High-Throughput Fabrication of Antibacterial Starch/PBAT/AgNPs@SiO2 Films for Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Starch/PBAT/AgNPs@SiO2 (SPA) Films
2.3. Characterization of Films
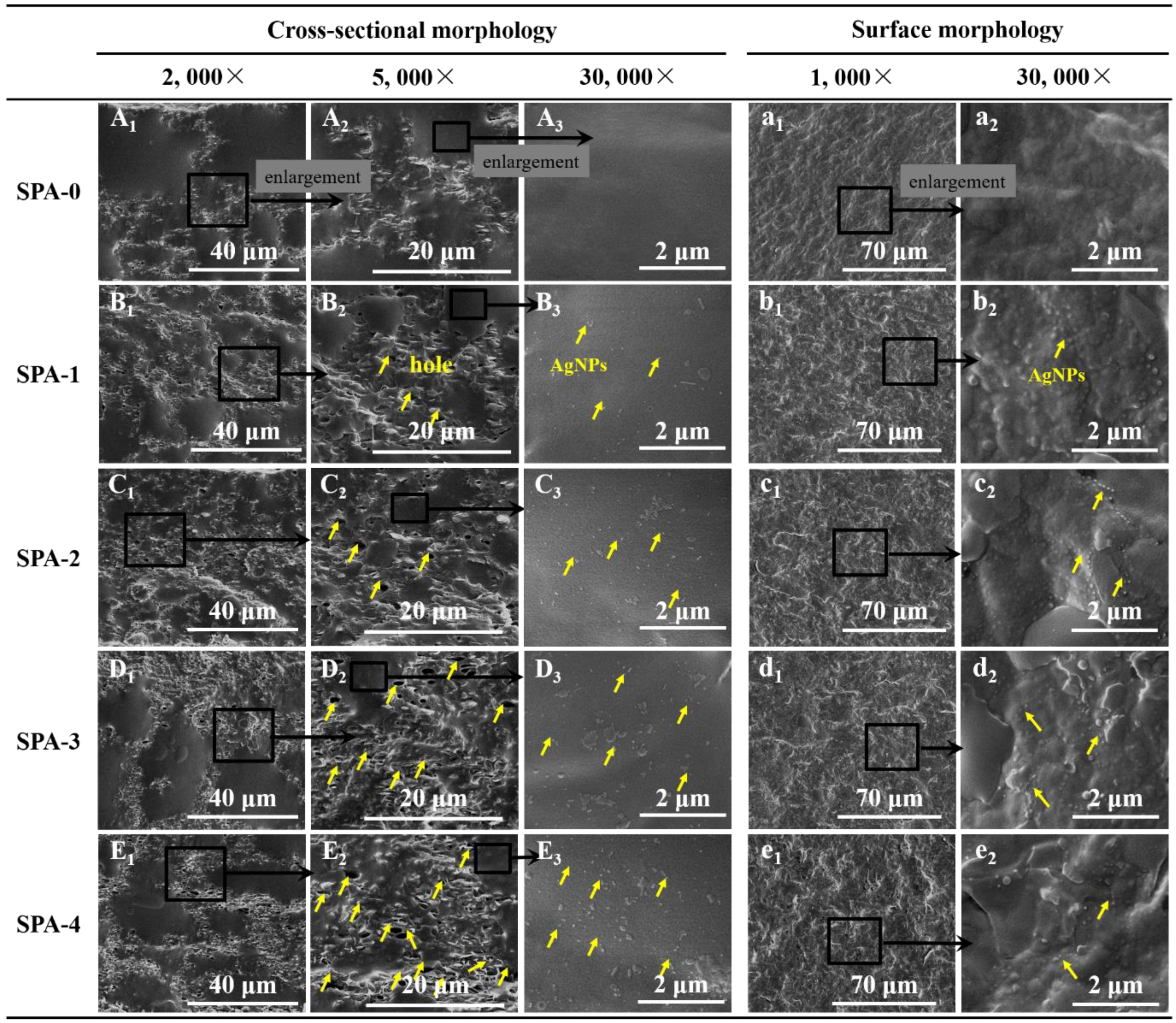
2.3.1. Scanning Electron Microscopy (SEM)
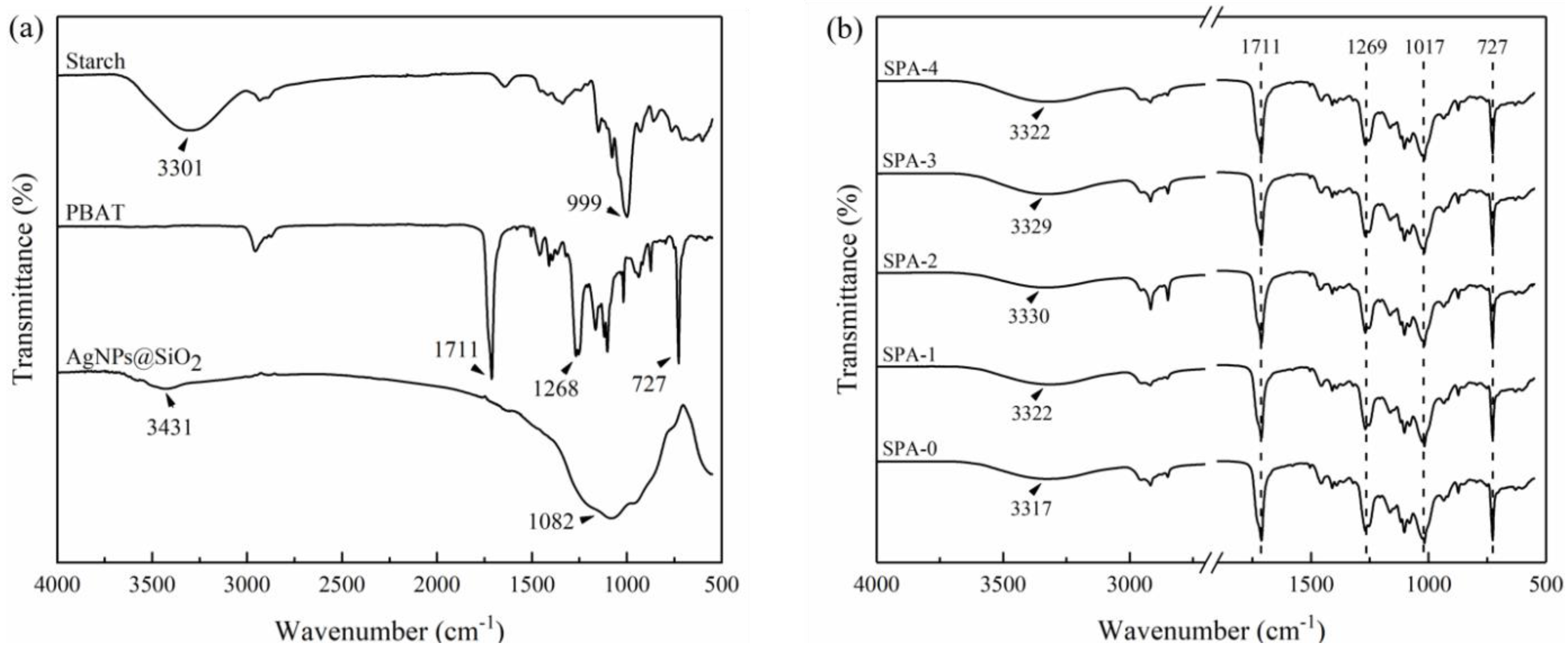
2.3.2. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
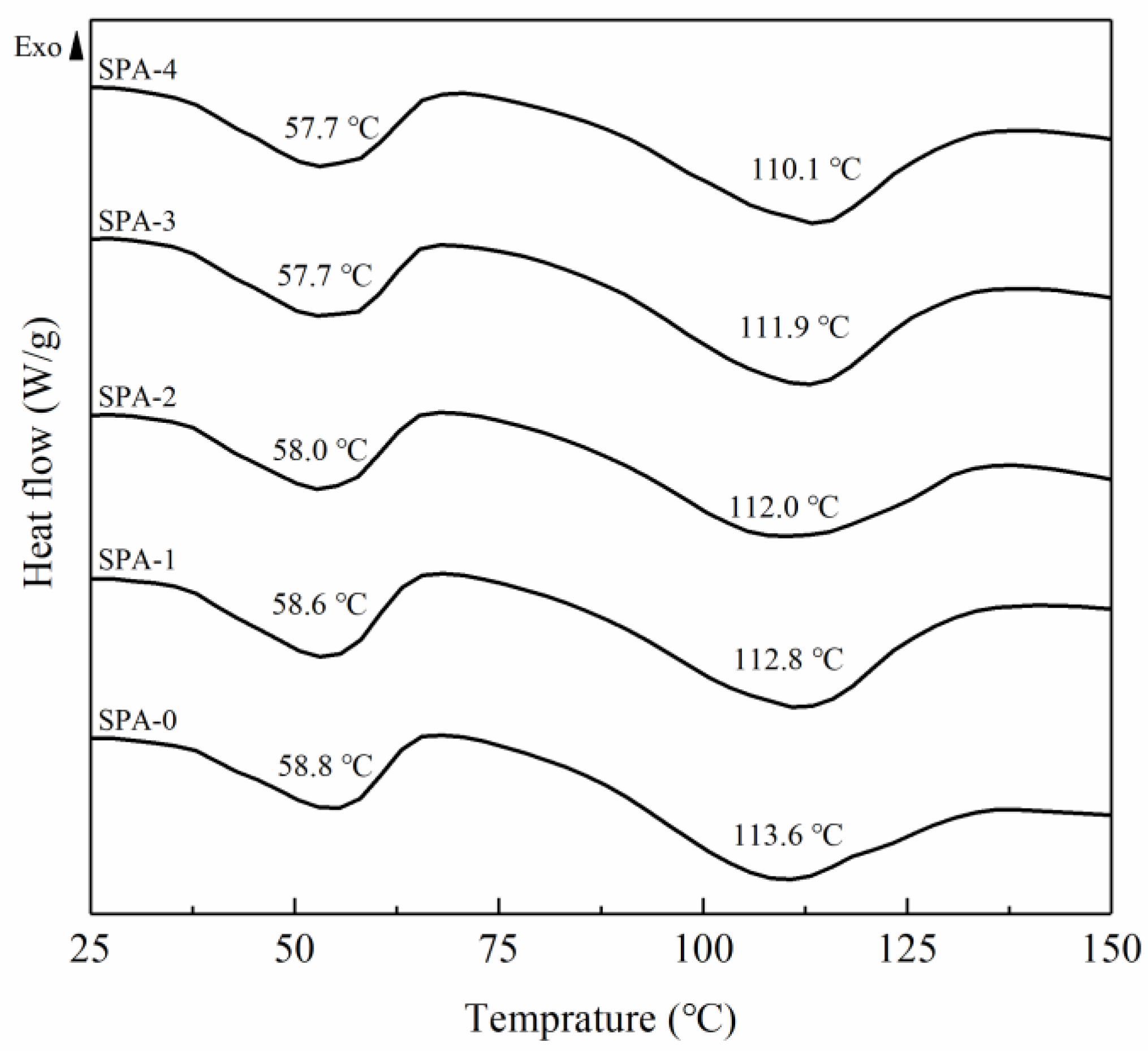
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Oxygen Permeability (OP)
2.3.5. Water Vapor Permeability (WVP)
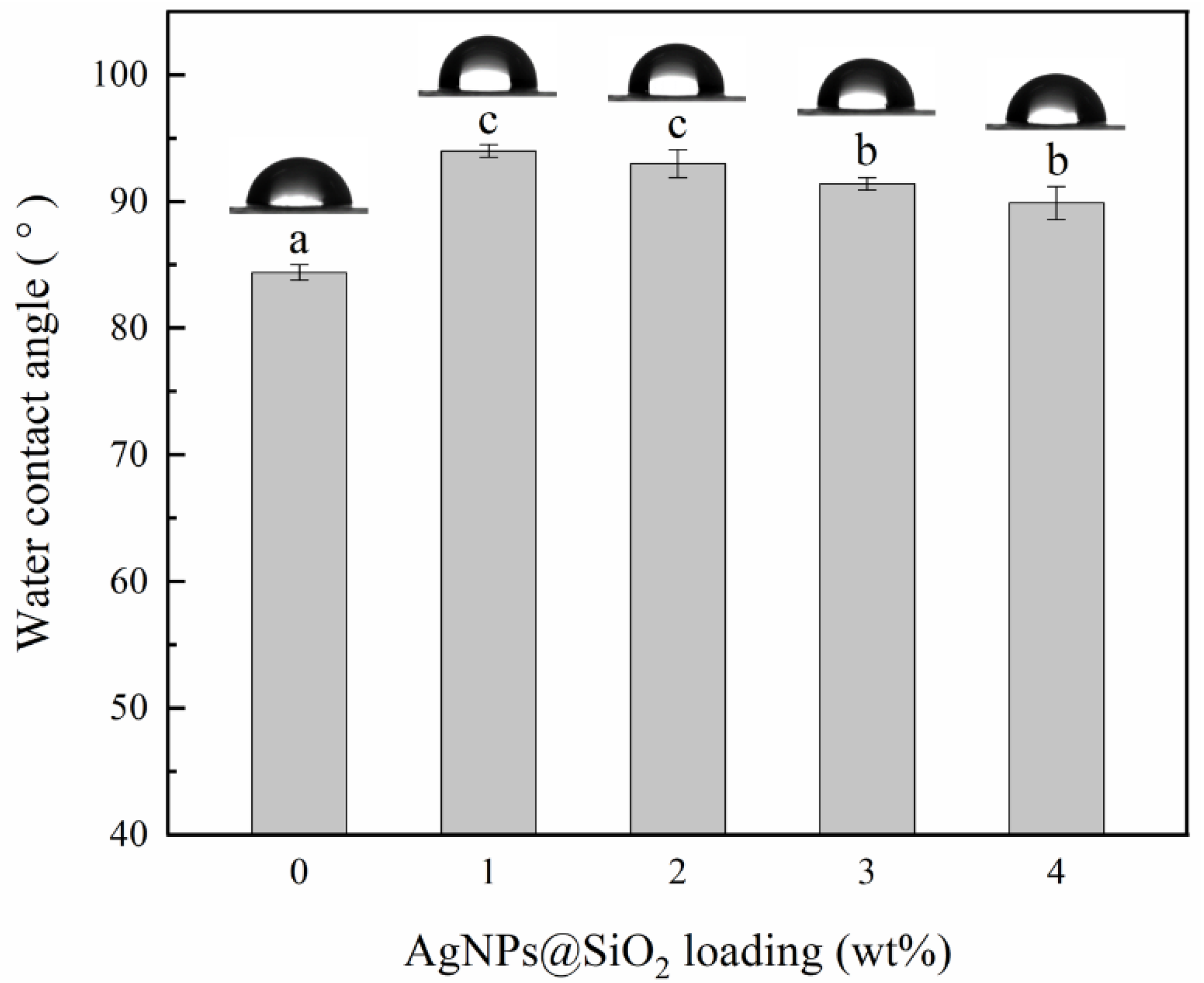
2.3.6. Water Contact Angle (WCA)
2.3.7. Mechanical Properties
2.3.8. Antimicrobial Activity
2.4. Preliminary Packaging Studies on Peach and Nectarine
2.5. Statistical Analysis
3. Results and Discussion
3.1. Morphology
3.2. ATR-FTIR
3.3. DSC
3.4. Mechanical Properties
3.5. Barrier Properties
3.6. Surface Hydrophilicity
3.7. Antimicrobial Activity
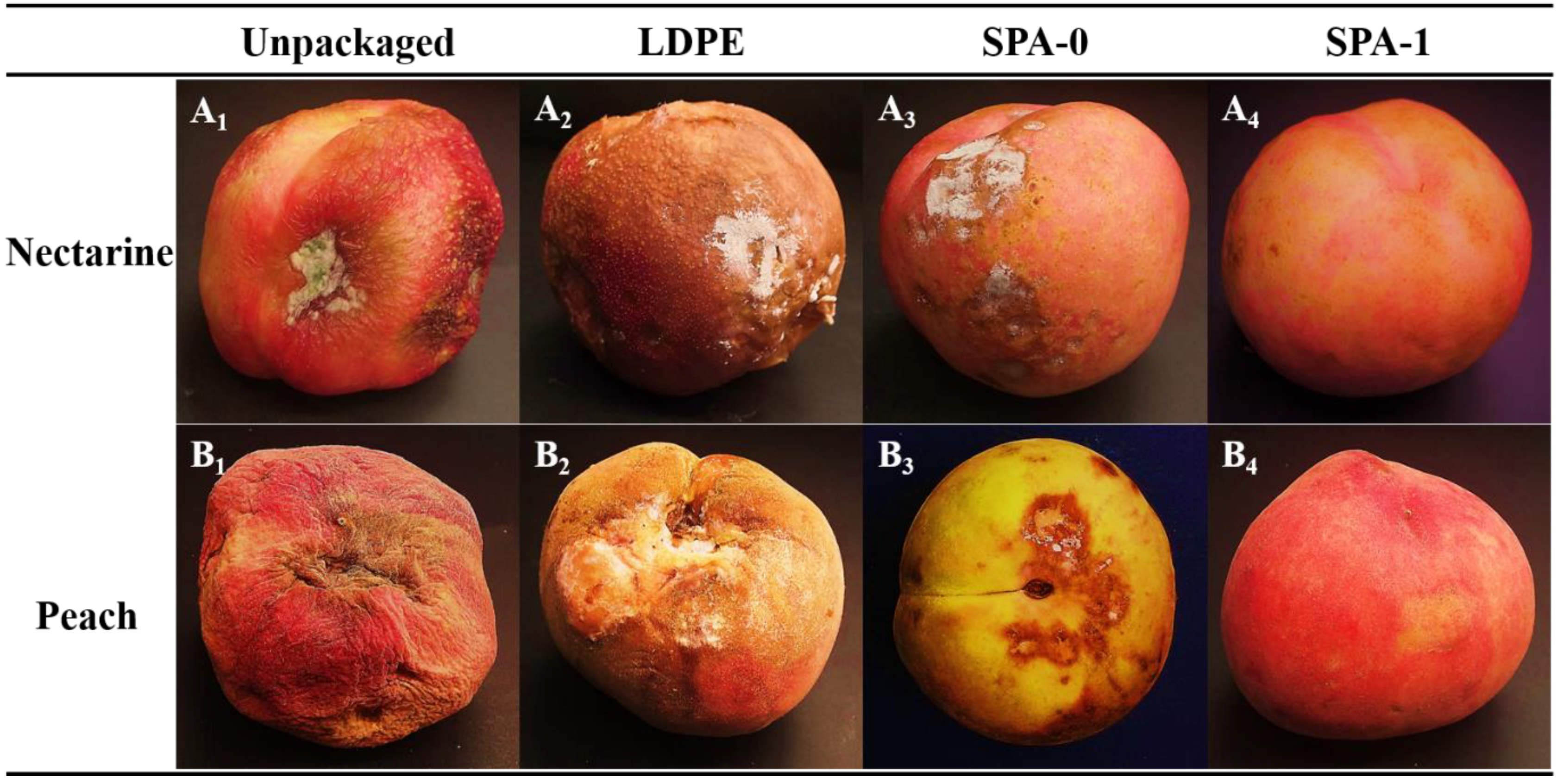
3.8. Preliminary Packaging Studies on Peach and Nectarine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology development in food packaging: A review. Trends Food Sci. Tech. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Anjugam, M.; Vaseeharan, B.; Iswarya, A.; Divya, M.; Prabhu, N.M.; Sankaranarayanan, K. Biological synthesis of silver nanoparticles using beta-1, 3 glucan binding protein and their antibacterial, antibiofilm and cytotoxic potential. Microb. Pathog. 2018, 115, 31–40. [Google Scholar] [CrossRef]
- Dhas, T.S.; Kumar, V.G.; Karthick, V.; Angel, K.J.; Govindaraju, K. Facile synthesis of silver chloride nanoparticles using marine alga and its antibacterial efficacy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 120, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ogunsona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekonnen, T.H. Engineered nanomaterials for antimicrobial applications: A review. Appl. Mater. Today 2020, 18, 100473. [Google Scholar] [CrossRef]
- Freire, P.L.; Albuquerque, A.J.; Farias, I.A.; da Silva, T.G.; Aguiar, J.S.; Galembeck, A. Antimicrobial and cytotoxicity evaluation of colloidal chitosan—Silver nanoparticles—Fluoride nanocomposites. Int. J. Biol. Macromol. 2016, 93, 896–903. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitra, G.; Franklin, D.S.; Sudarsan, S.; Sakthivel, M.; Guhanathan, S. Preparation, antimicrobial and antioxidant evaluation of indole-3-acetic acid-based pH-responsive bio-nanocomposites. Polym. Bull. 2017, 74, 3379–3398. [Google Scholar] [CrossRef]
- Shah, A.; Yameen, M.A.; Fatima, N.; Murtaza, G. Chemical synthesis of chitosan/silver nanocomposites films loaded with moxifloxacin: Their characterization and potential antibacterial activity. Int. J. Pharmacol. 2019, 561, 19–34. [Google Scholar] [CrossRef]
- Sherif, H.H.A.; Khalil, S.K.H.; Hegazi, A.G.; Khalil, W.A.; Moharram, M.A. Factors affecting the antibacterial activity of chitosan-silver nanocomposite. IET Nanobiotechnol. 2017, 11, 731–737. [Google Scholar] [CrossRef]
- Shah, A.; Hussain, I.; Murtaza, G. Chemical synthesis and characterization of chitosan/silver nanocomposites films and their potential antibacterial activity. Int. J. Biol. Macromol. 2018, 116, 520–529. [Google Scholar] [CrossRef]
- Hasan, M.M.; Zhou, Y.; Mahfuz, H.; Jeelani, S. Effect of SiO2 nanoparticle on thermal and tensile behavior of nylon-6. Mat. Sci. Eng. A-Struct. 2006, 429, 181–188. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Chang, M.; He, B.; Zhang, C. Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef]
- Tang, S.; Zou, P.; Xiong, H.; Tang, H. Effect of nano-SiO2 on the performance of starch/polyvinyl alcohol blend films. Carbohyd. Polym. 2008, 72, 521–526. [Google Scholar] [CrossRef]
- Katsikis, N.; Zahradnik, F.; Helmschrott, A.; Münstedt, H.; Vital, A. Thermal stability of poly(methyl methacrylate)/silica nano- and microcomposites as investigated by dynamic-mechanical experiments. Polym. Degrad. Stabil. 2007, 92, 1966–1976. [Google Scholar] [CrossRef]
- Zapata, P.A.; Larrea, M.; Tamayo, L.; Rabagliati, F.M.; Azocar, M.I.; Paez, M. Polyethylene/silver-nanofiber composites: A material for antibacterial films. Mater. Sci. Eng. C-Mater. 2016, 69, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Polat, S.; Fenercioğlu, H.; Güçlü, M. Effects of metal nanoparticles on the physical and migration properties of low density polyethylene films. J. Food Eng. 2018, 229, 32–42. [Google Scholar] [CrossRef]
- Liu, F.; Liu, H.; Li, X.; Zhao, H.; Zhu, D.; Zheng, Y. Nano-TiO2@Ag/PVC film with enhanced antibacterial activities and photocatalytic properties. Appl. Surf. Sci. 2012, 258, 4667–4671. [Google Scholar] [CrossRef]
- Mallakpour, S.; Barati, A. A straightforward preparation and characterization of novel poly(vinyl alcohol)/organoclay/silver tricomponent nanocomposite films. Prog. Org. Coat. 2014, 77, 1629–1634. [Google Scholar] [CrossRef]
- Moreira, R.C.L.; Oliveira, J.H.; Libel, G.P.; Amaral, P.E.R.; Pereira, E.C.A.; Siqueira, V.L.D. Modified polystyrene spheres/graphene oxide decorated with silver nanoparticles as bactericidal material. J. Mol. Struct. 2021, 1233, 130091. [Google Scholar] [CrossRef]
- Xie, J.Z.; Wang, Z.; Zhao, Q.H.; Yang, Y.C.; Xu, J.; Waterhouse, G.I.N.; Zhang, K.; Li, S.; Jin, P.; Jin, G. Scale-up fabrication of biodegradable poly(butylene adipate-co-terephthalate)/organophilic-clay nanocomposite films for potential packaging applications. ACS Omega 2018, 3, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Marrez, D.A.; Abdelhamid, A.E.; Darwesh, O.M. Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf 2019, 20, 100302. [Google Scholar] [CrossRef]
- Onofre-Cordeiro, N.A.; Silva, Y.E.O.; Solidonio, E.G.; de Sena, K.; Silva, W.E.; Santos, B.S. Agarose-silver particles films: Effect of calcium ascorbate in nanoparticles synthesis and film properties. Int. J. Biol. Macromol. 2018, 119, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Girdthep, S.; Worajittiphon, P.; Molloy, R.; Lumyong, S.; Leejarkpai, T.; Punyodom, W. Biodegradable nanocomposite blown films based on poly(lactic acid) containing silver-loaded kaolinite: A route to controlling moisture barrier property and silver ion release with a prediction of extended shelf life of dried longan. Polymer 2014, 55, 6776–6788. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Mariano, M.; Lepesqueur, L.S.S.; Pinheiro, I.F.; Santos, L.G.; Burga-Sanchez, J. Silver nanoparticles coated with dodecanethiol used as fillers in non-cytotoxic and antifungal PBAT surface based on nanocomposites. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Hou, H. Effects of high starch content on the physicochemical properties of starch/PBAT nanocomposite films prepared by extrusion blowing. Carbohydr. Polym. 2020, 239, 116231. [Google Scholar] [CrossRef]
- ASTM. Standard test method for tensile properties of thin plastic sheeting. In Annual Book of ASTM Standards; Standards Designations: D882-12; American Society for Testing and Material: Philadelphia, PA, USA, 2012. [Google Scholar]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Mohammadzadeh, P.P. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf 2019, 22, 100420. [Google Scholar] [CrossRef]
- ASTM. Standard test methods for water vapor transmission of materials. In Annual Book of ASTM Standards; Standards Designations: E96/E96M-16; American Society for Testing and Material: Philadelphia, PA, USA, 2016. [Google Scholar]
- Ahmed, J.; Arfat, Y.A.; Castro-Aguirre, E.; Auras, R. Mechanical, structural and thermal properties of Ag-Cu and ZnO reinforced polylactide nanocomposite films. Int. J. Biol. Macromol. 2016, 86, 885–892. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, C.; Zheng, S.; Li, W.; Li, B.; Xie, X. Poly (butylene adipate-co-terephthalate)/magnesium oxide/silver ternary composite biofilms for food packaging application. Food Packag. Shelf 2020, 24, 100487. [Google Scholar] [CrossRef]
- Wen, X.; Lin, Y.; Han, C.; Zhang, K.; Ran, X.; Li, Y. Thermomechanical and optical properties of biodegradable poly(L-lactide)/silica nanocomposites by melt compounding. J. Appl. Polym. Sci. 2009, 114, 3379–3388. [Google Scholar] [CrossRef]
- Park, J.T.; Seo, J.A.; Ahn, S.H.; Kim, J.H.; Kang, S.W. Surface modification of silica nanoparticles with hydrophilic polymers. J. Ind. Eng. Chem. 2010, 16, 517–522. [Google Scholar] [CrossRef]
- de Campos, S.S.; de Oliveira, A.; Moreira, T.F.M.; da Silva, T.B.V.; da Silva, M.V.; Pinto, J.A. TPCS/PBAT blown extruded films added with curcumin as a technological approach for active packaging materials. Food Packag. Shelf 2019, 22, 100424. [Google Scholar] [CrossRef] [Green Version]
- Luna, J.; Vílchez, A. Polymer Nanocomposites for Food Packaging. Emerg. Nanotechnol. Food Sci. 2017, 119–147. [Google Scholar]
- Cao, C.; Wang, Y.; Zheng, S.; Zhang, J.; Li, W.; Li, B. Poly (butylene adipate-co-terephthalate)/titanium dioxide/silver composite biofilms for food packaging application. LWT-Food Sci. Technol. 2020, 132, 109874. [Google Scholar] [CrossRef]
- Usman, A.; Hussain, Z.; Riaz, A.; Khan, A.N. Enhanced mechanical, thermal and antimicrobial properties of poly(vinyl alcohol)/graphene oxide/starch/silver nanocomposites films. Carbohydr. Polym. 2016, 153, 592–599. [Google Scholar] [CrossRef]
- Santos, R.A.L.; Muller, C.M.O.; Grossmann, M.V.E.; Mali, S.; Yamashita, F. Starch/poly (butylene adipate-co-terephthalate)/montmorillonite films produced by blow extrusion. Quím. Nova 2014, 6, 937–942. [Google Scholar] [CrossRef]
- Pilic, B.; Radusin, T.; Ristic, I.; Silvestre, C.; Lazic, V.; Balos, S. Hydrophobic silica nanoparticles as reinforcing filler for poly (lactic acid) polymer matrix. Hem. Ind. 2016, 70, 73–80. [Google Scholar] [CrossRef]
- Biswas, M.C.; Tiimob, B.J.; Abdela, W.; Jeelani, S.; Rangari, V.K. Nano silica-carbon-silver ternary hybrid induced antimicrobial composite films for food packaging application. Food Packag. Shelf 2019, 19, 104–113. [Google Scholar] [CrossRef]
- Ortega, F.; Arce, V.B.; Garcia, M.A. Nanocomposite starch-based films containing silver nanoparticles synthesized with lemon juice as reducing and stabilizing agent. Carbohydr. Polym. 2021, 252, 117208. [Google Scholar] [CrossRef]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active composite starch films containing green synthetized silver nanoparticles. Food Hydrocolloid. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr. Polym. 2015, 130, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ghoshal, G.; Goyal, M. Development and characterization of corn starch based nanocomposite film with AgNPs and plant extract. Mat. Sci. Energy Tech. 2020, 3, 672–678. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticle-loaded chitosan-starch based films: Fabrication and evaluation of tensile, barrier and antimicrobial properties. Mat. Sci. Eng. C-Mater. 2010, 30, 891–897. [Google Scholar] [CrossRef]
- Lazić, V.; Vivod, V.; Peršin, Z.; Stoiljković, M.; Ratnayake, I.S.; Ahrenkiel, P.S. Dextran-coated silver nanoparticles for improved barrier and controlled antimicrobial properties of nanocellulose films used in food packaging. Food Packag. Shelf 2020, 2, 100575. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Tocopherol-mediated synthesis of silver nanoparticles and preparation of antimicrobial PBAT/silver nanoparticles composite films. LWT-Food Sci. Technol. 2016, 72, 149–156. [Google Scholar] [CrossRef]
- Panrong, T.; Karbowiak, T.; Harnkarnsujarit, N. Effects of acetylated and octenyl-succinated starch on properties and release of green tea compounded starch/LLDPE blend films. J. Food Eng. 2020, 284, 110057. [Google Scholar] [CrossRef]
- He, T.; Liu, H.; Zhou, Y.; Yang, J.; Cheng, X.; Shi, H. Antibacterial effect and proteomic analysis of graphene-based silver nanoparticles on a pathogenic bacterium Pseudomonas aeruginosa. Biometals 2014, 27, 673–682. [Google Scholar] [CrossRef]
- Mathooko, F.M. Regulation of respiratory metabolism in fruits and vegetables by carbon dioxide. Postharvest Biol. Tech. 1996, 9, 247–264. [Google Scholar] [CrossRef]
- Akbudak, B.; Eris, A. Physical and chemical changes in peaches and nectarines during the modified atmosphere storage. Food Control 2004, 15, 307–313. [Google Scholar] [CrossRef]
- Fernández-Trujilio, J.P.; MartÍnez, J.A.; Artés, F. Modified atmosphere packaging affects the incidence of cold storage disorders and keeps ’flat’ peach quality. Food Res. Int. 1998, 31, 571–598. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Crisosto, C.H. Stone fruits: Peaches, nectarines, plums, apricots. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Academic Press: Cambridge, MA, USA, 2020; pp. P311–P322, Chapter 15. [Google Scholar] [CrossRef]


| Samples | Mechanical Properties | Barrier Properties | ||
|---|---|---|---|---|
| TS (MPa) | EAB (%) | WVP (×10−11 g·m·m−2·s−1·Pa−1) | OP (×10−14 cm3·cm·cm−2·s−1·Pa−1) | |
| SPA-0 | 10.27 ± 0.55 a,b | 656.63 ± 11.98 a | 3.58 ± 0.07 b | 6.26 ± 0.02 b |
| SPA-1 | 10.91 ± 0.66 b | 646.53 ± 7.39 a | 3.09 ± 0.08 a | 5.37 ± 0.20 a |
| SPA-2 | 11.29 ± 0.45 b | 774.40 ± 76.69 b | 3.07 ± 0.12 a | 5.48 ± 0.32 a |
| SPA-3 | 10.30 ± 0.45 a,b | 760.00 ± 37.76 b | 3.09 ± 0.17 a | 5.48 ± 0.53 a |
| SPA-4 | 9.64 ± 0.68 a | 751.00 ± 21.96 b | 3.14 ± 0.04 a | 6.31 ± 0.08 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zhai, X.; Zhang, R.; Wang, W.; Lim, L.-T.; Hou, H. High-Throughput Fabrication of Antibacterial Starch/PBAT/AgNPs@SiO2 Films for Food Packaging. Nanomaterials 2021, 11, 3062. https://doi.org/10.3390/nano11113062
Zhou S, Zhai X, Zhang R, Wang W, Lim L-T, Hou H. High-Throughput Fabrication of Antibacterial Starch/PBAT/AgNPs@SiO2 Films for Food Packaging. Nanomaterials. 2021; 11(11):3062. https://doi.org/10.3390/nano11113062
Chicago/Turabian StyleZhou, Shengxue, Xiaosong Zhai, Rui Zhang, Wentao Wang, Loong-Tak Lim, and Hanxue Hou. 2021. "High-Throughput Fabrication of Antibacterial Starch/PBAT/AgNPs@SiO2 Films for Food Packaging" Nanomaterials 11, no. 11: 3062. https://doi.org/10.3390/nano11113062
APA StyleZhou, S., Zhai, X., Zhang, R., Wang, W., Lim, L.-T., & Hou, H. (2021). High-Throughput Fabrication of Antibacterial Starch/PBAT/AgNPs@SiO2 Films for Food Packaging. Nanomaterials, 11(11), 3062. https://doi.org/10.3390/nano11113062







