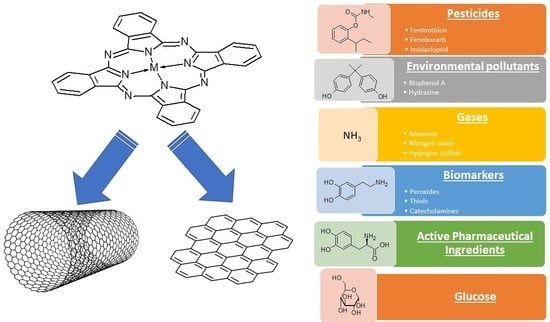
Azaporphyrins Embedded on Carbon-Based Nanomaterials for Potential Use in Electrochemical Sensing—A Review
Abstract
:1. Introduction
2. Food Pollutants and Components Sensing
3. Gas Sensing
4. Detection of Heavy Metals in Water Samples
5. Environmental Pollutant Sensing
6. Biomarkers Sensing
6.1. Peroxides
6.2. Thiols
6.3. Catecholamines
6.4. Other Biomarkers (NADH, Ascorbic Acid, Uric Acid and Urea, Nucleic Acids and Nucleotides)
7. Glucose Sensing
8. Active Pharmaceutical Ingredients (APIs) Sensing
9. Other Analytes’ Determination
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Al-Sagur, H.; Komathi, S.; Karakaş, H.; Atilla, D.; Gürek, A.; Basova, T.; Farmilo, N.; Hassan, A. A glucose biosensor based on novel Lutetium bis-phthalocyanine incorporated silica-polyaniline conducting nanobeads. Biosens. Bioelectron. 2018, 102, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Urban, G. Applications of Nanomaterials in Sensors and Diagnostics; Springer: New York, NY, USA, 2013; Volume 14, ISBN 978-3-642-36024-4. [Google Scholar]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Co-Ord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Basova, T.V.; Polyakov, M. Hybrid Materials Based on Carbon Nanotubes and Polyaromatic Molecules: Methods of Functionalization and Sensor Properties. Macroheterocycles 2020, 13, 91–112. [Google Scholar] [CrossRef]
- Michel, S.L.J.; Hoffman, B.M.; Baum, S.M.; Barrett, A.G.M. Peripherally Functionalized Porphyrazines: Novel Metallomacro-cycles with Broad, Untapped Potential. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons: New York, NY, USA, 2001; Volume 50, pp. 473–590. ISBN 0471435104. [Google Scholar]
- Rodríguez-Morgade, M.S.; Stuzhin, P.A. The chemistry of porphyrazines: An overview. J. Porphyr. Phthalocyanines 2004, 8, 1129–1165. [Google Scholar] [CrossRef]
- Pereira-Rodrigues, N.; Cofré, R.; Zagal, J.H.; Bedioui, F. Electrocatalytic activity of cobalt phthalocyanine CoPc adsorbed on a graphite electrode for the oxidation of reduced l-glutathione (GSH) and the reduction of its disulfide (GSSG) at physiological pH. Bioelectrochemistry 2007, 70, 147–154. [Google Scholar] [CrossRef]
- Sehlotho, N.; Nyokong, T. Effects of ring substituents on electrocatalytic activity of manganese phthalocyanines towards the reduction of molecular oxygen. J. Electroanal. Chem. 2006, 595, 161–167. [Google Scholar] [CrossRef]
- Bouvet, M.; Gaudillat, P.; Suisse, J.-M. Phthalocyanine-based hybrid materials for chemosensing. J. Porphyr. Phthalocyanines 2013, 17, 913–919. [Google Scholar] [CrossRef]
- Wu, H.; Guo, L.; Zhang, J.; Miao, S.; He, C.; Wang, B.; Wu, Y.; Chen, Z. Polyelectrolyte-free layer by layer self-assembled multilayer films of cationic phthalocyanine cobalt(II) and carbon nanotube for the efficient detection of 4-nitrophenol. Sens. Actuators B Chem. 2016, 230, 359–366. [Google Scholar] [CrossRef]
- Buber, E.; Yuzer, A.; Soylemez, S.; Kesik, M.; Ince, M.; Toppare, L. Construction and amperometric biosensing performance of a novel platform containing carbon nanotubes-zinc phthalocyanine and a conducting polymer. Int. J. Biol. Macromol. 2017, 96, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhang, H.; Li, N. An electrochemical biosensor for determination of ascorbic acid by cobalt (II) phthalocyanine–multi-walled carbon nanotubes modified glassy carbon electrode. Sens. Actuators B Chem. 2012, 161, 1074–1079. [Google Scholar] [CrossRef]
- Moraes, F.C.; Golinelli, D.L.C.; Mascaro, L.; Machado, S.A.S. Determination of epinephrine in urine using multi-walled carbon nanotube modified with cobalt phthalocyanine in a paraffin composite electrode. Sens. Actuators B Chem. 2010, 148, 492–497. [Google Scholar] [CrossRef]
- Jubete, E.; Żelechowska, K.; Loaiza, O.A.; Lamas, P.; Ochoteco, E.; Farmer, K.D.; Roberts, K.P.; Biernat, J.F. Derivatization of SWCNTs with cobalt phthalocyanine residues and applications in screen printed electrodes for electrochemical detection of thiocholine. Electrochim. Acta 2011, 56, 3988–3995. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Amr, A.E.-G.E.; Kamel, A.H.; Al-Omar, M.A.; Sayed, A.Y.A. Integrated all-solid-state sulfite sensors modified with two different ion-to-electron transducers: Rapid assessment of sulfite in beverages. RSC Adv. 2021, 11, 3783–3791. [Google Scholar] [CrossRef]
- Nooredeen, N.M.; El-Ghaffar, M.A.A.; Darwish, W.M.; Elshereafy, E.; Radwan, A.A.; Abbas, M.N.E. Graphene oxide with covalently attached zinc monoamino-phthalocyanine coated graphite electrode as a potentiometric platform for citrate sensing in pharmaceutical preparations. J. Solid State Electrochem. 2015, 19, 2141–2154. [Google Scholar] [CrossRef]
- Cerbin-Koczorowska, M.; Waszyk-Nowaczyk, M.; Bakun, P.; Goslinski, T.; Koczorowski, T. Current View on Green Tea Catechins Formulations, Their Interactions with Selected Drugs, and Prospective Applications for Various Health Conditions. Appl. Sci. 2021, 11, 4905. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Bahrim, G.; Rodriguez-Mendez, M.L. Electrochemical Study of Polyphenols with Amperometric Tyrosinase Based Biosensors. Rom. Biotechnol. Lett. 2012, 17, 7684–7693. [Google Scholar]
- Koçak, Ç.C.; Nas, A.; Kantekin, H.; Dursun, Z. Simultaneous determination of theophylline and caffeine on novel [Tetra-(5-chloroquinolin-8-yloxy) phthalocyanato] manganese(III)-Carbon nanotubes composite electrode. Talanta 2018, 184, 452–460. [Google Scholar] [CrossRef]
- Jerković, A.; Abou-Ahmed, S.; Ertl, P.; Stoeßl, B.; Lengauer, V.; Samphao, A.; Kalcher, K.; Leitinger, G.; Wernitznig, S.; Ortner, A. Development of a cobalt(II) phthalocyanine- MWCNT modified carbon paste electrode for the detection of polyunsaturated fatty acids. Anal. Chim. Acta 2018, 1038, 52–58. [Google Scholar] [CrossRef]
- Köksoy, B.; Akyüz, D.; Şenocak, A.; Durmuş, M.; Demirbas, E. Sensitive, simple and fast voltammetric determination of pesticides in juice samples by novel BODIPY-phthalocyanine-SWCNT hybrid platform. Food Chem. Toxicol. 2021, 147, 111886. [Google Scholar] [CrossRef]
- Canevari, T.; Prado, T.M.; Cincotto, F.H.; Machado, S.A. Immobilization of ruthenium phthalocyanine on silica-coated multi-wall partially oriented carbon nanotubes: Electrochemical detection of fenitrothion pesticide. Mater. Res. Bull. 2016, 76, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.-J.; Pan, M.-F.; Fang, G.-Z.; He, X.-L.; Xia, Y.-Q.; Wang, S. Electrochemical sensor based on a bilayer of PPY–MWCNTs–BiCoPc composite and molecularly imprinted PoAP for sensitive recognition and determination of metolcarb. RSC Adv. 2015, 5, 11498–11505. [Google Scholar] [CrossRef]
- Kunpatee, K.; Chamsai, P.; Mehmeti, E.; Stanković, D.; Ortner, A.; Kalcher, K.; Samphao, A. A highly sensitive fenobucarb electrochemical sensor based on graphene nanoribbons-ionic liquid-cobalt phthalocyanine composites modified on screen-printed carbon electrode coupled with a flow injection analysis. J. Electroanal. Chem. 2019, 855, 113630. [Google Scholar] [CrossRef]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.; Brett, C.M. Direct electrochemical determination of carbaryl using a multi-walled carbon nanotube/cobalt phthalocyanine modified electrode. Talanta 2009, 79, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.S.; Brett, C.M.A. Direct Electrochemical Determination of Glyphosate at Copper Phthalocyanine/Multiwalled Carbon Nanotube Film Electrodes. Electroanalysis 2010, 22, 1586–1591. [Google Scholar] [CrossRef]
- Ribeiro, F.W.P.; Lucas, F.W.D.S.; Mascaro, L.; Morais, S.; Casciano, P.N.D.S.; De Lima-Neto, P.; Correia, A. Electroanalysis of formetanate hydrochloride by a cobalt phthalocyanine functionalized multiwalled carbon nanotubes modified electrode: Characterization and application in fruits. Electrochim. Acta 2016, 194, 187–198. [Google Scholar] [CrossRef]
- Wong, A.; Sotomayor, M. Determination of carbofuran and diuron in FIA system using electrochemical sensor modified with organometallic complexes and graphene oxide. J. Electroanal. Chem. 2014, 731, 163–171. [Google Scholar] [CrossRef]
- Paula, S.A.; Ferreira, O.A.E.; César, P.A. Determination of Imidacloprid Based on the Development of a Glassy Carbon Electrode Modified with Reduced Graphene Oxide and Manganese (II) Phthalocyanine. Electroanalysis 2020, 32, 86–94. [Google Scholar] [CrossRef]
- Şenocak, A.; Tümay, S.O.; Makhseed, S.; Demirbas, E.; Durmuş, M. A synergetic and sensitive physostigmine pesticide sensor using copper complex of 3D zinc (II) phthalocyanine-SWCNT hybrid material. Biosens. Bioelectron. 2021, 174, 112819. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, F.I.; Colesniuc, C.N.; Park, J.; Ruidiaz, M.E.; Schuller, I.K.; Kummel, A.C.; Trogler, W. Comparative Gas Sensing in Cobalt, Nickel, Copper, Zinc, and Metal-Free Phthalocyanine Chemiresistors. J. Am. Chem. Soc. 2009, 131, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.; Kong, X.; Imran, M.; Mustafa, G.; Chen, Y. Excellent ambipolar gas sensing response of Eu[Pc(OC4H9)8]2/acidified multiwalled carbon nanotubes hybrid at room temperature. J. Porphyr. Phthalocyanines 2019, 23, 1455–1462. [Google Scholar] [CrossRef]
- Jassim, A.H.M.; Banimuslem, H.A. Characterization and sensing application of modified multi walled carbon nanotubes/metal phthalocyanine thin films. In Proceedings of the AIP Conference Proceedings, the 8th International Conference on Applied Science and Technology (ICAST 2020), Karbala, Iraq, 15–16 April 2020; Volume 2290, pp. 050009-1–050009-9. [Google Scholar] [CrossRef]
- Ridhi, R.; Gautam, S.; Saini, G.S.; Tripathi, S.K.; Rawat, J.S.; Jha, P. Amendment in Sensing Response of Single Walled Carbon Nanotube (SWCNT) Towards Ammonia Gas with Copper Phthalocyanine Functionalization. Mater. Today Proc. 2020, 28, 1759–1763. [Google Scholar] [CrossRef]
- Bonegardt, D.; Klyamer, D.; Köksoy, B.; Durmuş, M.; Basova, T. Hybrid materials of carbon nanotubes with fluoroalkyl- and alkyl-substituted zinc phthalocyanines. J. Mater. Sci. Mater. Electron. 2020, 31, 11021–11028. [Google Scholar] [CrossRef]
- Kang, D.; Wang, B.; Wang, X.; Li, Y.; Chen, Z.; He, C.; Wu, Y. Stably dispersed metallophthalocyanine noncovalently bonded to multiwalled carbon nanotubes for ammonia sensing at room temperature. Sens. Actuators B Chem. 2017, 246, 262–270. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Z.; Wu, H.; Guo, L.; He, C.; Wang, B.; Wu, Y. Enhanced NH 3 -sensing behavior of 2,9,16,23-tetrakis(2,2,3,3-tetrafluoropropoxy) metal(II) phthalocyanine/multi-walled carbon nanotube hybrids: An investigation of the effects of central metals. Carbon 2014, 80, 268–278. [Google Scholar] [CrossRef]
- Kaya, E.N.; Basova, T.; Polyakov, M.; Durmuş, M.; Kadem, B.; Hassan, A. Hybrid materials of pyrene substituted phthalocyanines with single-walled carbon nanotubes: Structure and sensing properties. RSC Adv. 2015, 5, 91855–91862. [Google Scholar] [CrossRef]
- Polyakov, M.; Basova, T.V. Hybrid Materials of Zinc(II) Tetra-tert-butylphthalocyanine and Zinc(II) Tetra-tert-butylnaphthalocyanine with Single Walled Carbon Nanotubes: Structure and Sensing Properties. Macroheterocycles 2017, 10, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, Y.; Wang, X.; Chen, Z.; He, C. Copper phthalocyanine noncovalent functionalized single-walled carbon nanotube with enhanced NH3 sensing performance. Sens. Actuators B Chem. 2014, 190, 157–164. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, X.; Wu, Y.; Chen, Z.; He, C. Lead phthalocyanine modified carbon nanotubes with enhanced NH3 sensing performance. Sens. Actuators B Chem. 2012, 171–172, 398–404. [Google Scholar] [CrossRef]
- Kareem, M.M.; Kadem, B.Y.; Mohammad, E.J.; Atiyah, A.J. Synthesis, Characterization and Gas Sensor Application of New Composite Based on MWCNTs:CoPc:Metal Oxide. Baghdad Sci. J. 2021, 18, 384. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Zhang, J.; Wu, F.; He, C.; Wu, Y.; Ren, Z. Phthalocyanine-mediated non-covalent coupling of carbon nanotubes with polyaniline for ultrafast NH3 gas sensors. J. Mater. Chem. A 2017, 5, 24493–24501. [Google Scholar] [CrossRef]
- Dehsari, H.S.; Gavgani, J.N.; Hasani, A.; Mahyari, M.; Shalamzari, E.K.; Salehi, A.; Taromi, F.A. Copper(ii) phthalocyanine supported on a three-dimensional nitrogen-doped graphene/PEDOT-PSS nanocomposite as a highly selective and sensitive sensor for ammonia detection at room temperature. RSC Adv. 2015, 5, 79729–79737. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, B.; Wang, X.; Li, Y.; Gai, S.; Wu, Y.; Cheng, X. A high-sensitive room temperature gas sensor based on cobalt phthalocyanines and reduced graphene oxide nanohybrids for the ppb-levels of ammonia detection. RSC Adv. 2019, 9, 37518–37525. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Wang, X.; Zhou, X.; Chen, Z.; He, C.; Yu, Z.; Wu, Y. Enhanced NH3-Sensitivity of Reduced Graphene Oxide Modified by Tetra-α-Iso-Pentyloxymetallophthalocyanine Derivatives. Nanoscale Res. Lett. 2015, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, X.; Li, X.; Guo, Z.; Zhou, X.; Wu, Y. The effects of amino substituents on the enhanced ammonia sensing performance of PcCo/rGO hybrids. RSC Adv. 2018, 8, 41280–41287. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, X.; Wang, B.; Chen, Z.; He, C.; Wu, Y. Preparation, characterization and NH3-sensing properties of reduced graphene oxide/copper phthalocyanine hybrid material. Sens. Actuators B Chem. 2014, 193, 340–348. [Google Scholar] [CrossRef]
- Brunet, J.; Pauly, A.; Dubois, M.; Rodriguez-Mendez, M.L.; Ndiaye, A.L.; Varenne, C.; Guérin, K. Improved selectivity towards NO2 of phthalocyanine-based chemosensors by means of original indigo/nanocarbons hybrid material. Talanta 2014, 127, 100–107. [Google Scholar] [CrossRef]
- Jha, P.; Sharma, M.; Chouksey, A.; Chaturvedi, P.; Kumar, D.; Upadhyaya, G.; Rawat, J.; Chaudhury, P. Functionalization of Carbon Nanotubes With Metal Phthalocyanine For SELECTIVE Gas Sensing Application. Synth. React. Inoorg. Met. Chem. 2014, 44, 1551–1557. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, Z.; Hu, X. Multivariate nanocomposites for electrochemical sensing in the application of food. TrAC Trends Anal. Chem. 2019, 118, 759–769. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, X.; Wang, T.; Li, B.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; Zhou, Z.; Yang, Z. Enhancing room-temperature NO2 gas sensing performance based on a metal phthalocyanine/graphene quantum dot hybrid material. RSC Adv. 2021, 11, 5618–5628. [Google Scholar] [CrossRef]
- Xu, H.; Liao, C.; Liu, Y.; Ye, B.-C.; Liu, B. Iron Phthalocyanine Decorated Nitrogen-Doped Graphene Biosensing Platform for Real-Time Detection of Nitric Oxide Released from Living Cells. Anal. Chem. 2018, 90, 4438–4444. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, Y.; Xie, H.; Shu, Q.; Yang, F.; Liu, Y.-L.; Liang, F.; Wang, H.; Huang, W.; Zhang, G.-J. A sensitive acupuncture needle microsensor for real-time monitoring of nitric oxide in acupoints of rats. Sci. Rep. 2017, 7, 6446. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Li, Y.-T.; Lei, Y.-M.; Liu, Y.-L.; Xiao, M.-M.; Gao, C.; Pang, D.-W.; Huang, W.-H.; Zhang, Z.-Y.; Zhang, G.-J. Real-Time Monitoring of Nitric Oxide at Single-Cell Level with Porphyrin-Functionalized Graphene Field-Effect Transistor Biosensor. Anal. Chem. 2016, 88, 11115–11122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Li, S.; Zhou, S.; Ren, N.; Ge, S.; Zhang, Y.; Wang, Y.; Yu, J. In situ grown COFs on 3D strutted graphene aerogel for electrochemical detection of NO released from living cells. Chem. Eng. J. 2021, 420, 127559. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Kang, S.; Gu, Z. Selective Voltammetric Determination of Nitrite Using Cobalt Phthalocyanine Modified on Multiwalled Carbon Nanotubes. J. Electrochem. Soc. 2020, 167, 046515. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Balamurugan, A.; Lai, Y.-H.; Ho, K.-C. A novel poly(3,4-ethylenedioxythiophene)/iron phthalocyanine/multi-wall carbon nanotubes nanocomposite with high electrocatalytic activity for nitrite oxidation. Talanta 2010, 82, 1905–1911. [Google Scholar] [CrossRef]
- Li, P.; Ding, Y.; Wang, A.; Zhou, L.; Wei, S.; Zhou, Y.; Tang, Y.; Chen, Y.; Cai, C.; Lu, T. Self-Assembly of Tetrakis (3-Trifluoromethylphenoxy) Phthalocyaninato Cobalt(II) on Multiwalled Carbon Nanotubes and Their Amperometric Sensing Application for Nitrite. ACS Appl. Mater. Interfaces 2013, 5, 2255–2260. [Google Scholar] [CrossRef]
- Rębiś, T.; Falkowski, M.; Kryjewski, M.; Popenda, L.; Sobotta, L.; Jurga, S.; Marszall, M.P.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Single-walled carbon nanotube/sulfanyl porphyrazine hybrids deposited on glassy carbon electrode for sensitive determination of nitrites. Dye Pigment. 2019, 171, 107660. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, N.; Sharma, A.K.; Mahajan, A.; Bedi, R.K. Improved Cl2 sensing characteristics of reduced graphene oxide when decorated with copper phthalocyanine nanoflowers. RSC Adv. 2017, 7, 25229–25236. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sharma, A.K.; Sohal, M.K.; Sharma, D.P.; Debnath, A.; Aswal, D.; Mahajan, A. Room temperature highly sensitive chlorine sensor based on reduced graphene oxide anchored with substituted copper phthalocyanine. Sens. Actuators B Chem. 2021, 327, 128925. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. CNTs based improved chlorine sensor from non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated cobalt phthalocyanines. RSC Adv. 2017, 7, 49675–49683. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Mahajan, A.; Saini, R.; Bedi, R.; Kumar, S.; Debnath, A.; Aswal, D. Reversible and fast responding ppb level Cl2 sensor based on noncovalent modified carbon nanotubes with Hexadecafluorinated copper phthalocyanine. Sens. Actuators B Chem. 2018, 255, 87–99. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.; Kumar, S.; Debnath, A.; Aswal, D. Non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated zinc phthalocyanine as ppb level chemiresistive chlorine sensor. Appl. Surf. Sci. 2018, 427, 202–209. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Tailoring of the chlorine sensing properties of substituted metal phthalocyanines non-covalently anchored on single-walled carbon nanotubes. RSC Adv. 2018, 8, 32719–32730. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, X.; Guo, Z.; Gai, S.; Li, Y.; Wu, Y. A highly sensitive ppb-level H2S gas sensor based on fluorophenoxy-substituted phthalocyanine cobalt/rGO hybrids at room temperature. RSC Adv. 2021, 11, 5993–6001. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Zhang, J.; Wu, F.; He, C.; Wang, B.; Wu, Y.; Ren, Z. Stably dispersed carbon nanotubes covalently bonded to phthalocyanine cobalt(ii) for ppb-level H2S sensing at room temperature. J. Mater. Chem. A 2016, 4, 1096–1104. [Google Scholar] [CrossRef]
- Ndiaye, A.L.; Brunet, J.; Varenne, C.; Pauly, A. Functionalized CNTs-Based Gas Sensors for BTX-Type Gases: How Functional Peripheral Groups Can Affect the Time Response through Surface Reactivity. J. Phys. Chem. C 2018, 122, 21632–21643. [Google Scholar] [CrossRef]
- Ndiaye, A.L.; Brunet, J.; Varenne, C.; Bonnet, P.; Pauly, A.; Dubois, M.; Guérin, K.; Lauron, B. Functionalized Carbon Nanotubes-Based Gas Sensors for Pollutants Detection: Investigation on the Use of a Double Transduction Mode. Key Eng. Mater. 2014, 605, 75–78. [Google Scholar] [CrossRef]
- Pauly, A.; Brunet, J.; Varenne, C.; Ndiaye, A. Insight in the interaction mechanisms between functionalized CNTs and BTX vapors in gas sensors: Are the functional peripheral groups the key for selectivity? Sens. Actuators B Chem. 2019, 298, 126768. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, N.; Zhou, Z.; Xu, D.; Wang, Z.; Yang, Z.; Wei, H.; Kong, E.S.-W.; Zhang, Y. Single-walled carbon nanotube/cobalt phthalocyanine derivative hybrid material: Preparation, characterization and its gas sensing properties. J. Mater. Chem. 2011, 21, 3779–3787. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, M.; Wang, T.; Chen, X.; Zeng, M.; Yang, J.; Zhou, Z.; Hu, N.; Su, Y.; Yang, Z. Room temperature DMMP gas sensing based on cobalt phthalocyanine derivative/graphene quantum dot hybrid materials. RSC Adv. 2021, 11, 14805–14813. [Google Scholar] [CrossRef]
- Tatu, G.L.A.; Van Staden, J.F. Phthalocyanine Modified Electrodes Based on Reduced Graphene Oxide for Determination of Lead in Different Types of Water. Rev. Roum. Chim. 2019, 64, 887–892. [Google Scholar] [CrossRef]
- Van Staden, J.F.; Tatu, G.-L.A. Modified graphite/graphene dot microsensors for the assay of trace amounts of lead and cadmium in water catchments areas using differential pulse anodic stripping voltammetry. Rev. Roum. Chim. 2019, 64, 867–877. [Google Scholar] [CrossRef]
- De Oliveira, D.P.C.; Ribeiro, F.W.P.; Becker, H.; De Lima-Neto, P.; Correia, A. An Electrochemical Biosensor Based on The Tyrosinase Enzyme For The Determination Of Phenol In Wastewater. Química Nova 2015, 38, 924–931. [Google Scholar] [CrossRef]
- Hou, K.; Huang, L.; Qi, Y.; Huang, C.; Pan, H.; Du, M. A bisphenol A sensor based on novel self-assembly of zinc phthalocyanine tetrasulfonic acid-functionalized graphene nanocomposites. Mater. Sci. Eng. C 2015, 49, 640–647. [Google Scholar] [CrossRef]
- Jilani, B.S.; Mruthyunjayachari, C.D.; Malathesh, P.; Sharankumar, T.M.; Reddy, K.V. Electrochemical sensing based MWCNT-Cobalt tetra substituted sorbaamide phthalocyanine onto the glassy carbon electrode towards the determination of 2-Amino phenol: A voltammetric study. Sens. Actuators B Chem. 2019, 301, 127078. [Google Scholar] [CrossRef]
- Silva, S.M.; de Oliveira, F.M.; Justino, D.D.; Kubota, L.T.; Tanaka, A.A.; Damos, F.S.; Luz, R.D.C.S. A Novel Sensor Based on Manganese azo-Macrocycle/Carbon Nanotubes to Perform the Oxidation and Reduction Processes of Two Diphenol Isomers. Electroanalysis 2014, 26, 602–611. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Guo, L.; Wang, Y.; Du, S.; Wu, Y.; Ren, Z. Direct Coupling of Phthalocyanine Cobalt(II) and Graphene via Self-Driven Layer-by-Layer Assembly for Efficient Electrochemical Detection of Catechol. J. Electrochem. Soc. 2020, 167, 027533. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Q.; Xia, Z.; Gui, G.; Deng, F. Fulvic Acid Reduced GO and Phthalocyanine Nanorods as Reaction Platform for Simultaneous Determination of Catechol, Hydroquinone, Phenol and p-nitrophenol. J. Electrochem. Soc. 2019, 166, B1293–B1299. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.M.; Manibalan, K.; Huang, S.T. Determination of 4-Nitrophenol at Iron Phthalocy-anine Decorated Graphene Nanosheets Film Modified Electrode. Int. J. Electrochem. Sci. 2015, 10, 1384–1392. [Google Scholar]
- Cesarino, I.; Moraes, F.C.; Ferreira, T.; Lanza, M.; Machado, S.A. Real-time electrochemical determination of phenolic compounds after benzene oxidation. J. Electroanal. Chem. 2012, 672, 34–39. [Google Scholar] [CrossRef]
- Nemakal, M.; Aralekallu, S.; Mohammed, I.; Swamy, S.; Sannegowda, L.K. Electropolymerized octabenzimidazole phthalocyanine as an amperometric sensor for hydrazine. J. Electroanal. Chem. 2019, 839, 238–246. [Google Scholar] [CrossRef]
- Rebis, T.; Lijewski, S.; Nowicka, J.; Popenda, L.; Sobotta, L.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Electrochemical properties of metallated porphyrazines possessing isophthaloxybutylsulfanyl substituents: Application in the electrocatalytic oxidation of hydrazine. Electrochim. Acta 2015, 168, 216–224. [Google Scholar] [CrossRef]
- Mani, V.; Ezhil Vilian, A.T.; Chen, S.M. Graphene Oxide Dispersed Carbon Nanotube and Iron Phthalocyanine Composite Modified Electrode for the Electrocatalytic Determination of Hydrazine. Int. J. Electrochem. Sci. 2012, 7, 12774–12785. [Google Scholar]
- Gündoğan-Paul, M.; Çelebi, S.S.; Özyörük, H.; Yıldız, A. Amperometric enzyme electrode for organic peroxides determination prepared from horseradish peroxidase immobilized in poly(vinylferrocenium) film. Biosens. Bioelectron. 2002, 17, 875–881. [Google Scholar] [CrossRef]
- Wang, H.; Bu, Y.; Dai, W.; Li, K.; Wang, H.; Zuo, X. Well-dispersed cobalt phthalocyanine nanorods on graphene for the electrochemical detection of hydrogen peroxide and glucose sensing. Sens. Actuators B Chem. 2015, 216, 298–306. [Google Scholar] [CrossRef]
- Mashazi, P.; Mugadza, T.; Sosibo, N.; Mdluli, P.; Vilakazi, S.; Nyokong, T. The effects of carbon nanotubes on the electrocatalysis of hydrogen peroxide by metallo-phthalocyanines. Talanta 2011, 85, 2202–2211. [Google Scholar] [CrossRef]
- Pillay, J.; Ozoemena, K.I. Layer-by-layer self-assembled nanostructured phthalocyaninatoiron(II)/SWCNT-poly(m-aminobenzenesulfonic acid) hybrid system on gold surface: Electron transfer dynamics and amplification of H2O2 response. Electrochim. Acta 2009, 54, 5053–5059. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Wu, H.; Wu, F.; He, C.; Wang, B.; Wu, Y.; Ren, Z. An electrochemical bifunctional sensor for the detection of nitrite and hydrogen peroxide based on layer-by-layer multilayer films of cationic phthalocyanine cobalt(ii) and carbon nanotubes. J. Mater. Chem. B 2016, 4, 1310–1317. [Google Scholar] [CrossRef]
- Falkowski, M.; Rebis, T.; Piskorz, J.; Popenda, L.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Improved electrocatalytic response toward hydrogen peroxide reduction of sulfanyl porphyrazine/multiwalled carbon nanotube hybrids deposited on glassy carbon electrodes. Dye Pigment. 2016, 134, 569–579. [Google Scholar] [CrossRef]
- Gorduk, O.; Gorduk, S.; Sahin, Y. Fabrication of Tetra-Substituted Copper(II) Phthalocyanine-Graphene Modified Pencil Graphite Electrode for Amperometric Detection of Hydrogen Peroxide. ECS J. Solid State Sci. Technol. 2020, 9, 061003. [Google Scholar] [CrossRef]
- Yu, Z.; Zou, L.; Chen, Y.; Jiang, J. (Pc)Eu(Pc)Eu[trans-T(COOCH3)2PP]/GO Hybrid Film-Based Nonenzymatic H2O2 Electrochemical Sensor with Excellent Performance. ACS Appl. Mater. Interfaces 2016, 8, 30398–30406. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qian, J.; Wang, K.; Yang, X.; Dong, X.; Qiu, B. Fabrication of multifunctional magnetic FePc@Fe3O4/reduced graphene oxide nanocomposites as biomimetic catalysts for organic peroxide sensing. J. Electroanal. Chem. 2013, 693, 79–85. [Google Scholar] [CrossRef]
- Cui, L.; Chen, L.; Xu, M.; Su, H.; Ai, S. Nonenzymatic amperometric organic peroxide sensor based on nano-cobalt phthalocyanine loaded functionalized graphene film. Anal. Chim. Acta 2012, 712, 64–71. [Google Scholar] [CrossRef]
- Silva, N.; Castro-Castillo, C.; Oyarzún, M.; Ramírez, S.; Gutierrez-Ceron, C.; Marco, J.; Silva, J.; Zagal, J. Modulation of the electrocatalytic activity of Fe phthalocyanine to carbon nanotubes: Electrochemistry of l-cysteine and l-cystine. Electrochim. Acta 2019, 308, 295–306. [Google Scholar] [CrossRef]
- Falkowski, M.; Rebis, T.; Kryjewski, M.; Popenda, L.; Lijewski, S.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. An enhanced electrochemical nanohybrid sensing platform consisting of reduced graphene oxide and sulfanyl metalloporphyrazines for sensitive determination of hydrogen peroxide and l -cysteine. Dye Pigment. 2017, 138, 190–203. [Google Scholar] [CrossRef]
- Koczorowski, T.; Rębiś, T.; Szczolko, W.; Antecka, P.; Teubert, A.; Milczarek, G.; Goslinski, T. Reduced graphene oxide/iron(II) porphyrazine hybrids on glassy carbon electrode for amperometric detection of NADH and L-cysteine. J. Electroanal. Chem. 2019, 848, 848. [Google Scholar] [CrossRef]
- Mani, V.; Huang, S.-T.; Devasenathipathy, R.; Yang, T.C.K. Electropolymerization of cobalt tetraamino-phthalocyanine at reduced graphene oxide for electrochemical determination of cysteine and hydrazine. RSC Adv. 2016, 6, 38463–38469. [Google Scholar] [CrossRef]
- Indherjith, S.; Selvakumar, K. Combining cross-reactivity of an electrode array with the selective thiol reporting process of redox indicators: Targeted sensing of biothiols. Anal. Methods 2018, 10, 3602–3615. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, J.; Liu, B.; Griveau, S.; Bedioui, F. Enhanced electrochemical sensing of thiols based on cobalt phthalocyanine immobilized on nitrogen-doped graphene. Biosens. Bioelectron. 2015, 66, 438–444. [Google Scholar] [CrossRef]
- Hosseini, H.; Mahyari, M.; Bagheri, A.; Shaabani, A. A novel bioelectrochemical sensing platform based on covalently attachment of cobalt phthalocyanine to graphene oxide. Biosens. Bioelectron. 2014, 52, 136–142. [Google Scholar] [CrossRef]
- Moya, P.M.O.; Alfaro, M.M.; Kazemi, R.; Alpuche-Avilés, M.A.; Griveau, S.; Bedioui, F.; Granados, S.G. Simultaneous Electrochemical Speciation of Oxidized and Reduced Glutathione. Redox Profiling of Oxidative Stress in Biological Fluids with a Modified Carbon Electrode. Anal. Chem. 2017, 89, 10726–10733. [Google Scholar] [CrossRef] [PubMed]
- Luz, R.C.S.; Maroneze, C.; Tanaka, A.; Kubota, L.T.; Gushikem, Y.; Damos, F.S. The electrocatalytic activity of a supramolecular assembly of CoTsPc/FeT4MPyP on multi-walled carbon nanotubes towards L-glutathione, and its determination in human erythrocytes. Microchim. Acta 2010, 171, 169–178. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.M.; Manibalan, K.; Huang, S.T. Determination of L-Cysteine at Iron Tetrasulfonated Phthalocyanine Decorated Multiwalled Carbon Nanotubes Film Modified Electrode. Int. J. Electrochem. Sci. 2015, 10, 682–690. [Google Scholar]
- Kang, T.-F.; Shen, G.-L.; Yu, R.-Q. Voltammetric behaviour of dopamine at nickel phthalocyanine polymer modified electrodes and analytical applications. Anal. Chim. Acta 1997, 354, 343–349. [Google Scholar] [CrossRef]
- Siqueira, J.J.R.; Gasparotto, L.; Oliveira, J.O.N.; Zucolotto, V. Processing of Electroactive Nanostructured Films Incorporating Carbon Nanotubes and Phthalocyanines for Sensing. J. Phys. Chem. C 2008, 112, 9050–9055. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, J.; Yan, L.; Zhu, L.; Liu, B. An electrochemical sensor for selective detection of dopamine based on nickel tetrasulfonated phthalocyanine functionalized nitrogen-doped graphene nanocomposites. J. Electroanal. Chem. 2016, 779, 92–98. [Google Scholar] [CrossRef]
- Diab, N.; Morales, D.M.; Andronescu, C.; Masoud, M.; Schuhmann, W. A sensitive and selective graphene/cobalt tetrasulfonated phthalocyanine sensor for detection of dopamine. Sens. Actuators B Chem. 2019, 285, 17–23. [Google Scholar] [CrossRef]
- Moraes, F.C.; Cabral, M.F.; Machado, S.A.S.; Mascaro, L.H. Electrocatalytic Behavior of Glassy Carbon Electrodes Modified with Multiwalled Carbon Nanotubes and Cobalt Phthalocyanine for Selective Analysis of Dopamine in Presence of Ascorbic Acid. Electroanalysis 2008, 20, 851–857. [Google Scholar] [CrossRef]
- Sameenoi, Y.; Mensack, M.M.; Boonsong, K.; Ewing, R.; Dungchai, W.; Chailapakul, O.; Cropek, D.M.; Henry, C.S. Poly(dimethylsiloxane) cross-linked carbon paste electrodes for microfluidic electrochemical sensing. Analysis 2011, 136, 3177–3184. [Google Scholar] [CrossRef]
- de Souza, A.P.R.; Bertotti, M.; Foster, C.W.; Banks, C.E. Back-to-Back Screen-Printed Electroanalytical Sensors: Extending the Potential Applications of the Simplistic Design. Electroanalysis 2015, 27, 2295–2301. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, N.; Li, K.; Bu, Y.; Le Dai, W.; Zuo, X. Multiwalled Carbon Nanotubes Covered with Cobalt (II) Phthalocyanine by In Situ Synthesis and its Electrochemical Sensing Performance towards DA and UA. Mater. Sci. Forum 2014, 809–810, 43–52. [Google Scholar] [CrossRef]
- Patrascu, D.; David, I.; David, V.; Mihailciuc, C.; Stamatin, I.; Ciurea, J.; Nagy, L.; Nagy, G.; Ciucu, A.A. Selective voltammetric determination of electroactive neuromodulating species in biological samples using iron(II) phthalocyanine modified multi-wall carbon nanotubes paste electrode. Sens. Actuators B Chem. 2011, 156, 731–736. [Google Scholar] [CrossRef]
- Ndebele, N.; Sen, P.; Nyokong, T. Electrochemical detection of dopamine using phthalocyanine-nitrogen-doped graphene quantum dot conjugates. J. Electroanal. Chem. 2021, 886, 115111. [Google Scholar] [CrossRef]
- Pari, M.; Reddy, K.V.; Fasiulla, K.B.C. Amperometric determination of dopamine based on an interface platform comprising tetra-substituted Zn2+ phthalocyanine film layer with embedment of reduced graphene oxide. Sens. Actuators A Phys. 2020, 316, 112377. [Google Scholar] [CrossRef]
- Agboola, B.O.; Mocheko, A.; Pillay, J.; Ozoemena, K.I. Nanostructured cobalt phthalocyanine single-walled carbon nanotube platform: Electron transport and electrocatalytic activity on epinephrine. J. Porphyr. Phthalocyanines 2008, 12, 1289–1299. [Google Scholar] [CrossRef]
- Rębiś, T.; Falkowski, M.; Milczarek, G.; Goslinski, T. Electrocatalytic NADH Sensing using Electrodes Modified with 2-[2-(4-Nitrophenoxy)ethoxy]ethylthio-Substituted Porphyrazine/Single-Walled Carbon Nanotube Hybrids. ChemElectroChem 2020, 7, 2838–2850. [Google Scholar] [CrossRef]
- Ribeiro, I.; Yotsumoto-Neto, S.; Dos Santos, W.; Fernandes, R.; Goulart, M.; Damos, F.; Luz, R. Improved NADH Electroanalysis on Nickel(II) Phthalocyanine Tetrasulfonic Acid/ Calf Thymus Deoxyribonucleic Acid/Reduced Graphene Oxide Composite. J. Braz. Chem. Soc. 2017, 28, 1768–1778. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Mensing, J.P.; Phokharatkul, D.; Lomas, T.; Tuantranont, A. Highly selective electrochemical sensor for ascorbic acid based on a novel hybrid graphene-copper phthalocyanine-polyaniline nanocomposites. Electrochim. Acta 2014, 133, 294–301. [Google Scholar] [CrossRef]
- Zuo, X.; Li, N.; Zhang, H. Direct electrochemical determination of ascorbic acid by a cobalt(II) tetra-neopentyloxy phthalocyanine-multi-walled carbon nanotubes glassy carbon electrode. J. Mater. Sci. 2012, 47, 2731–2735. [Google Scholar] [CrossRef]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M. A simple sonochemical approach to fabricate a urea biosensor based on zinc phthalocyanine/graphene oxide/urease bioelectrode. Ultrason. Sonochem. 2018, 42, 183–192. [Google Scholar] [CrossRef]
- Giarola, J.; Pereira, A.C. Development and Application of a Sensor Based on Carbonaceous Materials and Cobalt Phthalocyanine Composite for Electrochemical Determination of Uric Acid. Electroanalysis 2016, 28, 1348–1355. [Google Scholar] [CrossRef]
- Nemakal, M.; Aralekallu, S.; Mohammed, I.; Prabhu C.P., K.; Sannegowda, L.K. Chemisorbed palladium phthalocyanine for simultaneous determination of biomolecules. Microchem. J. 2018, 143, 82–91. [Google Scholar] [CrossRef]
- Balan, I.; David, I.G.; David, V.; Stoica, A.-I.; Mihailciuc, C.; Stamatin, I.; Ciucu, A.A. Electrocatalytic voltammetric determination of guanine at a cobalt phthalocyanine modified carbon nanotubes paste electrode. J. Electroanal. Chem. 2011, 654, 8–12. [Google Scholar] [CrossRef]
- Zhu, X.; Ai, S.; Chen, Q.; Yin, H.; Xu, J. Label-free electrochemical detection of Avian Influenza Virus genotype utilizing multi-walled carbon nanotubes–cobalt phthalocyanine–PAMAM nanocomposite modified glassy carbon electrode. Electrochem. Commun. 2009, 11, 1543–1546. [Google Scholar] [CrossRef]
- Nxele, S.R.; Nyokong, T. The electrochemical detection of prostate specific antigen on glassy carbon electrode modified with combinations of graphene quantum dots, cobalt phthalocyanine and an aptamer. J. Inorg. Biochem. 2021, 221, 111462. [Google Scholar] [CrossRef]
- Al-Ogaidi, A.J.M.; Staden, R.-I.S.-V.; Gugoasa, L.A.; Rosu, M.-C.; Socaci, C. Electrochemical Determination of the KRAS Genetic Marker for Colon Cancer with Modified Graphete and Graphene Paste Electrodes. Anal. Lett. 2018, 51, 2822–2834. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, S.; Xu, G.; Peng, Y.; Gong, L.; Li, X.; Li, Y.; Lin, Y.; Chen, G. Highly photoactive heterojunction based on g-C3N4 nanosheets decorated with dendritic zinc(ii) phthalocyanine through axial coordination and its ultrasensitive enzyme-free sensing of choline. RSC Adv. 2014, 4, 58226–58230. [Google Scholar] [CrossRef]
- Claussen, J.C.; Kumar, A.; Jaroch, D.B.; Khawaja, M.H.; Hibbard, A.B.; Porterfield, D.M.; Fisher, T.S. Nanostructuring Platinum Nanoparticles on Multilayered Graphene Petal Nanosheets for Electrochemical Biosensing. Adv. Funct. Mater. 2012, 22, 3399–3405. [Google Scholar] [CrossRef]
- Chaiyo, S.; Mehmeti, E.; Siangproh, W.; Hoang, T.L.; Nguyen, H.P.; Chailapakul, O.; Kalcher, K. Non-enzymatic electrochemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine–ionic liquid–graphene composite. Biosens. Bioelectron. 2018, 102, 113–120. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Chen, D.; Meng, X.; Tang, F.; Du, A.; Zhang, L. Amperometric glucose biosensor based on a gold nanorods/cellulose acetate composite film as immobilization matrix. Colloids Surfaces B Biointerfaces 2009, 72, 188–192. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Y.; Kan, J. A noninterference polypyrrole glucose biosensor. Biosens. Bioelectron. 2006, 22, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xue, H.; Zhang, Y.; Shen, Z. A glucose biosensor based on microporous polyacrylonitrile synthesized by single rare-earth catalyst. Biosens. Bioelectron. 2002, 17, 541–545. [Google Scholar] [CrossRef]
- Mani, V.; Devasenathipathy, R.; Chen, S.-M.; Huang, S.-T.; Vasantha, V. Immobilization of glucose oxidase on graphene and cobalt phthalocyanine composite and its application for the determination of glucose. Enzym. Microb. Technol. 2014, 66, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Rattanarat, P.; Teengam, P.; Siangproh, W.; Ishimatsu, R.; Nakano, K.; Chailapakul, O.; Imato, T. An Electrochemical Compact Disk-type Microfluidics Platform for Use as an Enzymatic Biosensor. Electroanalysis 2015, 27, 703–712. [Google Scholar] [CrossRef]
- Al-Sagur, H.; Komathi, S.; Khan, M.; Gurek, A.; Hassan, A. A novel glucose sensor using lutetium phthalocyanine as redox mediator in reduced graphene oxide conducting polymer multifunctional hydrogel. Biosens. Bioelectron. 2017, 92, 638–645. [Google Scholar] [CrossRef]
- Al-Sagur, H.; Sundaram, K.S.; Kaya, E.; Durmuş, M.; Basova, T.; Hassan, A. Amperometric glucose biosensing performance of a novel graphene nanoplatelets-iron phthalocyanine incorporated conducting hydrogel. Biosens. Bioelectron. 2019, 139, 111323. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Fan, Y.-J.; Cheng, L.; Fan, L.-L.; Wang, Z.-Y.; Zhong, J.-P.; Wu, L.-N.; Shen, X.-C.; Shi, Z.-J. A novel glucose biosensor based on the immobilization of glucose oxidase on layer-by-layer assembly film of copper phthalocyanine functionalized graphene. Electrochim. Acta 2013, 104, 178–184. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Karuppiah, C.; Chen, S.-M.; Palanisamy, S.; Lou, B.-S.; Ali, M.A.; Al-Hemaid, F.M.A. A sensitive and selective enzyme-free amperometric glucose biosensor using a composite from multi-walled carbon nanotubes and cobalt phthalocyanine. RSC Adv. 2015, 5, 26762–26768. [Google Scholar] [CrossRef]
- Kang, J.-S.; Lee, M.-H. Overview of Therapeutic Drug Monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- de Holanda, L.F.; Ribeiro, F.W.P.; Sousa, C.P.; Casciano, P.N.D.S.; de Lima-Neto, P.; Correia, A.N. Multi-walled carbon nanotubes–cobalt phthalocyanine modified electrode for electroanalytical determination of acetaminophen. J. Electroanal. Chem. 2016, 772, 9–16. [Google Scholar] [CrossRef]
- Kantize, K.; Booysen, I.N.; Mambanda, A. Electrochemical sensing of acetaminophen using nanocomposites comprised of cobalt phthalocyanines and multiwalled carbon nanotubes. J. Electroanal. Chem. 2019, 850, 113391. [Google Scholar] [CrossRef]
- Andrei, C.C.; Bala, D.; Ciucu, A.A.; Ciurea, A.; Mihailciuc, C. Electrochemical Determination of L-Dopa in Pharmaceutical Samples Using Metallophtalocyanines Modified Carbon Nanotubes Paste Electrodes. Rev. Roum. Chim. 2014, 59, 835–843. [Google Scholar]
- Aragão, J.S.; Ribeiro, F.W.; Portela, R.R.; Santos, V.N.; Sousa, C.P.; Becker, H.; Correia, A.N.; de Lima-Neto, P. Electrochemical determination diethylstilbestrol by a multi-walled carbon nanotube/cobalt phthalocyanine film electrode. Sens. Actuators B Chem. 2017, 239, 933–942. [Google Scholar] [CrossRef]
- Meenakshi, S.; Kaladevi, G.; Pandian, K.; Wilson, P. Cobalt phthalocyanine tagged graphene nanoflakes for enhanced electrocatalytic detection of N-acetylcysteine by amperometry method. Ionics 2018, 24, 2807–2819. [Google Scholar] [CrossRef]
- Peng, J.; Huang, Q.; Zhuge, W.; Liu, Y.; Zhang, C.; Yang, W.; Xiang, G. Blue-light photoelectrochemical sensor based on nickel tetra-amined phthalocyanine-graphene oxide covalent compound for ultrasensitive detection of erythromycin. Biosens. Bioelectron. 2018, 106, 212–218. [Google Scholar] [CrossRef]
- Peng, J.; Huang, Q.; Liu, Y.; Liu, F.; Zhang, C.; Huang, Y.; Huang, W. The synthesis of graphene oxide covalently linked with nickel tetraamino phthalocyanine: A photoelectrochemical sensor for the analysis of rifampicin irradiated with blue light. J. Chin. Chem. Soc. 2019, 66, 1311–1317. [Google Scholar] [CrossRef]
- Porto, L.; Da Silva, D.N.; Silva, M.C.; Pereira, A.C. Electrochemical Sensor Based on Multi-walled Carbon Nanotubes and Cobalt Phthalocyanine Composite for Pyridoxine Determination. Electroanalysis 2019, 31, 820–828. [Google Scholar] [CrossRef]
- Spindola, R.F.; Zanin, H.; Macena, C.S.; Contin, A.; Luz, R.; Damos, F.S. Evaluation of a novel composite based on functionalized multi-walled carbon nanotube and iron phthalocyanine for electroanalytical determination of isoniazid. J. Solid State Electrochem. 2016, 21, 1089–1099. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, G.; Wang, X.; Pan, M.; Qian, H.; Liu, H.; Wang, S. Sensitive and selective electrochemical determination of quinoxaline-2-carboxylic acid based on bilayer of novel poly(pyrrole) functional composite using one-step electro-polymerization and molecularly imprinted poly(o-phenylenediamine). Anal. Chim. Acta 2014, 806, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Americo da Silva, T.; Braunger, M.L.; Neris Coutinho, M.A.; Rios do Amaral, L.; Rodrigues, V.; Riul, A. 3D-Printed Graphene Electrodes Applied in an Impedimetric Electronic Tongue for Soil Analysis. Chemosensors 2019, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Guiterrez, A.P.; Granados, S.G.; Ordaz, A.A.; Griveau, S.; Richard, C.; Zagal, J.; Bedioui, F. Preparation and Characterization of Modified Electrodes Based on Carbon Nanotubes/Pyrrole/Cobalt Phthalocyanine for the Development of Hybrid Materials for the Electrochemical Activation of 2-mercaptoethanol. ECS Trans. 2008, 15, 133–141. [Google Scholar] [CrossRef]
- Shaik, M.; Rao, V.; Gupta, M.; Pandey, P. Layer-by-layer self-assembling copper tetrasulfonated phthalocyanine on carbon nanotube modified glassy carbon electrode for electro-oxidation of 2-mercaptoethanol. Thin Solid Films 2012, 526, 256–260. [Google Scholar] [CrossRef]










| Analyzed Drug | Type of CBM | Azaporphyrin Derivative | Type of Electrode | Reference |
|---|---|---|---|---|
| L-dopa | Multi-walled carbon nanotubes (MWCNTs) | Unsubstituted Fe(II)Pc and Co(II) Pc | Carbon-nanotube paste electrode | [149] |
| Diethylstilbestrol—DES(synthetic estrogen) | MWCNTs functionalized with gold nanoparticles | Unsubstituted Co(II)Pc | GCE | [150] |
| N-acetylcysteine | Graphene nanoflakes | Unsubstituted Co(II)Pc | Carbon paste electrode | [151] |
| Erythromycin | GO | Tetraamiono-substituted nickel(II) phthalocyanine | Indium tin oxide coated electrode (photoelectrochemical sensor) | [152] |
| Rifampicin | GO | Tetraamiono-substituted nickel(II) phthalocyanine | Indium tin oxide coated electrode (photoelectrochemical sensor) | [153] |
| Pyridoxine (vitamin B6) | MWCNTs | Unsubstituted Co(II)Pc | Pyrolytic graphite electrode (PGE) | [154] |
| Isoniazid | Graphene-functionalized oxidized MWCNTs | Unsubstituted Fe(II)Pc | GCE | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koczorowski, T.; Cerbin-Koczorowska, M.; Rębiś, T. Azaporphyrins Embedded on Carbon-Based Nanomaterials for Potential Use in Electrochemical Sensing—A Review. Nanomaterials 2021, 11, 2861. https://doi.org/10.3390/nano11112861
Koczorowski T, Cerbin-Koczorowska M, Rębiś T. Azaporphyrins Embedded on Carbon-Based Nanomaterials for Potential Use in Electrochemical Sensing—A Review. Nanomaterials. 2021; 11(11):2861. https://doi.org/10.3390/nano11112861
Chicago/Turabian StyleKoczorowski, Tomasz, Magdalena Cerbin-Koczorowska, and Tomasz Rębiś. 2021. "Azaporphyrins Embedded on Carbon-Based Nanomaterials for Potential Use in Electrochemical Sensing—A Review" Nanomaterials 11, no. 11: 2861. https://doi.org/10.3390/nano11112861
APA StyleKoczorowski, T., Cerbin-Koczorowska, M., & Rębiś, T. (2021). Azaporphyrins Embedded on Carbon-Based Nanomaterials for Potential Use in Electrochemical Sensing—A Review. Nanomaterials, 11(11), 2861. https://doi.org/10.3390/nano11112861