Up-Converting Luminescence and Temperature Sensing of Er3+/Tm3+/Yb3+ Co-Doped NaYF4 Phosphors Operating in Visible and the First Biological Window Range
Abstract
:1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.1.1. Materials
2.1.2. Preparation of NaYF4: 2 mol% Er3+, 20 mol% Yb3+ and NaYF4: 2 mol% Er3+, 0.5 mol% Tm3+, 20 mol% Yb3+ Phosphors
2.2. Instruments
3. Results and Discussions
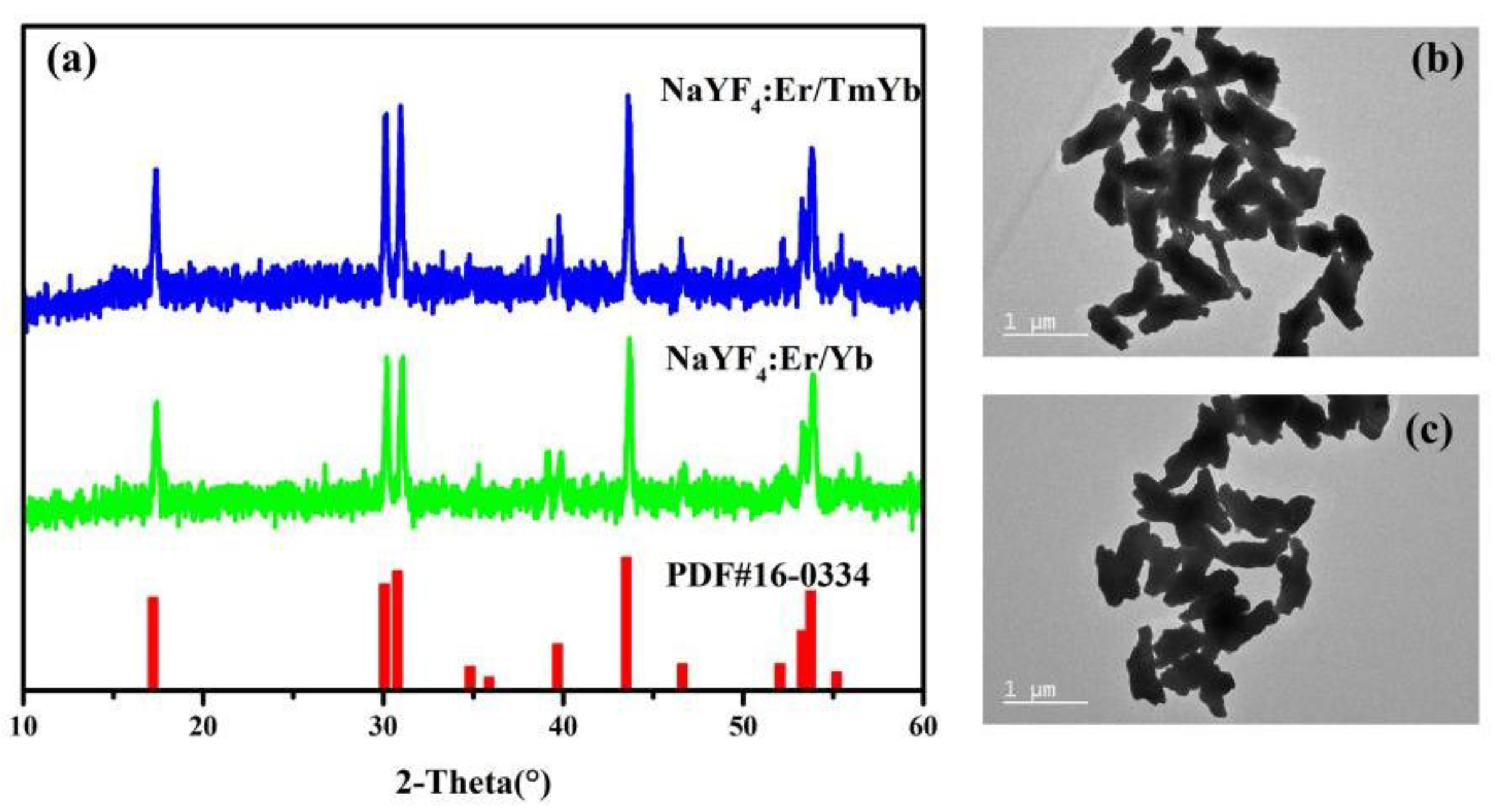
3.1. XRD Analysis
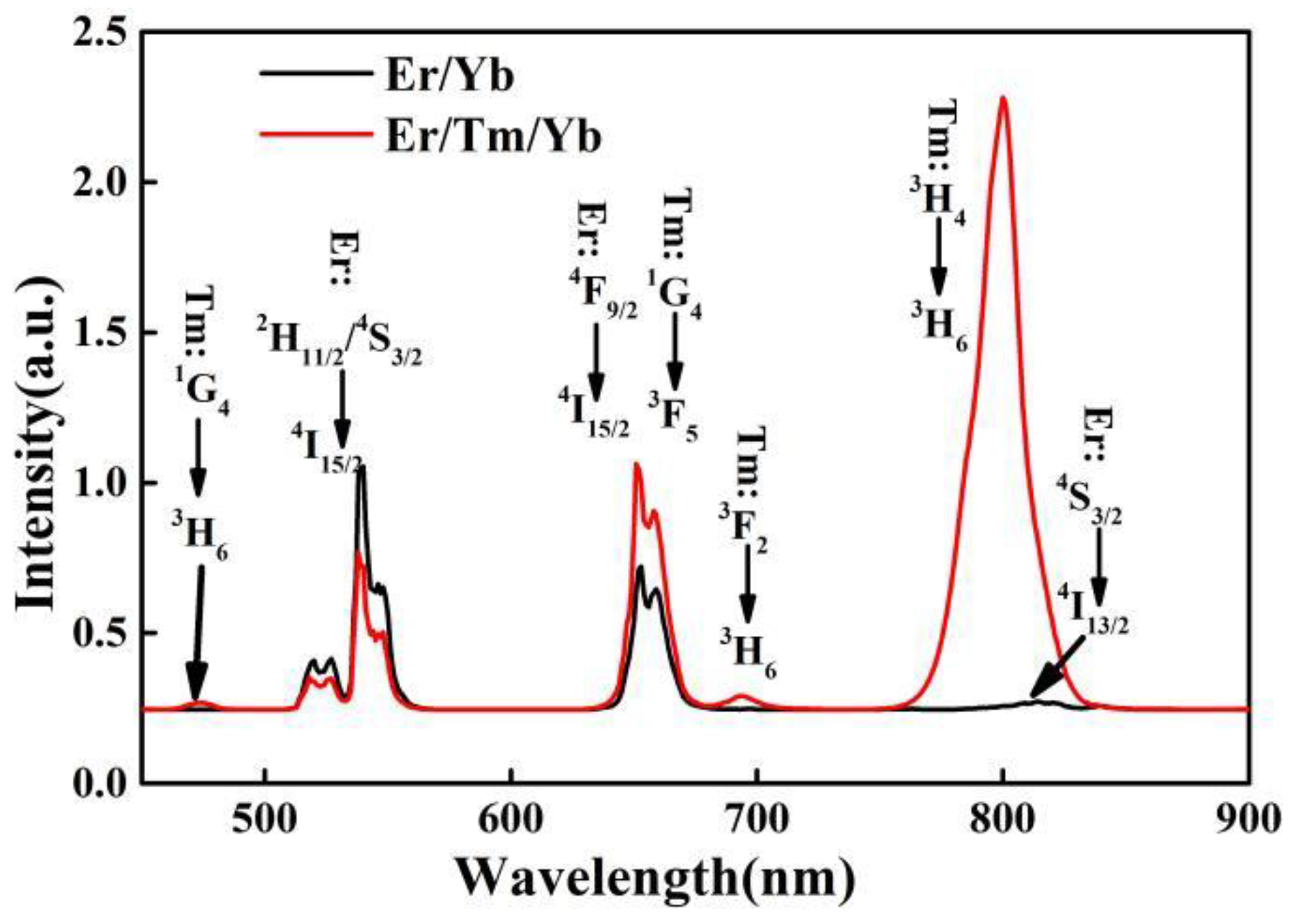
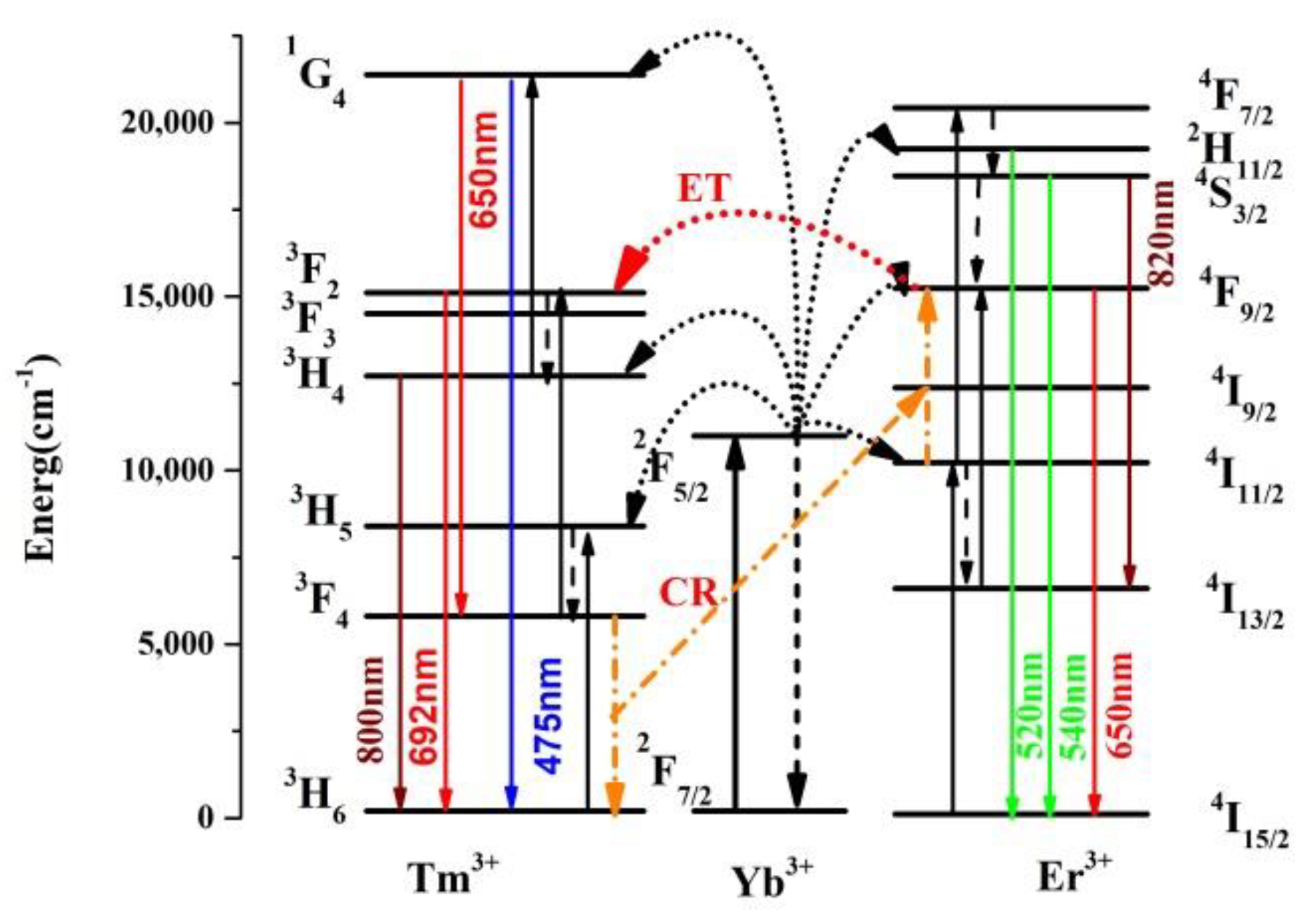
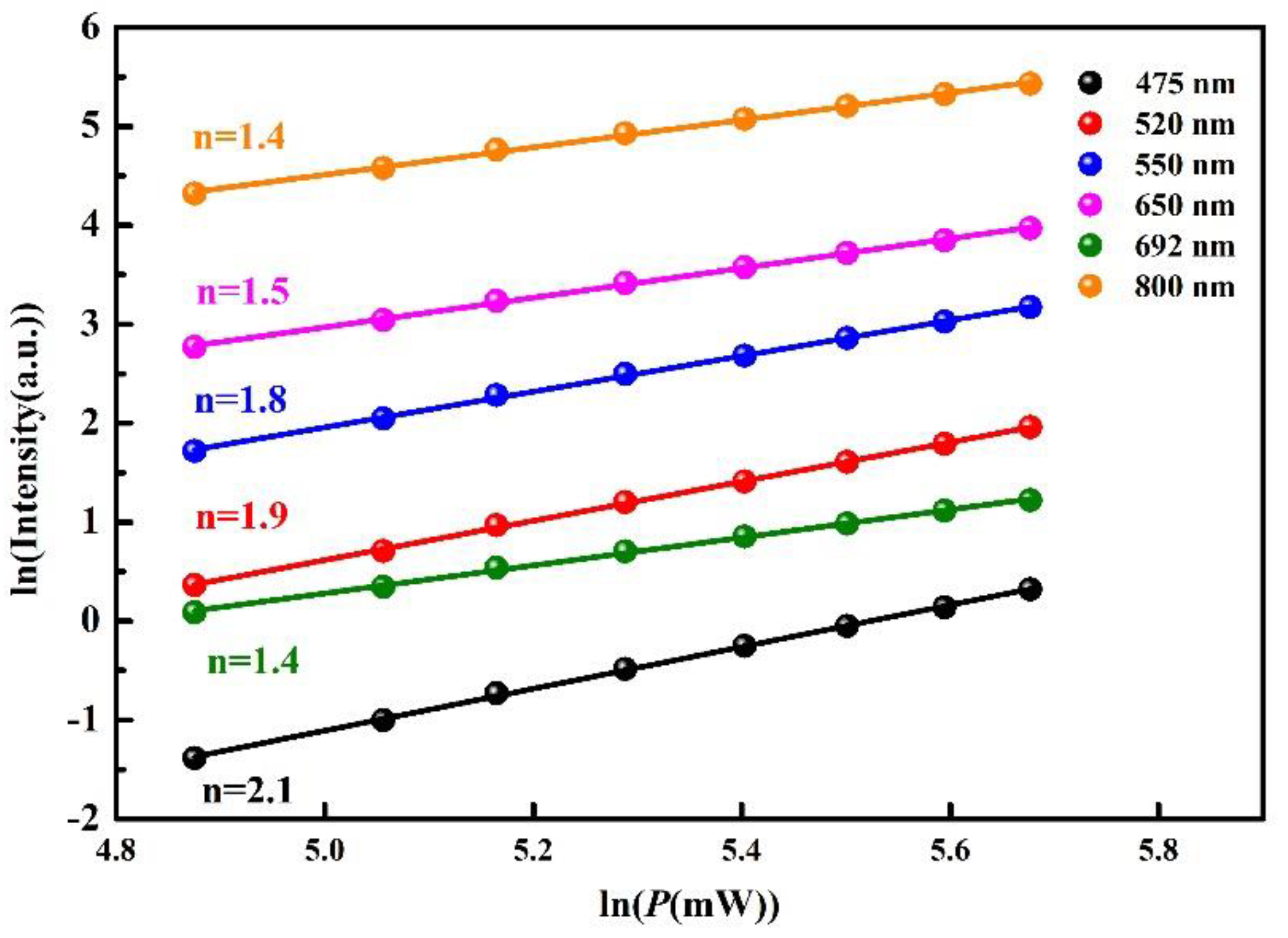
3.2. Power Dependent Up-Conversion Luminescence
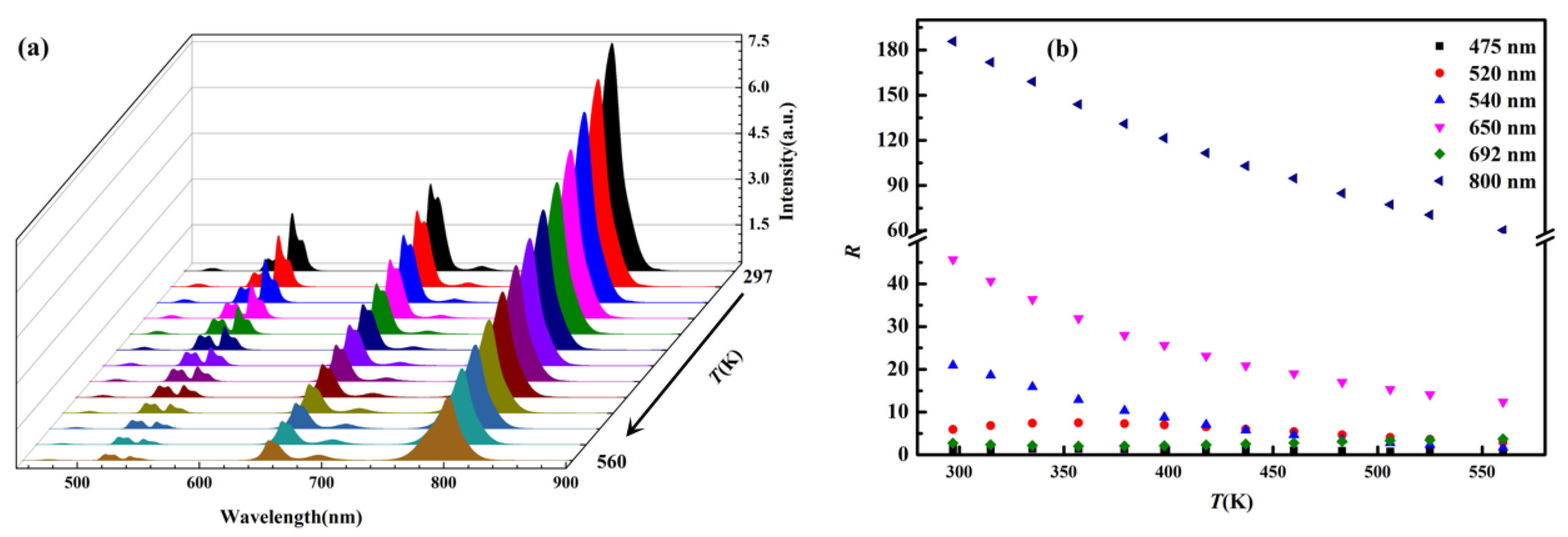
3.3. Temperature-Dependent Up-Conversion Luminescence
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.D.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef]
- Usamentiaga, R.; Venegas, P.; Guerediaga, J.; Vega, L.; Molleda, J.; Bulnes, F.G. Infrared thermography for temperature measurement and non-destructive testing. Sensors 2014, 14, 12305–12348. [Google Scholar] [CrossRef] [Green Version]
- Artlett, C.P.; Pask, H.M. Optical remote sensing of water temperature using Raman spectroscopy. Opt. Express 2015, 23, 31844–31856. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Polakova, K.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zboril, R. Carbon dot nanothermometry: Intracellular photoluminescence lifetime thermal sensing. ACS Nano 2017, 11, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Acosta-Mora, P.; Mendez-Ramos, J.; Fischer, S. Optical nanoprobes for biomedical applications: Shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy. J. Mater. Chem. B 2017, 5, 4365–4392. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, J.; Ma, J.; Liu, Y.; Wang, Y.; Wu, D. Ratiometric nanothermometer based on rhodamine dye-incorporated F127-melamine-formaldehyde polymer nanoparticle: Preparation, characterization, wide-range temperature sensing, and precise intracellular thermometry. ACS Appl. Mater. Interfaces 2016, 8, 14396–14405. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.; Lima, P.P.; Silva, N.J.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Jiang, S.; Wei, X.; Chen, Y.; Duan, C.; Yin, M. Optical thermometry based on upconversion luminescence in Yb3+/Ho3+ co-doped NaLuF4. J. Alloys Compd. 2014, 588, 654–657. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, G.; Li, X.; Jiang, S.; Wei, X.; Chen, Y.; Yin, M.; Duan, C. Strategy for thermometry via Tm3+-doped NaYF4 core-shell nanoparticles. Opt. Lett. 2014, 39, 6687–6690. [Google Scholar] [CrossRef] [PubMed]
- Kusama, H.; Sovers, O.J.; Yoshioka, T. Line shift method for phosphor temperature measurements. Jpn. J. Appl. Phys. 1976, 15, 2349. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Peng, H.; Stich, M.I. Luminescent terbium and europium probes for lifetime based sensing of temperature between 0 and 70 °C. J. Mater. Chem. 2010, 20, 6975–6981. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, H.; Fu, Z. Investigating the luminescence behaviors and temperature sensing properties of rare-earth-doped Ba2In2O5 phosphors. Inorg. Chem. 2018, 57, 8841–8849. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Yu, J.S. Effect of molybdenum on upconversion emission and temperature sensing properties in Na0.5Bi0.5TiO3:Er/Yb ceramics. Ceram. Inter. 2015, 41, 6710–6714. [Google Scholar] [CrossRef]
- Belkhir, N.H.; Toncelli, A.; Parchur, A.K.; Alves, E.; Maalej, R. Efficient temperature sensing using photoluminescence of Er/Yb implanted GaN thin films. Sens. Actuat. B Chem. 2017, 248, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Liu, J.; Pei, L.; Xu, Y.; Xia, Z. Enhanced up-conversion luminescence and optical temperature sensing in graphitic C3N4 quantum dots grafted with BaWO4: Yb3+, Er3+ phosphors. J. Mater. Chem. C 2019, 7, 6112–6119. [Google Scholar] [CrossRef]
- Gao, X.; Song, F.; Ju, D.; Zhou, A.; Khan, A.; Chen, Z.; Feng, M.; Liu, L. Room-temperature ultrafast synthesis, morphology and upconversion luminescence of K0.3Bi0.7F2.4:Yb3+/Er3+ nanoparticles for temperature-sensing application. CrystEngComm 2020, 22, 7066–7074. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Zhang, S.; Zhang, H. Stimuli-responsive nanotheranostics based on lanthanide-doped upconversion nanoparticles for cancer imaging and therapy: Current advances and future challenges. Nano Today 2019, 25, 38–67. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Lu, K.; Wen, S.; Chen, H.; Shahzad, M.K.; Liu, L. Sub-10 nm NaNdF4 nanoparticles as near-infrared photothermal probes with self-temperature feedback. ACS Appl. Nano Mater. 2020, 3, 2517–2526. [Google Scholar] [CrossRef]
- Joshi, R.; Perala, R.S.; Shelar, S.B.; Ballal, A.; Singh, B.P.; Ningthoujam, R.S. Super Bright Red Upconversion in NaErF4:0.5%Tm@NaYF4:20%Yb Nanoparticles for Anti-counterfeit and Bioimaging Applications. ACS Appl. Mater. Interfaces 2020, 13, 3481–3490. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, H.; Fu, Z. Investigation on the up-conversion luminescence and temperature sensing properties based on non-thermally coupled levels of rare earth ions doped Ba2In2O5 phosphor. J. Lumin. 2019, 206, 273–277. [Google Scholar] [CrossRef]
- de Sousa Filho, P.C.; Alain, J.; Lemenager, G.; Larquet, E.; Fick, J.; Serra, O.A.; Gacoin, T. Colloidal rare earth vanadate single crystalline particles as ratiometric luminescent thermometers. J. Phys. Chem. C 2019, 123, 2441–2450. [Google Scholar] [CrossRef]
- Wang, J.; Lin, H.; Cheng, Y.; Cui, X.; Gao, Y.; Ji, Z.; Xu, J.; Wang, Y. A novel high-sensitive upconversion thermometry strategy: Utilizing synergistic effect of dual-wavelength lasers excitation to manipulate electron thermal distribution. Sens. Actuat. B Chem. 2019, 278, 165–171. [Google Scholar] [CrossRef]
- Runowski, M.; Bartkowiak, A.; Majewska, M.; Martín, I.R.; Lis, S. Upconverting lanthanide doped fluoride NaLuF4:Yb3+-Er3+-Ho3+-optical sensor for multi-range fluorescence intensity ratio (FIR) thermometry in visible and NIR regions. J. Lumin. 2018, 201, 104–109. [Google Scholar] [CrossRef]
- Suyver, J.F.; Grimm, J.; Van Veen, M.K.; Biner, D.; Krämer, K.W.; Güdel, H.U. Upconversion spectroscopy and properties of NaYF4 doped with Er3+, Tm3+ and/or Yb3+. J. Lumin. 2006, 117, 1–12. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, G.; Zhu, Y.; Huo, L.; Mao, W.; Zou, D.; Sun, X.; Wu, E.; Zhang, H.; Zhang, J.; et al. Intense multiphoton upconversion of Yb3+-Tm3+ doped β-NaYF4 individual nanocrystals by saturation excitation. J. Mater. Chem. C 2015, 3, 364–369. [Google Scholar] [CrossRef]
- Maurya, A.; Bahadur, A.; Dwivedi, A.; Choudhary, A.K.; Yadav, T.P.; Vishwakarma, P.K.; Rai, S.B. Optical properties of Er3+, Yb3+ co-doped calcium zirconate phosphor and temperature sensing efficiency: Effect of alkali ions (Li+, Na+ and K+). J. Phys. Chem. Solids 2018, 119, 228–237. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, X.; Wang, Y.; Liang, L. Color modulation and temperature sensing investigation of Gd2O3: 1 mol% Er3+, 1 mol% Yb3+ phosphors under different excitation condition. J. Lumin. 2019, 215, 116556. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, C. Electronic structure, upconversion luminescence and optical temperature sensing behavior of Yb3+-Er3+/Ho3+ doped NaLaMgWO6. J. Alloys Compd. 2019, 783, 84–94. [Google Scholar] [CrossRef]
- Lu, H.; Hao, H.; Gao, Y.; Li, D.; Shi, G.; Song, Y.; Wang, Y.; Zhang, X. Optical sensing of temperature based on non-thermally coupled levels and upconverted white light emission of a Gd2(WO4)3 phosphor co-doped with in Ho(III), Tm(III), and Yb(III). Microchim. Acta 2017, 184, 641–646. [Google Scholar] [CrossRef]
- Mikalauskaite, I.; Pleckaityte, G.; Skapas, M.; Zarkov, A.; Katelnikovas, A.; Beganskiene, A. Emission spectra tuning of upconverting NaGdF4: 20%Yb, 2%Er nanoparticles by Cr3+ co-doping for optical temperature sensing. J. Lumin. 2019, 213, 210–217. [Google Scholar] [CrossRef]
- Dantelle, G.; Reita, V.; Delacour, C. Luminescent Yb3+,Er3+-Doped α-La(IO3)3 Nanocrystals for Neuronal Network Bio-Imaging and Nanothermometry. Nanomaterials 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, J.; Li, X. Upconversion luminescence of Ba3La(PO4)3:Yb3+-Er3+/Tm3+ phosphors for optimal temperature sensing. Appl. Opt. 2018, 57, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Stopikowska, N.; Runowski, M.; Woźny, P.; Goderski, S.; Lis, S. Improving temperature resolution of luminescent nanothermometers working in the near-infrared range using non-thermally coupled levels of Yb3+ & Tm3+. J. Lumin. 2020, 228, 117643. [Google Scholar]
- Lu, K.; Yi, Y.; Xu, L.; Sun, X.; Liu, L.; Li, H. Temperature-independent lifetime and thermometer operated in a biological window of upconverting NaErF4 nanocrystals. Nanomaterials 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinichev, A.A.; Kurochkin, M.A.; Kolomytsev, A.Y.; Khasbieva, R.S.; Kolesnikov, E.Y.; Lähderanta, E.; Kolesnikov, I.E. Yb3+/Er3+- codoped GeO2-PbO-PbF2 glass ceramics for ratiometric upconversion temperature sensing based on thermally and non-thermally coupled levels. Opt. Mater. 2019, 90, 200–207. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, D.; Zhu, X.; Zheng, L.; Zhang, Z.; Cao, W. NIR to NIR luminescence thermometry in core/multishells-structured nanoparticles operating in the biological window. J. Lumin. 2020, 225, 117358. [Google Scholar] [CrossRef]
- Chen, G.; Lei, R.; Wang, H.; Huang, F.; Zhao, S.; Xu, S. Temperature-dependent emission color and temperature sensing behavior in Tm3+/Yb3+: Y2O3 nanoparticles. Opt. Mater. 2018, 77, 233–239. [Google Scholar] [CrossRef]
- Chen, S.; Song, W.; Cao, J.; Hu, F.; Guo, H. Highly sensitive optical thermometer based on FIR technique of transparent NaY2F7:Tm3+/Yb3+ glass ceramic. J. Alloys Compd. 2020, 825, 154011. [Google Scholar] [CrossRef]
- Li, Z.; Lin, L.; Feng, Z.; Huang, L.; Wang, Z.; Zheng, Z. Wide-range temperature sensing of NaYF4: Ho3+ nanoparticles by multi-emissions in dual spectral ranges. J. Lumin. 2021, 232, 117873. [Google Scholar] [CrossRef]
- Xia, H.; Lei, L.; Xia, J.; Hua, Y.; Deng, D.; Xu, S. Yb/Er/Tm tri-doped Na3ZrF7 upconversion nanocrystals for high performance temperature sensing. J. Lumin. 2019, 209, 8–13. [Google Scholar] [CrossRef]

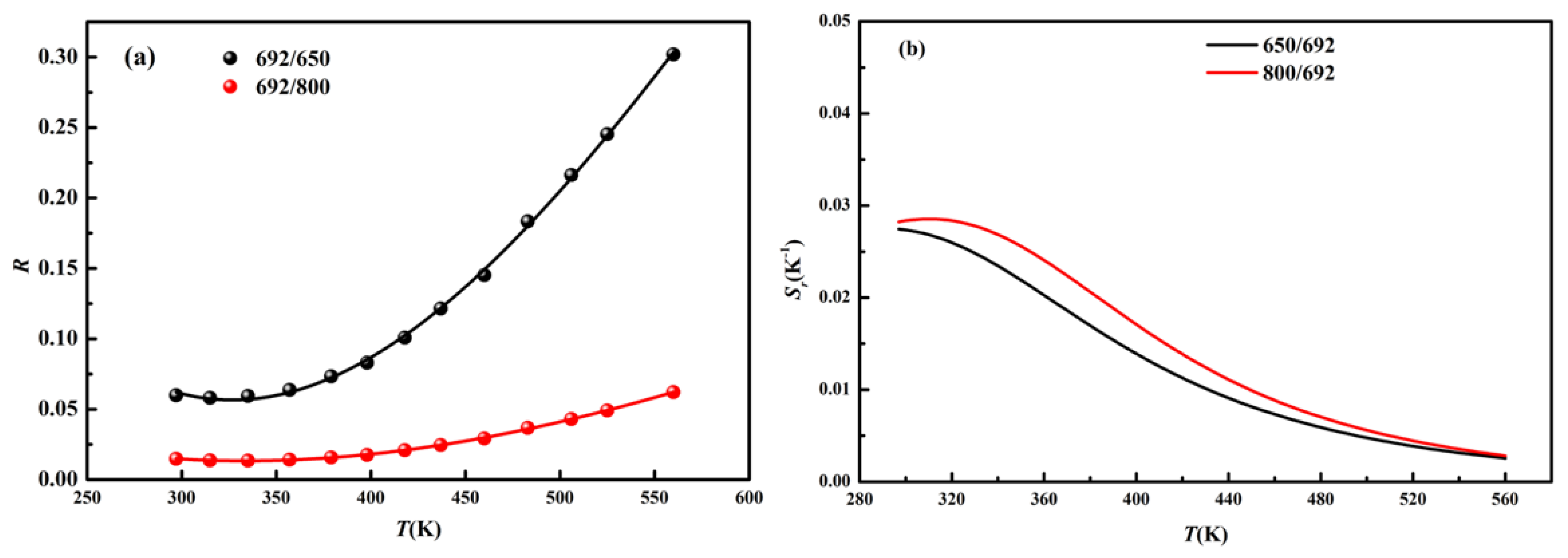
| Parameter | R692/650 | R692/800 |
|---|---|---|
| B0 | 0.94 | 0.17 |
| B1 | −0.0061 | −0.0010 |
| B2 | 1.3 × 10−5 | 1.9 × 10−6 |
| B3 | −6.7 × 10−9 | −7.9 × 10−10 |
| Materials | Wavelengths (nm) | Temperature Range (K) | Sr(K−1) (Temperature, K) | Ref. |
|---|---|---|---|---|
| NaGdF4: Yb/Er | 520/550 | 77–500 | 0.0112 (298) | [30] |
| La(IO3)3: Yb/Er | 520/550 | 298–343 | 0.012 (298) | [31] |
| Ba3La(PO4)3: Yb/Er | 520/550 | 298–498 | 0.0117 (298) | [32] |
| Ba3La(PO4)3: Yb/Tm | 690/792 | 303–503 | 0.0211 (303) | [32] |
| YF3: Yb/Tm | 940/800 | 303–345 | 0.008 (310) | [33] |
| NaErF4@NaGdF4 | 806/654 | 303–593 | 0.0058 (303) | [34] |
| GeO2-PbO-PbF2: Yb/Er | 520/650 | 300–466 | 0.01 (300) | [35] |
| NaYbF4/NaYF4: Tm/Yb/NaYF4 | 800/1800 | 303–423 | 0.0033 (300) | [36] |
| Y2O3: Yb/Tm | 684/490 | 303–573 | 0.0151 (445) | [37] |
| NaY2F7: Yb/Tm | 678/700 | 307–567 | 0.016 (415) | [38] |
| NaYF4: Ho | 961/1183 | 113–473 | 0.008 (367) | [39] |
| Na3ZrF7: Yb/Er/Tm | 800/673 | 313–393 | 0.0176 (313) | [40] |
| NaYF4: Er/Tm/Yb | 520/550 | 297–560 | 0.013 (297) | This work |
| 692/650 | 297–560 | 0.027 (297) | This work | |
| 692/800 | 297–560 | 0.028 (297) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Zhang, X.; Li, L.; Hao, H. Up-Converting Luminescence and Temperature Sensing of Er3+/Tm3+/Yb3+ Co-Doped NaYF4 Phosphors Operating in Visible and the First Biological Window Range. Nanomaterials 2021, 11, 2660. https://doi.org/10.3390/nano11102660
Li J, Wang Y, Zhang X, Li L, Hao H. Up-Converting Luminescence and Temperature Sensing of Er3+/Tm3+/Yb3+ Co-Doped NaYF4 Phosphors Operating in Visible and the First Biological Window Range. Nanomaterials. 2021; 11(10):2660. https://doi.org/10.3390/nano11102660
Chicago/Turabian StyleLi, Jingyun, Yuxiao Wang, Xueru Zhang, Liang Li, and Haoyue Hao. 2021. "Up-Converting Luminescence and Temperature Sensing of Er3+/Tm3+/Yb3+ Co-Doped NaYF4 Phosphors Operating in Visible and the First Biological Window Range" Nanomaterials 11, no. 10: 2660. https://doi.org/10.3390/nano11102660
APA StyleLi, J., Wang, Y., Zhang, X., Li, L., & Hao, H. (2021). Up-Converting Luminescence and Temperature Sensing of Er3+/Tm3+/Yb3+ Co-Doped NaYF4 Phosphors Operating in Visible and the First Biological Window Range. Nanomaterials, 11(10), 2660. https://doi.org/10.3390/nano11102660






