Construction of Functional Materials in Various Material Forms from Cellulosic Cholesteric Liquid Crystals
Abstract
:1. General Introduction: Need for and Overview of Cellulosic Liquid-Crystals as Functional Materials
1.1. Introduction
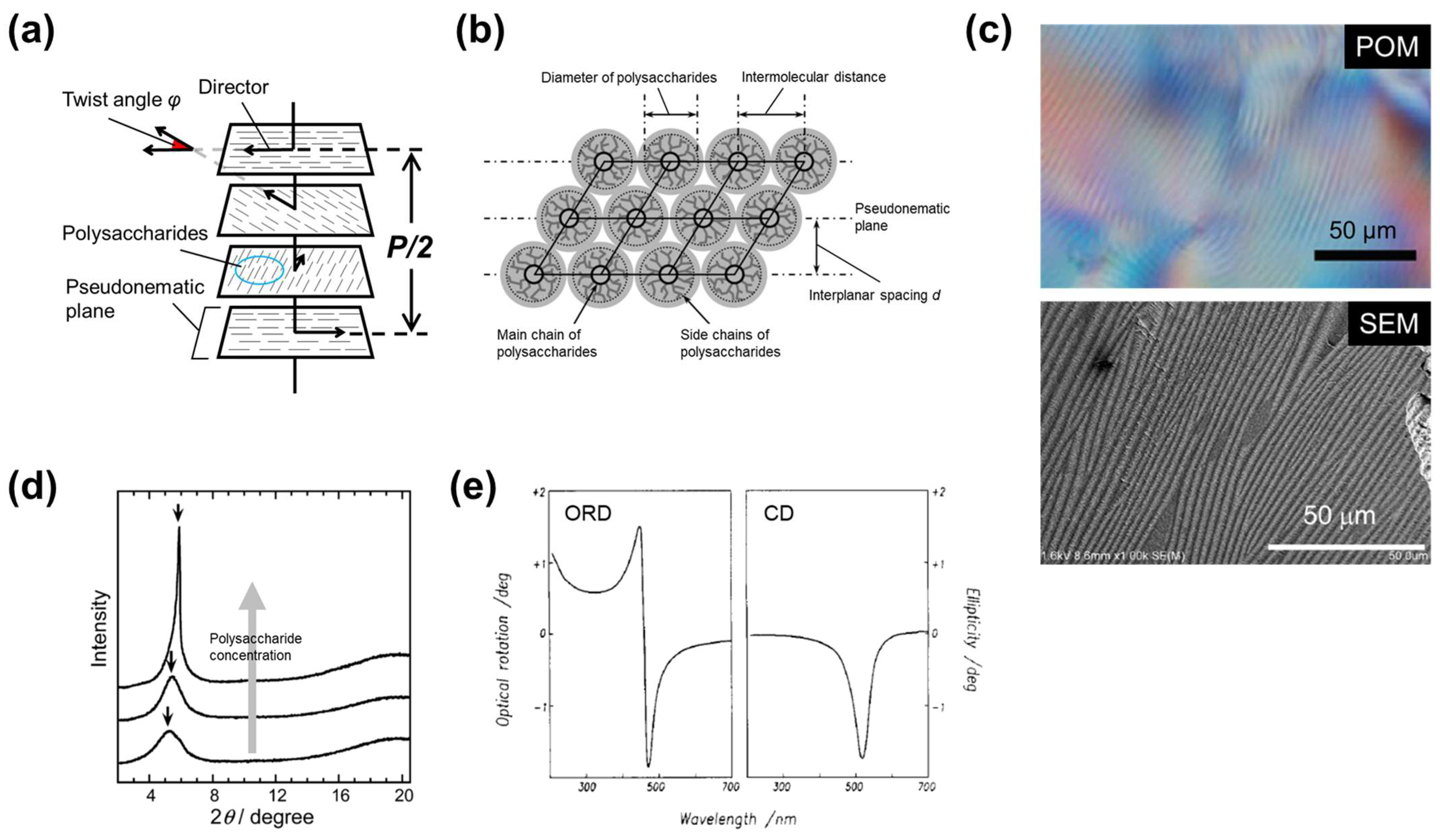
1.2. Cellulosic Liquid-Crystals
2. Cellulosic ChLC-Based Solid Functional Materials
2.1. Cellulosic ChLC Films
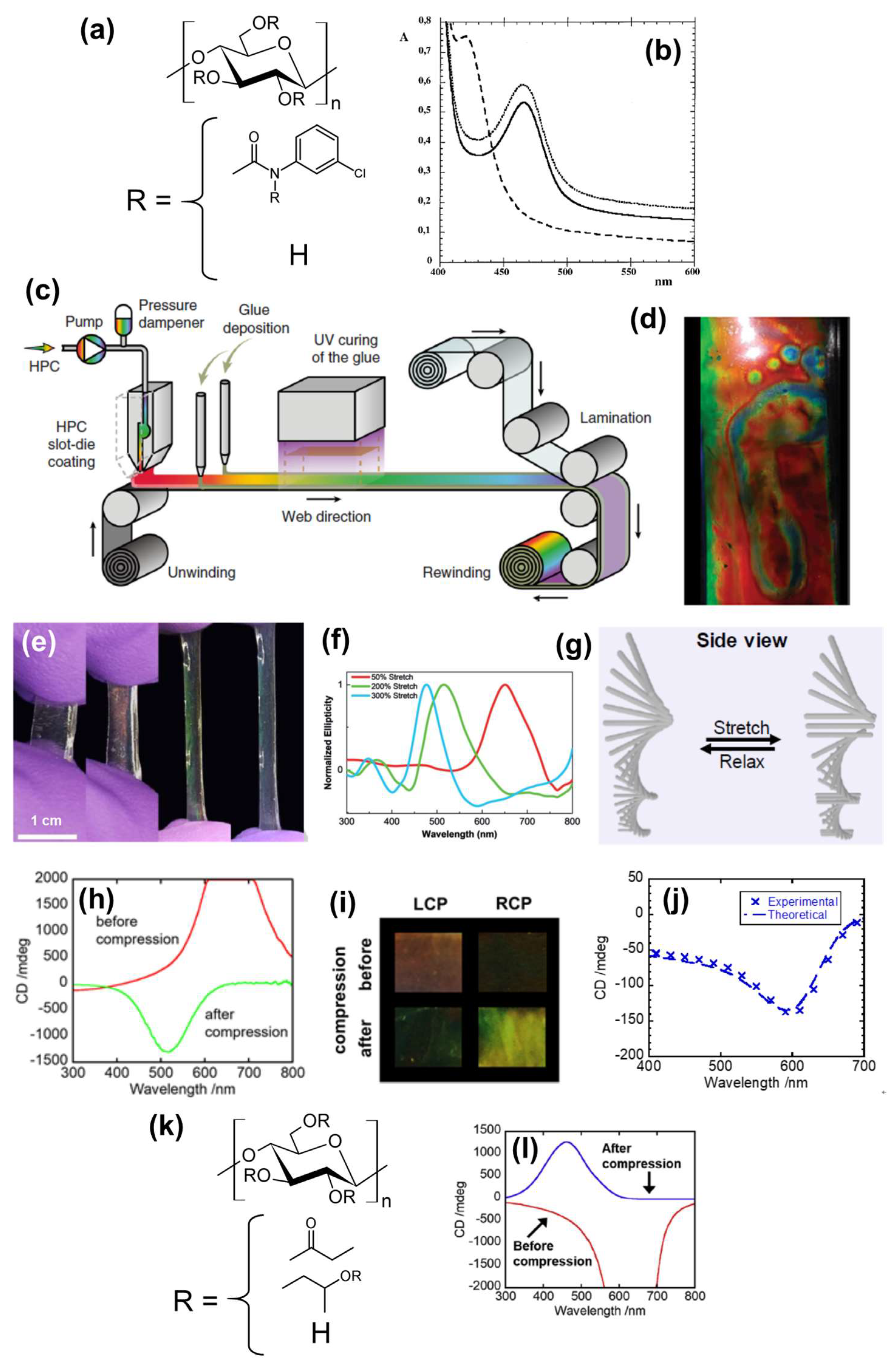
2.1.1. Mechanochromic Films
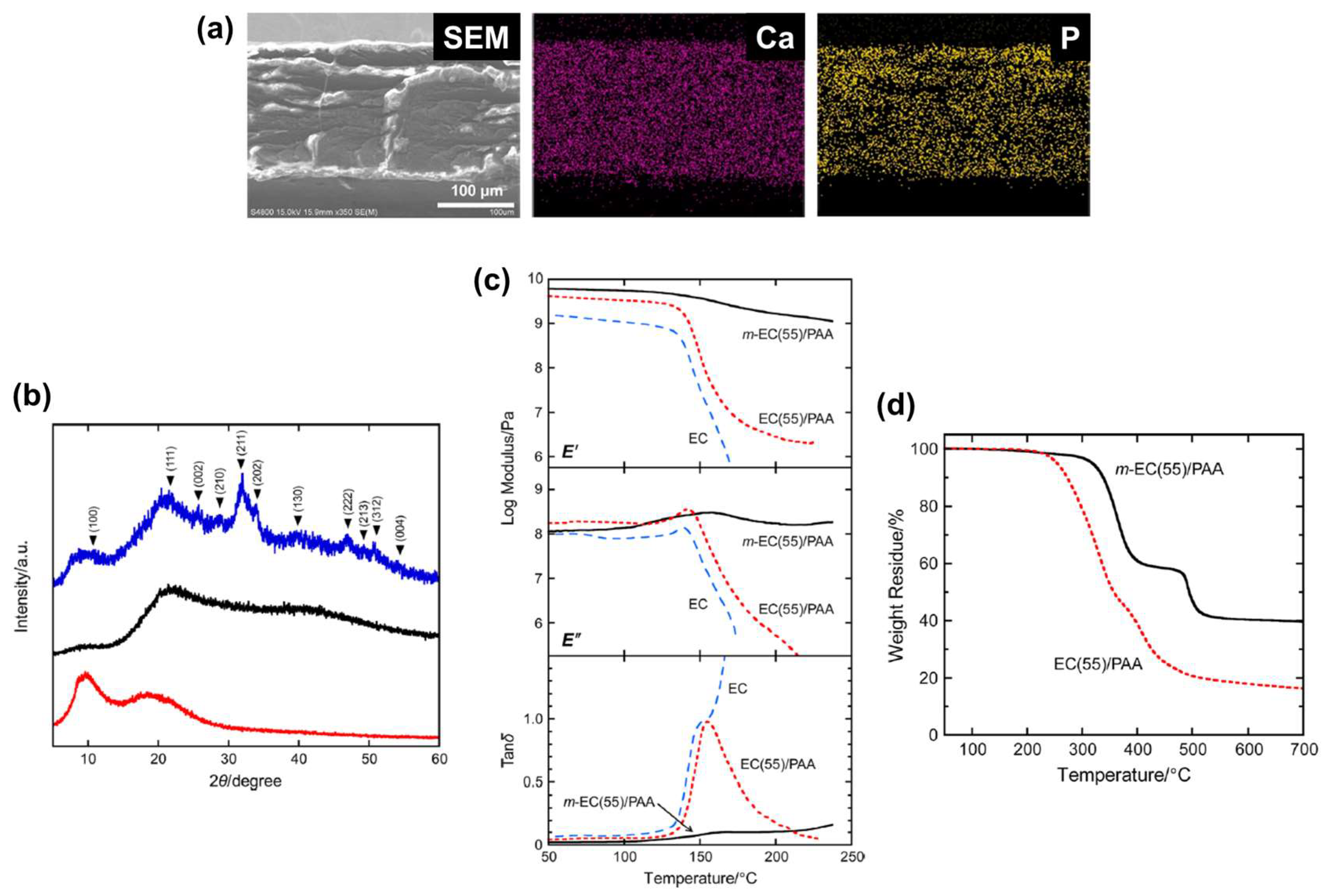
2.1.2. Biomineralized Films
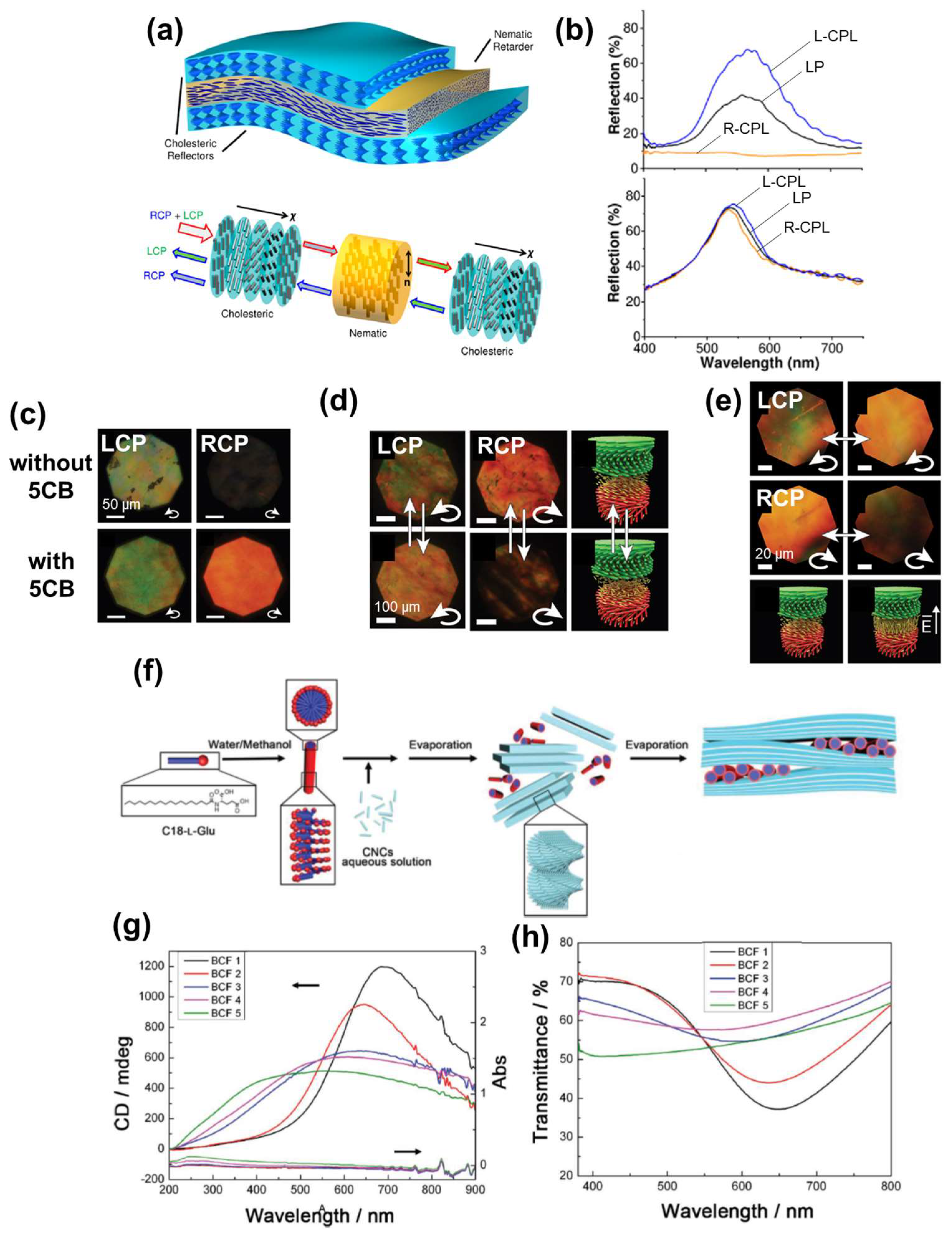
2.1.3. Optical Filters
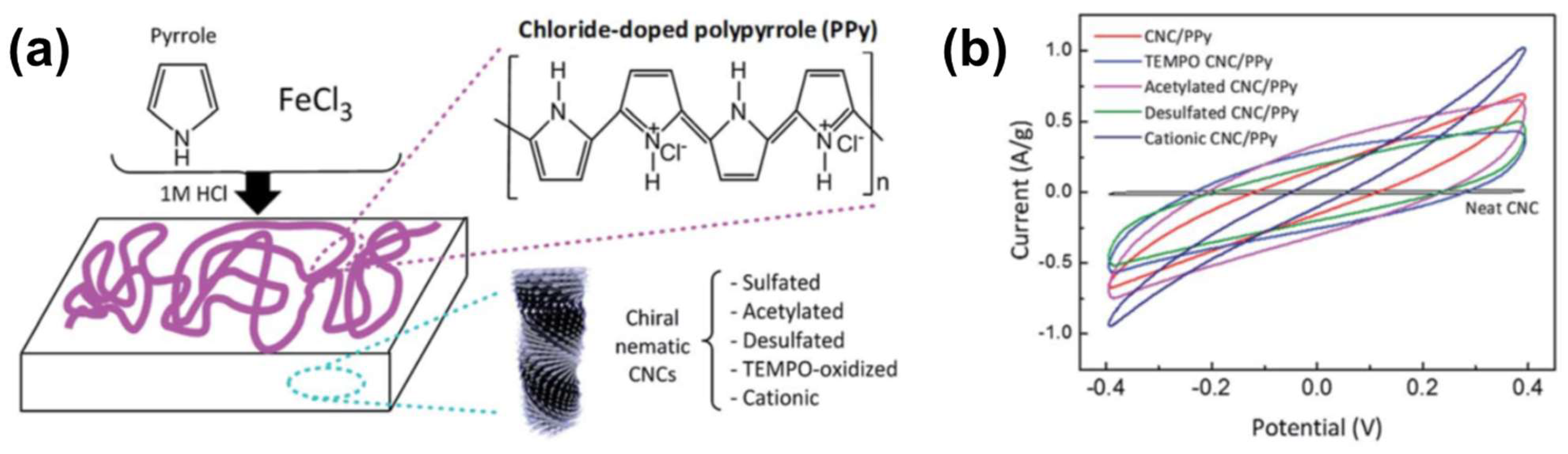
2.1.4. Conducting Films
2.2. Cellulosic ChLC Gels
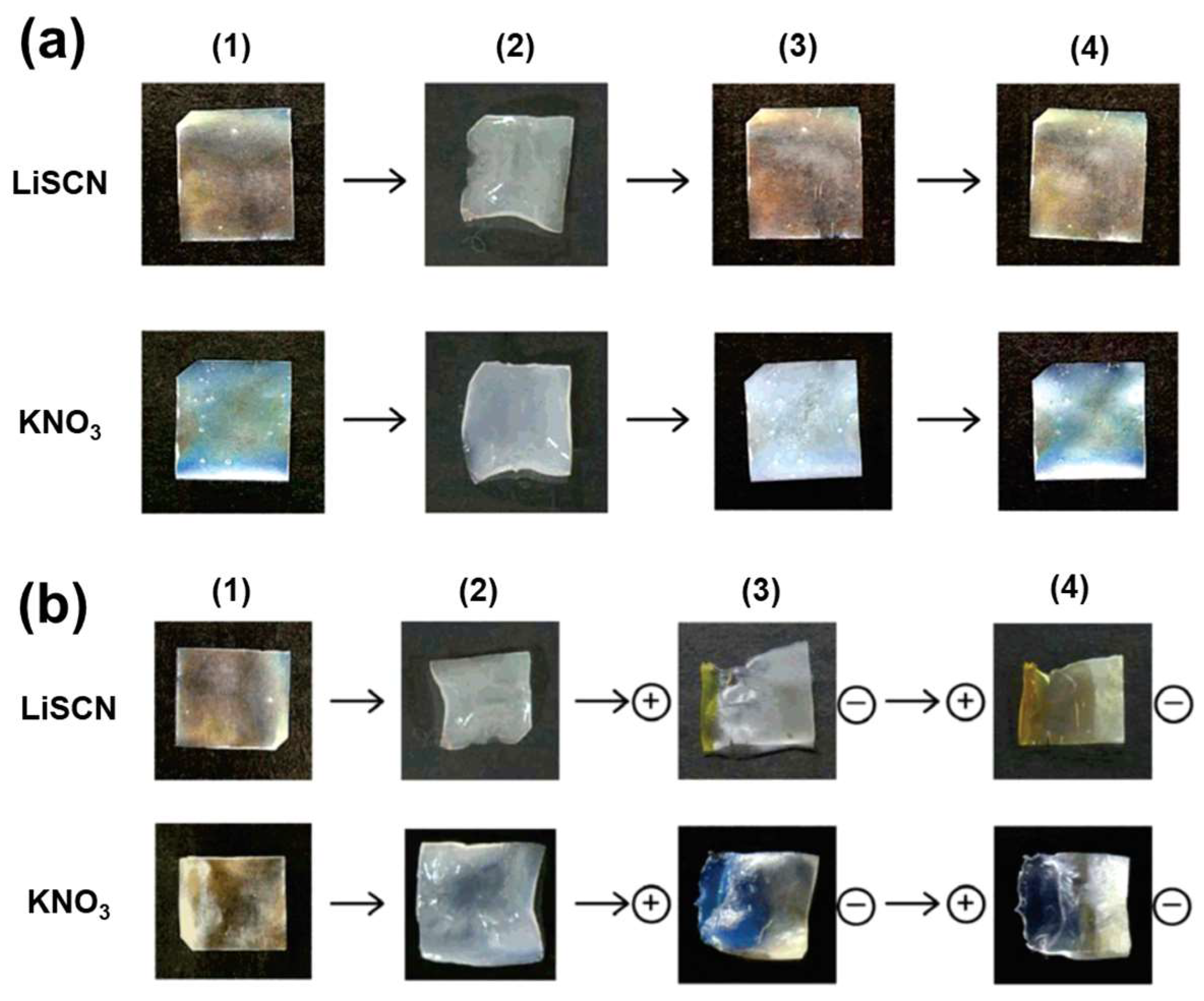
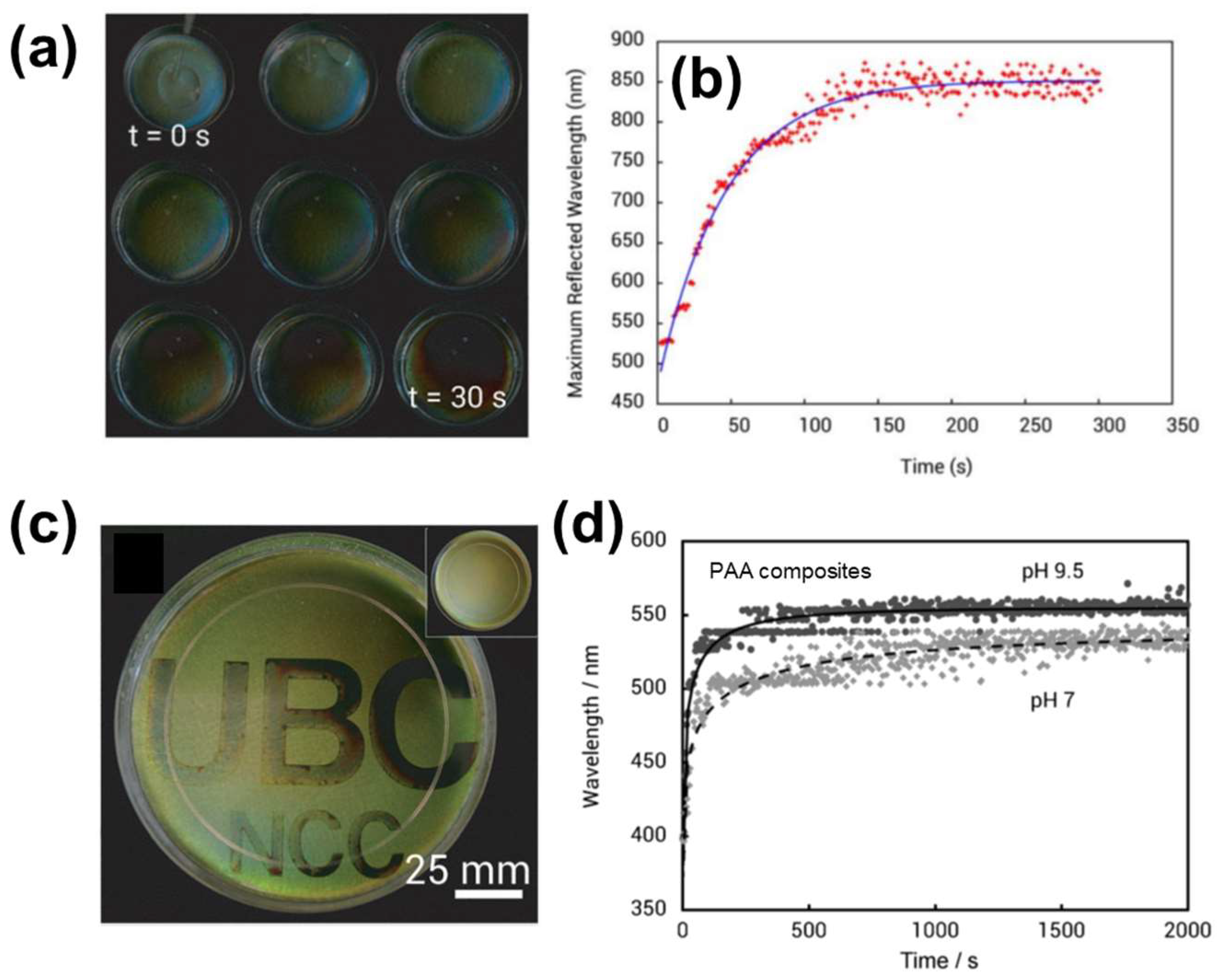
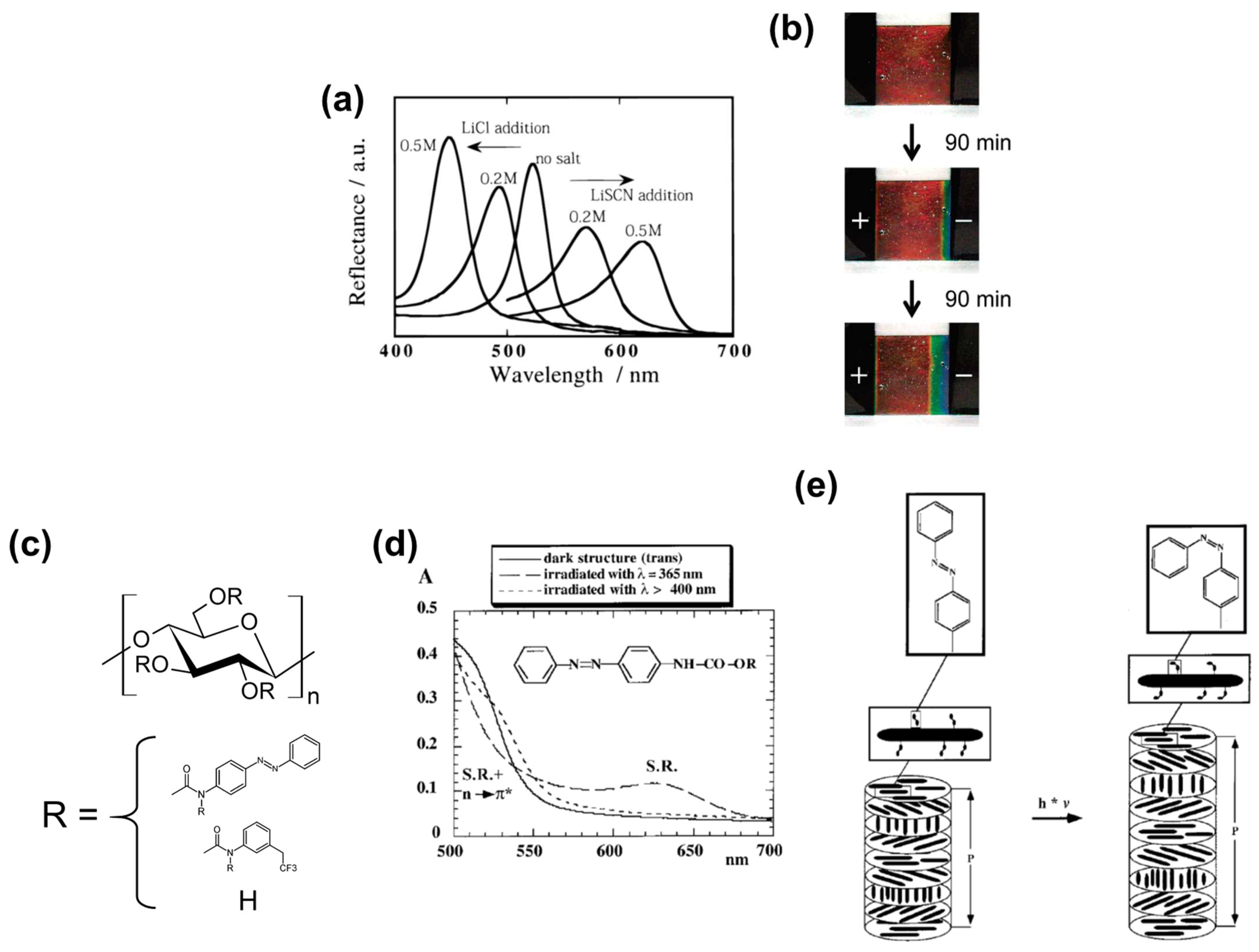
2.2.1. Salt-Responsive Color Gels
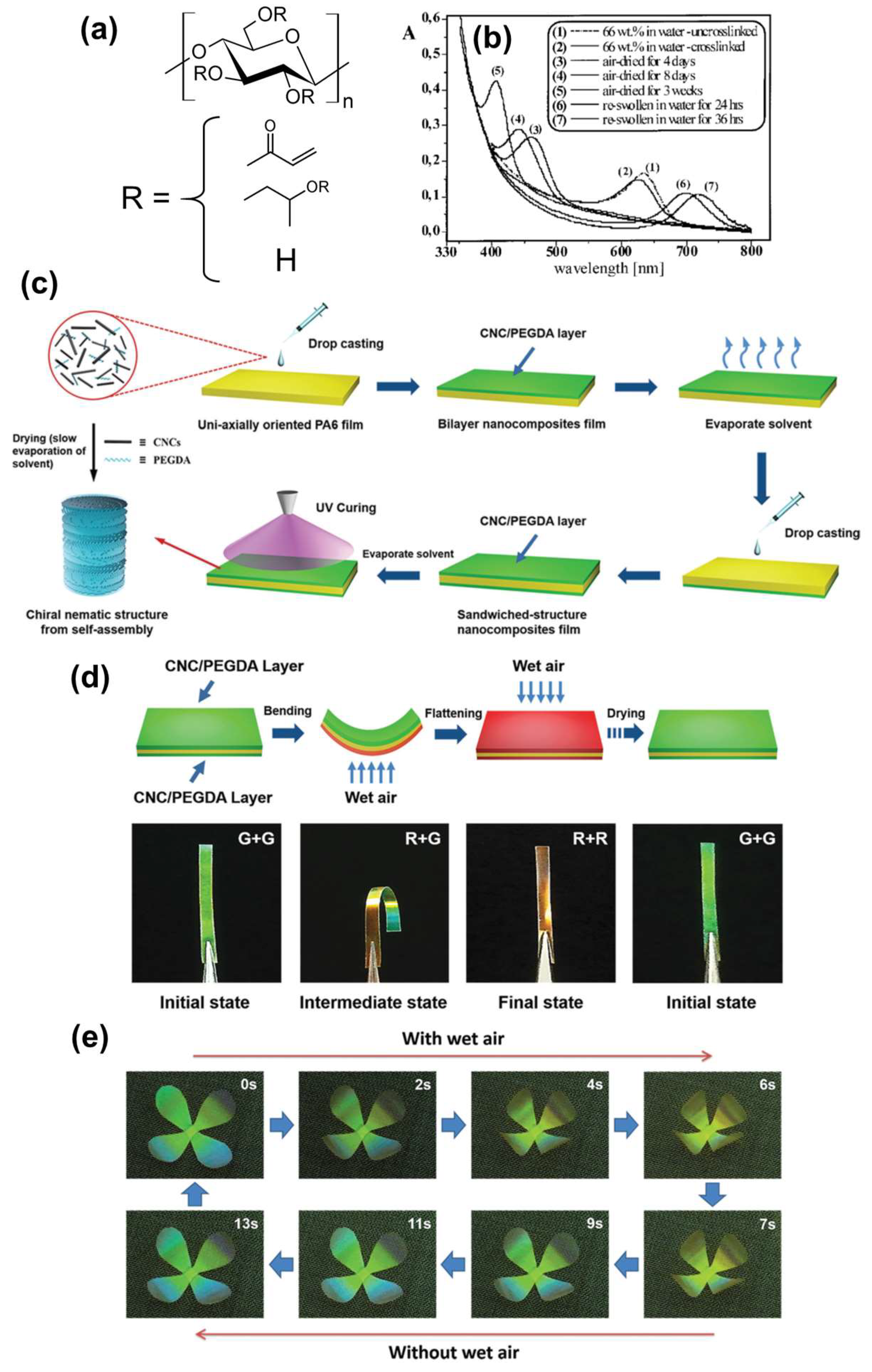
2.2.2. Humidity-Responsive Color Gels
2.2.3. Colored Gels with Tailored Stimuli-Responsivity
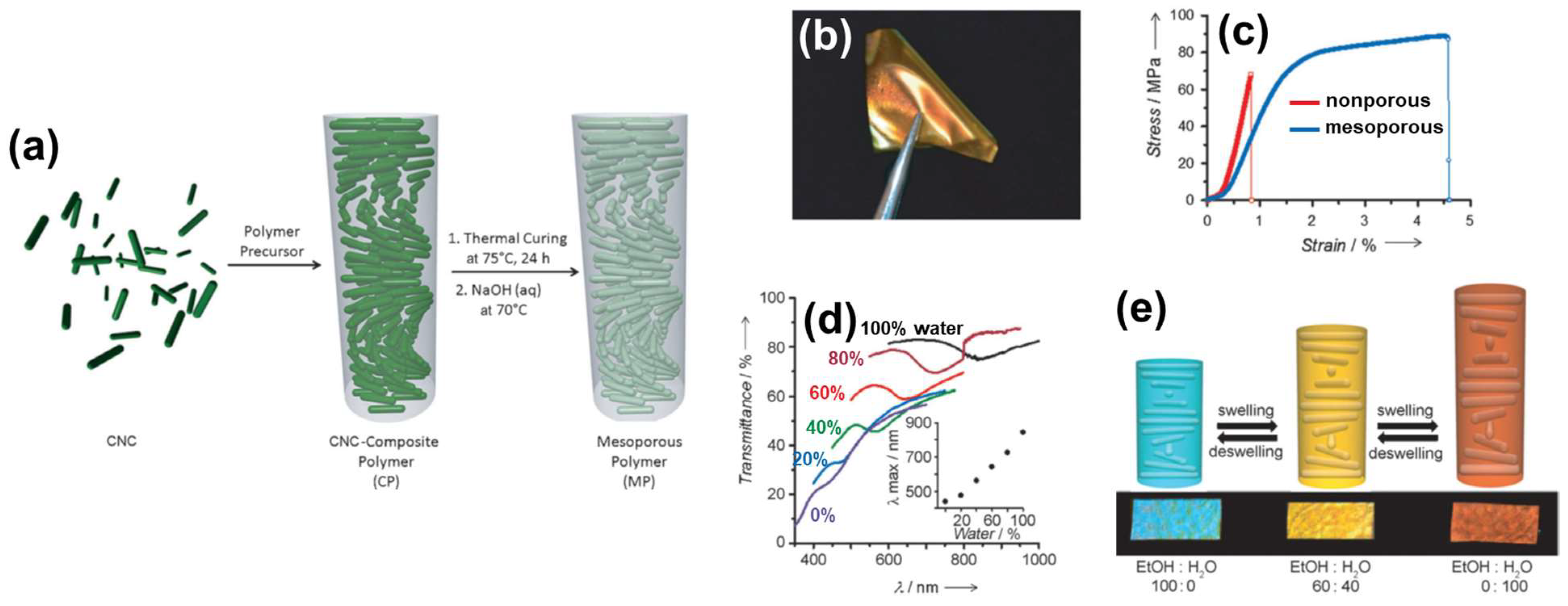
2.3. Cellulosic ChLC Mesoporous Materials
2.3.1. Flexible and Solvent-Responsive Mesoporous Films
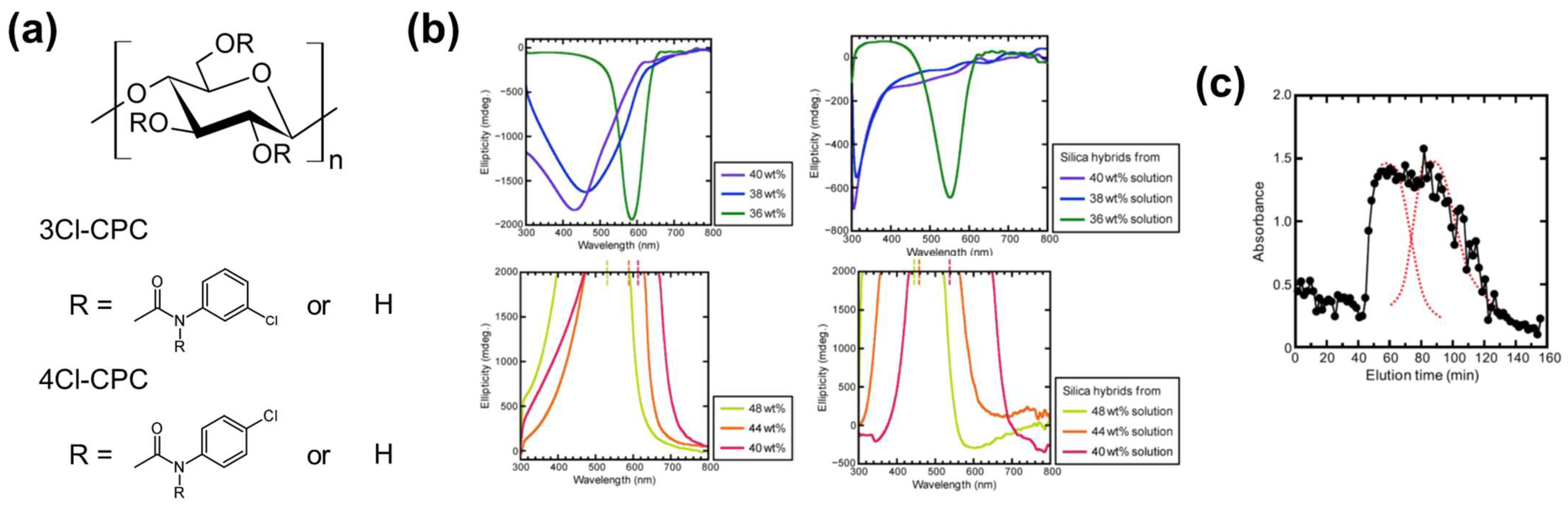
2.3.2. Cellulose Derivative–Silica Mesoporous Hybrids for Chiral Chromatography
2.4. Other Cellulosic ChLC Solid Functional Materials
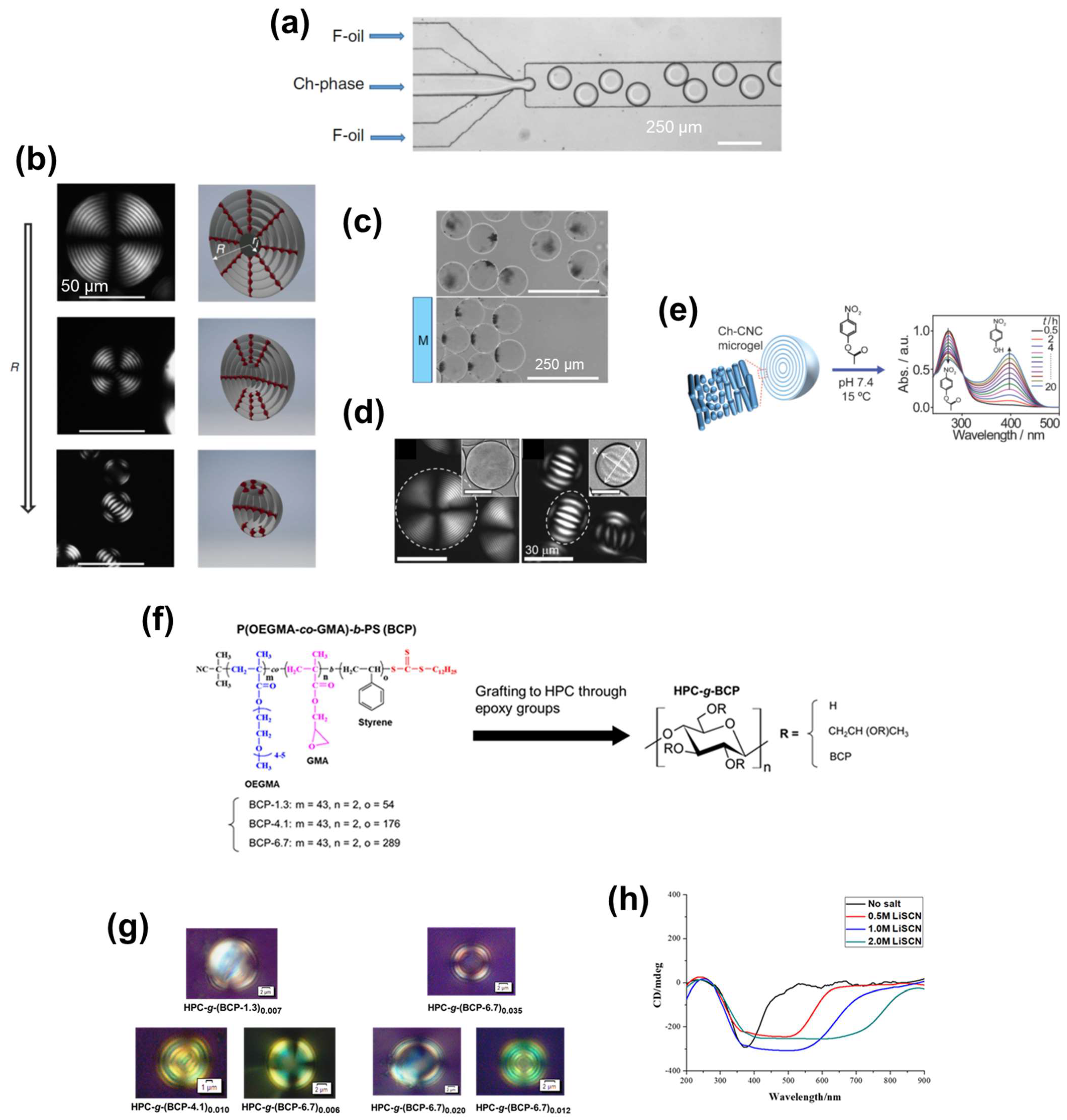
3. Cellulosic ChLC-Based Liquid Functional Materials
3.1. Cellulosic ChLC Solutions
3.2. Cellulosic ChLC Emulsions
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gray, D.G. Chiral nematic ordering of polysaccharides. Carbohydr. Polym. 1994, 25, 277–284. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Park, J.H.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Schütz, C.; Bruckner, J.R.; Honorato-Rios, C.; Tosheva, Z.; Anyfantakis, M.; Lagerwall, J.P.F. From equilibrium liquid crystal formation and kinetic arrest to photonic bandgap films using suspensions of cellulose nanocrystals. Crystals 2020, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Giese, M.; Blusch, L.K.; Khan, M.K.; MacLachlan, M.J. Functional materials from cellulose-derived liquid-crystal templates. Angew. Chem. Int. Ed. 2015, 54, 2888–2910. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Sato, J.; Sugimura, K. Liquid Crystals of Cellulosics: Fascinating Ordered Structures for the Design of Functional Material Systems. Adv. Polym. Sci. 2016, 271, 241–286. [Google Scholar] [CrossRef]
- de Vries, H. Rotatory power and other optical properties of certain liquid crystals. Acta Crystallogr. 1951, 4, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Kuse, Y.; Asahina, D.; Nishio, Y. Molecular structure and liquid-crystalline characteristics of chitosan phenylcarbamate. Biomacromolecules 2009, 10, 166–173. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Teramoto, Y. Recent advances in nanocellulose composites with polymers: A guide for choosing partners and how to incorporate them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef] [Green Version]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.C. Structure of native cellulose microfibrils, the starting point for nanocellulose manufacture. Philos. Trans. R. Soc. A 2018, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozdyniewicz, D.J.; Nieminen, K.; Kraft, G.; Sixta, H. Degradation of viscose fibers during acidic treatment. Cellulose 2016, 23, 213–229. [Google Scholar] [CrossRef]
- Gray, D. Recent Advances in Chiral Nematic Structure and Iridescent Color of Cellulose Nanocrystal Films. Nanomaterials 2016, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Canejo, J.P.; Monge, N.; Echeverria, C.; Fernandes, S.N.; Godinho, M.H. Cellulosic liquid crystals for films and fibers. Liq. Cryst. Rev. 2017, 5, 86–110. [Google Scholar] [CrossRef]
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Xue, M.D.; Kimura, T.; Revol, J.F.; Gray, D.G. Effects of ionic strength on the isotropic-chiral nematic phase transition of suspensions of cellulose crystallites. Langmuir 1996, 12, 2076–2082. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.F.; Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Biréfringent glassy phase of a cellulose microcrystal suspension. Langmuir 2000, 16, 2413–2415. [Google Scholar] [CrossRef]
- Araki, J.; Kuga, S. Effect of trace electrolyte on liquid crystal type of cellulose microcrystals. Langmuir 2001, 17, 4493–4496. [Google Scholar] [CrossRef]
- Viet, D.; Beck-Candanedo, S.; Gray, D.G. Dispersion of cellulose nanocrystals in polar organic solvents. Cellulose 2007, 14, 109–113. [Google Scholar] [CrossRef]
- Yi, J.; Xu, Q.; Zhang, X.; Zhang, H. Temperature-induced chiral nematic phase changes of suspensions of poly(N,N-dimethylaminoethyl methacrylate)-grafted cellulose nanocrystals. Cellulose 2009, 16, 989–997. [Google Scholar] [CrossRef]
- Teramoto, Y. Functional thermoplastic materials from derivatives of cellulose and related structural polysaccharides. Molecules 2015, 20, 5487–5527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werbowyj, R.S.; Gray, D.G. Liquid Crystalline Structure in Aqueous Hydroxypropyl Cellulose Solutions. Mol. Cryst. Liq. Cryst. 1976, 34, 97–103. [Google Scholar] [CrossRef]
- Werbowyj, R.S.; Gray, D.G. Optical Properties of (Hydroxypropy)cellulose Liquid Crystals. Cholesteric Pitch and Polymer Concentration. Macromolecules 1984, 17, 1512–1520. [Google Scholar] [CrossRef]
- Guo, J.-X.; Gray, D.G. Effect of Degree of Acetylation and Solvent on the Chiroptical Properties of Lyotropic (Acetyl)(Ethyl)Cellulose Solutions. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 2529–2537. [Google Scholar] [CrossRef]
- Guo, J.-X.; Gray, D.G. Chiroptical Behavior of (Acetyl)(ethyl)cellulose Liquid Crystalline Solutions in Chloroform. Macromolecules 1989, 22, 2086–2090. [Google Scholar] [CrossRef]
- Siekmeyer, M.; Zugenmaier, P. Solvent dependence of lyotropic liquid-crystalline phases of cellulose tricarbanilate. Die Makromol. Chemie 1990, 191, 1177–1196. [Google Scholar] [CrossRef]
- Ishii, H.; Sugimura, K.; Nishio, Y. Thermotropic liquid crystalline properties of (hydroxypropyl)cellulose derivatives with butyryl and heptafluorobutyryl substituents. Cellulose 2019, 26, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, T.; Sugiura, M.; Takada, A.; Sato, T.; Miyamoto, T. Characteristics of Cellulosic Thermotropics. Bull. Inst. Chem. Res. Kyoto Univ. 1991, 69, 211–218. [Google Scholar]
- Huang, B.; Ge, J.J.; Li, Y.; Hou, H. Aliphatic acid esters of (2-hydroxypropyl) cellulose-Effect of side chain length on properties of cholesteric liquid crystals. Polymer 2007, 48, 264–269. [Google Scholar] [CrossRef]
- Nishio, Y.; Nada, T.; Hirata, T.; Fujita, S.; Sugimura, K.; Kamitakahara, H. Handedness Inversion in Chiral Nematic (Ethyl)cellulose Solutions: Effects of Substituents and Temperature. Macromolecules 2021, 54, 6014–6027. [Google Scholar] [CrossRef]
- Osipov, M.A. Theory for cholesteric ordering in lyotropic liquid crystals. Nuovo Cim. D 1988, 10, 1249–1262. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry. Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Tatsumi, M.; Teramoto, Y.; Nishio, Y. Polymer composites reinforced by locking-in a liquid-crystalline assembly of cellulose nanocrystallites. Biomacromolecules 2012, 13, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Ritcey, A.M.; Gray, D.G. Circular reflectivity from the cholesteric liquid crystalline phase of (2-ethoxypropyl)cellulose. Macromolecules 1988, 21, 1251–1255. [Google Scholar] [CrossRef]
- Suto, S.; Suzuki, K. Crosslinked hydroxypropyl cellulose films retaining cholesteric liquid crystalline order: 2. Anisotropic swelling behaviour in water. Polymer 1997, 38, 391–396. [Google Scholar] [CrossRef]
- Charlet, G.; Gray, D. Solid Cholesteric Films Cast from Aqueous (Hydroxypropyl)cellulose. Macromolecules 1987, 20, 33–38. [Google Scholar] [CrossRef]
- Suto, S.; Suzuki, K. Crosslinked hydroxypropyl cellulose films retaining cholesteric liquid crystalline order. I. Effects of cast conditions and heat treatment on the textures and order of films. J. Appl. Polym. Sci. 1995, 55, 139–151. [Google Scholar] [CrossRef]
- Nishio, Y.; Suzuki, S.; Takahashi, T. Structural Investigation of Liquid-Crystalline Ethylcellulose. Polym. J. 1985, 17, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Nishio, Y.; Yamane, T.; Takahashi, T. Morphological Studies of Liquid-Crystalline Cellulose Derivatives. I. Liquid-Crystalline Characteristics of Hydroxypropyl Cellulose in 2-Hydroxyethyl Methacrylate Solutions and in Polymer Composites Prepared by Bulk Polymerization. J. Polym. Sci. Part B Polym. Phys. 1985, 23, 1043–1052. [Google Scholar] [CrossRef]
- Nishio, Y.; Yamane, T.; Takahashi, T.; Engineering, F. Morphological Studies of Liquid-Crystalline Cellulose Derivatives. II. Hydroxypropyl Cellulose Films Prepared from Liquid-Crystalline Aqueous Solutions. J. Polym. Sci. Part B Polym. Phys. 1985, 23, 1053–1064. [Google Scholar] [CrossRef]
- Müller, M.; Zentel, R.; Keller, H. Solid Opalescent Films Originating from Urethanes of Cellulose. Adv. Mater. 1997, 9, 159–162. [Google Scholar] [CrossRef]
- Aoki, R.; Fukawa, M.; Furumi, S. Preparation of the color films from cellulose derivatives in a diacrylate liquid. J. Photopolym. Sci. Technol. 2019, 32, 651–656. [Google Scholar] [CrossRef]
- Shimokawa, H.; Hayata, K.; Fukawa, M.; Furumi, S. Fabrication of reflective color films from cellulose derivatives. J. Photopolym. Sci. Technol. 2020, 33, 467–471. [Google Scholar] [CrossRef]
- Mu, X.; Gray, D.G. Formation of chiral nematic films from cellulose nanocrystal suspensions is a two-stage process. Langmuir 2014, 30, 9256–9260. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surf. A Physicochem. Eng. Asp. 1998, 142, 75–82. [Google Scholar] [CrossRef]
- Chen, G.; Hong, W. Mechanochromism of Structural-Colored Materials. Adv. Opt. Mater. 2020, 8, 1–28. [Google Scholar] [CrossRef]
- Ito, T.; Katsura, C.; Sugimoto, H.; Nakanishi, E.; Inomata, K. Strain-responsive structural colored elastomers by fixing colloidal crystal assembly. Langmuir 2013, 29, 13951–13957. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Kurokawa, T.; Haque, M.; Nakajima, T.; Nonoyama, T.; Li, X.; Kajiwara, I.; Gong, J. Mechano-actuated ultrafast full-colour switching in layered photonic hydrogels. Nat. Commun. 2014, 5, 4659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, I.R.; Li, C.; Colella, N.S.; Ito, K.; Watkins, J.J. Strain-Tunable One Dimensional Photonic Crystals Based on Zirconium Dioxide/Slide-Ring Elastomer Nanocomposites for Mechanochromic Sensing. ACS Appl. Mater. Interfaces 2015, 7, 3641–3646. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Choi, T.M.; Kim, B.; Han, S.H.; Lee, J.M.; Kim, S.H. Chameleon-Inspired Mechanochromic Photonic Films Composed of Non-Close-Packed Colloidal Arrays. ACS Nano 2017, 11, 11350–11357. [Google Scholar] [CrossRef]
- Müller, M.; Zentel, R. Cholesteric phases and films from cellulose derivatives. Macromol. Chem. Phys. 2000, 201, 2055–2063. [Google Scholar] [CrossRef]
- Liang, H.L.; Bay, M.M.; Vadrucci, R.; Barty-King, C.H.; Peng, J.; Baumberg, J.J.; De Volder, M.F.L.; Vignolini, S. Roll-to-roll fabrication of touch-responsive cellulose photonic laminates. Nat. Commun. 2018, 9, 4632. [Google Scholar] [CrossRef]
- Boott, C.E.; Tran, A.; Hamad, W.Y.; MacLachlan, M.J. Cellulose Nanocrystal Elastomers with Reversible Visible Color. Angew. Chem. Int. Ed. 2020, 59, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagi, K.; Teramoto, Y. Dual mechanochromism of cellulosic cholesteric liquid-crystalline films: Wide-ranging colour control and circular dichroism inversion by mechanical stimulus. J. Mater. Chem. C 2018, 6, 1370–1376. [Google Scholar] [CrossRef]
- Ritcey, A.M.; Charlet, G.; Gray, D.G. Effect of residual linear orientation on the optical properties of cholesteric films. Can. J. Chem. 1988, 66, 2229–2233. [Google Scholar] [CrossRef]
- Miyagi, K.; Teramoto, Y. Elucidation of the Mechanism of Stress-Induced Circular Dichroic Inversion of Cellulosic/Polymer Liquid Crystalline Composites. Macromolecules 2020, 53, 3250–3254. [Google Scholar] [CrossRef]
- Miyagi, K.; Teramoto, Y. Exploration of immobilization conditions of cellulosic lyotropic liquid crystals in monomeric solvents by in situ polymerization and achievement of dual mechanochromism at room temperature. RSC Adv. 2018, 8, 24724–24730. [Google Scholar] [CrossRef] [Green Version]
- Miyagi, K.; Teramoto, Y. Function extension of dual-mechanochromism of acylated hydroxypropyl cellulose/synthetic polymer composites achieved by “moderate” compatibility as well as hydrogen bonding. Polymer 2019, 174, 150–158. [Google Scholar] [CrossRef]
- Miyagi, K.; Teramoto, Y. Facile design of pressure-sensing color films of liquid crystalline cellulosic/synthetic polymer composites that function at desired temperatures. Cellulose 2019, 26, 9673–9685. [Google Scholar] [CrossRef]
- Fukawa, M.; Suzuki, K.; Furumi, S. Disappearance of reflection color by photopolymerization of lyotropic cholesteric liquid crystals from cellulose derivatives. J. Photopolym. Sci. Technol. 2018, 31, 563–567. [Google Scholar] [CrossRef]
- Arakaki, A.; Shimizu, K.; Oda, M.; Sakamoto, T.; Nishimura, T.; Kato, T. Biomineralization-inspired synthesis of functional organic/inorganic hybrid materials: Organic molecular control of self-organization of hybrids. Org. Biomol. Chem. 2015, 13, 974–989. [Google Scholar] [CrossRef] [Green Version]
- Nudelman, F.; Sommerdijk, N.A.J.M. Biomineralization as an inspiration for materials chemistry. Angew. Chem. Int. Ed. 2012, 51, 6582–6596. [Google Scholar] [CrossRef]
- Ogiwara, T.; Katsumura, A.; Sugimura, K.; Teramoto, Y.; Nishio, Y. Calcium Phosphate Mineralization in Cellulose Derivative/Poly(acrylic acid) Composites Having a Chiral Nematic Mesomorphic Structure. Biomacromolecules 2015, 16, 3959–3969. [Google Scholar] [CrossRef]
- Katsumura, A.; Sugimura, K.; Nishio, Y. Calcium carbonate mineralization in chiral mesomorphic order-retaining ethyl cellulose/poly(acrylic acid) composite films. Polymer 2018, 139, 26–35. [Google Scholar] [CrossRef]
- Nakao, Y.; Sugimura, K.; Nishio, Y. CaCO3 mineralization in polymer composites with cellulose nanocrystals providing a chiral nematic mesomorphic structure. Int. J. Biol. Macromol. 2019, 141, 783–791. [Google Scholar] [CrossRef]
- De La Cruz, J.A.; Liu, Q.; Senyuk, B.; Frazier, A.W.; Peddireddy, K.; Smalyukh, I.I. Cellulose-Based Reflective Liquid Crystal Films as Optical Filters and Solar Gain Regulators. ACS Photonics 2018, 5, 2468–2477. [Google Scholar] [CrossRef]
- Fernandes, S.N.; Almeida, P.L.; Monge, N.; Aguirre, L.E.; Reis, D.; de Oliveira, C.L.P.; Neto, A.M.F.; Pieranski, P.; Godinho, M.H. Mind the Microgap in Iridescent Cellulose Nanocrystal Films. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Hamad, W.Y.; MacLachlan, M.J. Broadband Circular Polarizing Film Based on Chiral Nematic Liquid Crystals. Adv. Opt. Mater. 2018, 6, 1800412. [Google Scholar] [CrossRef]
- Lizundia, E.; Nguyen, T.D.; Vilas, J.L.; Hamad, W.Y.; MacLachlan, M.J. Chiroptical, morphological and conducting properties of chiral nematic mesoporous cellulose/polypyrrole composite films. J. Mater. Chem. A 2017, 5, 19184–19194. [Google Scholar] [CrossRef]
- Chiba, R.; Nishio, Y.; Sato, Y.; Ohtaki, M.; Miyashita, Y. Preparation of cholesteric (hydroxypropyl) cellulose/polymer networks and ion-mediated control of their optical properties. Biomacromolecules 2006, 7, 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, J.; Li, J.; Ye, S.; Wei, J.; Guo, J. A bio-inspired cellulose nanocrystal-based nanocomposite photonic film with hyper-reflection and humidity-responsive actuator properties. J. Mater. Chem. C 2016, 4, 9687–9696. [Google Scholar] [CrossRef]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.Y.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Kwon, S.; Lee, W.; Ko, S. Optical response of photonic cellulose nanocrystal film for a novel humidity indicator. Int. J. Biol. Macromol. 2019, 140, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Cao, Y.; Ji, H.; Chen, J.; He, Z.; Long, Z.; Dong, C. Fabrication of environmental humidity-responsive iridescent films with cellulose nanocrystal/polyols. Carbohydr. Polym. 2020, 240, 116281. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Lee, W.; Chung, U.; Ko, S. Study of humidity-responsive behavior in chiral nematic cellulose nanocrystal films for colorimetric response. Cellulose 2018, 25, 305–317. [Google Scholar] [CrossRef]
- Chen, H.; Hou, A.; Zheng, C.; Tang, J.; Xie, K.; Gao, A. Light- and Humidity-Responsive Chiral Nematic Photonic Crystal Films Based on Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 24505–24511. [Google Scholar] [CrossRef]
- He, Y.D.; Zhang, Z.L.; Xue, J.; Wang, X.H.; Song, F.; Wang, X.L.; Zhu, L.L.; Wang, Y.Z. Biomimetic Optical Cellulose Nanocrystal Films with Controllable Iridescent Color and Environmental Stimuli-Responsive Chromism. ACS Appl. Mater. Interfaces 2018, 10, 5805–5811. [Google Scholar] [CrossRef]
- Khan, M.K.; Giese, M.; Yu, M.; Kelly, J.A.; Hamad, W.Y.; Maclachlan, M.J. Flexible mesoporous photonic resins with tunable chiral nematic structures. Angew. Chem. Int. Ed. 2013, 52, 8921–8924. [Google Scholar] [CrossRef]
- Sato, J.; Sugimura, K.; Teramoto, Y.; Nishio, Y. Preparation and chiroptical properties of cellulose chlorophenylcarbamate–silica hybrids having a chiral nematic mesomorphic structure. Polymer 2019, 173, 172–181. [Google Scholar] [CrossRef]
- Anyfantakis, M.; Jampani, V.S.R.; Kizhakidathazhath, R.; Binks, B.P.; Lagerwall, J.P.F. Responsive Photonic Liquid Marbles. Angew. Chem. Int. Ed. 2020, 59, 19260–19267. [Google Scholar] [CrossRef]
- Trindade, A.C.; Carreto, M.; Helgesen, G.; Knudsen, K.D.; Puchtler, F.; Breu, J.; Fernandes, S.; Godinho, M.H.; Fossum, J.O. Photonic composite materials from cellulose nanorods and clay nanolayers. Eur. Phys. J. Spec. Top. 2020, 229, 2741–2755. [Google Scholar] [CrossRef]
- Yi, H.; Lee, S.H.; Ko, H.; Lee, D.; Bae, W.G.; Kim, T.; Hwang, D.S.; Jeong, H.E. Ultra-Adaptable and Wearable Photonic Skin Based on a Shape-Memory, Responsive Cellulose Derivative. Adv. Funct. Mater. 2019, 29, 1902720. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Wang, Y.; Zhao, Y. Bioinspired conductive cellulose liquid-crystal hydrogels as multifunctional electrical skins. Proc. Natl. Acad. Sci. USA 2020, 117, 18310–18316. [Google Scholar] [CrossRef] [PubMed]
- Grey, P.; Fernandes, S.N.; Gaspar, D.; Fortunato, E.; Martins, R.; Godinho, M.H.; Pereira, L. Field-Effect Transistors on Photonic Cellulose Nanocrystal Solid Electrolyte for Circular Polarized Light Sensing. Adv. Funct. Mater. 2019, 29, 1805279. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, W.; Wang, C.; Zheng, X.; Wang, D.; Zhang, X.; Wang, X.; Xue, X.; Qing, G. Highly Strong and Solvent-Resistant Cellulose Nanocrystal Photonic Films for Optical Coatings. ACS Appl. Mater. Interfaces 2021, 13, 17118–17128. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Kimura, F.; Kimura, T.; Teramoto, Y.; Nishio, Y. Anisotropic Polymer Composites Synthesized by Immobilizing Cellulose Nanocrystal Suspensions Specifically Oriented under Magnetic Fields. Biomacromolecules 2014, 15, 4579–4589. [Google Scholar] [CrossRef]
- Xu, M.; Ma, C.; Zhou, J.; Liu, Y.; Wu, X.; Luo, S.; Li, W.; Yu, H.; Wang, Y.; Chen, Z.; et al. Assembling semiconductor quantum dots in hierarchical photonic cellulose nanocrystal films: Circularly polarized luminescent nanomaterials as optical coding labels. J. Mater. Chem. C 2019, 7, 13794–13802. [Google Scholar] [CrossRef]
- Cao, Y.; Lewis, L.; Hamad, W.Y.; MacLachlan, M.J. Pressure-Responsive Hierarchical Chiral Photonic Aerogels. Adv. Mater. 2019, 31, 1808186. [Google Scholar] [CrossRef]
- Zhao, T.H.; Parker, R.M.; Williams, C.A.; Lim, K.T.P.; Frka-Petesic, B.; Vignolini, S. Printing of Responsive Photonic Cellulose Nanocrystal Microfilm Arrays. Adv. Funct. Mater. 2019, 29, 1804531. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Feng, D.; Chen, G.; Chen, X.; Hong, W. Re-Printable Chiral Photonic Paper with Invisible Patterns and Tunable Wettability. Adv. Funct. Mater. 2021, 31, 2009916. [Google Scholar] [CrossRef]
- Nishio, Y.; Kai, T.; Kimura, N.; Oshima, K.; Suzuki, H. Controlling the selective light reflection of a cholesteric liquid crystal of (hydroxypropyl) cellulose by electrical stimulation. Macromolecules 1998, 31, 2384–2386. [Google Scholar] [CrossRef]
- Chiba, R.; Nishio, Y.; Miyashita, Y. Electrooptical Behavior of Liquid-Crystalline (Hydroxypropyl) cellulose/Inorganic Salt Aqueous Solutions. Macromolecules 2003, 36, 1706–1712. [Google Scholar] [CrossRef]
- Sivakumar, S.; Wark, K.L.; Gupta, J.K.; Abbott, N.L.; Caruso, F. Liquid crystal emulsions as the basis of biological sensors for the optical detection of bacteria and viruses. Adv. Funct. Mater. 2009, 19, 2260–2265. [Google Scholar] [CrossRef]
- Khan, M.; Park, S.Y. General Liquid-crystal droplets produced by microfluidics for urea detection. Sens. Actuators B Chem. 2014, 202, 516–522. [Google Scholar] [CrossRef]
- Lee, H.G.; Munir, S.; Park, S.Y. Cholesteric Liquid Crystal Droplets for Biosensors. ACS Appl. Mater. Interfaces 2016, 8, 26407–26417. [Google Scholar] [CrossRef]
- Niu, X.; Luo, D.; Chen, R.; Wang, F.; Sun, X.; Dai, H. Optical biosensor based on liquid crystal droplets for detection of cholic acid. Opt. Commun. 2016, 381, 286–291. [Google Scholar] [CrossRef]
- Uchida, Y.; Takanishi, Y.; Yamamoto, J. Controlled fabrication and photonic structure of cholesteric liquid crystalline shells. Adv. Mater. 2013, 25, 3234–3237. [Google Scholar] [CrossRef]
- Iwai, Y.; Kaji, H.; Uchida, Y.; Nishiyama, N. Chemiluminescence emission in cholesteric liquid crystalline core–shell microcapsules. J. Mater. Chem. C 2014, 2, 4904–4908. [Google Scholar] [CrossRef]
- Iwai, Y.; Kaji, H.; Uchida, Y.; Nishiyama, N. Temperature-dependent Color Change of Cholesteric Liquid Crystalline Core-shell Microspheres. Mol. Cryst. Liq. Cryst. 2015, 615, 9–13. [Google Scholar] [CrossRef]
- Humar, M.; Muševič, I. 3D microlasers from self-assembled cholesteric liquid-crystal microdroplets. Opt. Express 2010, 18, 26995–27003. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Kim, S.K.; Won, J.C.; Kim, Y.H.; Kim, S.-H. Reconfigurable Photonic Capsules Containing Cholesteric Liquid Crystals with Planar Alignment. Angew. Chem. 2015, 127, 15481–15485. [Google Scholar] [CrossRef]
- Schwartz, M.; Lenzini, G.; Geng, Y.; Rønne, P.B.; Ryan, P.Y.A.; Lagerwall, J.P.F. Cholesteric Liquid Crystal Shells as Enabling Material for Information-Rich Design and Architecture. Adv. Mater. 2018, 30, 1707382. [Google Scholar] [CrossRef]
- Li, Y.; Jun-Yan Suen, J.; Prince, E.; Larin, E.M.; Klinkova, A.; Thérien-Aubin, H.; Zhu, S.; Yang, B.; Helmy, A.S.; Lavrentovich, O.D.; et al. Colloidal cholesteric liquid crystal in spherical confinement. Nat. Commun. 2016, 7, 12520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.; Li, Y.; Seo, M.; Kumacheva, E. Nanofibrillar Stimulus-Responsive Cholesteric Microgels with Catalytic Properties. Angew. Chem. Int. Ed. 2016, 55, 14014–14018. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.X.; Hamad, W.Y.; MacLachlan, M.J. Polymer and Mesoporous Silica Microspheres with Chiral Nematic Order from Cellulose Nanocrystals. Angew. Chem. Int. Ed. 2016, 55, 12460–12464. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Miyagi, K.; Maiti, M.; Teramoto, Y. Topological Transition in Spontaneously Formed Cellulosic Liquid-Crystalline Microspheres in a w/o Emulsion. Biomacromolecules 2018, 19, 4650–4657. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Zimmerman, J.S.; De Pablo, J.J.; Caruso, F.; Abbott, N.L. Characterization of adsorbate-induced ordering transitions of liquid crystals within monodisperse droplets. Langmuir 2009, 25, 9016–9024. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyagi, K.; Teramoto, Y. Construction of Functional Materials in Various Material Forms from Cellulosic Cholesteric Liquid Crystals. Nanomaterials 2021, 11, 2969. https://doi.org/10.3390/nano11112969
Miyagi K, Teramoto Y. Construction of Functional Materials in Various Material Forms from Cellulosic Cholesteric Liquid Crystals. Nanomaterials. 2021; 11(11):2969. https://doi.org/10.3390/nano11112969
Chicago/Turabian StyleMiyagi, Kazuma, and Yoshikuni Teramoto. 2021. "Construction of Functional Materials in Various Material Forms from Cellulosic Cholesteric Liquid Crystals" Nanomaterials 11, no. 11: 2969. https://doi.org/10.3390/nano11112969
APA StyleMiyagi, K., & Teramoto, Y. (2021). Construction of Functional Materials in Various Material Forms from Cellulosic Cholesteric Liquid Crystals. Nanomaterials, 11(11), 2969. https://doi.org/10.3390/nano11112969





