Sono-Chemical Synthesis of Silver Quantum Dots Immobilized on Exfoliated Graphitic Carbon Nitride Nanostructures Using Ginseng Extract for Photocatalytic Hydrogen Evolution, Dye Degradation, and Antimicrobial Studies
Abstract
:1. Introduction
2. Materials and Methods
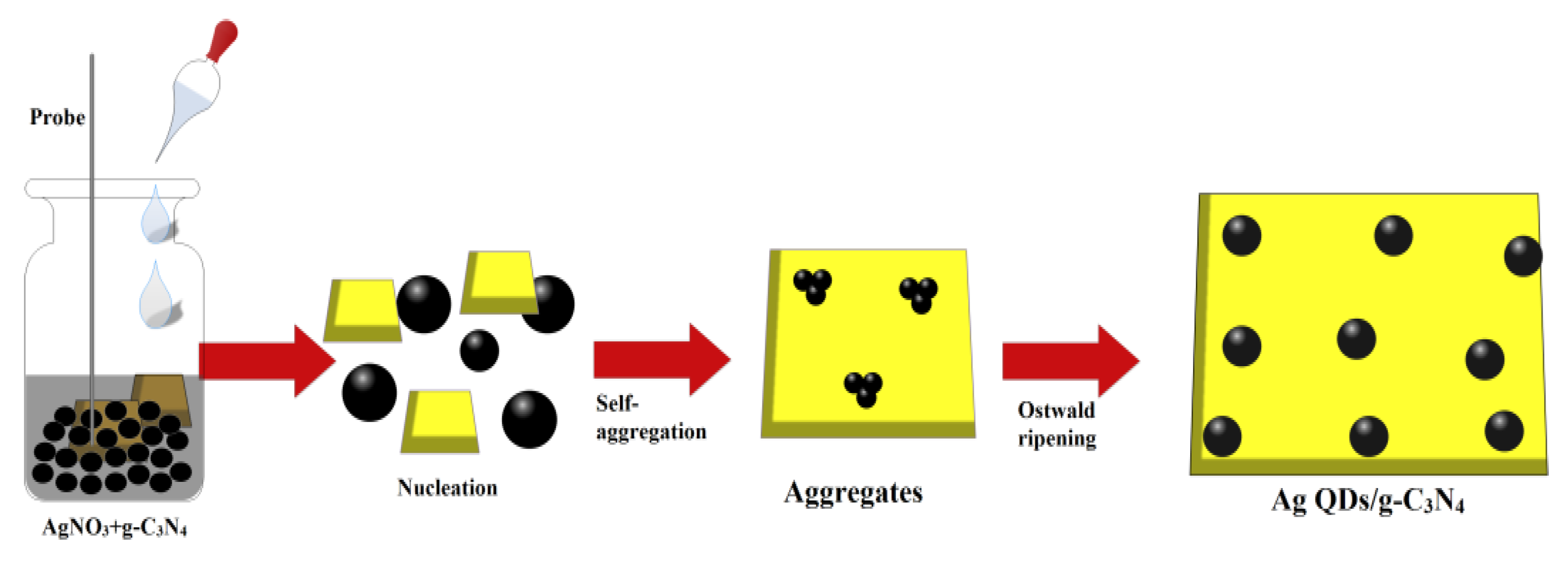
2.1. Preparation Methods
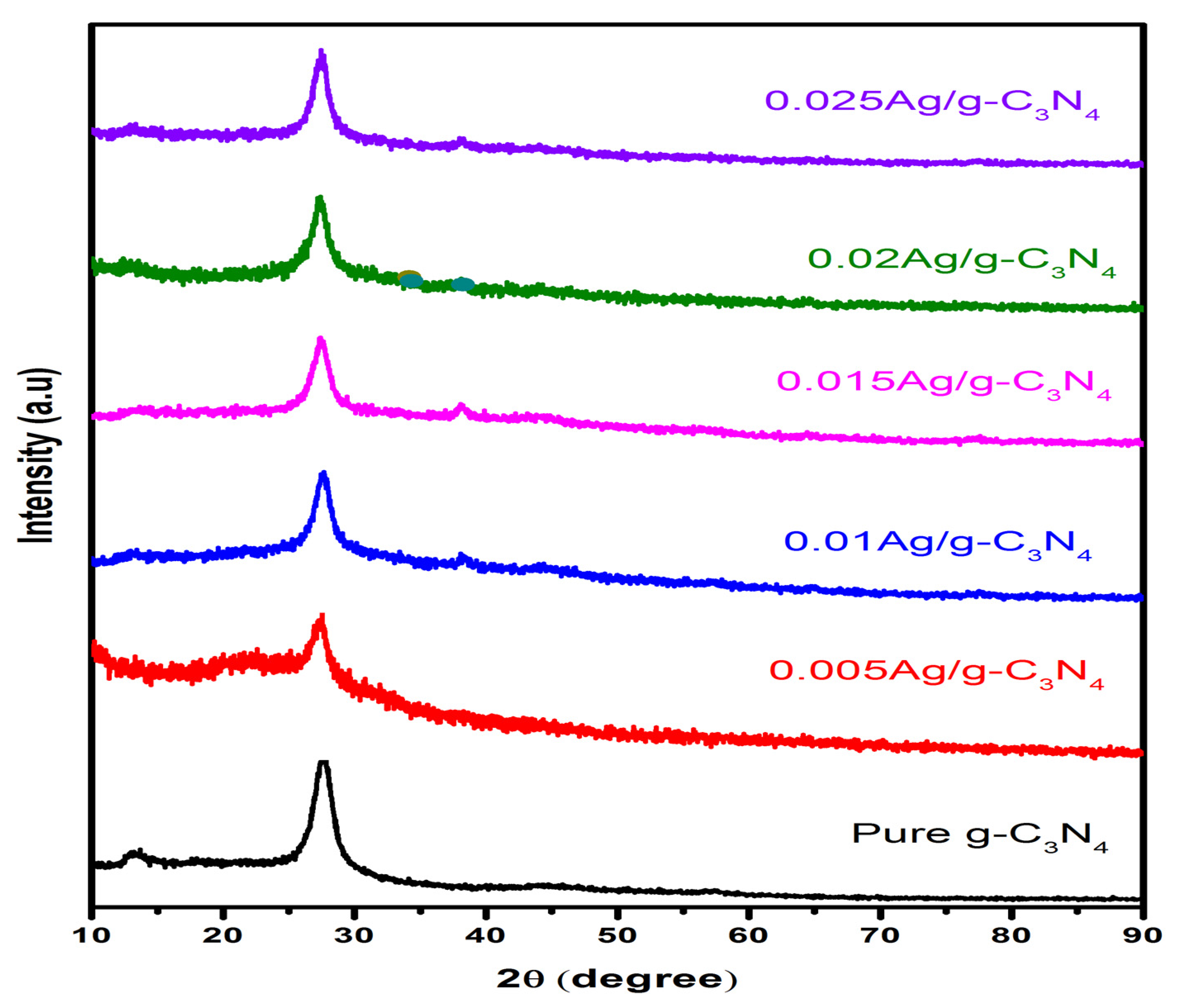
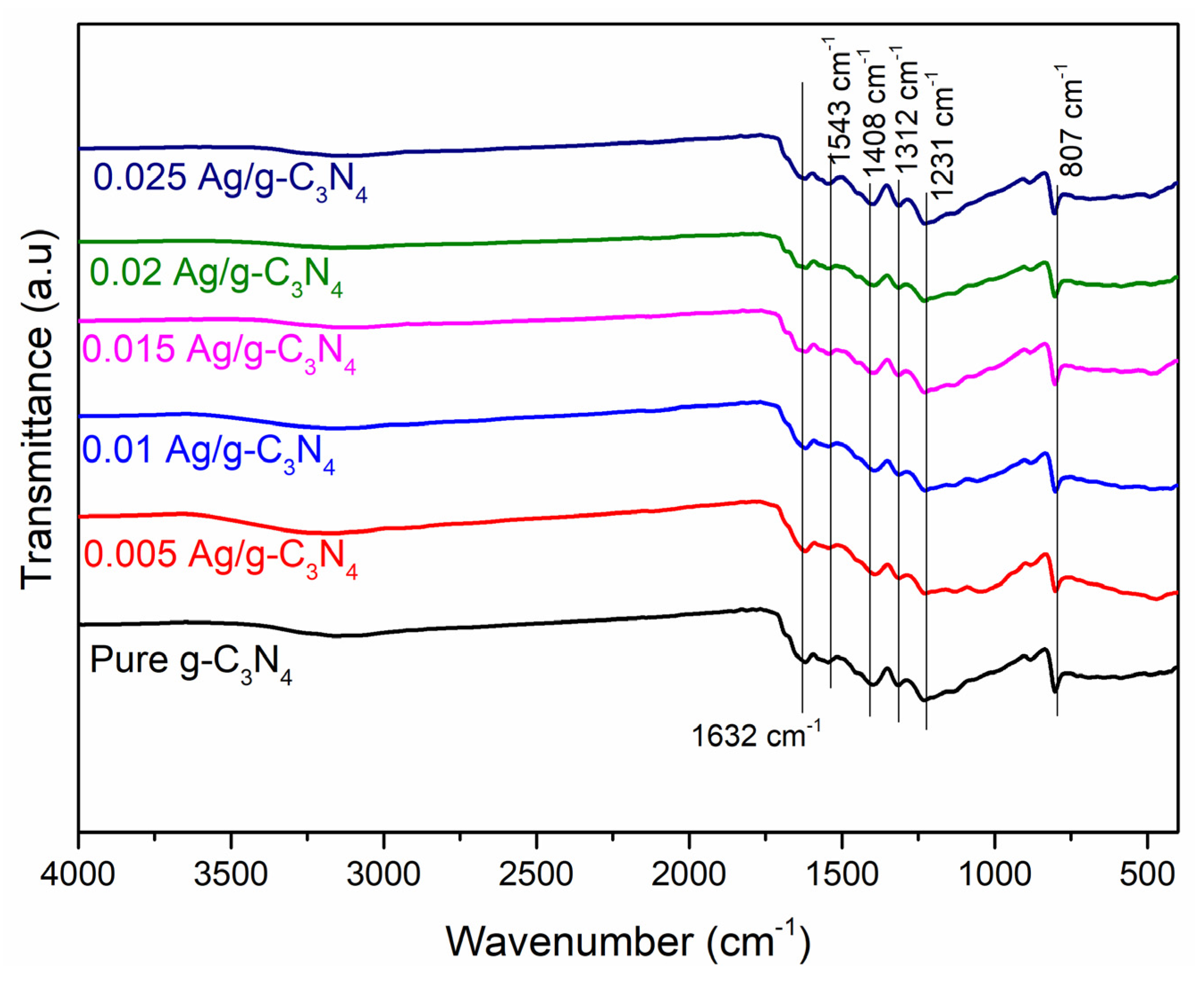
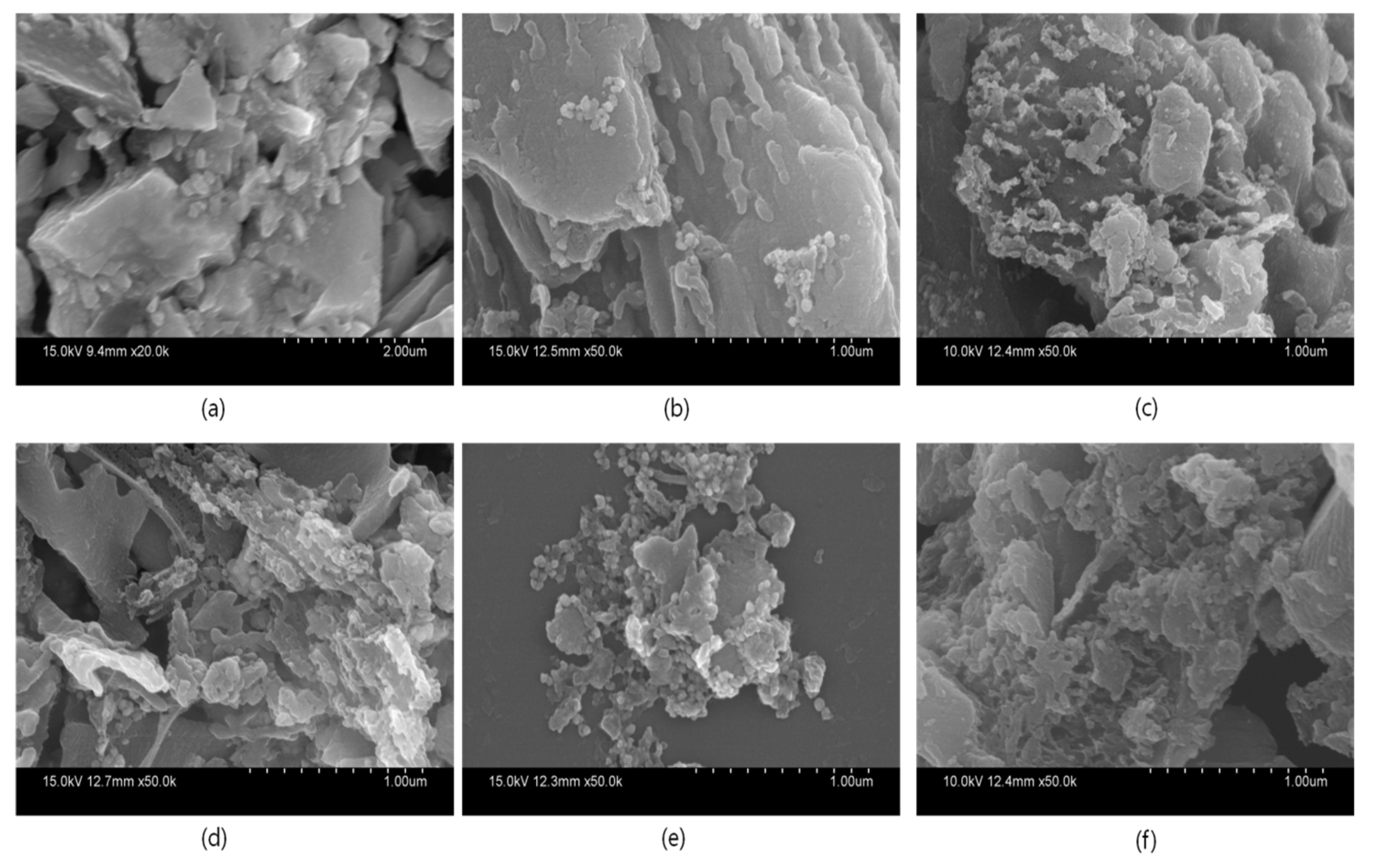
2.2. Characterization
2.3. Photocatalytic Dye Degradation
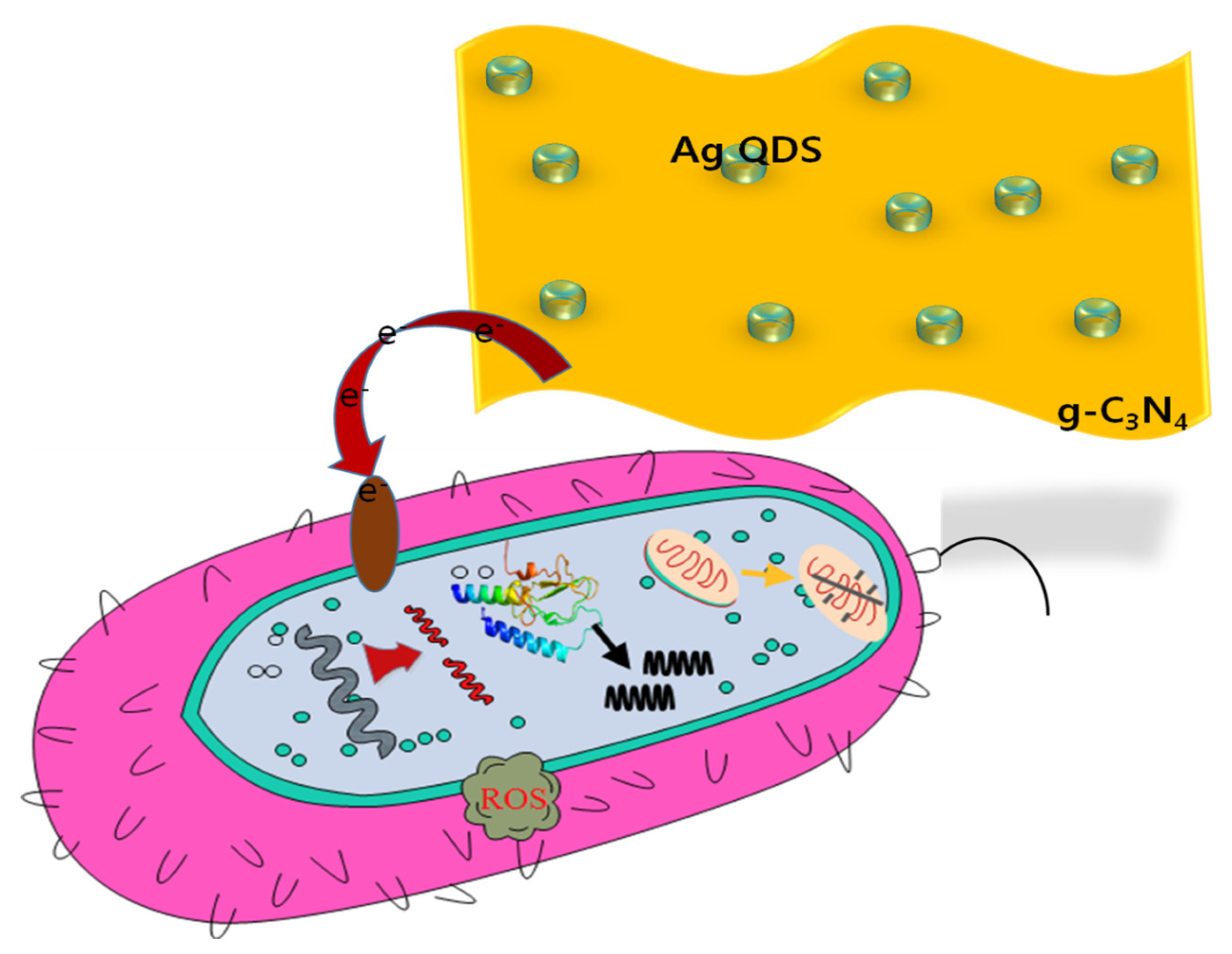
2.4. Antibacterial Activities
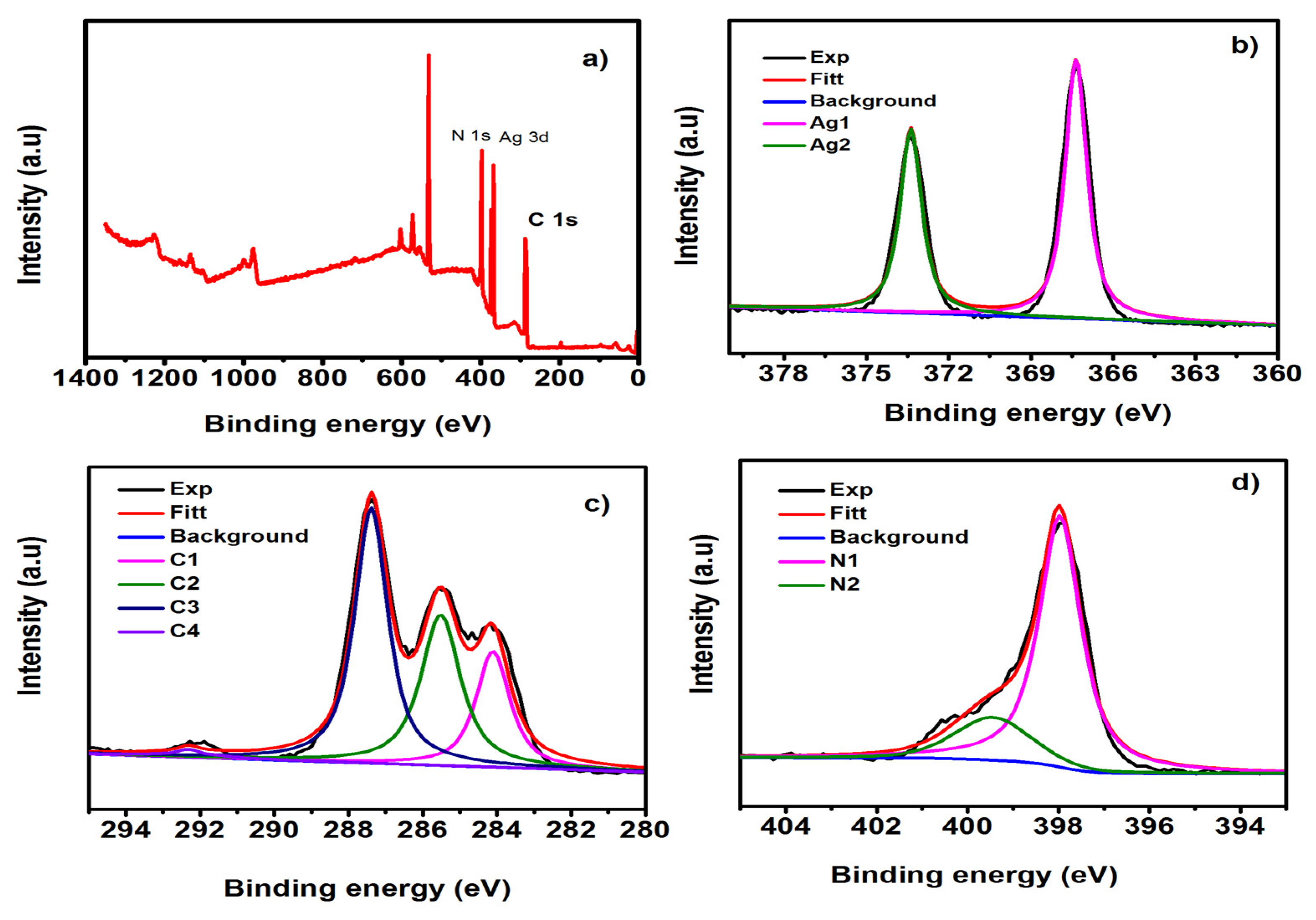
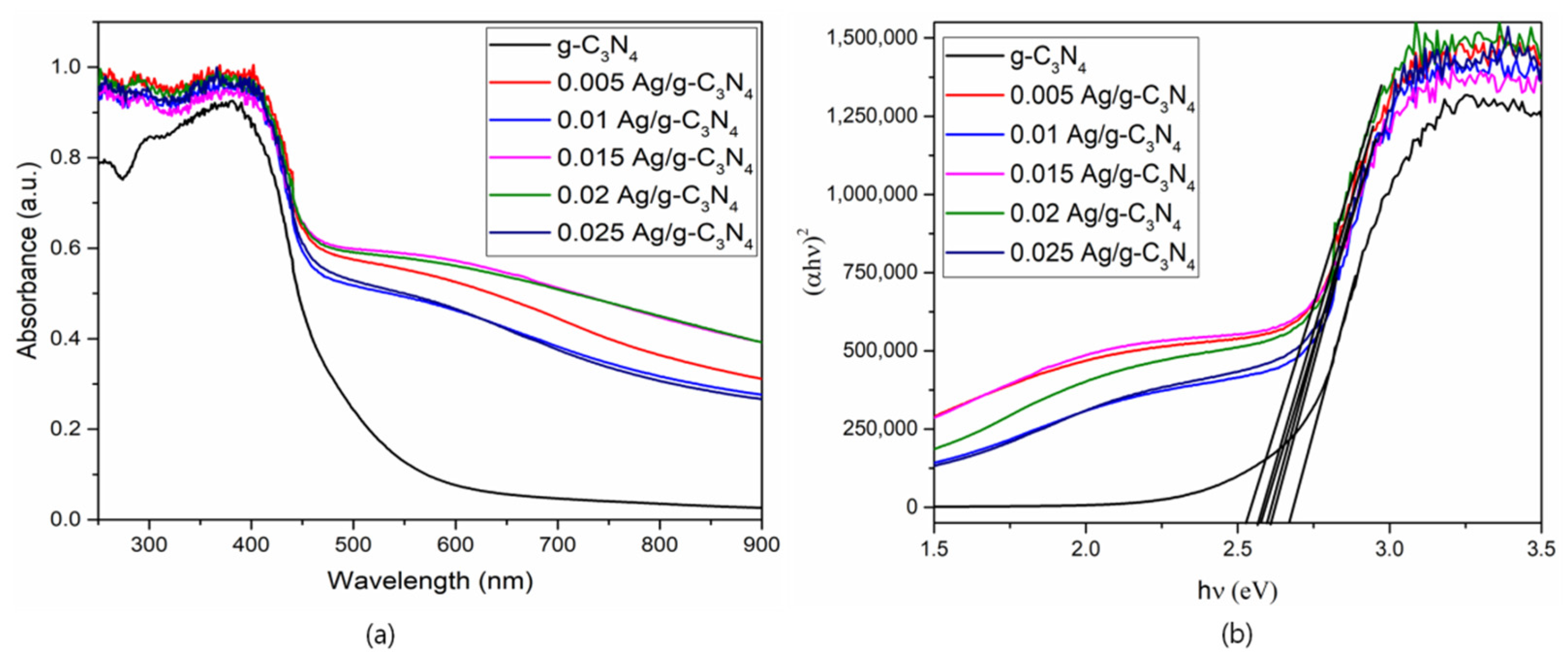
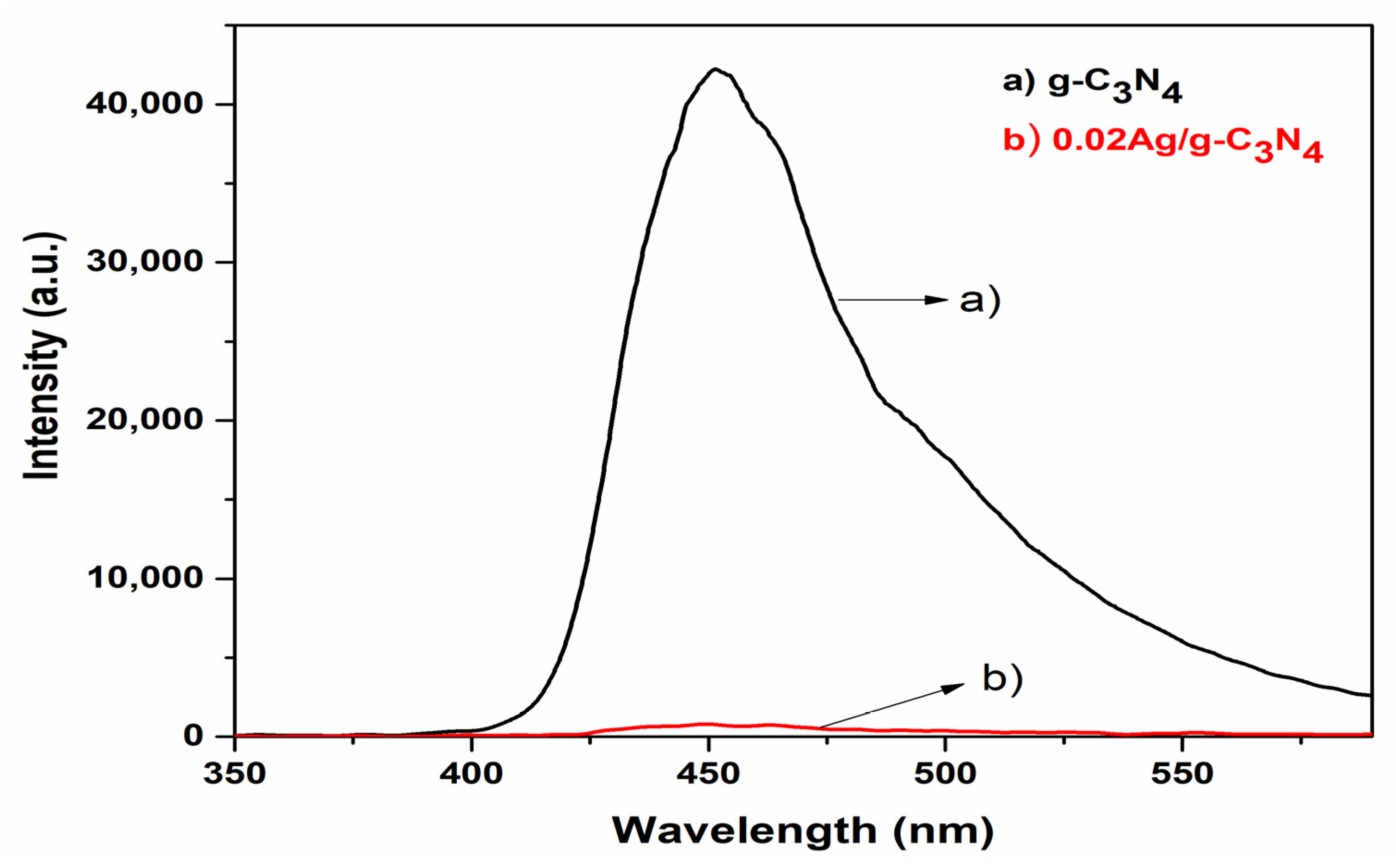
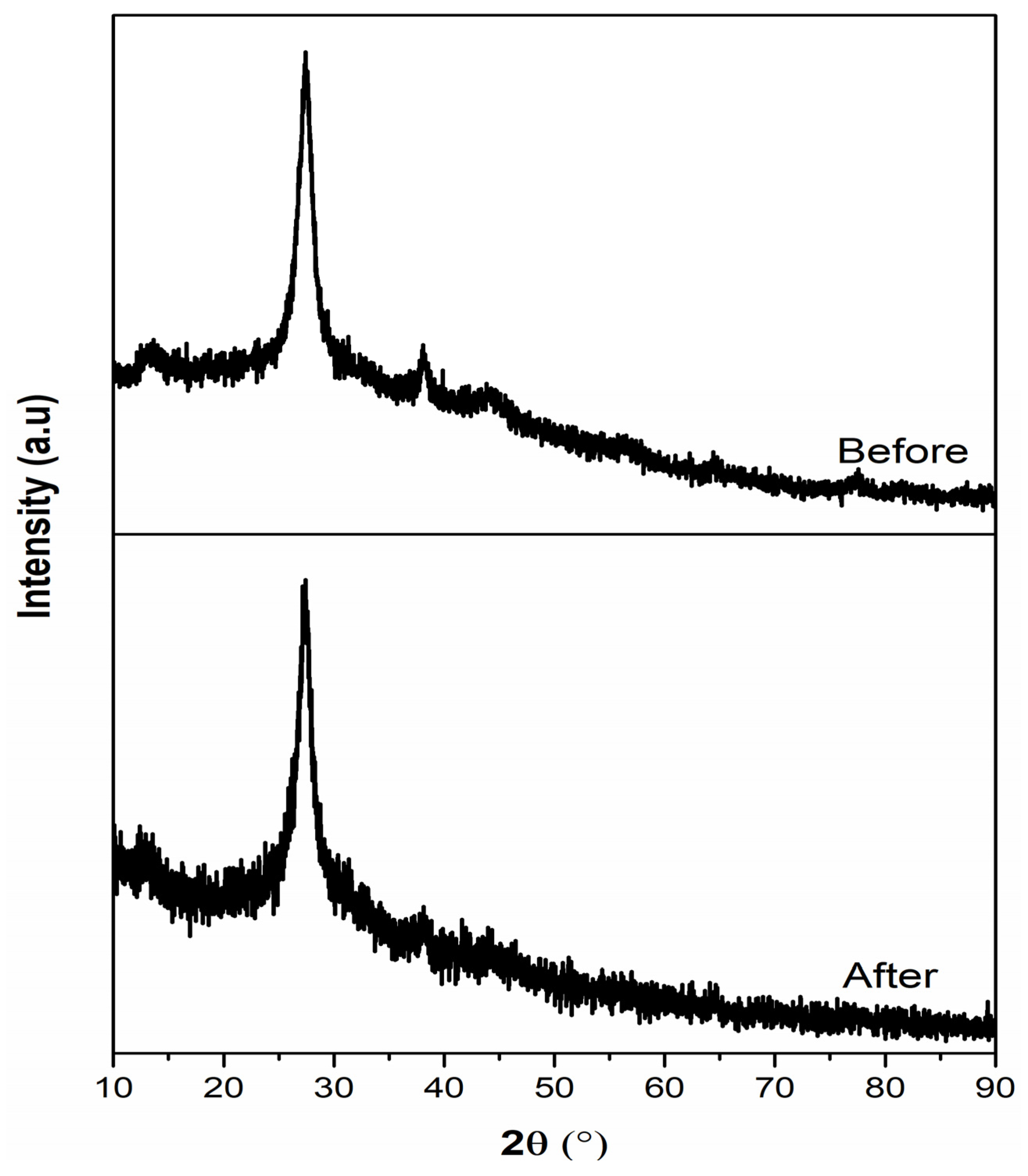
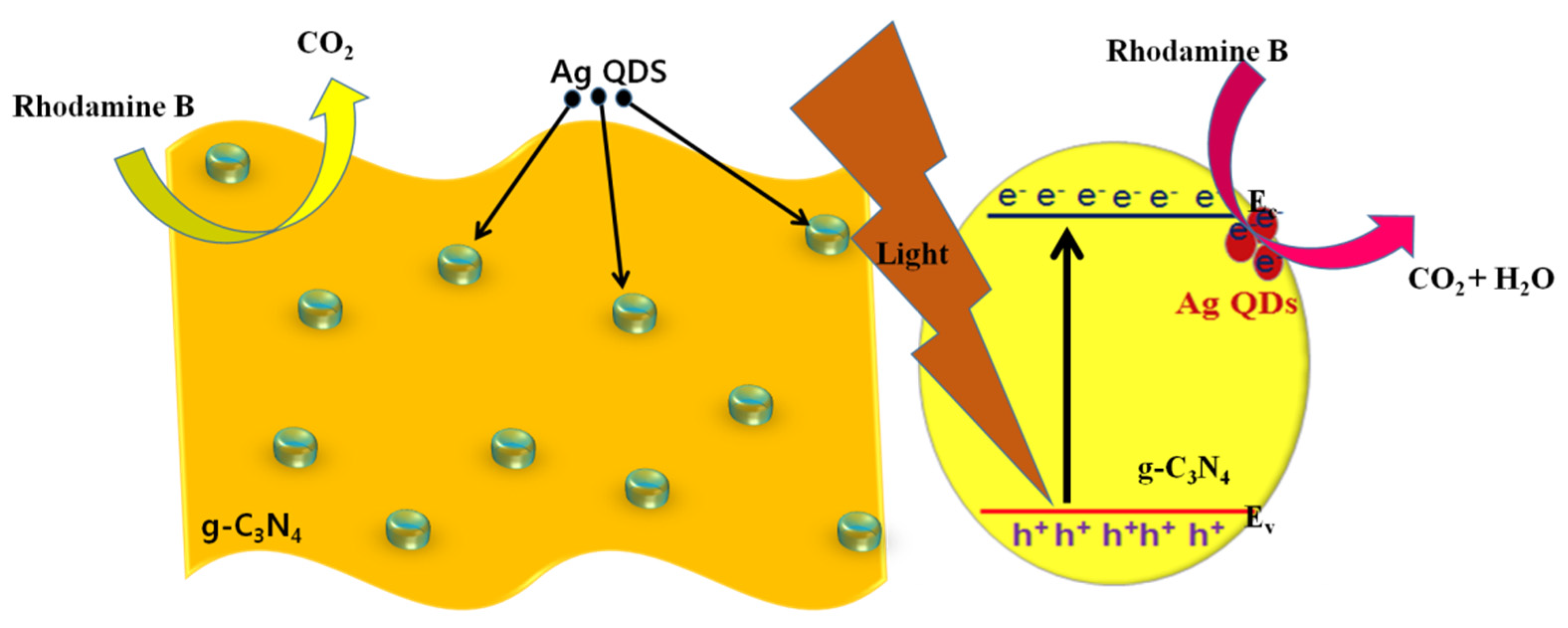
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bommireddy, P.R.; Kumar, M.; Lee, Y.W.; Manne, R.; Suh, Y.; Park, S.H. Prussian blue analogue Co3(Co(CN)6)2 cuboids as an electrode material for high-performance supercapacitor. J. Power Sour. 2021, 513, 230521. [Google Scholar] [CrossRef]
- Mallem, S.P.R.; Koduru, M.; Chandrasekhar, K.; Vattikuti, S.V.P.; Manne, R.; Reddy, V.R.; Lee, J.H. Potato chip-like 0D interconnected ZnCo2O4 nanoparticles for high-performance supercapacitors. Crystals 2021, 11, 469. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Pant, H.R.; Kim, J.H.; Kim, H.J.; Park, C.H.; Kim, C.S. One-pot synthesis and characterization of Ag-ZnO/g-C3N4 photocatalyst with improved photoactivity and antibacterial properties. Colloid Surf. Phys. Chem. Eng. Asp. 2015, 482, 477–484. [Google Scholar] [CrossRef]
- Wang, P.; Zhan, S.; Wang, H.; Xia, Y.; Hou, Q.; Zhou, Q. Cobalt phosphide nanowires as efficient co-catalyst for photocatalytic hydrogen evolution over Zn0.5Cd0.5S. Appl. Catal. B Environ. 2018, 230, 210–219. [Google Scholar] [CrossRef]
- Sun, K.; Shen, J.; Liu, Q.; Tang, H.; Zhang, M.; Zulfiqar, S. Synergistic effect of Co(II)-hole and Pt-electron co-catalysts for enhanced photocatalytic hydrogen evolution performance of P-doped g-C3N4. Chin. J. Catal. 2020, 4, 72–81. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhera, S.K.; Shankar, M.V.; Neppolian, B. Synergetic improvement in charge carrier transport and light-harvesting over ternary InVO4- g-C3N4/rGO hybrid nanocomposite for hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 7530–7540. [Google Scholar] [CrossRef]
- El-Daly, S.A.; Rahman, M.M.; Alamry, K.A.; Asiri, A.M. Fluorescence quenching of Perylene DBPI dye by colloidal low-dimensional gold nanoparticles. J. Fluoresc. 2015, 25, 973–978. [Google Scholar] [CrossRef]
- Subhan, Md.A.; Jhuma, S.S.; Saha, P.C.; Ahmed, J.; Asiri, A.M.; Rifat, T.P.; Raihan, T.; Azad, A.K.; Rahman, M.M. Photocatalysis, enhanced anti-bacterial performance and discerning thiourea sensing of Ag2O·SnO2·TiO2 heterostructure. J. Environ. Chem. Eng. 2020, 8, 104051. [Google Scholar] [CrossRef]
- Subhan, Md.A.; Rifat, T.P.; Saha, P.C.; Alam, M.M.; Asiri, A.M.; Rahman, M.M.; Akter, S.; Raihan, T.; Azad, A.K.; Uddin, J. Enhanced visible light-mediated photocatalysis, antibacterial functions and fabrication of a 3-chlorophenol sensor based on ternary Ag2O·SrO·CaO. RSC Adv. 2020, 10, 11274–11291. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Yang, H.; Luo, W.; Wang, P.; Yu, H. Selective adsorption of thiocyanate anions on Ag-modified g-C3N4 for enhanced photocatalytic hydrogen evolution. Chin. J. Catal. 2017, 38, 1990–1998. [Google Scholar] [CrossRef]
- Ravichandran, K.; Sindhuja, E. Fabrication of cost-effective g-C3N4/Ag activated ZnO photocatalyst in thin-film form for enhanced visible light-responsive dye degradation. Mater. Chem. Phys. 2019, 221, 203–215. [Google Scholar] [CrossRef]
- Kwon, KW.; Shim, M. γ-Fe2O3/II-VI sulfide nanocrystal heterojunctions. J. Am. Chem. Soc. 2005, 127, 10269–10275. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Pellegrino, T.; Manna, L. Synthesis, properties, and perspectives of hybrid nanocrystal structures. Chem. Soc. Rev. 2006, 35, 1195–1208. [Google Scholar] [CrossRef]
- Wang, G.X.; Shen, X.P.; Yao, J.; Park, J. Graphene nanosheets for enhanced lithium storage in lithium-ion batteries. Carbon 2009, 47, 2049–2053. [Google Scholar] [CrossRef]
- Akhavan, O. Graphene nano-mesh by ZnO nanorod photocatalysts. ACS Nano 2010, 4, 4174–4180. [Google Scholar] [CrossRef]
- Babu, B.; Mallikarjuna, K.; Reddy, Ch. V.; Park, J. Facile synthesis of Cu@TiO2 core shell nanowires for efficient photocatalysis. Mater. Lett. 2016, 176, 265–269. [Google Scholar] [CrossRef]
- Feng, H.; Guo, Q.; Xu, Y.; Chen, T.; Zhou, Y.; Wang, Y. Surface non-polarization of g-C3N4 by decoration with sensitized quantum dots for improved CO2 photoreduction. ChemSusChem 2018, 11, 4256–4261. [Google Scholar] [CrossRef]
- Akhund, A.; Habibi-Yangjeh, A.; Abitorabi, M.; Rahim Pouran, S. Review on the photocatalytic conversion of carbon dioxide to value-added compounds and renewable fuels by graphitic carbon nitride-based photocatalysts. Catal. Rev. 2019, 61, 595–628. [Google Scholar] [CrossRef]
- Babu, B.; Shim, J.; Yoo, K. Efficient solar-light-driven photoelectrochemical water oxidation of one-step in-situ synthesized Co-doped g-C3N4 nanolayers. Ceram. Int. 2020, 46, 16422–16430. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, R.; Wu, M.Z.; Yuan, Y.P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar]
- Vesali-Kermani, E.; Habibi-Yangjeh, A.; Ghosh, S. Visible-light induced nitrogen photo-fixation ability of g-C3N4 nano-sheets decorated with MgO nanoparticles. J. Ind. Eng. Chem. 2020, 84, 185–195. [Google Scholar] [CrossRef]
- Ghann, W.E.; Kang, H.; Uddin, J.; Chowdhury, F.A.; Khondaker, S.I.; Moniruzzaman, M.; Kabir, Md.H.; Rahman, M.M. Synthesis and characterization of reduced graphene oxide and their application in dye-sensitized solar cells. Chem. Eng. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Ghann, W.; Sharma, V.; Kang, H.; Karim, F.; Richards, B.; Mobin, S.M.; Uddin, J.; Rahman, M.M.; Hossain, F.; Kabir, H.; et al. The synthesis and characterization of carbon dots and their application in dye sensitized solar cell. Int. J. Hydrog. Energy 2019, 44, 14580–14587. [Google Scholar] [CrossRef]
- Akter, N.; Hossain, Md.A.; Hassan, M.J.; Amin, M.K.; Elias, M.; Rahman, M.M.; Asiri, A.M.; Siddiquey, I.A.; Hasnat, M.A. Amine modified tannin gel for adsorptive removal of Brilliant Green dye. J. Environ. Chem. Eng. 2016, 4, 1231–1241. [Google Scholar] [CrossRef]
- Tang, H.; Wang, R.; Zhao, C.; Chen, Z.; Yang, X.; Bukhvalov, D. Oxamide-modified g-C3N4 nanostructures: Tailoring surface topography for high-performance visible light photocatalysis. Chem. Eng. J. 2019, 374, 1064–1075. [Google Scholar] [CrossRef]
- Tian, H.; Liu, M.; Zheng, W. Constructing 2D graphitic carbon nitride nanosheets/layered MoS2/graphene ternary-nanojunction with enhanced photocatalytic activity. Appl. Catal. B Environ. 2018, 225, 468–476. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Pouran, R.S. Review on magnetically separable graphitic carbon nitride-based nanocomposites as promising visible-light-driven photocatalysts. J. Mater. Sci. Mater. Electron. 2018, 29, 1719–1747. [Google Scholar] [CrossRef]
- Saiganesh, S.; Krishnan, T.; Mallikarjuna, K.; Reddy, L.V.; Reddy, M.S.P. Phytogenic generation of NiO nanoparticles using Stevia leaf extract and evaluation of their In-Vitro antioxidant and antimicrobial properties. Biomolecules 2020, 10, 89. [Google Scholar]
- Bicheng, Z.; Xia, P.; Li, Y.; Ho, W.; Jiaguo, Y. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl. Surf. Sci. 2017, 391, 175–183. [Google Scholar]
- Mallikarjuna, K.; Kumar, M.K.; Kim, H. Synthesis of oxygen-doped-g-C3N4/WO3 porous structures for visible driven photocatalytic H2 production. Physica E 2021, 126, 114428. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Wang, X.; Huang, Y.; Zeng, M.; Xu, J. In situ ion exchange synthesis of strongly coupled Ag@AgCl/g-C3N4 porous nanosheets as plasmonic photocatalyst for highly efficient visible-light photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 22116–22125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, H.C. Size tuning, functionalization, and reactivation of Au in TiO2 nanoreactors. Angew. Chem. Int. Ed. 2005, 44, 4342–4345. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, K.; Kim, H. Bandgap-tuned ultra-small SnO2-nanoparticle-decorated 2D-Bi2WO6 nanoplates for visible-light-driven photocatalytic applications. Chemosphere 2021, 263, 128185. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Safwan, J.A.; Islam, M.S.; Rahman, Z.; Karim, M.R.; Pirzada, T.J.; Samed, A.J.; Rahman, M.M. Electrochemical decolorization of Methylene blue at Pt electrode in KCl solution for environmental remediation. J. Ind. Eng. Chem. 2015, 21, 787–791. [Google Scholar] [CrossRef]
- Rahman, M.M.; Asiri, A.M.; Youssef, T.E.; Marwani, H.M. Photocatalytic degradation of remazol brilliant orange 3R using wet-chemically prepared CdO-ZnO nanofibers for environmental remediation. Mater. Express 2016, 6, 137–148. [Google Scholar] [CrossRef]
- Kim, T.Y.; Song, D.; Barea, E.M.; Lee, J.H.; Kim, Y.R.; Cho, W.; Lee, S.; Rahman, M.M.; Bisquert, J.; Kang, Y.S. Origin of high open-circuit voltage in solid state dye-sensitized solar cells employing polymer electrolyte. Nano Energy 2016, 28, 455–461. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Sun, Z.; Wei, J.; Zheng, G.; Asiri, A.M.; Khan, S.B.; Rahman, M.M.; Zhao, D. Hierarchical Cu2S microsponges constructed from nanosheets for efficient photocatalysis. Small 2013, 9, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.E.; Han, T.H.; Khan, M.M.; Karim, M.R.; Cho, M.H. Environmentally Sustainable Fabrication of Ag@g-C3N4 Nanostructures and Their Multifunctional Efficacy as Antibacterial Agents and Photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2912–2922. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Yu, P.; Li, Z.; Zheng, T. Characteristics, mechanisms, and bacteria behavior of photocatalysis with a solid Z-scheme Ag/AgBr/g-C3N4 nanosheet in water disinfection. Appl. Catal. A Gen. 2020, 590, 117282. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, L.V. Jayashree, L.N.; Venkata Reddy, Ch.; Cho, M.; Kim, D.; Shim, J.S. Vanadium-doped graphitic carbon nitride for multifunctional applications: Photoelectrochemical water splitting and antibacterial activities. Chemosphere 2021, 264, 128593. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Reddy, L.V.; Devarayapalli, K.C.; Prabhakar Vattikuti, S.V.; Wee, Y.J.; Shim, J.S. Environmentally Friendly Synthesis: Photocatalytic Dye Degradation and Bacteria Inactivation Using Ag/f-MWCNTs Composite. J. Clust. Sci. 2020, 32, 711–718. [Google Scholar] [CrossRef]



| Compound | Zone of inhibition (activity) (mm) | |||
|---|---|---|---|---|
| E. coli | B. subtilis | L. monocytogenes | S. aureus | |
| a | 12 | 10 | - | 10 |
| b | 15 | 12 | 10 | 12 |
| c | 15 | 13 | 12 | 12 |
| d | 16 | 15 | 12 | 14 |
| e | 20 | 18 | 16 | 16 |
| f | 20 | 19 | 16 | 17 |
| Chloramphenicol | 22 | 24 | 22 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallikarjuna, K.; Vattikuti, S.V.P.; Manne, R.; Manjula, G.; Munirathnam, K.; Mallapur, S.; Marraiki, N.; Mohammed, A.; Reddy, L.V.; Rajesh, M.; et al. Sono-Chemical Synthesis of Silver Quantum Dots Immobilized on Exfoliated Graphitic Carbon Nitride Nanostructures Using Ginseng Extract for Photocatalytic Hydrogen Evolution, Dye Degradation, and Antimicrobial Studies. Nanomaterials 2021, 11, 2918. https://doi.org/10.3390/nano11112918
Mallikarjuna K, Vattikuti SVP, Manne R, Manjula G, Munirathnam K, Mallapur S, Marraiki N, Mohammed A, Reddy LV, Rajesh M, et al. Sono-Chemical Synthesis of Silver Quantum Dots Immobilized on Exfoliated Graphitic Carbon Nitride Nanostructures Using Ginseng Extract for Photocatalytic Hydrogen Evolution, Dye Degradation, and Antimicrobial Studies. Nanomaterials. 2021; 11(11):2918. https://doi.org/10.3390/nano11112918
Chicago/Turabian StyleMallikarjuna, Koduru, Surya Veerendra Prabhakar Vattikuti, Ravi Manne, Gangarapu Manjula, Keelapattu Munirathnam, Srinivas Mallapur, Najat Marraiki, Arifullah Mohammed, Lebaka Veeranjaneya Reddy, Megala Rajesh, and et al. 2021. "Sono-Chemical Synthesis of Silver Quantum Dots Immobilized on Exfoliated Graphitic Carbon Nitride Nanostructures Using Ginseng Extract for Photocatalytic Hydrogen Evolution, Dye Degradation, and Antimicrobial Studies" Nanomaterials 11, no. 11: 2918. https://doi.org/10.3390/nano11112918








