S.; Ma, C.; Villani, M.; Zuverza-Mena, N.; Pagano, L.; Huang, Y.; Zappettini, A.; Keller, A.A.; Marmiroli, N.; Dhankher, O.P. Surface coating determines the response of soybean plants to cadmium sulfide quantum dots. NanoImpact 2019, 14, 100151. [Google Scholar] [CrossRef]
- Majumdar, S.; Pagano, L.; Wohlschlegel, J.A.; Villani, M.; Zappettini, A.; White, J.C.; Keller, A.A. Proteomic, gene and metabolite characterization reveal the uptake and toxicity mechanisms of cadmium sulfide quantum dots in soybean plants. Environ. Sci. Nano 2019, 6, 3010–3026. [Google Scholar] [CrossRef]
- Liu, W.; Majumdar, S.; Li, W.; Keller, A.A.; Slaveykova, V.I. Metabolomics for early detection of stress in freshwater alga Poterioochromonas malhamensis exposed to silver nanoparticles. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Huang, Y.; Adeleye, A.S.; Zhao, L.; Minakova, A.S.; Anumol, T.; Keller, A.A. Antioxidant response of cucumber (Cucumis sativus) exposed to nano copper pesticide: Quantitative determination via LC-MS/MS. Food Chem. 2019, 270, 47–52. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Minakova, A.S.; Anumol, T.; Keller, A.A. Quantitative analysis of changes in amino acids levels for cucumber (Cucumis sativus) exposed to nano copper. NanoImpact 2018, 12, 9–17. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Ngaire Brady, J.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Han, L.; Huang, B. Membrane fatty acid composition and saturation levels associated with leaf dehydration tolerance and post-drought rehydration in Kentucky bluegrass. Crop Sci. 2011, 51, 273–281. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Siebert, D.; Heineke, D.; Scharf, H.; Schultz, G. Pyruvate-derived amino acids in spinach chloroplasts: Synthesis and regulation during photosynthetic carbon metabolism. Plant Physiol. 1984, 76, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Mahil, E.; Kumar, B. Foliar application of nanofertilizers in agricultural crops–A review. J. Farm Sci. 2019, 32, 239–249. [Google Scholar]
- Zhao, L.; Huang, Y.; Adeleye, A.S.; Keller, A.A. Metabolomics reveals Cu(OH)2 nanopesticide-activated anti-oxidative pathways and decreased beneficial antioxidants in spinach leaves. Environ. Sci. Technol. 2017, 51, 10184–10194. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, B.; Plaxton, W. The central role of glutamate and aspartate in the post-translational control of respiration and nitrogen assimilation in plant cells. Amino Acids High. Plant CAB Int. 2015, 16, 277–297. [Google Scholar]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Wang, L.; Li, X.; Wang, H.; Jiang, Y.; Wang, W. Responses of seed germination and shoot metabolic profiles of maize (Zea mays L.) to Y2O3 nanoparticle stress. RSC Adv. 2019, 9, 27720–27731. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Huang, M.; Zhang, W.; Gardea-Torresdey, J.L.; White, J.C.; Ji, R.; Zhao, L. Silver Nanoparticles Alter Soil Microbial Community Compositions and Metabolite Profiles in Unplanted and Cucumber-Planted Soils. Environ. Sci. Technol. 2020, 54, 3334–3342. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Zhou, H.; Adeleye, A.S.; Wang, H.; Ortiz, C.; Mazer, S.J.; Keller, A.A. GC-TOF-MS based metabolomics and ICP-MS based metallomics of cucumber (Cucumis sativus) fruits reveal alteration of metabolites profile and biological pathway disruption induced by nano copper. Environ. Sci. Nano 2016, 3, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, H.; White, J.C.; Chen, X.; Li, H.; Qu, X.; Ji, R. Metabolomics reveals that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways. Environ. Sci. Nano 2019, 6, 1716–1727. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Zhao, L.; Ortiz, C.; Adeleye, A.S.; Hu, Q.; Zhou, H.; Huang, Y.; Keller, A.A. Metabolomics to detect response of lettuce (Lactuca sativa) to Cu(OH)2 nanopesticides: Oxidative stress response and detoxification mechanisms. Environ. Sci. Technol. 2016, 50, 9697–9707. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Zhao, X.; Zhao, S.; Gu, X.; Du, W.; Wei, H.; Ji, R.; Zhao, L. Metabolomics reveals the “invisible” responses of spinach plants exposed to CeO2 nanoparticles. Environ. Sci. Technol. 2019, 53, 6007–6017. [Google Scholar] [CrossRef]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, L.; Le, X.C. Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.). Environ. Pollut. 2017, 230, 302–310. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. Arab. Book 2010, 8, e0132. [Google Scholar] [CrossRef] [Green Version]

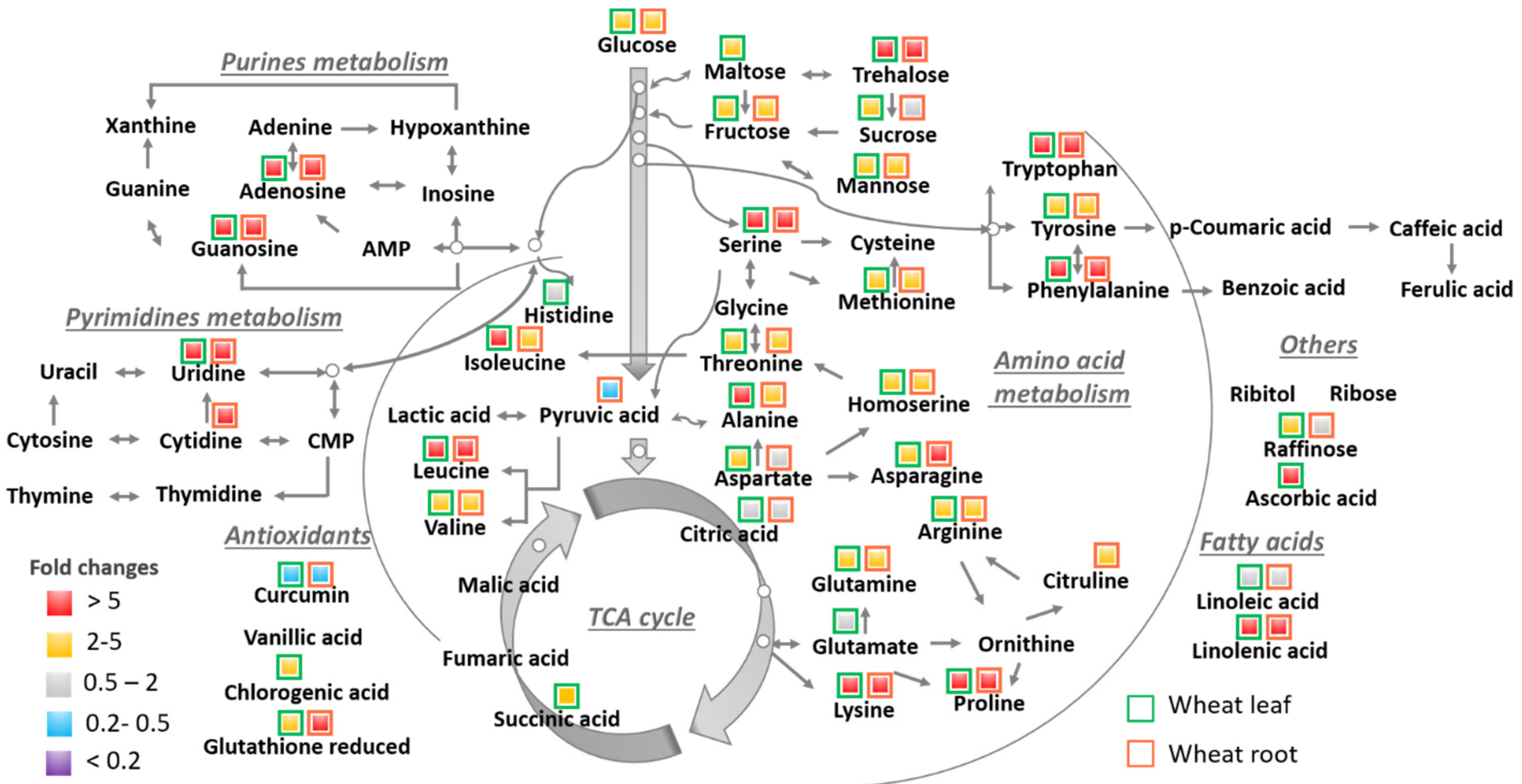
 wheat leaves exposed to Cu(OH)2 NMs;
wheat leaves exposed to Cu(OH)2 NMs;  wheat leaves exposed to MoO3 NMs;
wheat leaves exposed to MoO3 NMs;  wheat roots exposed to Cu(OH)2 NMs;
wheat roots exposed to Cu(OH)2 NMs;  wheat roots exposed to MoO3 NMs.
wheat roots exposed to MoO3 NMs.
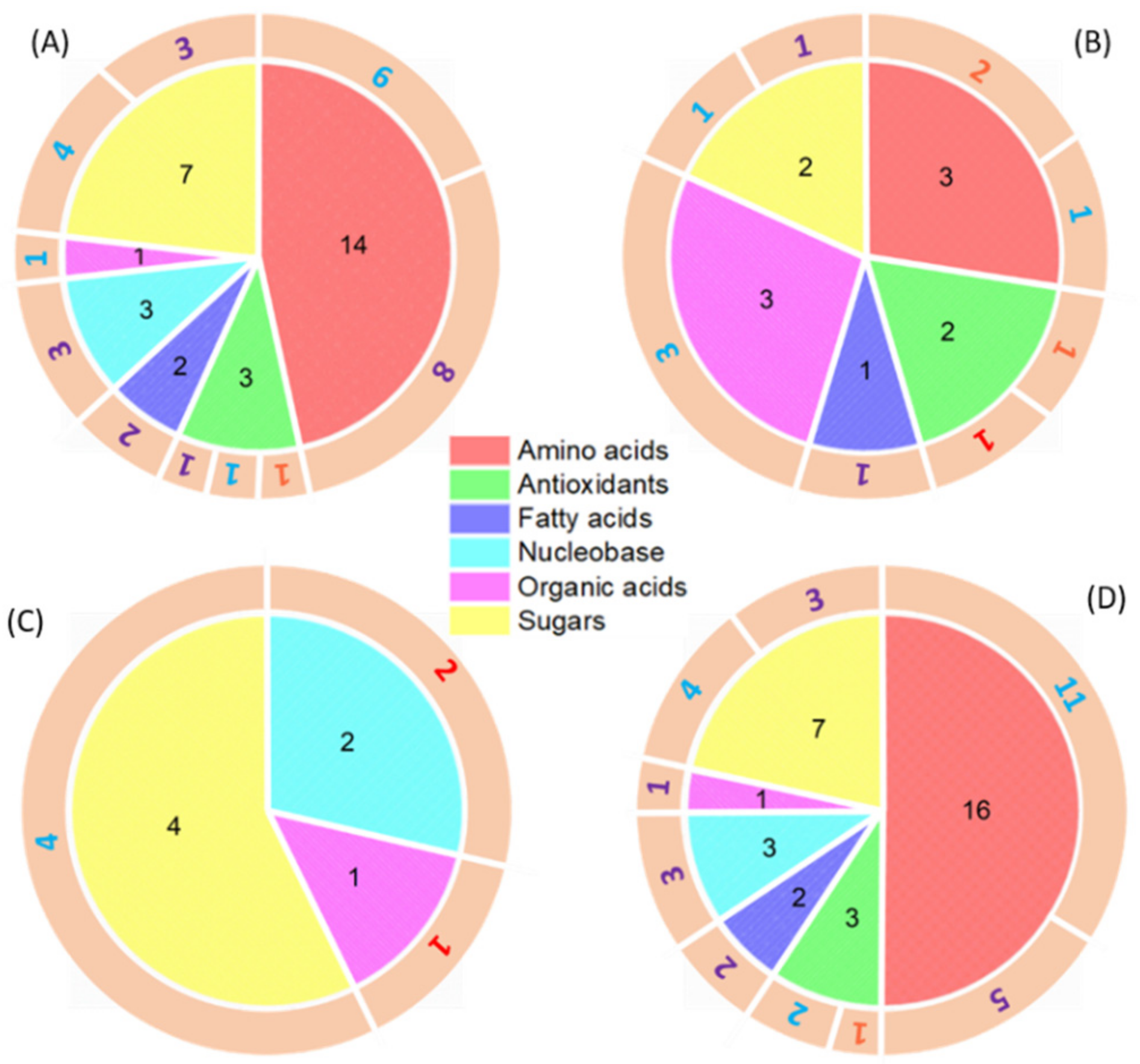
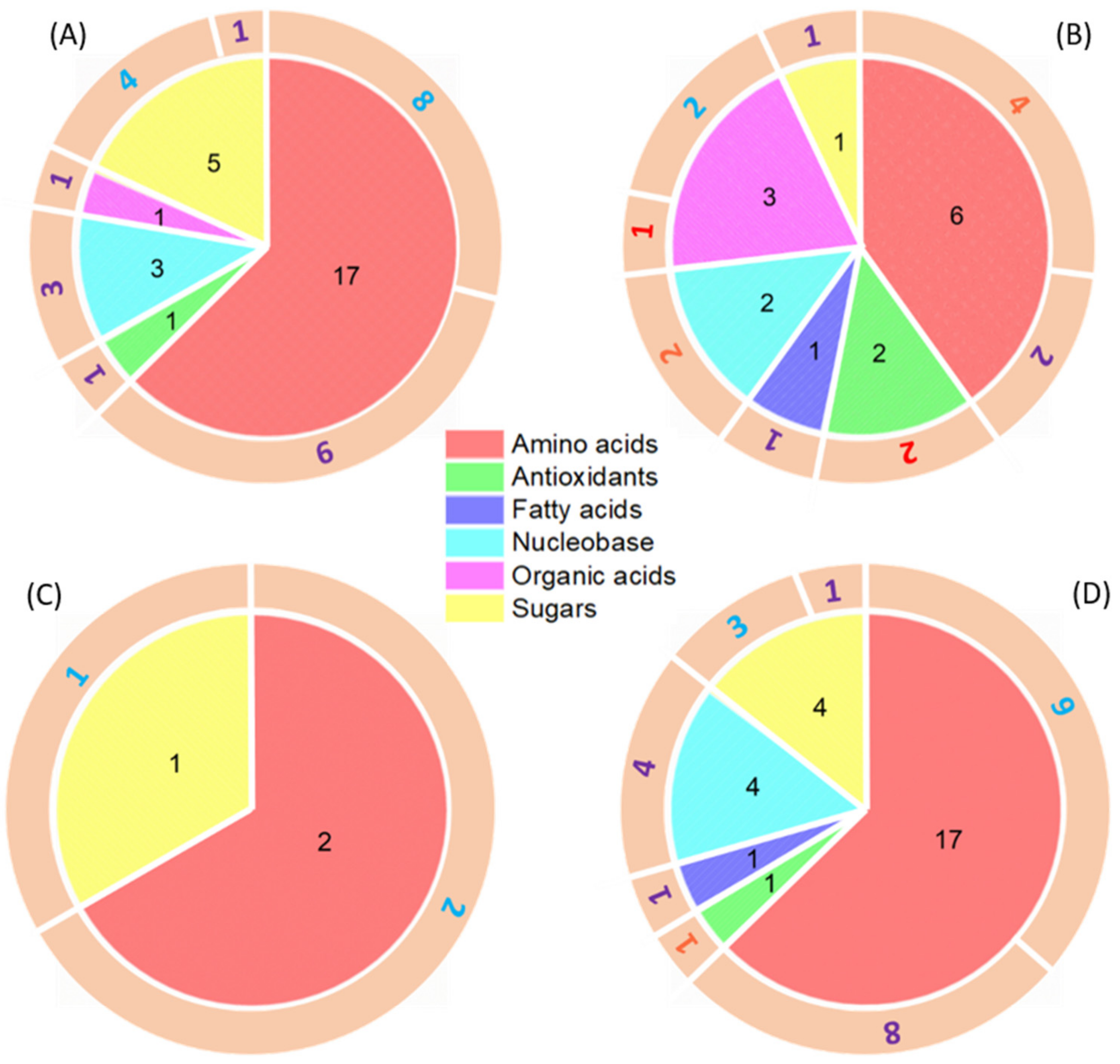
 wheat leaves exposed to Cu(OH)2 NMs;
wheat leaves exposed to Cu(OH)2 NMs;  wheat leaves exposed to MoO3 NMs;
wheat leaves exposed to MoO3 NMs;  wheat roots exposed to Cu(OH)2 NMs;
wheat roots exposed to Cu(OH)2 NMs;  wheat roots exposed to MoO3 NMs.
wheat roots exposed to MoO3 NMs.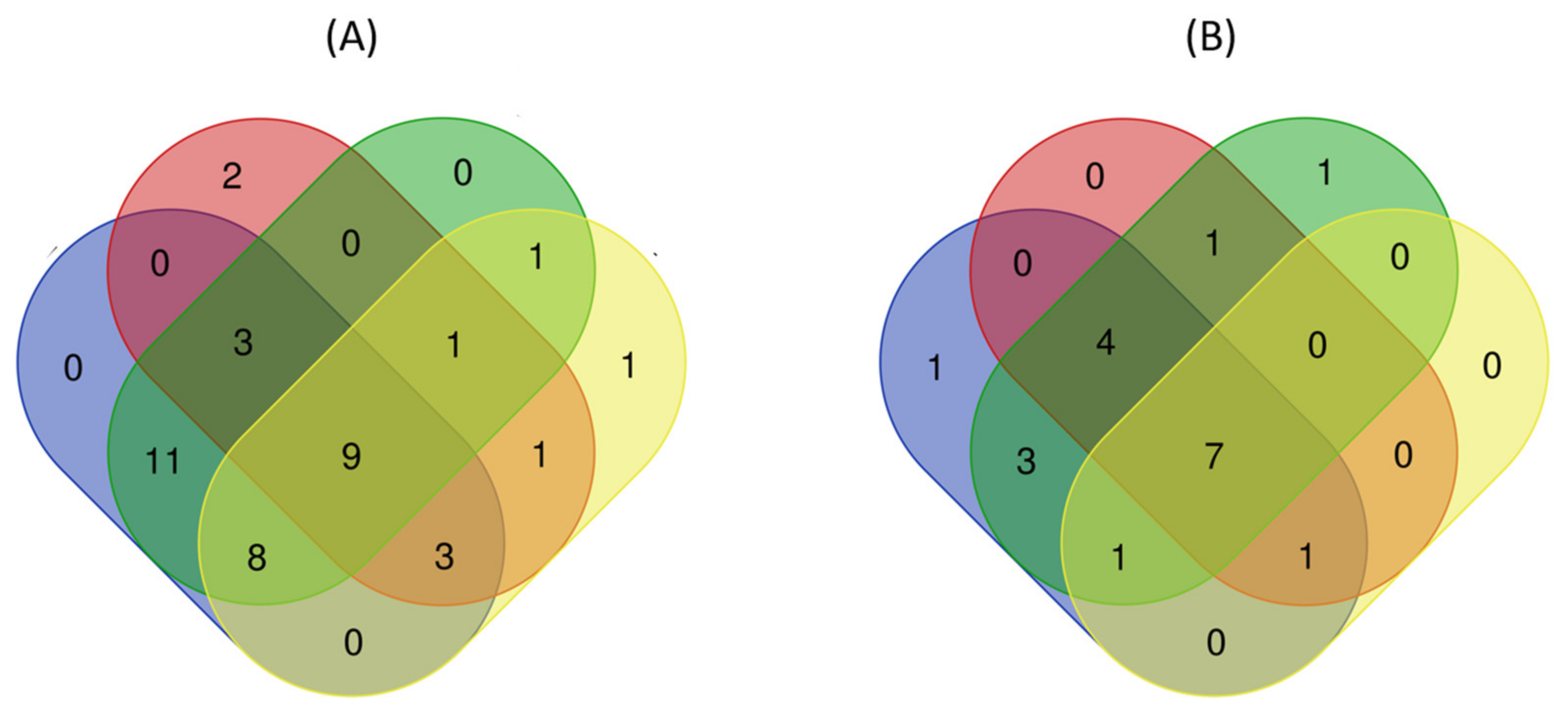
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Keller, A.A. Metabolomic Response of Early-Stage Wheat (Triticum aestivum) to Surfactant-Aided Foliar Application of Copper Hydroxide and Molybdenum Trioxide Nanoparticles. Nanomaterials 2021, 11, 3073. https://doi.org/10.3390/nano11113073
Huang X, Keller AA. Metabolomic Response of Early-Stage Wheat (Triticum aestivum) to Surfactant-Aided Foliar Application of Copper Hydroxide and Molybdenum Trioxide Nanoparticles. Nanomaterials. 2021; 11(11):3073. https://doi.org/10.3390/nano11113073
Chicago/Turabian StyleHuang, Xiangning, and Arturo A. Keller. 2021. "Metabolomic Response of Early-Stage Wheat (Triticum aestivum) to Surfactant-Aided Foliar Application of Copper Hydroxide and Molybdenum Trioxide Nanoparticles" Nanomaterials 11, no. 11: 3073. https://doi.org/10.3390/nano11113073
APA StyleHuang, X., & Keller, A. A. (2021). Metabolomic Response of Early-Stage Wheat (Triticum aestivum) to Surfactant-Aided Foliar Application of Copper Hydroxide and Molybdenum Trioxide Nanoparticles. Nanomaterials, 11(11), 3073. https://doi.org/10.3390/nano11113073