A Redox-Mediator-Integrated Flexible Micro-Supercapacitor with Improved Energy Storage Capability and Suppressed Self-Discharge Rate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrications of HQ-MSCs
2.2. Characterization and Electrochemical Tests of HQ-MSCs
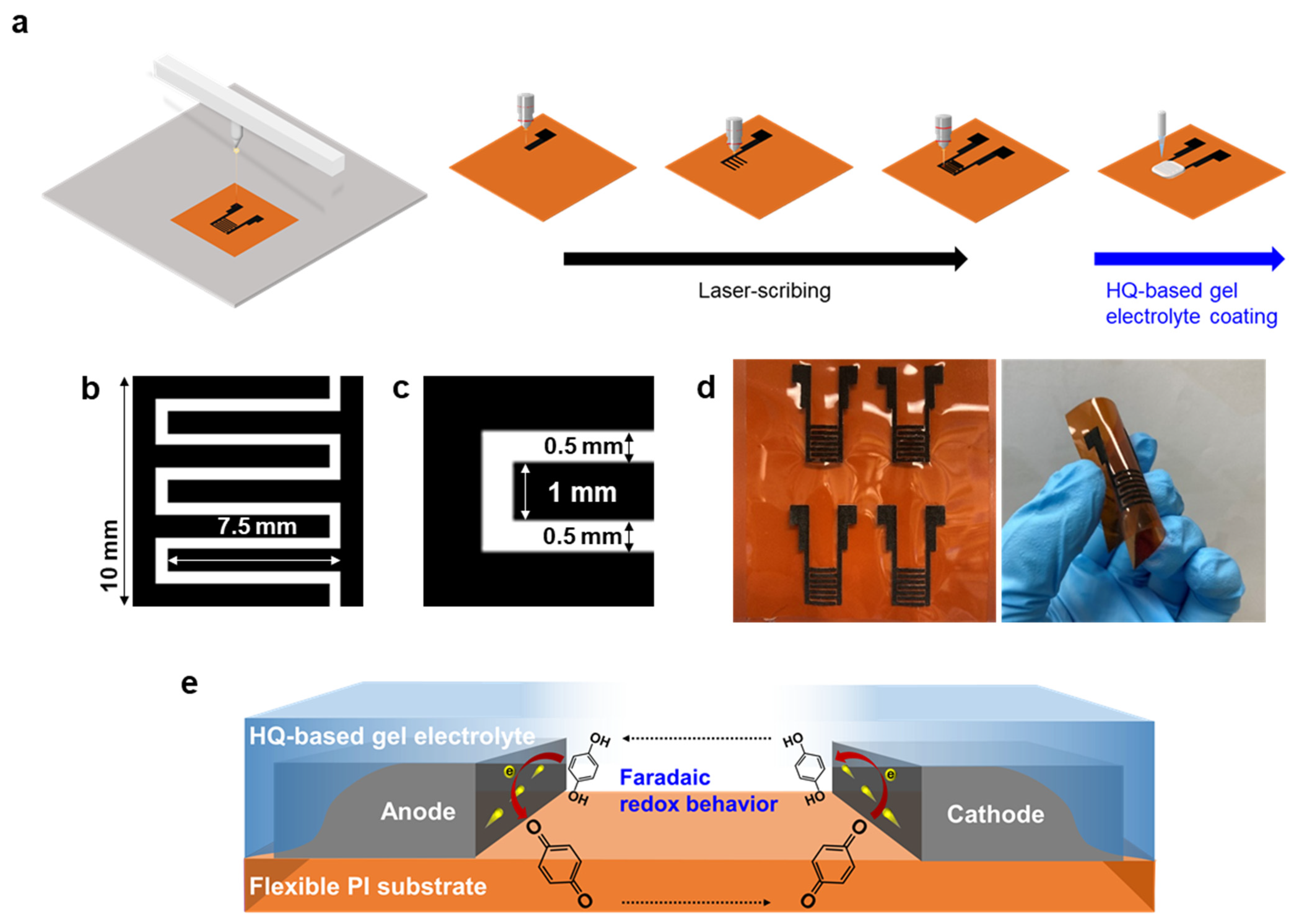
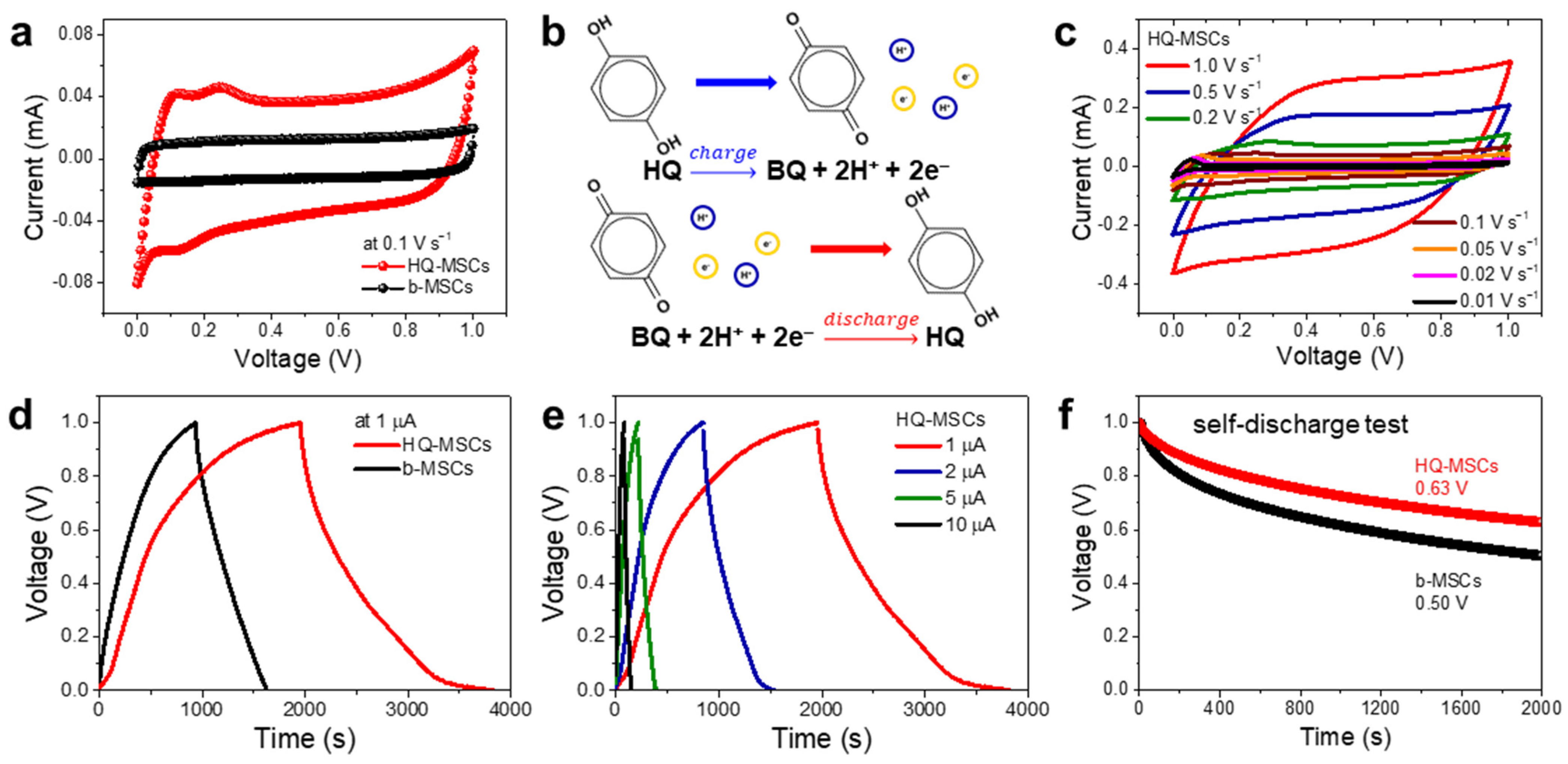
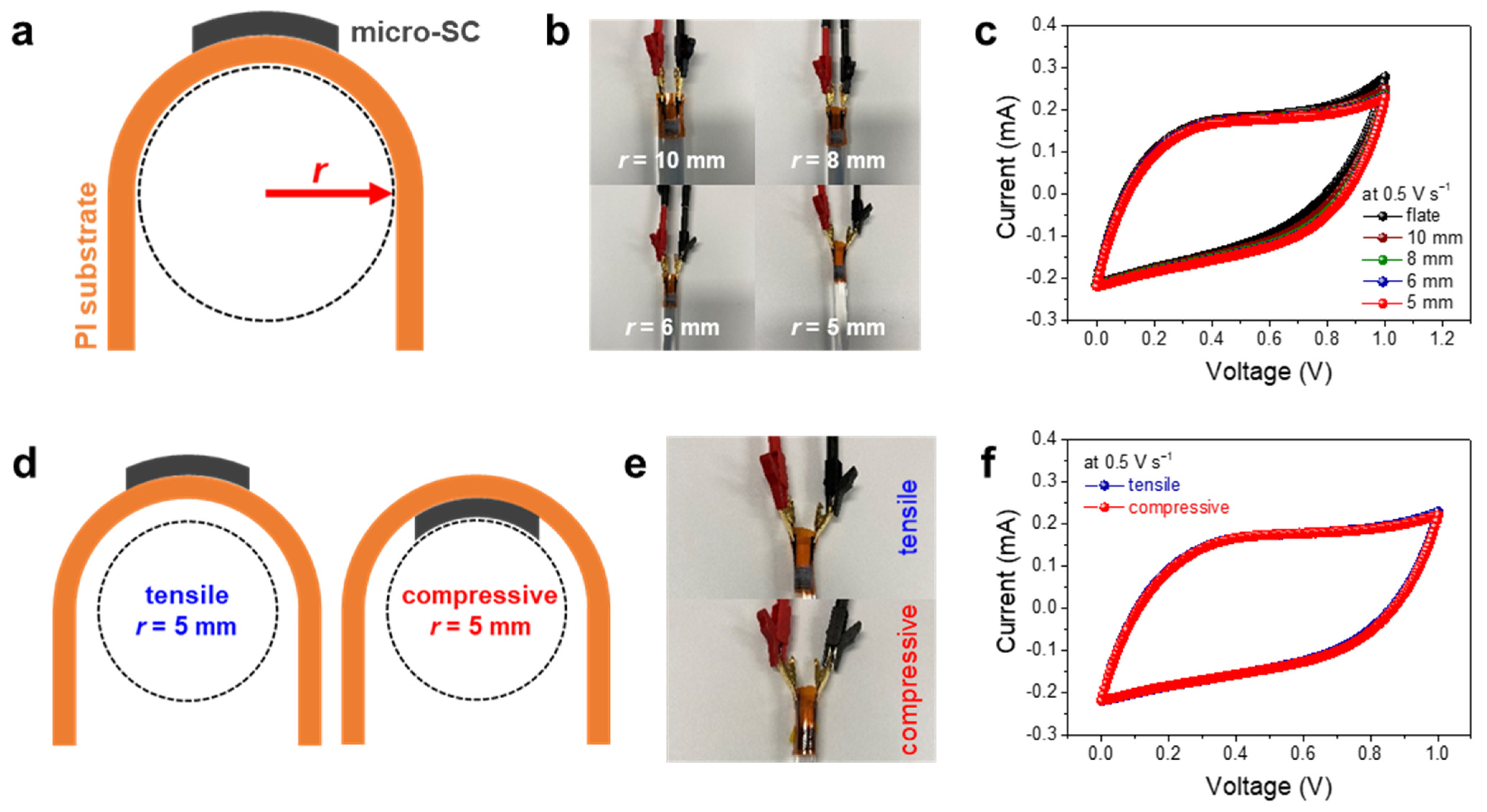
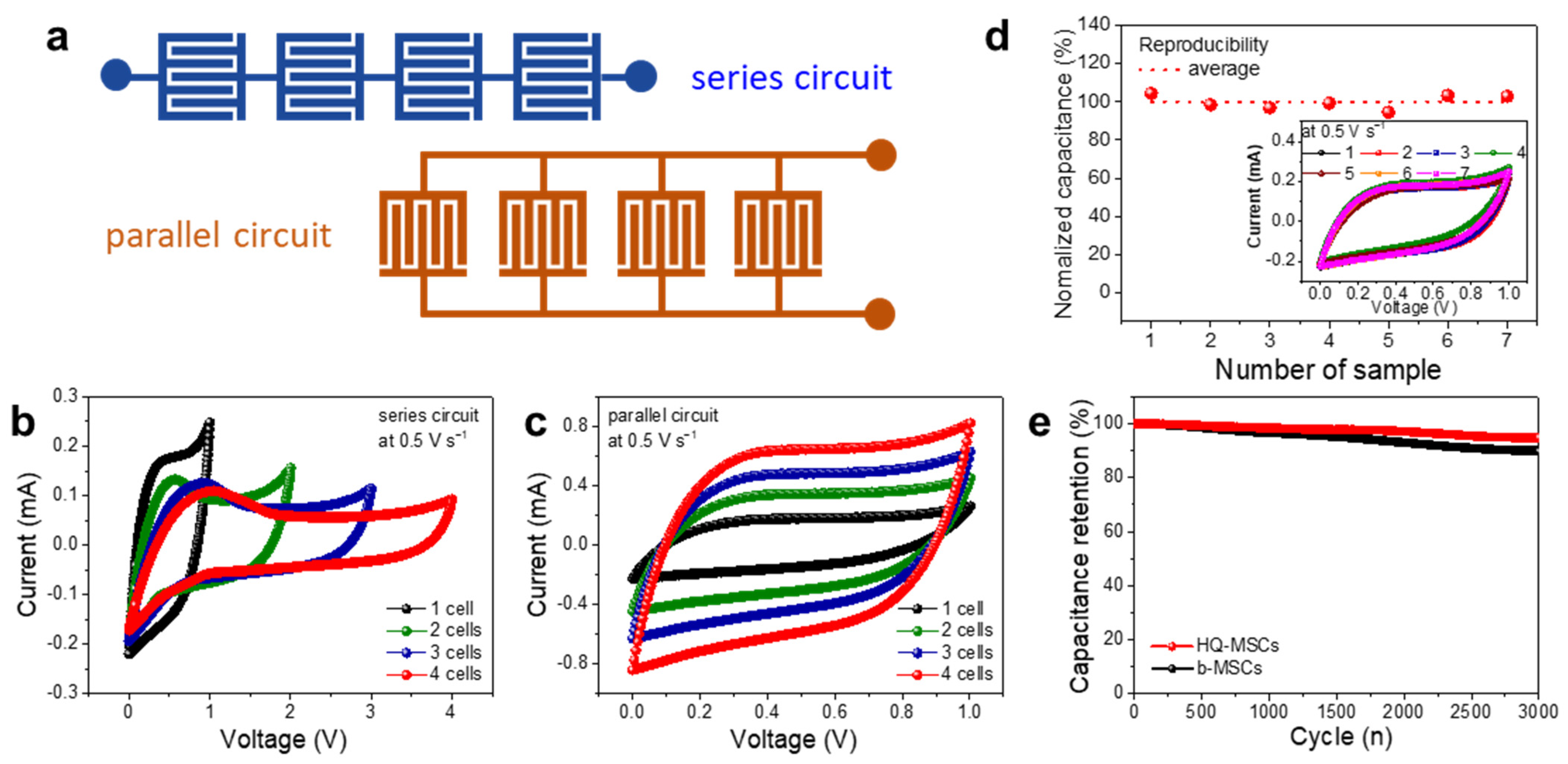
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, F.; Li, Y.; Qu, J.; Wang, J.; Bandari, V.K.; Zhu, F.; Schmidt, O.G. Recent developments of stamped planar micro-supercapacitors: Materials, fabrication and perspectives. Nano Mater. Sci. 2021, 3, 154–169. [Google Scholar] [CrossRef]
- Bu, F.; Zhou, W.; Xu, Y.; Du, Y.; Guan, C.; Huang, W. Recent developments of advanced micro-supercapacitors: Design, fabrication and applications. NPJ Flex. Electron. 2020, 4, 31. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Chee, M.O.L.; Dong, P.; Ye, M.; Shen, J. Recent advances in micro-supercapacitors. Nanoscale 2019, 11, 5807–5821. [Google Scholar] [CrossRef]
- Maphiri, V.M.; Rutavi, G.; Sylla, N.F.; Adewinbi, S.A.; Fasakin, O.; Manyala, N. Novel thermally reduced graphene oxide microsupercapacitor fabricated via mask—Free AxiDraw direct writing. Nanomaterials 2021, 11, 1909. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, D.; Yu, Y.; Liu, L.; Wu, Y. Carbon-based flexible micro-supercapacitor fabrication via mask-free ambient micro-plasma-jet etching. Carbon 2017, 111, 121–127. [Google Scholar] [CrossRef]
- Smith, A.D.; Li, Q.; Vyas, A.; Haque, M.M.; Wang, K.; Velasco, A.; Zhang, X.; Thurakkal, S.; Quellmalz, A.; Niklaus, F.; et al. Carbon-based electrode materials for microsupercapacitors in self-powering sensor networks: Present and future development. Sensors 2019, 19, 4231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.-Q.; Tu, Q.-M.; Geng, C.-L.; Huang, J.-L.; Gu, Y.; Lin, J.-M.; Huang, Y.-F.; Wu, J.-H. High energy density and low self-discharge of a quasi-solid-state supercapacitor with carbon nanotubes incorporated redox-active ionic liquid-based gel polymer electrolyte. Electrochim. Acta 2020, 331, 135425. [Google Scholar] [CrossRef]
- Singh, B.K.; Shaikh, A.; Dusane, R.O.; Parida, S. Nanoporous gold–Nitrogen–doped carbon nano-onions all-solid-state micro-supercapacitor. Nano Struct. Nano Objects 2019, 17, 239–247. [Google Scholar] [CrossRef]
- Peng, Z.; Ye, R.; Mann, J.A.; Zakhidov, D.; Li, Y.; Smalley, P.R.; Lin, J.; Tour, J.M. Flexible boron-doped laser-induced graphene microsupercapacitors. ACS Nano 2015, 9, 5868–5875. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripthi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef] [Green Version]
- Rani, J.R.; Thangavel, R.; Oh, S.-I.; Lee, Y.S.; Jang, J.-H. An ultra-high-energy density supercapacitor; fabrication based on thiol-functionalized graphene oxide scrolls. Nanomaterials 2019, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Liu, J. Definitions of pseudocapacitive materials: A brief review. Energy Envion. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, Y.; Yang, C.; Fu, Y.; Guo, Y.; Ma, X. High-performance ionic liquid-based gel polymer electrolyte incorporating anion-trapping boron sites for all-solid-state supercapacitor application. ACS Appl. Mater. Interfaces 2018, 10, 39570–39580. [Google Scholar] [CrossRef] [PubMed]
- Seol, M.-L.; Nam, I.; Sdatian, E.; Dutta, N.; Han, J.-W.; Meyyappan, M. Printable gel polymer electrolytes for solid-state printed supercapacitors. Nanomaterials 2021, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dou, Q.; Sun, Y.; Lu, Y.; Lu, Y.; Zhang, Q.; Meng, J.; Zhang, X.; Shi, S.; Yan, X. A moisture absorbing gel electrolyte enables aqueous and flexible supercapacitors operating at high temperatures. J. Mater. Chem. A 2019, 7, 20398–20404. [Google Scholar] [CrossRef]
- Chen, L.; Bai, H.; Huang, Z.; Li, L. Mechanism investigation and suppression of self-discharge in active electrolyte enhanced supercapacitors. Energy Environ. Sci. 2014, 7, 1750–1759. [Google Scholar] [CrossRef] [Green Version]
- Xia, M.; Nie, J.; Zhang, Z.; Lu, X.; Wang, Z.L. Suppressing self-discharge of supercapacitors via electrorheological effect of liquid crystals. Nano Energy 2018, 47, 43–50. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commnun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Tran, T.S.; Dutta, N.K.; Choudhury, N.R. Graphene-based inks for printing of planar micro-supercapacitors: A review. Materials 2019, 12, 978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Kang, D.-Y.; Moon, J.H. Full lithographic fabrication of boron-doped 3D porous carbon patterns for high volumetric energy density microsupercapacitors. Nano Energy 2018, 53, 182–188. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, L.-Y. Investigating the redox behavior of activated carbon supercapacitors with hydroquinone and p-phenylenediamine dual redox additives in the electrolyte. J. Colloid Interface Sci. 2019, 537, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Nagar, B.; Dubal, D.P.; Pies, L.; Merkoçi, A.; Gómez-Romero, P. Design and fabrication of printed paper-based hybrid micro-supercapacitor by using graphene and redox-active electrolyte. ChemSusChem 2018, 11, 1849–1856. [Google Scholar] [CrossRef]
- Akinwolemiwa, B.; Peng, C.; Chen, G.Z. Redox electrolytes in supercapacitors. J. Electrochem. Soc. 2015, 162, A5054. [Google Scholar] [CrossRef]
- Frackowiak, E.; Meller, M.; Menzel, J.; Gastorl, D.; Fic, K. Redox-active electrolyte for supercapacitor application. Faraday Discuss. 2014, 172, 179–198. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Chang, J.; Zhao, D.; Wang, J.; Yang, C.; Cao, B. A review of redox electrolytes for supercapacitors. Front. Chem. 2020, 8, 413. [Google Scholar] [CrossRef]
- Qin, W.; Zhou, N.; Wu, C.; Xie, M.; Sun, H.; Guo, Y.; Pan, L. Mini-review on the redox additives in aqueous electrolyte for high performance supercapacitor. ACS Omega 2020, 5, 3801–3808. [Google Scholar] [CrossRef] [Green Version]
- Bataev, I.; Panagiotopoulos, N.T.; Charlot, F.; Jorge Junior, A.M.; Pons, M.; Evangelakis, G.A.; Yavari, A.R. Structure and deformation behavior of Zr–Cu thin films deposited on Kapton substrates. Surf. Coat. Technol. 2014, 239, 171–176. [Google Scholar] [CrossRef]
- Siburian, R.; Sihotang, H.; Raja, S.L.; Supeno, M.; Simanjuntak, C. New route to synthesize of graphene nano sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.-F.; Yang, C.-H.; Peng, Y.-Y.; Pu, N.-W.; Ger, M.-D.; Hsieh, C.-T.; Chang, J.-K. Graphene nanosheets, carbon nanotubes, graphite, and activated carbon as anode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 10320–10326. [Google Scholar] [CrossRef]
- Rosher, S.; Hoffmann, R.; Ambacher, O. Determination of the graphene–graphite ratio of graphene powder by Raman 2D band symmetry analysis. Anal. Methods 2019, 11, 1224–1228. [Google Scholar] [CrossRef] [Green Version]
- Chong, L.; Guo, H.; Zhang, Y.; Hu, Y.; Zhang, Y. Raman study of Sstrain relaxation from grain boundaries in epitaxial graphene grown by chemical vapor deposition on SiC. Nanomaterials 2019, 9, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Chakraborty, B.; Sood, A.K. Raman spectroscopy of graphene on different substrates and influence of defects. Bull. Mater. Sci. 2008, 31, 579–584. [Google Scholar] [CrossRef]
- Stubrov, Y.; Nikolenko, A.; Gubanov, V.; Strelchuk, V. Manifestation of structure of electron bands in double-resonant raman spectra of single-walled carbon nanotubes. Nanoscale Res. Lett. 2016, 11, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, T.; Georgiev, G.L.; Auner, G.; Newaz, G.; Herfurth, H.J.; Ratwa, R. XPS analysis of laser transmission micro-joint between poly (vinylidene fluoride) and titanium. Appl. Surf. Sci. 2008, 255, 2569–2573. [Google Scholar] [CrossRef]
- Morgan, D.J. Comments on the XPS analysis of carbon materials. C 2021, 7, 51. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Benturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Senthikumar, S.T.; Selvan, R.K.; Melo, J.S. Redox additive/active electrolytes: A novel approach to enhance the performance of supercapacitors. J. Mater. Chem. A 2013, 1, 12386–12394. [Google Scholar] [CrossRef]
- Park, Y.; Choi, H.; Lee, D.-G.; Kim, M.-C.; Tran, N.A.T.; Cho, Y.; Lee, Y.-W.; Sohn, J.I. Rational design of electrochemical iodine-based redox mediators for water-proofed flexible fiber supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 2409–2415. [Google Scholar] [CrossRef]
- Choi, H.; Kim, M.-C.; Park, Y.; Lee, S.; Ahn, W.; Hong, J.; Soh, J.I.; Jang, A.-R.; Lee, Y.-W. Electrochemically active hydroquinone-based redox mediator for flexible energy storage system with improved charge storing ability. J. Colloid Interface Sci. 2021, 588, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, H.; Kim, M.-C.; Tran, N.A.T.; Cho, Y.; Sohn, J.I.; Hong, J.; Lee, Y.-W. Effect of ionic conductivity in polymer-gel electrolytes containing iodine-based redox mediators for efficient, flexible energy storage systems. J. Ind. Eng. Chem. 2021, 94, 384–389. [Google Scholar] [CrossRef]
- Boota, M.; Hatzell, K.B.; Kumbur, E.C.; Gogotsi, Y. Towards high-energy-density pseudocapacitive flowable electrodes by the incorporation of hydroquinone. ChemSusChem 2015, 8, 835–843. [Google Scholar] [CrossRef]
- Geng, C.-L.; Fan, L.-Q.; Wang, C.-Y.; Wang, Y.-L.; Sun, S.-J.; Song, Z.-Y.; Liu, N.; Wu, J.-H. High energy density and high working voltage of a quasi-solid-state supercapacitor with a redox-active ionic liquid added gel polymer electrolyte. New J. Chem. 2019, 43, 18935–18942. [Google Scholar] [CrossRef]
- Jinisha, B.; Anilkumar, K.M.; Manoj, M.; Ashraf, C.M.; Pradeep, V.S.; Jayalekshmi, S. Solid-state supercapacitor with impressive performance characteristics, assembled using redox-mediated gel polymer electrolyte. J. Solid State Electrochem. 2019, 23, 3343–3353. [Google Scholar] [CrossRef]
- Wada, H.; Yoshikawa, K.; Nohara, S.; Furukawa, N.; Inoue, H.; Sugoh, N.; Iwasaki, H.; Iwakura, C. Electrochemical characteristics of new electric double layer capacitor with acidic polymer hydrogel electrolyte. J. Power Source 2006, 159, 1464–1467. [Google Scholar] [CrossRef]
- Mourad, E.; Coustan, L.; Lannelongue, P.; Zigah, D.; Mehdi, A.; Vioux, A.; Freunberger, S.A.; Favier, F.; Fontaine, O. Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors. Nat. Mater. 2017, 16, 446–453. [Google Scholar] [CrossRef]
- Yu, F.; Huang, M.; Wu, J.; Qiu, z.; Fan, L.; Lin, J.; Lin, Y. A redox-mediator-doped gel polymer electrolyte applied in quasi-solid-state supercapacitors. J. Appl. Polym. Sci. 2014, 131, 39784. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wi, S.M.; Kim, J.; Lee, S.; Choi, Y.-R.; Kim, S.H.; Park, J.B.; Cho, Y.; Ahn, W.; Jang, A.-R.; Hong, J.; et al. A Redox-Mediator-Integrated Flexible Micro-Supercapacitor with Improved Energy Storage Capability and Suppressed Self-Discharge Rate. Nanomaterials 2021, 11, 3027. https://doi.org/10.3390/nano11113027
Wi SM, Kim J, Lee S, Choi Y-R, Kim SH, Park JB, Cho Y, Ahn W, Jang A-R, Hong J, et al. A Redox-Mediator-Integrated Flexible Micro-Supercapacitor with Improved Energy Storage Capability and Suppressed Self-Discharge Rate. Nanomaterials. 2021; 11(11):3027. https://doi.org/10.3390/nano11113027
Chicago/Turabian StyleWi, Sung Min, Jihong Kim, Suok Lee, Yu-Rim Choi, Sung Hoon Kim, Jong Bae Park, Younghyun Cho, Wook Ahn, A-Rang Jang, John Hong, and et al. 2021. "A Redox-Mediator-Integrated Flexible Micro-Supercapacitor with Improved Energy Storage Capability and Suppressed Self-Discharge Rate" Nanomaterials 11, no. 11: 3027. https://doi.org/10.3390/nano11113027
APA StyleWi, S. M., Kim, J., Lee, S., Choi, Y.-R., Kim, S. H., Park, J. B., Cho, Y., Ahn, W., Jang, A.-R., Hong, J., & Lee, Y.-W. (2021). A Redox-Mediator-Integrated Flexible Micro-Supercapacitor with Improved Energy Storage Capability and Suppressed Self-Discharge Rate. Nanomaterials, 11(11), 3027. https://doi.org/10.3390/nano11113027





