Smart Design of Mitochondria-Targeted and ROS-Responsive CPI-613 Delivery Nanoplatform for Bioenergetic Pancreatic Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
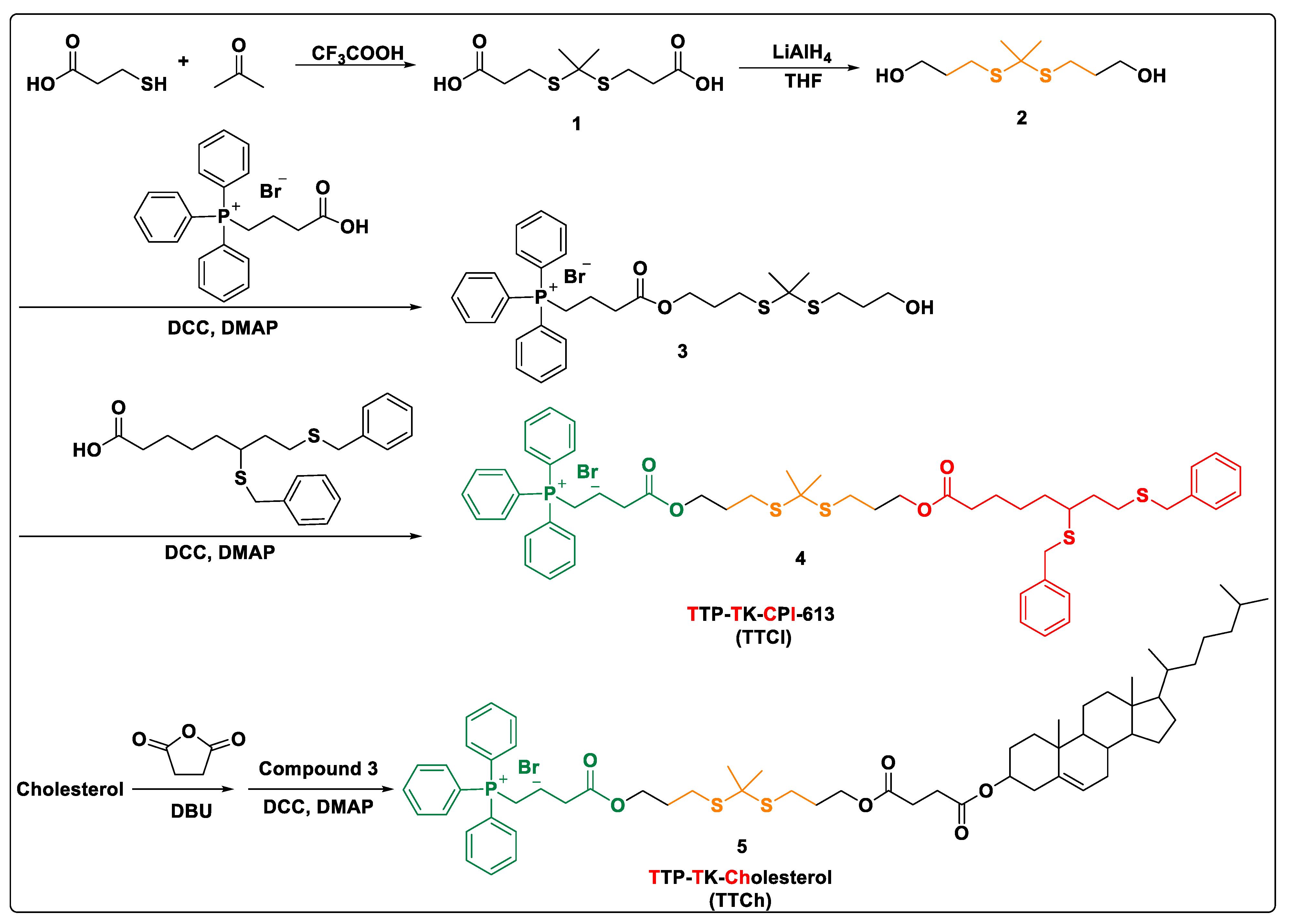
2.1. Synthesis of Prodrug TTCI
2.1.1. Synthesis of Compounds 1 and 2
2.1.2. Synthesis of Compound 3
2.1.3. Synthesis of Prodrug TTCI
2.1.4. Synthesis of Cholesterol Analogue TTCh
2.2. Preparation of Nanoparticles (NPs)
2.3. Characterization of TTCI NPs
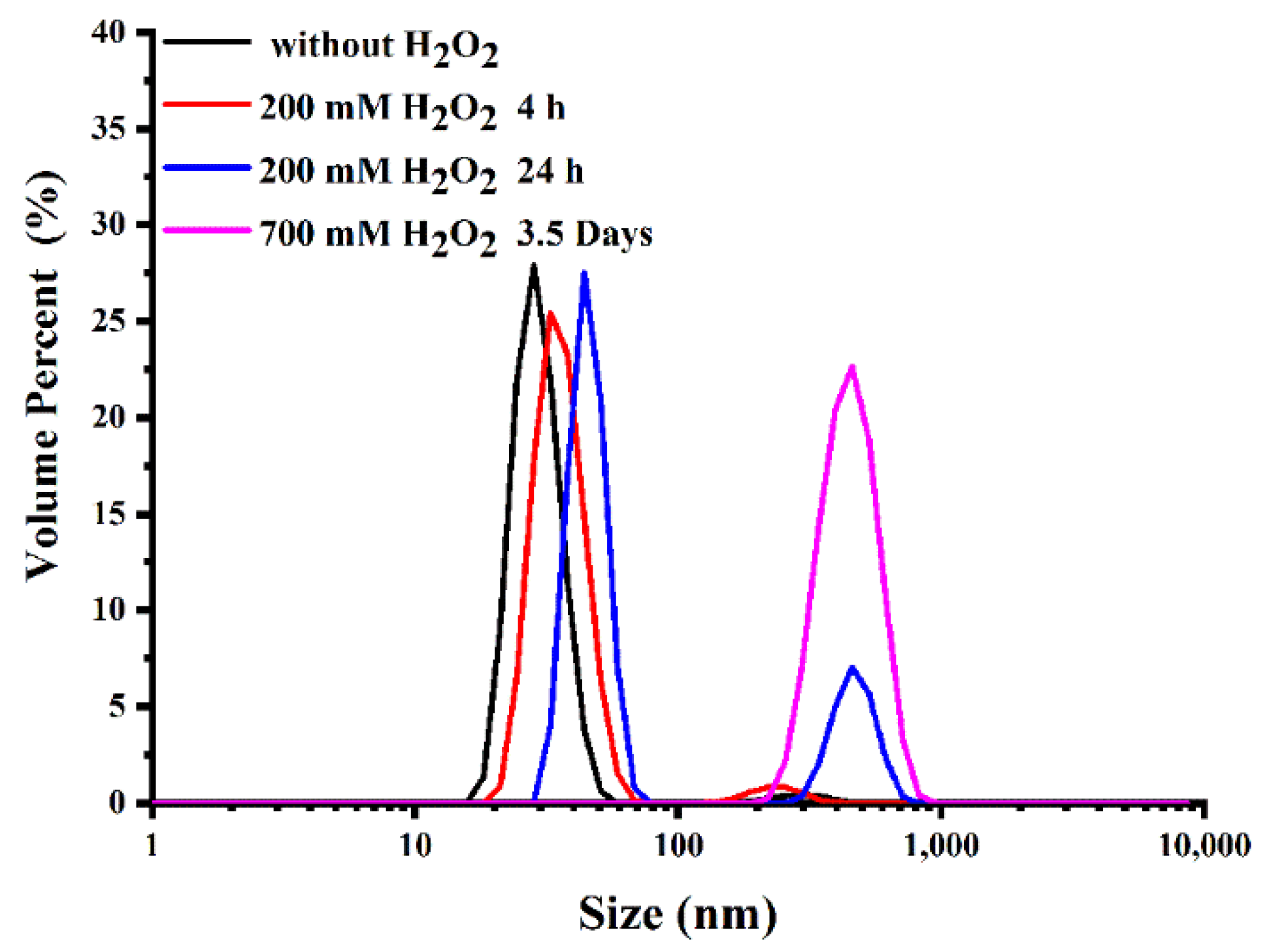
2.4. ROS-Triggered Disassembly Studies of TTCI NPs
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Cell Proliferation and Growth
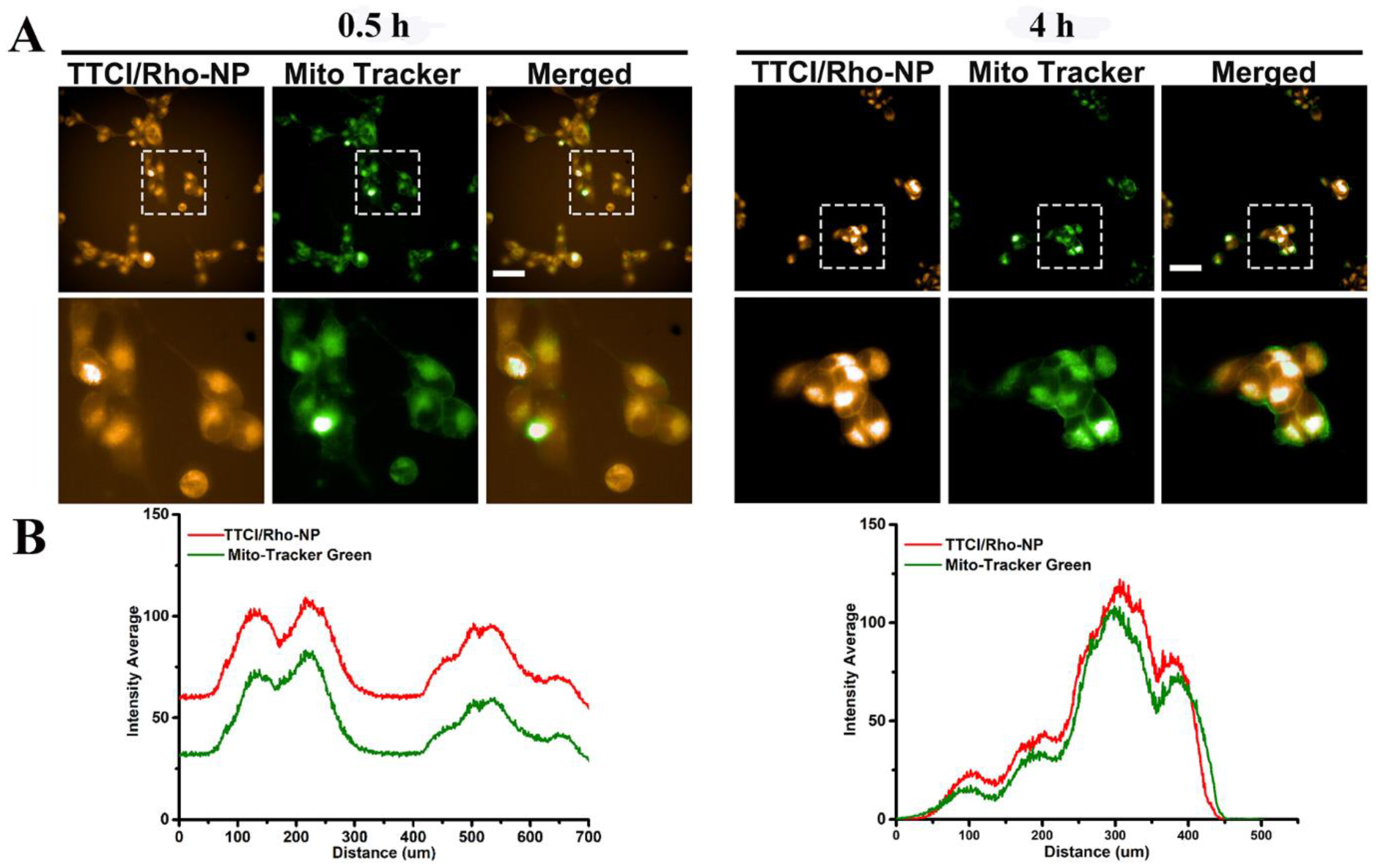
2.8. Intracellular Distributions
2.9. Mitochondria Targeted Imaging
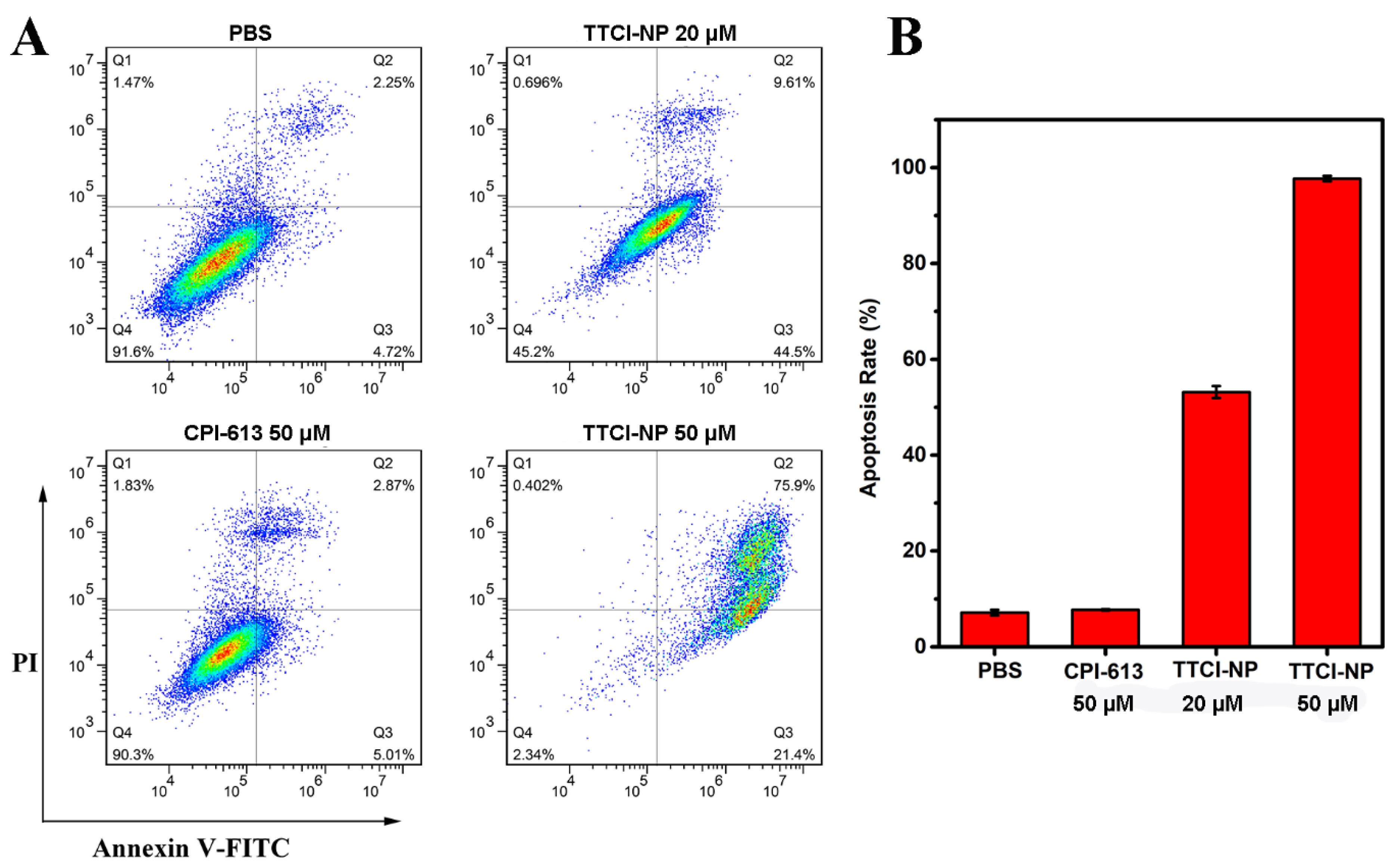
2.10. Cell Apoptosis Assay
3. Results and Discussion
3.1. Synthesis and Characterization of the Lipid Prodrug
3.2. Preparation and Characterization of CPI-613-Prodrug NPs
3.3. Measurement of ROS-Responsive TTCI NPs Degradation In Vitro
3.4. Cellular Internalization and the Specific Mitochondria-Targeting Function of the TTCI NPs
3.5. In Vitro Antitumor Activity
3.6. Cell Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Xu, Z.; Huang, Z.; Tang, Y.; Yang, D.; Huang, J.; He, L.; Liu, M.; Chen, Z.; Teng, Y. CPI-613 rewires lipid metabolism to enhance pancreatic cancer apoptosis via the AMPK-ACC signaling. J. Exp. Clin. Cancer Res. 2020, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sausen, M.; Phallen, J.; Adleff, V.; Jones, S.; Leary, R.J.; Barrett, M.T.; Anagnostou, V.; Parpart-Li, S.; Murphy, D.; Kay Li, Q.; et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat. Commun. 2015, 6, 7686. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Maturo, C.; Perera, C.N.; Luddy, J.; Rodriguez, R.; Shorr, R. Translational assessment of mitochondrial dysfunction of pan-creatic cancer from in vitro gene microarray and animal efficacy studies, to early clinical studies, via the novel tumor-specific an-ti-mitochondrial agent, CPI-613. Ann. Transl. Med. 2014, 2. [Google Scholar] [CrossRef]
- Alistar, A.; Morris, B.B.; Desnoyer, R.; Klepin, H.D.; Hosseinzadeh, K.; Clark, C.; Cameron, A.; Leyendecker, J.; D’Agostino, R.; Topaloglu, U.; et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: A single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017, 18, 770–778. [Google Scholar] [CrossRef]
- Zachar, Z.; Marecek, J.; Maturo, C.; Gupta, S.; Stuart, S.D.; Howell, K.; Schauble, A.; Lem, J.; Piramzadian, A.; Karnik, S.; et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J. Mol. Med. 2011, 89, 1137–1148. [Google Scholar] [CrossRef]
- Pardee, T.S.; Lee, K.; Luddy, J.; Maturo, C.; Rodriguez, R.; Isom, S.; Miller, L.; Stadelman, K.M.; Levitan, D.; Hurd, D.; et al. A Phase I Study of the First-in-Class Antimitochondrial Metabolism Agent, CPI-613, in Patients with Advanced Hematologic Malignancies. Clin. Cancer Res. 2014, 20, 5255–5264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lycan, T.W.; Pardee, T.S.; Petty, W.J.; Bonomi, M.; Alistar, A.; Lamar, Z.S.; Isom, S.; Chan, M.D.; Miller, A.A.; Ruiz, J. A Phase II Clinical Trial of CPI-613 in Patients with Relapsed or Refractory Small Cell Lung Carcinoma. PLoS ONE 2016, 11, e0164244. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, Z.; Liu, H.; Fetse, J.P.; Jain, A.; Lin, C.-Y.; Cheng, K. Development of a Tumor-Responsive Nanopolyplex Targeting Pancreatic Cancer Cells and Stroma. ACS Appl. Mater. Interfaces 2019, 11, 45390–45403. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.-L.; Liu, Y.-H.; Wu, W.-X.; Liu, B.-Y.; Wang, N.; Yu, X.-Q. Chemoenzymatic synthesis of dual-responsive graft copolymers for drug delivery: Long-term stability, high loading and cell selectivity. J. Mater. Chem. B 2018, 6, 6993–7003. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-Y.; Wu, W.-X.; Liu, Y.-H.; Jia, C.; Yang, X.-L.; Li, J.; Wang, N.; Yu, X.-Q. Water-soluble mitochondria-targeting polymeric prodrug micelles for fluorescence monitoring and high intracellular anticancer efficiency. Polym. Chem. 2017, 8, 5982–5987. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Khaw, B.-A.; Weissig, V. Intracellular targets for DNA delivery: Nuclei and mitochondria. Somat. Cell Mol. Genet. 2002, 27, 49–64. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Meng, S.; Li, H.; Yang, Q.; Xu, Z.; Chen, X.; Sun, Z.; Jiang, B.; Li, C. Nanoplatform based on GSH-responsive mesoporous silica nanoparticles for cancer therapy and mitochondrial targeted imaging. Microchim. Acta 2021, 188, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Sun, Z.; Li, C.; Jiang, B. Recent progress on mitochondrial targeted cancer therapy based on inorganic nanomaterials. Mater. Today Chem. 2019, 12, 240–260. [Google Scholar] [CrossRef]
- Sung, J.; Rho, J.G.; Jeon, G.G.; Chu, Y.; Min, J.S.; Lee, S.; Kim, J.H.; Kim, W.; Kim, E. A New Infrared Probe Targeting Mitochondria via Regulation of Molecular Hydrophobicity. Bioconjugate Chem. 2018, 30, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.S.; Qin, X.; Zhou, J.; Li, L.; Huang, W.; Yao, S.Q. Smart Design of Nanomaterials for Mitochondria-Targeted Nanotherapeutics. Angew. Chem. Int. Ed. 2021, 60, 2232–2256. [Google Scholar] [CrossRef]
- Jhaveri, A.; Torchilin, V. Intracellular delivery of nanocarriers and targeting to subcellular organelles. Expert Opin. Drug Deliv. 2016, 13, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Bruno, B.J.; Rabenau, M.; Lim, C.S. Delivery of drugs and macromolecules to the mitochondria for cancer therapy. J. Control. Release 2016, 240, 38–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, R.; Albuquerque, T.; Neves, A.R.; Bhatt, H.; Biswas, S.; Cardoso, A.M.; de Lima, M.C.P.; Jurado, A.S.; Costa, D. Physicochemical characterization and targeting performance of triphenylphosphonium nano-polyplexes. J. Mol. Liq. 2020, 316, 113873. [Google Scholar] [CrossRef]
- Mani, S.; Swargiary, G.; Tyagi, S.; Singh, M.; Jha, N.K.; Singh, K.K. Nanotherapeutic approaches to target mitochondria in cancer. Life Sci. 2021, 281, 119773. [Google Scholar] [CrossRef]
- Yousif, L.F.; Stewart, K.M.; Kelley, S.O. Targeting Mitochondria with Organelle-Specific Compounds: Strategies and Applications. ChemBioChem 2009, 10, 1939–1950. [Google Scholar] [CrossRef]
- Bielski, E.R.; Zhong, Q.; Brown, M.; da Rocha, S.R.P. Effect of the Conjugation Density of Triphenylphosphonium Cation on the Mitochondrial Targeting of Poly(amidoamine) Dendrimers. Mol. Pharm. 2015, 12, 3043–3053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhang, P.-Y. [Retracted] Mitochondria targeting nano agents in cancer therapeutics (Review). Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Ozsvari, B.; Sotgia, F.; Lisanti, M.P. Exploiting mitochondrial targeting signal(s), TPP and bis-TPP, for eradicating cancer stem cells (CSCs). Aging 2018, 10, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P.; Holmgren, A.; Larsson, N.-G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the Bio-logical Roles of Reactive Oxygen Species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Wang, K.; Zhang, D.; Sun, B.; Ji, B.; Wei, L.; Li, Z.; Wang, M.; Zhang, X.; Zhang, H.; et al. Light-activatable dual-source ROS-responsive prodrug nanoplatform for synergistic chemo-photodynamic therapy. Biomater. Sci. 2018, 6, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Feito, M.J.; González-Mayorga, A.; Diez-Orejas, R.; Matesanz, M.C.; Portolés, M.T. Response of macrophages and neural cells in contact with reduced graphene oxide microfibers. Biomater. Sci. 2018, 6, 2987–2997. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Xia, Y. A Reactive Oxygen Species (ROS)-Responsive Polymer for Safe, Efficient, and Targeted Gene Delivery in Cancer Cells. Angew. Chem. Int. Ed. 2013, 52, 6926–6929. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, C.; Gabriel, A.; Yang, Y.; Jiang, X.; Yang, Y.; Pan, F.; Fuente, J.M.d.l.; Cui, D. Near-Infrared Light Triggered ROS-activated Theranostic Platform based on Ce6-CPT-UCNPs for Simultaneous Fluorescence Imaging and Chemo-Photodynamic Combined Therapy. Theranostics 2016, 6, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, Y.; You, X.; Zhang, F.; Shah, V.; Chen, X.; Tong, D.; Wei, X.; Yin, L.; Wu, J.; et al. Development of a reactive oxygen species (ROS)-responsive nanoplatform for targeted oral cancer therapy. J. Mater. Chem. B 2016, 4, 4675–4682. [Google Scholar] [CrossRef]
- Lyu, Y.; He, S.; Li, J.; Jiang, Y.; Sun, H.; Miao, Y.; Pu, K. A Photolabile Semiconducting Polymer Nanotransducer for Near-Infrared Regulation of CRISPR/Cas9 Gene Editing. Angew. Chem. Int. Ed. 2019, 58, 18197–18201. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, B.; Yang, G.; Ouyang, Y.; Tian, J.; Zhang, W. NIR-Triggered Multifunctional and Degradable Nanoplatform Based on an ROS-Sensitive Block Copolymer for Imaging-Guided Chemo-Phototherapy. Biomacromolecules 2019, 20, 4218–4229. [Google Scholar] [CrossRef] [PubMed]
- Van der Meel, R.; Fens, M.H.A.M.; Vader, P.; van Solinge, W.W.; Eniola-Adefeso, O.; Schiffelers, R.M. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J. Control. Release 2014, 195, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhao, D.-M.; Deng, X.; Zhang, J.; Zhang, Y.-M.; Yu, X.-Q. Functionalized Asymmetric Bola-Type Amphiphiles for Efficient Gene and Drug Delivery. Nanomaterials 2018, 8, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-M.; Xia, M.; Ao, R.; Gao, L.-X.; Tang, Y.; Huang, J.-H.; Luo, Y.-F.; Chen, Z.-Z.; Wang, B.-C.; Huang, Z. Smart Design of Mitochondria-Targeted and ROS-Responsive CPI-613 Delivery Nanoplatform for Bioenergetic Pancreatic Cancer Therapy. Nanomaterials 2021, 11, 2875. https://doi.org/10.3390/nano11112875
Zhang Y-M, Xia M, Ao R, Gao L-X, Tang Y, Huang J-H, Luo Y-F, Chen Z-Z, Wang B-C, Huang Z. Smart Design of Mitochondria-Targeted and ROS-Responsive CPI-613 Delivery Nanoplatform for Bioenergetic Pancreatic Cancer Therapy. Nanomaterials. 2021; 11(11):2875. https://doi.org/10.3390/nano11112875
Chicago/Turabian StyleZhang, Yi-Mei, Meng Xia, Rui Ao, Li-Xia Gao, Yan Tang, Jiu-Hong Huang, Ya-Fei Luo, Zhong-Zhu Chen, Bo-Chu Wang, and Zheng Huang. 2021. "Smart Design of Mitochondria-Targeted and ROS-Responsive CPI-613 Delivery Nanoplatform for Bioenergetic Pancreatic Cancer Therapy" Nanomaterials 11, no. 11: 2875. https://doi.org/10.3390/nano11112875
APA StyleZhang, Y.-M., Xia, M., Ao, R., Gao, L.-X., Tang, Y., Huang, J.-H., Luo, Y.-F., Chen, Z.-Z., Wang, B.-C., & Huang, Z. (2021). Smart Design of Mitochondria-Targeted and ROS-Responsive CPI-613 Delivery Nanoplatform for Bioenergetic Pancreatic Cancer Therapy. Nanomaterials, 11(11), 2875. https://doi.org/10.3390/nano11112875





