Emerging MXene–Polymer Hybrid Nanocomposites for High-Performance Ammonia Sensing and Monitoring
Abstract
:1. Exploring Needs of Ammonia Detection and Monitoring: Introduction
2. Exploring MXP-NCs for Efficient NH3 Sensing
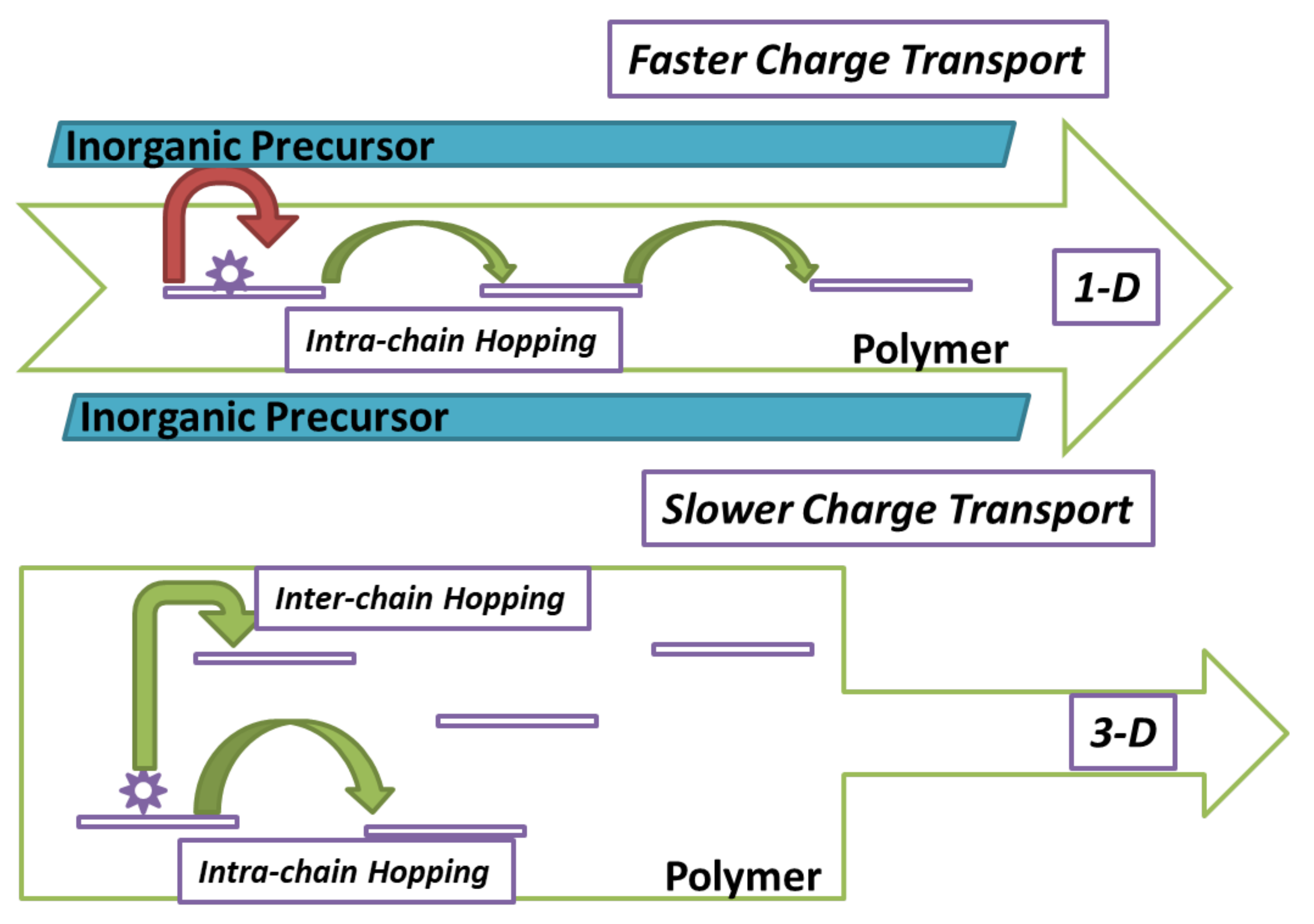
3. Classification of MXP-NCs Functional Structures
4. Fabrication of MXP-NCs-Based High-Performance NH3 Sensors
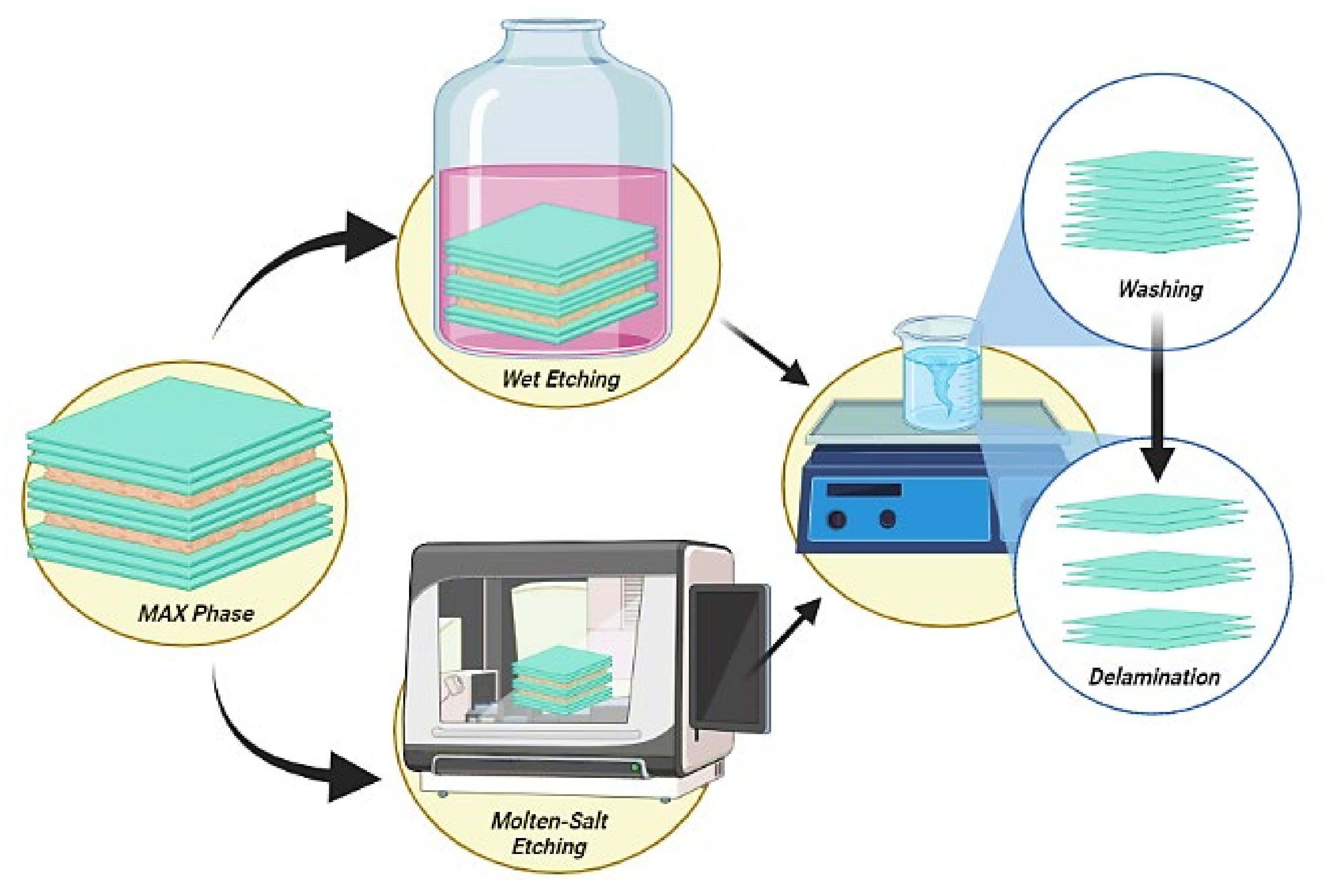
4.1. Stage-1: Synthesis of MXene
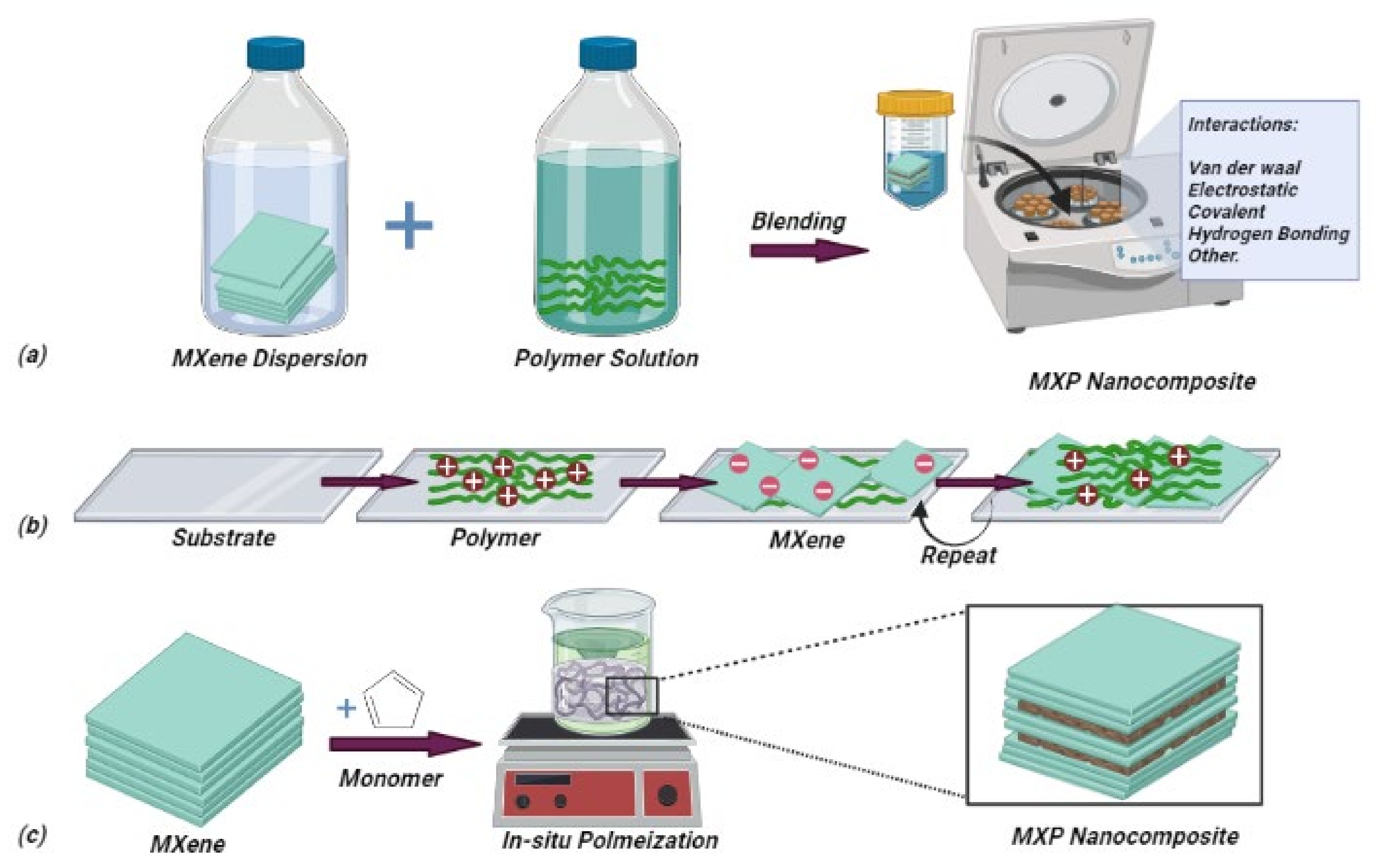
4.2. Stage-2: Synthesis of MXP-NCs
4.2.1. Ex situ Routes
4.2.2. In situ Routes
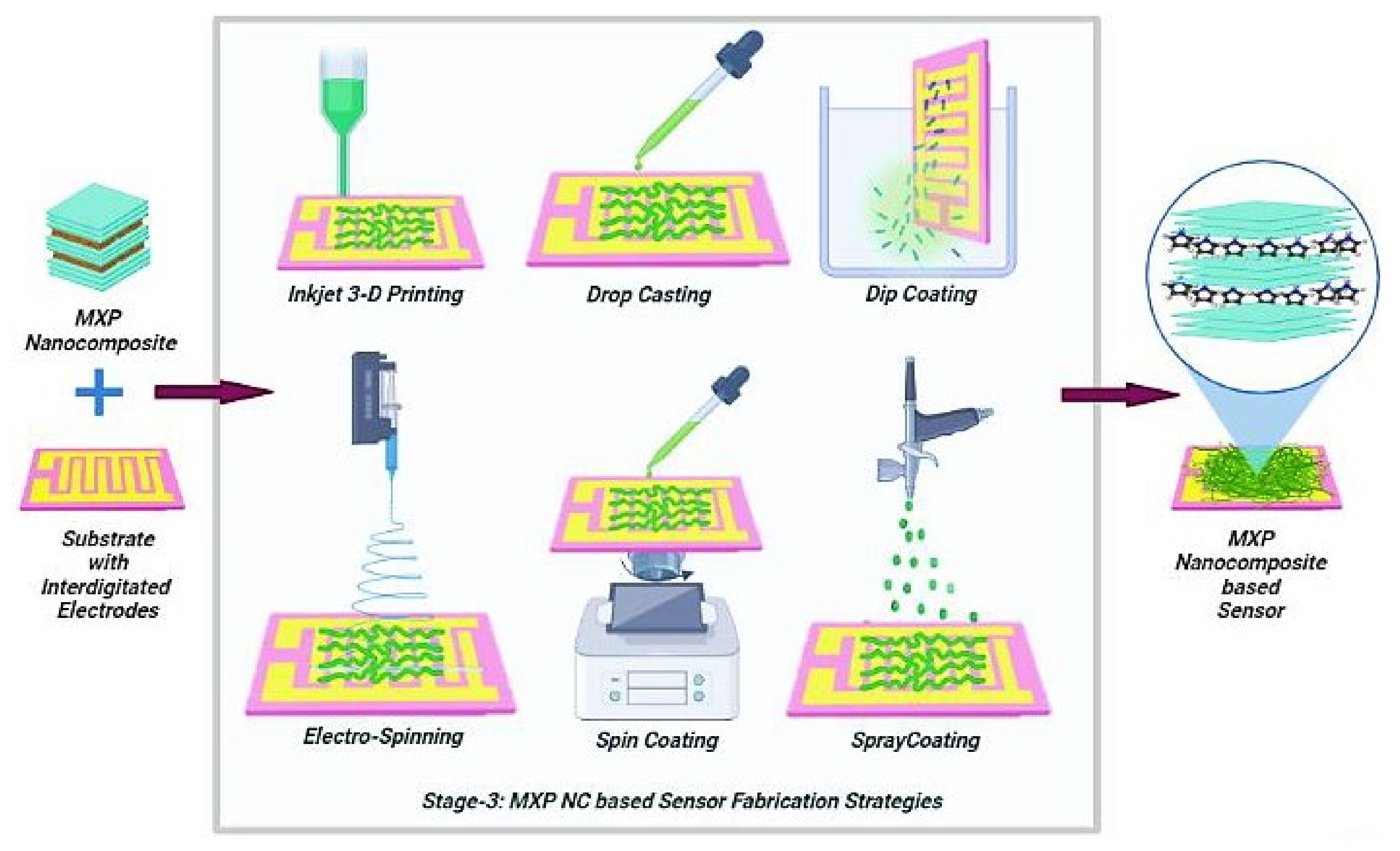
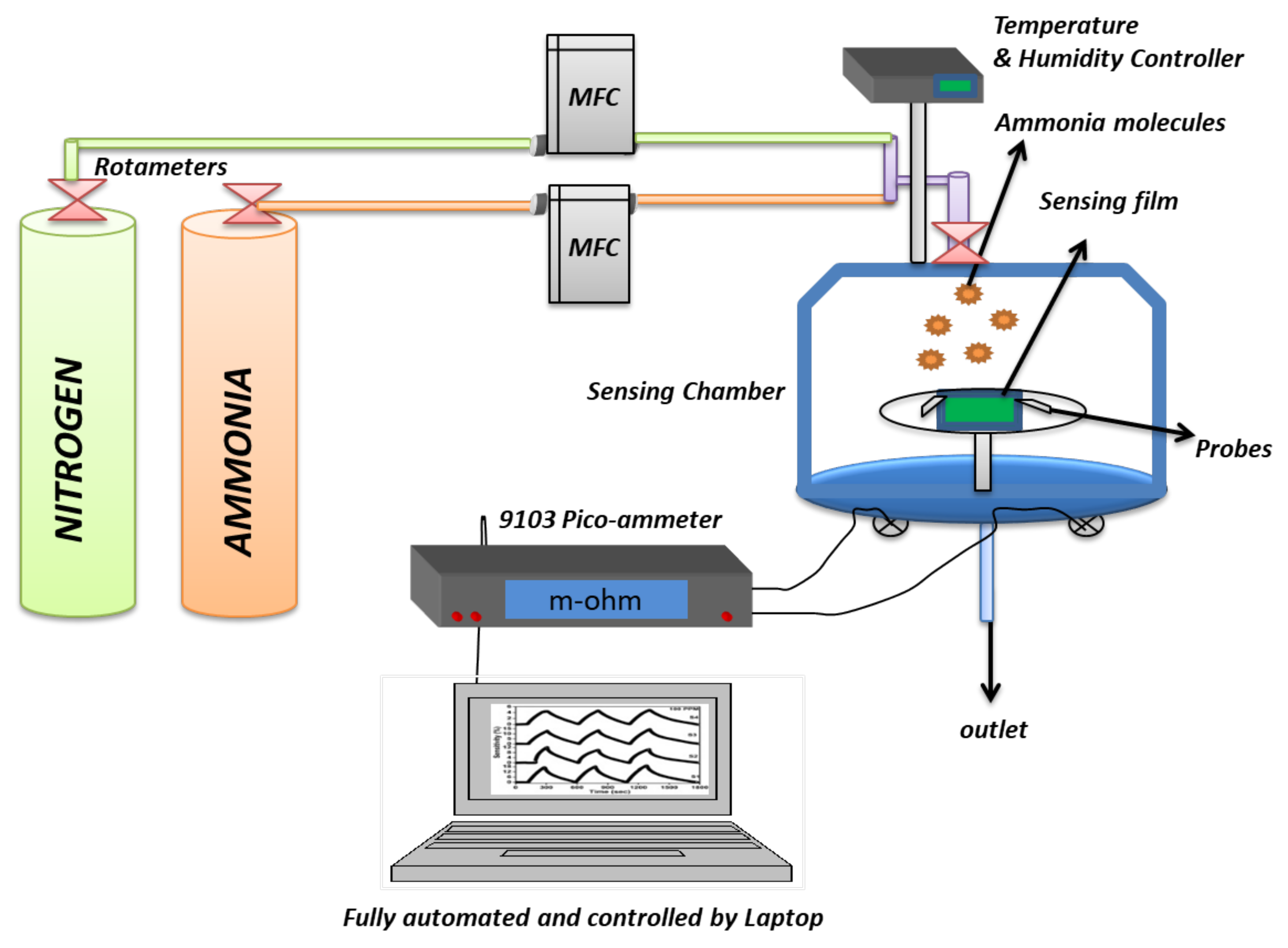
4.3. Stage-3: Fabrication of Chemiresistor
5. Unique Properties of MXP-NCs for NH3 Sensing
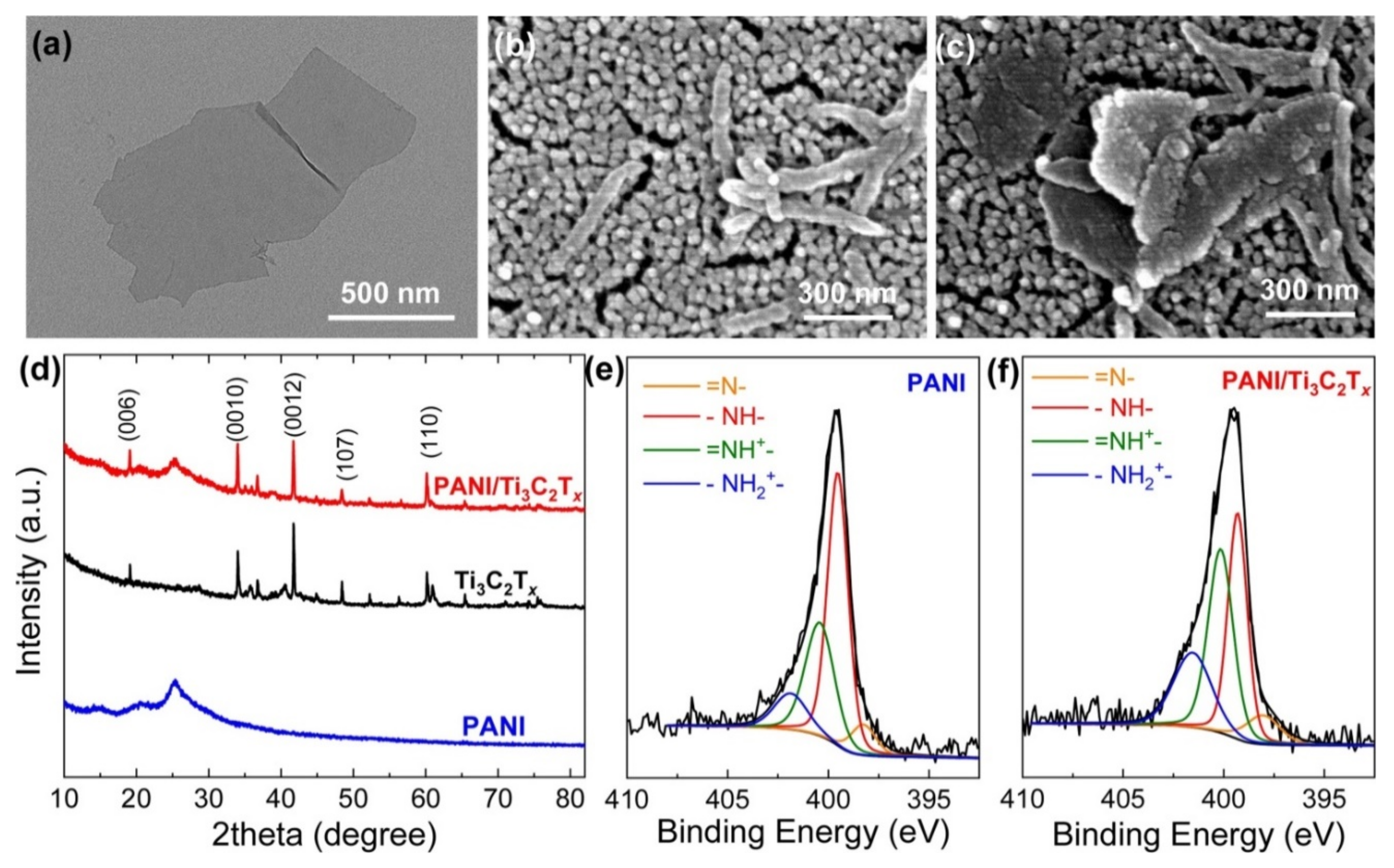
5.1. Morphological Properties and Molecular Interactions
5.2. Electrical Properties of MXP-NCs
5.3. Thermal Properties of MXP-NCs
5.4. Other Advanced Properties
6. Approaches for NH3 Gas Sensing
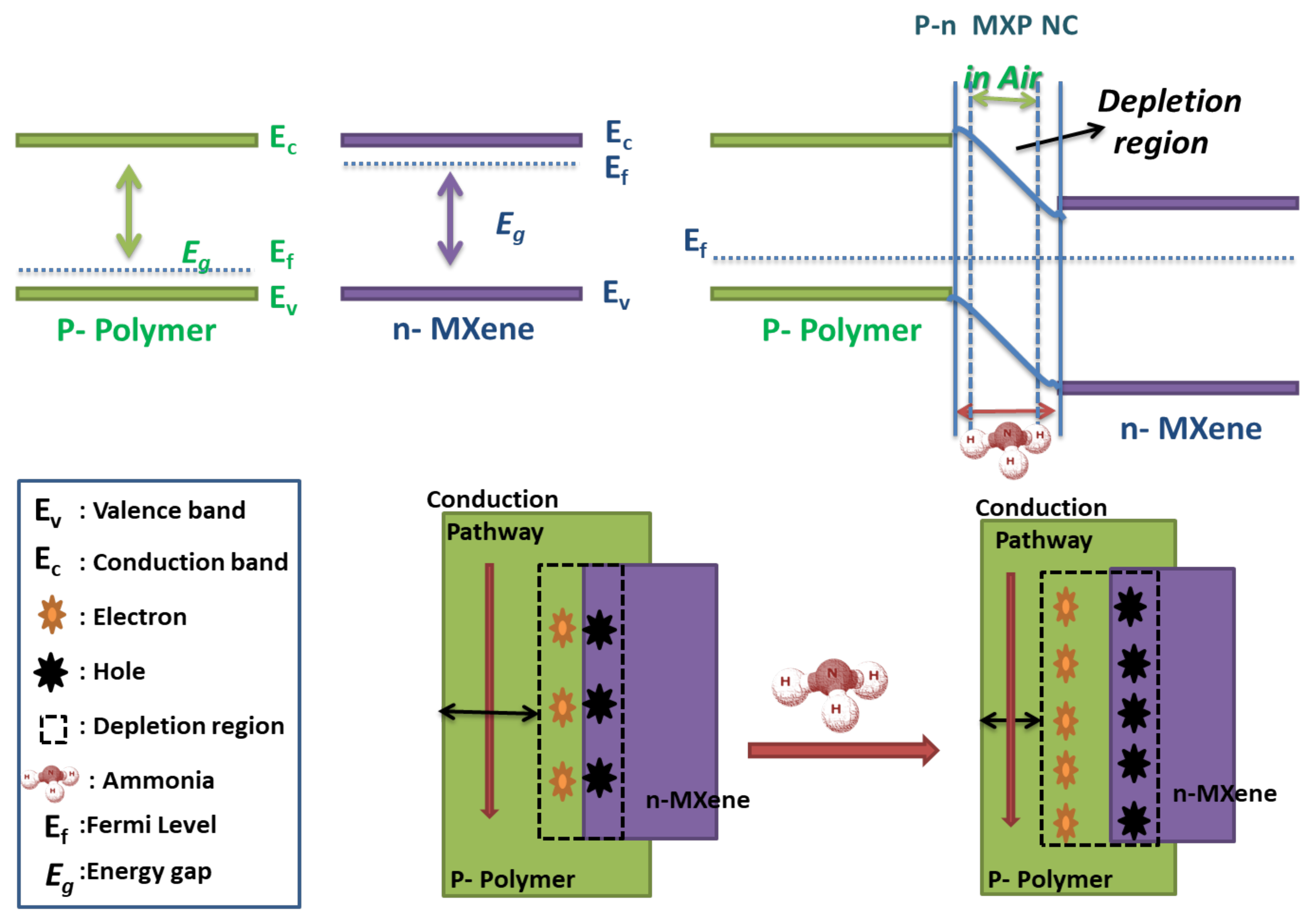
7. NH3 Sensing Mechanism Supported by MXP-NCs
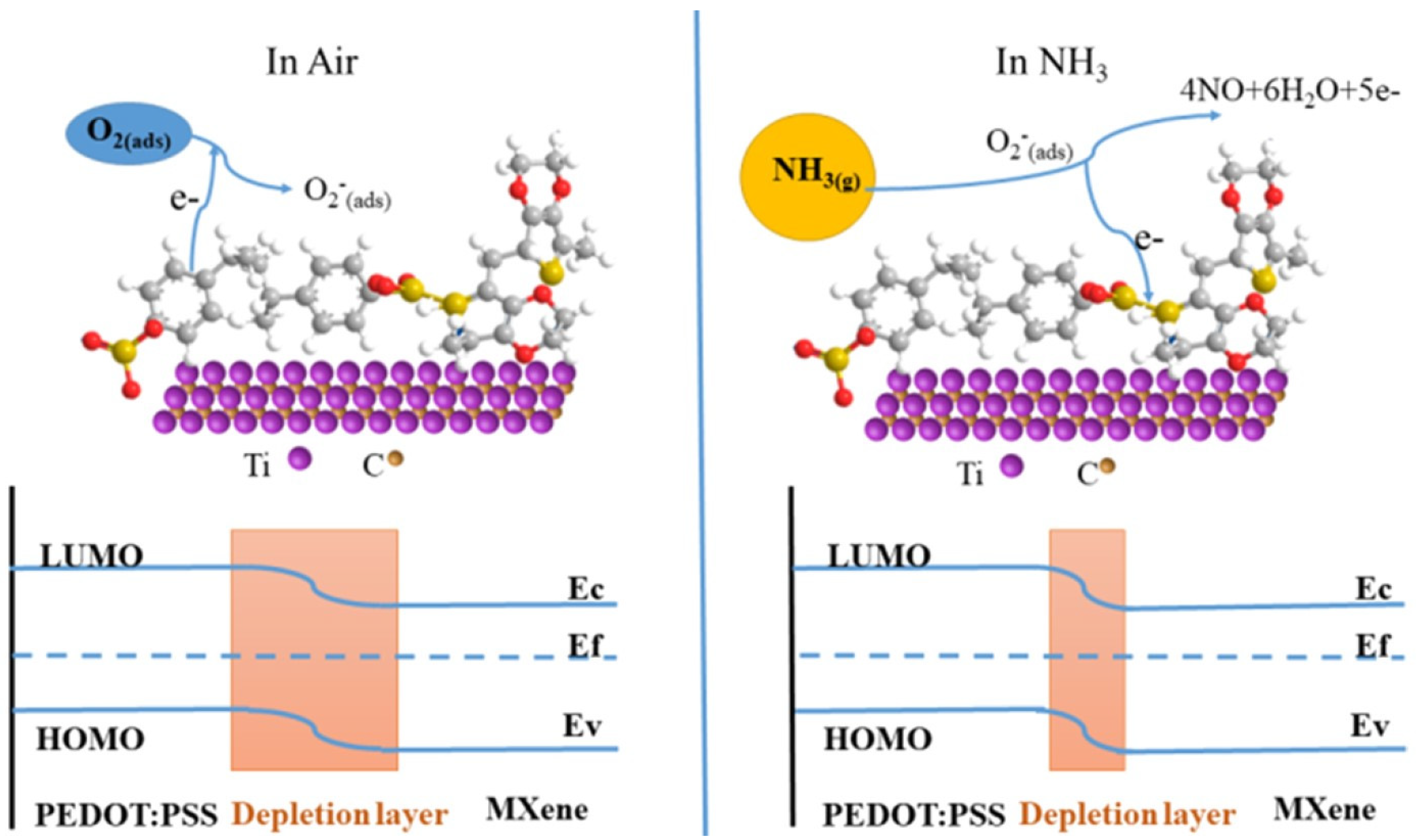
7.1. Ammonia Sensing Mechanism in Pristine Precursors of MXP-NCs
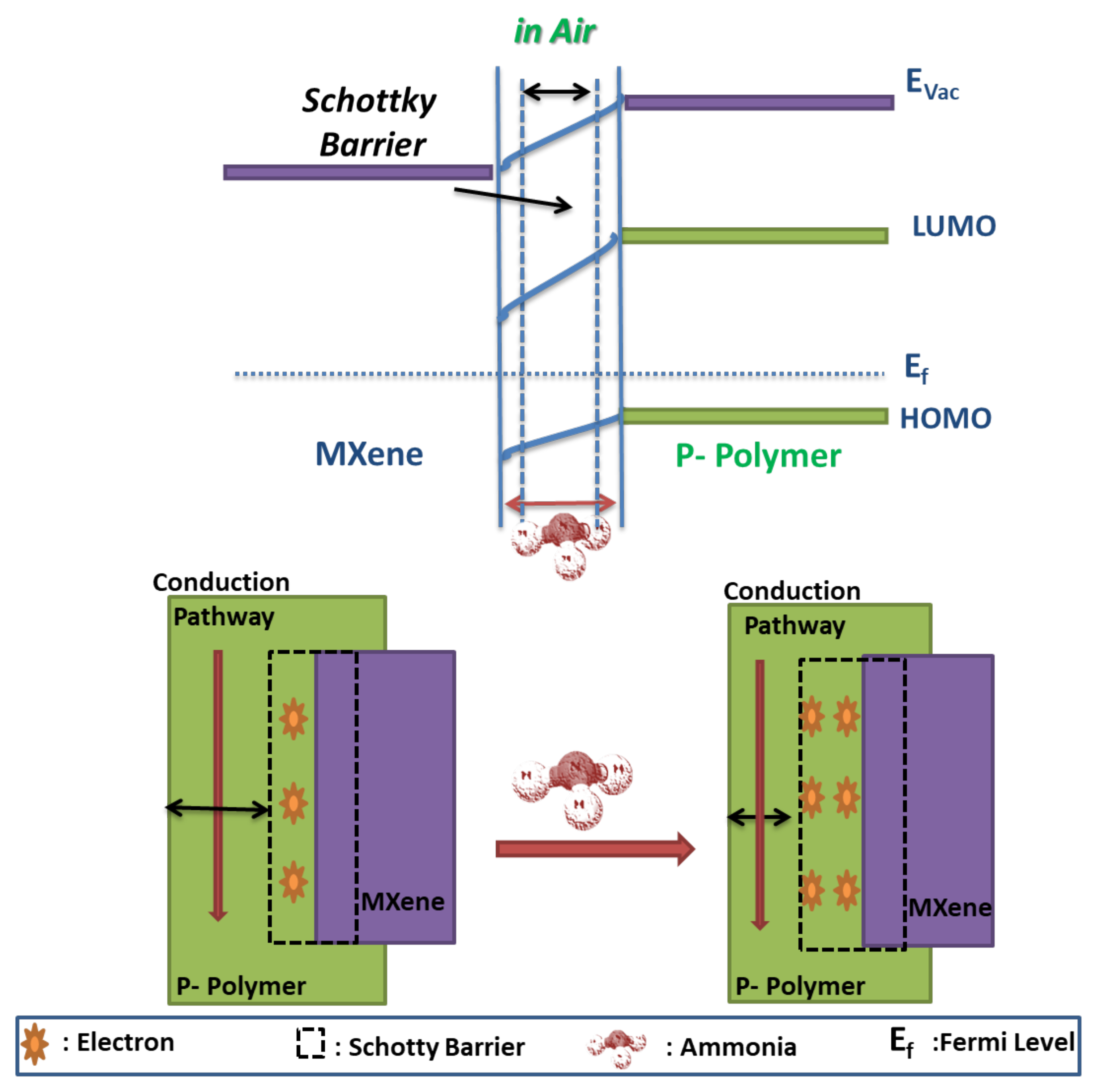
7.2. Ammonia Sensing Mechanism in MXP-NCs
7.2.1. Chemisorption Based Ammonia Sensing
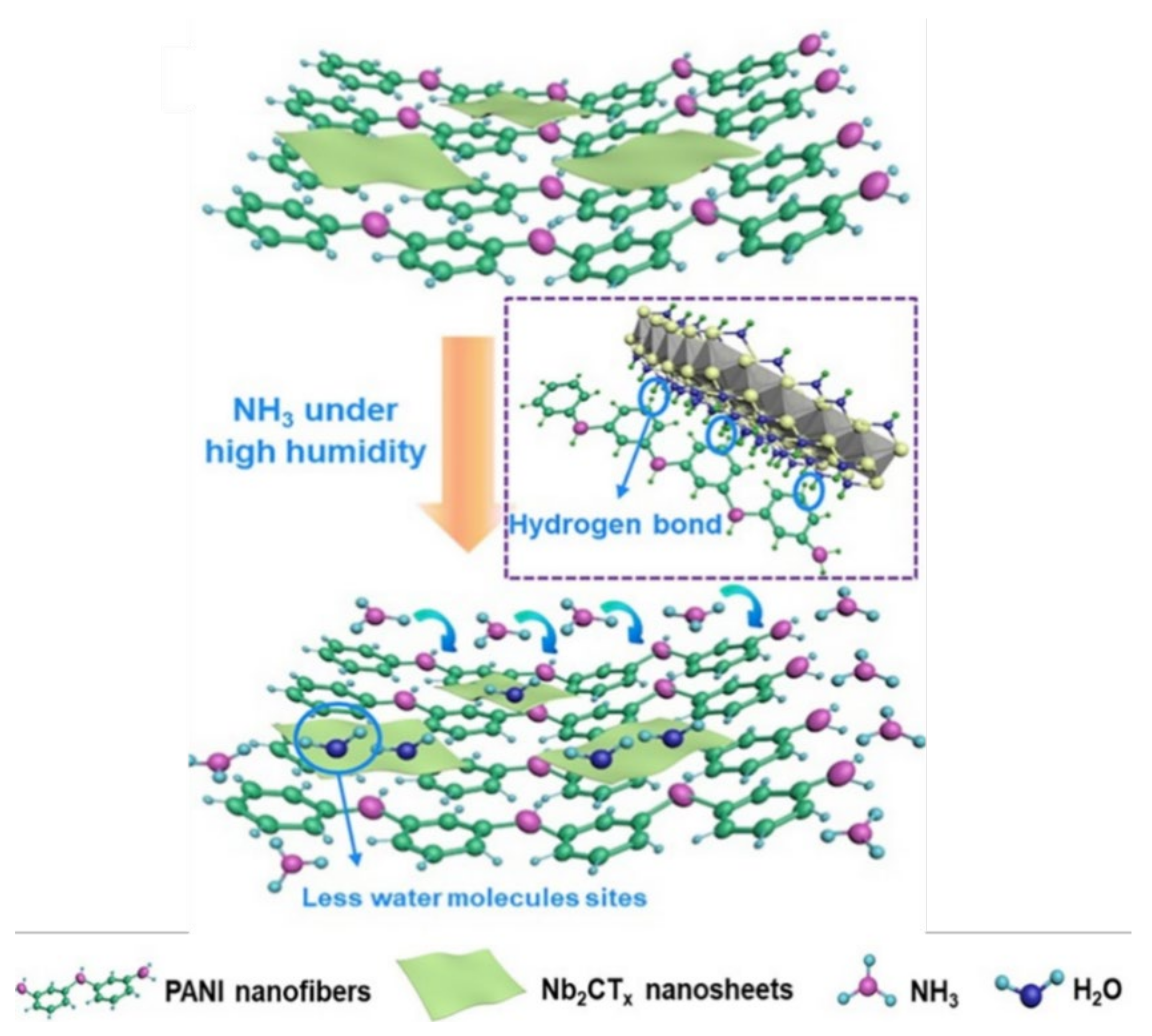
7.2.2. Formation of Hydrogen Bonds
7.2.3. Formation of Unique Hetero-Interfacial Functional Groups
7.2.4. Physisorption-Based Gas Sensing
8. MXP-NCs-Based NH3 Sensing Performance
8.1. Sensitivity, Room Temperature Operation, and Low Detection Limit
8.2. Response-Recovery Times, Repeatability, and Detection Range
8.3. Selectivity Demonstrated by MXP-NCs-Based NH3 Sensors
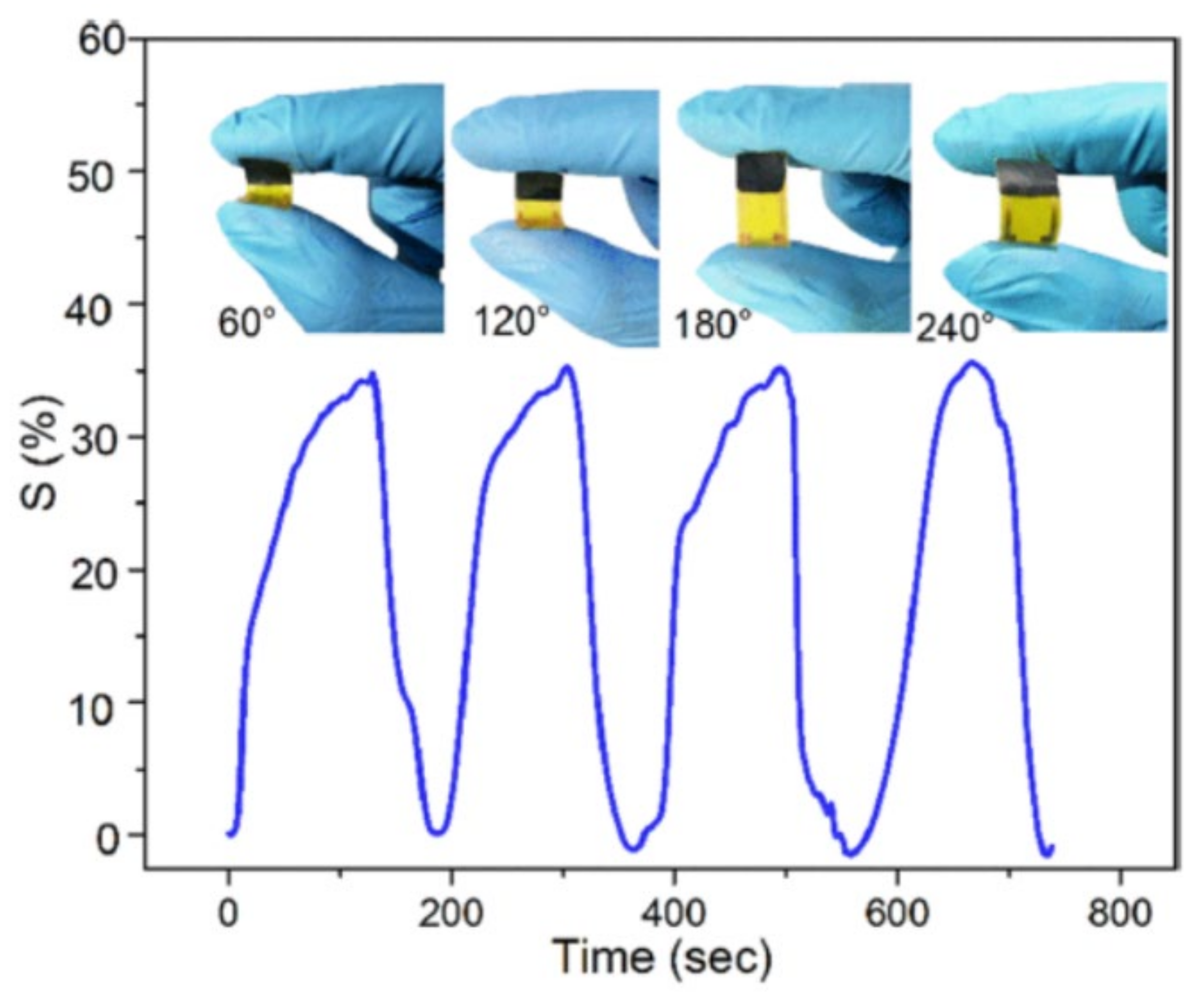
8.4. Mechanical Flexibility Demonstrated by MXP-NCs-Based NH3 Sensors
8.5. Stability-Based on the Effect of Varying Environmental Conditions
9. Advancements in NH3 Detection for Applications Point-of-View
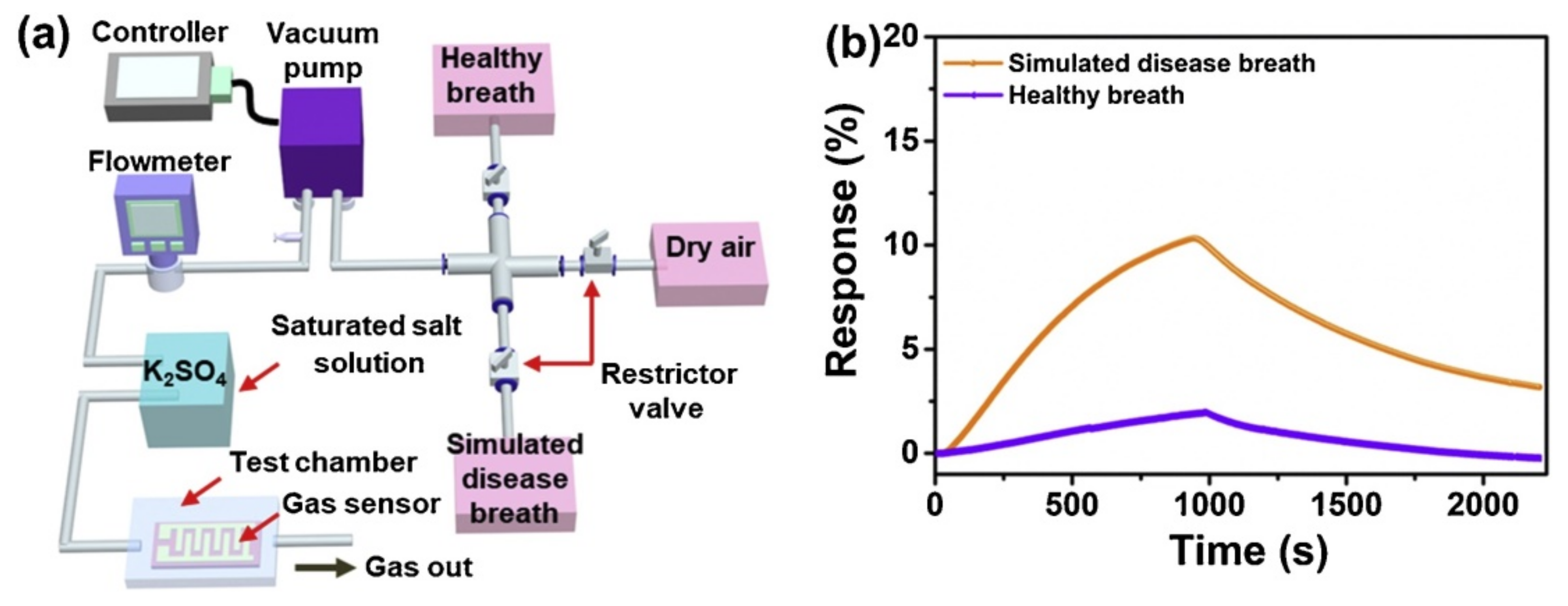
9.1. Human Breath Analysis Based on NH3 Detection
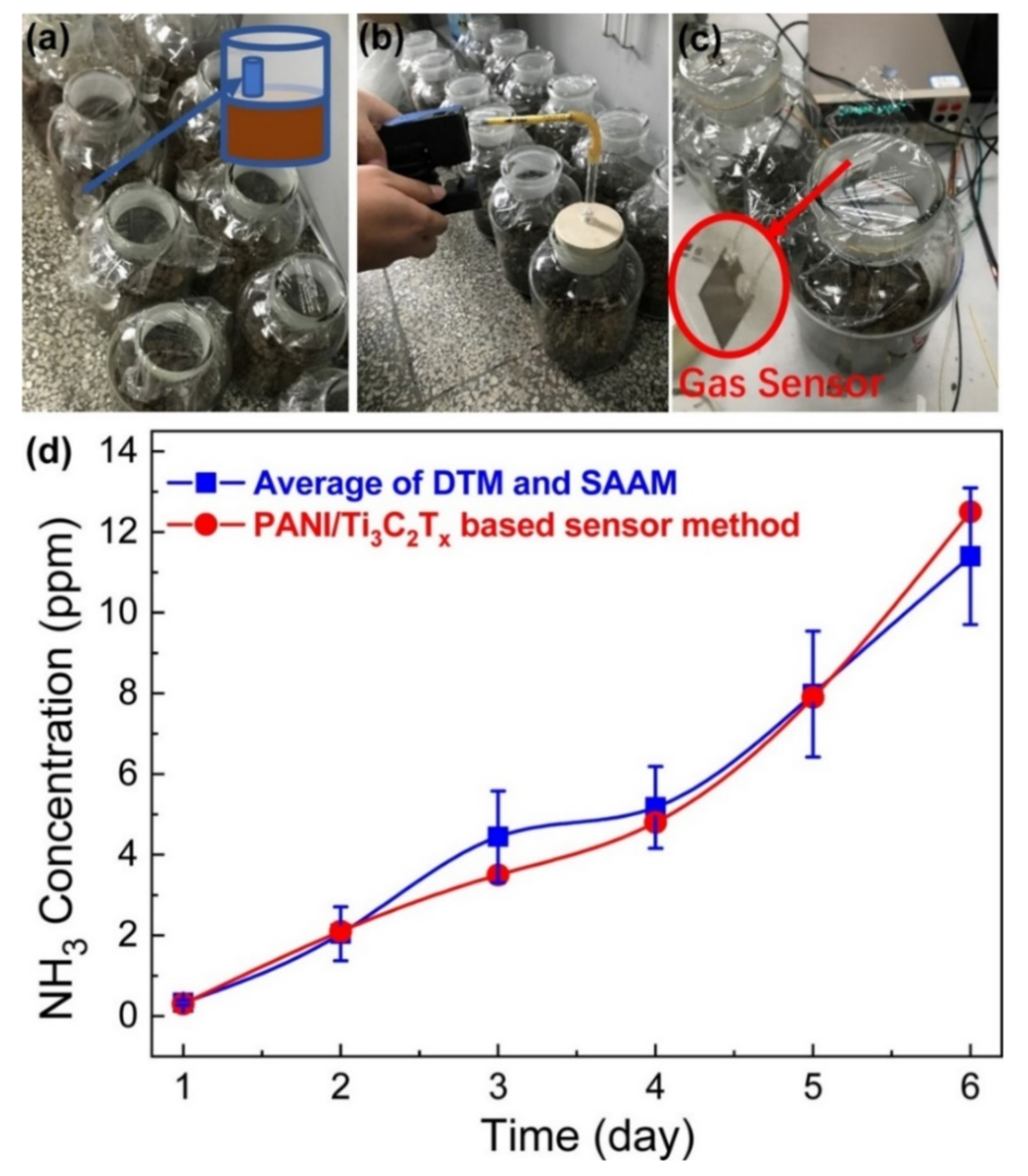
9.2. Detecting Volatilization of Agricultural NH3
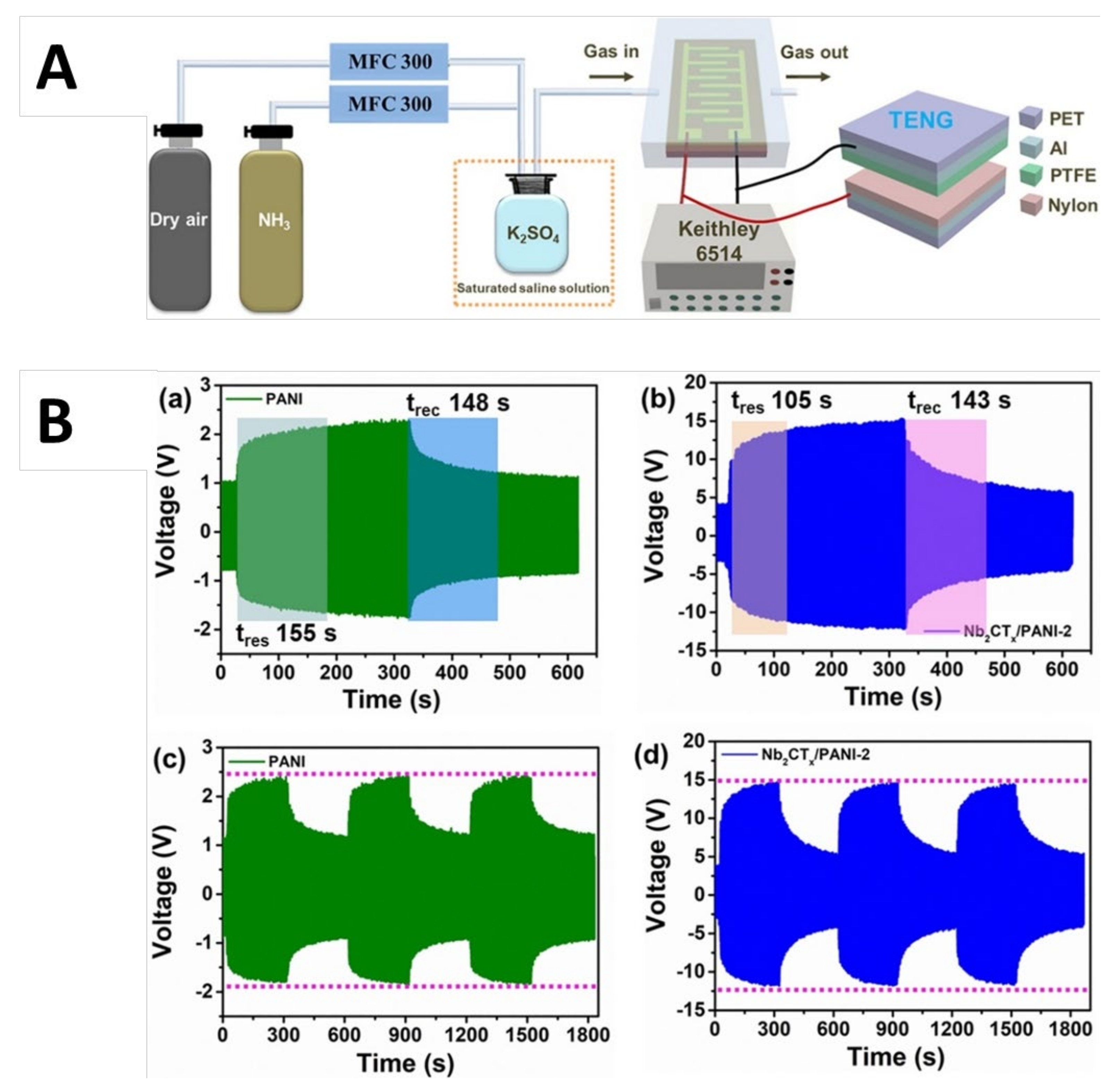
9.3. Self-Driven NH3 Sensing
9.4. Monitoring of Environmental Contaminated by Atmospheric NH3
10. Challenges and Alternative Approaches
10.1. Optimization of Concentration of Precursors
10.2. Slower Response
10.3. Mass Production
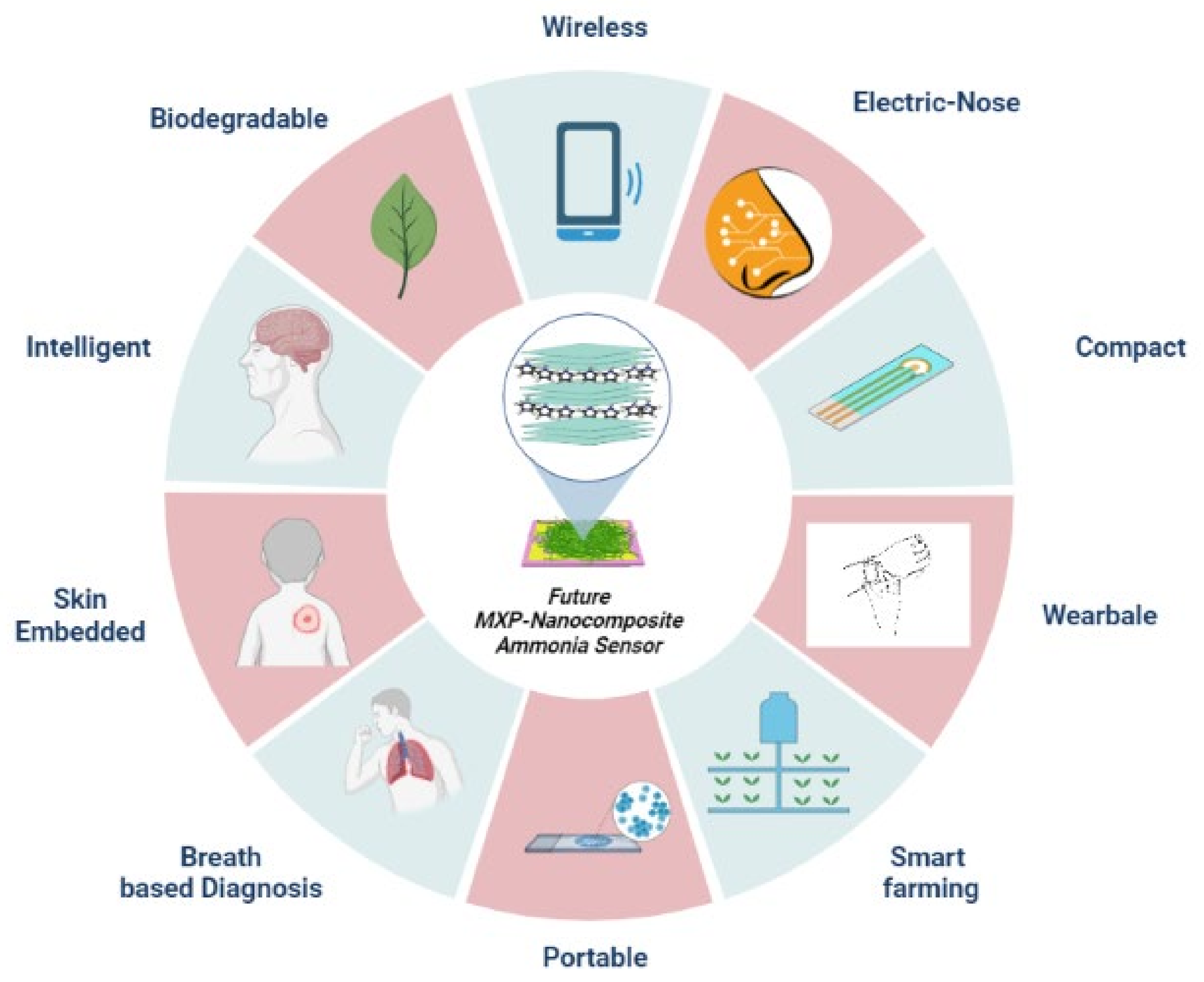
11. Conclusions, Prospects, and Viewpoints
Funding
Acknowledgments
Conflicts of Interest
References
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, V.; Kaur, A. Enhanced and selective ammonia sensing behaviour of poly(aniline co-pyrrole) nanospheres chemically oxidative polymerized at low temperature. J. Ind. Eng. Chem. 2015, 26, 143–148. [Google Scholar] [CrossRef]
- Insausti, M.; Timmis, R.; Kinnersley, R.; Rufino, M. Advances in sensing ammonia from agricultural sources. Sci. Total Environ. 2020, 706, 135124. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, B.; Erisman, J.W.; Reis, A.; Fang, Y.; Lu, X.; Zhang, X. PM2.5 pollution is substantially affected by ammonia emissions in China. Environ. Pollut. 2016, 218, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and COVID Total 19: The Role of Particulate Matter in the Spread and Increase of COVID-19’s Morbidity and Mortality. Int. J. Environ. Res. Public Health 2020, 17, 4487. [Google Scholar] [CrossRef] [PubMed]
- Ricci, P.P.; Gregory, O.J. Sensors for the detection of ammonia as a potential biomarker for health screening. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Gupta, S.; Avasthi, D.K.; Adelung, R.; Mishra, Y.K.; Jain, U. Zinc Oxide Tetrapods Based Biohybrid Interface for Voltammetric Sensing of Helicobacter pylori. ACS Appl. Mater. Interfaces 2018, 10, 30631–30639. [Google Scholar] [CrossRef]
- Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Ultrahigh Selective Room-Temperature Ammonia Gas Sensor Based on Tin–Titanium Dioxide/reduced Graphene/Carbon Nanotube Nanocomposites by the Solvothermal Method. ACS Omega 2019, 4, 16916–16924. [Google Scholar] [CrossRef] [Green Version]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Aarya, S.; Kumar, Y.; Chahota, R.K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym. Mater. 2019, 30, 269–290. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, D.; Wang, X.; Yu, X.; Yu, J. Biocompatible and degradable gelatin dielectric based low-operating voltage organic transistors for ultra-high sensitivity NH3 detection. Appl. Phys. Lett. 2018, 113, 263301. [Google Scholar] [CrossRef] [Green Version]
- Alrammouz, R.; Podlecki, J.; Abboud, P.; Sorli, B.; Habchi, R. A review on flexible gas sensors: From materials to devices. Sens. Actuators A Phys. 2018, 284, 209–231. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S.-H. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Mishra, R.K.; Choi, G.J.; Mishra, Y.K.; Kaushik, A.; Sohn, Y.; Lee, S.H.; Gwag, J.S. A highly stable, selective, and high-performance VOC sensor using a SnS2 nano-lotus structure. J. Mater. Chem. C 2021, 9, 7713. [Google Scholar] [CrossRef]
- González-Garnica, M.; Galdámez-Martínez, A.; Malagón, F.; Ramos, C.; Santana, G.; Abolhassani, R.; Panda, P.K.; Kaushik, A.; Mishra, Y.K.; Karthik, T.V.; et al. One dimensional Au-ZnO hybrid nanostructures based CO2 detection: Growth mechanism and role of the seed layer on sensing performance. Sens. Actuators B Chem. 2021, 337, 129765. [Google Scholar] [CrossRef]
- Khan, S.B. Gas Sensors, 1st ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Anichini, C.; Czepa, W.; Pakulski, D.; Aliprandi, A.; Ciesielski, A.; Samorì, P. Chemical sensing with 2D materials. Chem. Soc. Rev. 2018, 47, 4860–4908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, M.; Yang, B.; Tan, J.; Ding, X.; Li, W. Recent Advances in Multidimensional (1D, 2D, and 3D) Composite Sensors Derived from MXene: Synthesis, Structure, Application, and Perspective. Small Methods 2021, 5, 2100409. [Google Scholar] [CrossRef]
- Mathew, M.; Shinde, P.V.; Samal, R.; Rout, C.S. A review on mechanisms and recent developments in p-n heterojunctions of 2D materials for gas sensing applications. J. Mater. Sci. 2021, 56, 9575–9604. [Google Scholar] [CrossRef]
- Rathee, G.; Bartwal, G.; Rathee, J.; Mishra, Y.K.; Kaushik, A.; Solanki, P.R. Emerging Multimodel Zirconia Nanosystems for High-Performance Biomedical Applications. Adv. NanoBiomed Res. 2021, 1, 2100039. [Google Scholar] [CrossRef]
- Chaudhary, V. High performance X-band electromagnetic shields based on methyl-orange assisted polyaniline-silver core-shell nanocomposites. Polym. Technol. Mater. 2021, 60, 1–10. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. A Review outlines the development and potential applications of a wide range of 2D metal carbides and nitrides. Science 2021, 372, 6547. [Google Scholar] [CrossRef]
- Aghaei, S.M.; Aasi, A.; Panchapakesan, B. Experimental and Theoretical Advances in MXene-Based Gas Sensors. ACS Omega 2021, 6, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Riazi, H.; Taghizadeh, G.; Soroush, M. MXene-Based Nanocomposite Sensors. ACS Omega 2021, 6, 11103–11112. [Google Scholar] [CrossRef] [PubMed]
- Zamhuri, A.; Lim, G.P.; Ma, N.L.; Tee, K.S.; Soon, C.F. MXene in the lens of biomedical engineering: Synthesis, applications and future outlook. Biomed. Eng. Online 2021, 20, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2019, 5, 235–258. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Review—Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. J. Electrochem. Soc. 2019, 167, 037503. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [Green Version]
- Ansari, N.; Lone, M.Y.; Shumaila; Ali, J.; Zulfequar, M.; Husain, M.; Islam, S.S.; Husain, S. Trace level toxic ammonia gas sensing of single-walled carbon nanotubes wrapped polyaniline nanofibers. J. Appl. Phys. 2020, 127, 044902. [Google Scholar] [CrossRef]
- Baker, C.O.; Huang, X.; Nelson, W.; Kaner, R.B. Polyaniline nanofibers: Broadening applications for conducting polymers. Chem. Soc. Rev. 2017, 46, 1510–1525. [Google Scholar] [CrossRef]
- Chaudhary, V.; Royal, A.; Chavali, M.; Yadav, S.K. Advancements in research and development to combat COVID-19 using nanotechnology. Nanotechnol. Environ. Eng. 2021, 6, 1–15. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Sun, Z.; Huang, P.; Li, Y.-Q.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaur, A. Enhanced room temperature sulfur dioxide sensing behaviour of in situ polymerized polyaniline–tungsten oxide nanocomposite possessing honeycomb morphology. RSC Adv. 2015, 5, 73535–73544. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, R.; Tian, J.; Huang, W.; Liu, J. MXene-Based Nanocomposites for Energy Conversion and Storage Applications. Chem. A Eur. J. 2020, 26, 6342–6359. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Hossain, M.; Chowdhury, H.K. Two-dimensional MXene-based flexible nanostructures for functional nanodevices: A review. J. Mater. Chem. A 2021, 9, 3231–3269. [Google Scholar] [CrossRef]
- Ling, Z.; Ren, C.E.; Zhao, M.-Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 111–16676. [Google Scholar] [CrossRef] [Green Version]
- Carey, M.; Barsoum, M. MXene polymer nanocomposites: A review. Mater. Today Adv. 2021, 9, 100120. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Li, L.; Wang, Y.; Wang, J.; Xiong, J.; Du, S.; Zhang, P.; Shi, X.; Yu, J. MXene/Polymer Nanocomposites: Preparation, Properties, and Applications. Polym. Rev. 2021, 61, 80–115. [Google Scholar] [CrossRef]
- Kausar, A. Polymer/MXene nanocomposite—A new age for advanced materials. Polym. Technol. Mater. 2021, 60, 1377–1392. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.H.; Choi, Y.Y.; Jo, S.B.; Myoung, J.; Cho, J.H. Sensing with MXenes: Progress and Prospects. Adv. Mater. 2021, 2005846. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Wang, K.; Wei, W.; Wang, L.; Han, W. High-performance flexible sensing devices based on polyaniline/MXene nanocomposites. InfoMat 2019, 1, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic–Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef]
- Chen, J.; Chen, K.; Tong, D.; Huang, Y.; Zhang, J.; Xue, J.; Huang, Q.; Chen, T. CO2 and temperature dual responsive “Smart” MXene phases. Chem. Commun. 2015, 51, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yun, J.; Wu, S.; Li, L.; Yu, L.; Kim, K.H. Architecturally Robust Graphene-Encapsulated MXene Ti2CTx@Polyaniline Composite for High-Performance Pouch-Type Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 34212–34221. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Li, K.; Li, X.; Gogotsi, Y. Ti3C2T/PEDOT:PSS hybrid materials for room-temperature methanol sensor. Chin. Chem. Lett. 2020, 31, 1018–1021. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Deng, Q.; Zhou, J.; Pei, Z.; Xue, Q.; Wang, Z.; Li, H.; Huang, Q.; Zhi, C. Highly Flexible, Freestanding Supercapacitor Electrode with Enhanced Performance Obtained by Hybridizing Polypyrrole Chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.-J.; Li, P.; Zhao, Y.; Niu, Q.J. Fabrication of novel MXene (Ti3C2)/polyacrylamide nanocomposite hydrogels with enhanced mechanical and drug release properties. Soft Matter 2020, 16, 162–169. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Jiang, Y.; He, Z.; Liu, B.; Xie, H.; Li, H.; Li, Z.; Wang, Y.; Tai, H. Toward agricultural ammonia volatilization monitoring: A flexible polyaniline/Ti3C2T hybrid sensitive films based gas sensor. Sens. Actuators B Chem. 2020, 316, 128144. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-Dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.; Kim, D.-J. Review—Recent Exploration of Two-Dimensional MXenes for Gas Sensing: From a Theoretical to an Experimental View. J. Electrochem. Soc. 2020, 167, 037515. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, B.; Duan, Z.; Zhao, Q.; Zhang, Y.; Xie, G.; Jiang, Y.; Li, S.; Tai, H. PANI nanofibers-supported Nb2CTx nanosheets-enabled selective NH3 detection driven by TENG at room temperature. Sens. Actuators B Chem. 2021, 327, 128923. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Liu, B.; Duan, Z.; Pan, H.; Yuan, Z.; Xie, G.; Wang, J.; Fang, Z.; Tai, H. Ultrathin Nb2CT nanosheets-supported polyaniline nanocomposite: Enabling ultrasensitive NH3 detection. Sens. Actuators B Chem. 2021, 343, 130069. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Wang, X.; Li, A.; Li, F.; Zhou, Y. Corrosion behavior of selected Mn+1AXn phases in hot concentrated HCl solution: Effect of A element and MX layer. Corros. Sci. 2012, 60, 129–135. [Google Scholar] [CrossRef]
- Peng, J.; Chen, X.; Ong, W.-J.; Zhao, X.; Li, N. Surface and Heterointerface Engineering of 2D MXenes and Their Nanocomposites: Insights into Electro- and Photocatalysis. Chem 2019, 5, 18–50. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zheng, Y.; Wang, K.; Lv, C.; Wei, W.; Wang, L.; Han, W. Highly Stable Cross-Linked Cationic Polyacrylamide/Ti3C2Tx MXene Nanocomposites for Flexible Ammonia-Recognition Devices. Adv. Mater. Technol. 2020, 5, 2000248. [Google Scholar] [CrossRef]
- Naguib, M.; Saito, T.; Lai, S.; Rager, M.S.; Aytug, T.; Paranthaman, M.P.; Zhao, M.-Q.; Gogotsi, Y. Ti3C2Tx (MXene)–Polyacrylamide nanocomposite films. RSC Adv. 2016, 6, 72069–72073. [Google Scholar] [CrossRef]
- Yu, B.; Tawiah, B.; Wang, L.-Q.; Yuen, A.C.Y.; Zhang, Z.-C.; Shen, L.-L.; Lin, B.; Fei, B.; Yang, W.; Li, A.; et al. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef]
- Sheng, X.; Zhao, Y.; Zhang, L.; Lu, X. Properties of two-dimensional Ti3C2 MXene/thermoplastic polyurethane nanocomposites with effective reinforcement via melt blending. Compos. Sci. Technol. 2019, 181, 107710. [Google Scholar] [CrossRef]
- Jin, L.; Wu, C.; Wei, K.; He, L.; Gao, H.; Zhang, H.; Zhang, K.; Asiri, A.M.; Alamry, K.A.; Yang, L.; et al. Polymeric Ti3C2Tx MXene Composites for Room Temperature Ammonia Sensing. ACS Appl. Nano Mater. 2020, 3, 12071–12079. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; Liu, X.; Fahlman, M.; Halim, J.; Persson, P.O.; Rosen, J.; Zhang, F. Polymer-MXene composite films formed by MXene-facilitated electrochemical polymerization for flexible solid-state microsupercapacitors. Nano Energy 2019, 60, 734–742. [Google Scholar] [CrossRef]
- Carey, M.; Hinton, Z.; Sokol, M.; Alvarez, N.J.; Barsoum, M.W. Nylon-6/Ti3C2Tz MXene Nanocomposites Synthesized by in Situ Ring Opening Polymerization of ε-Caprolactam and Their Water Transport Properties. ACS Appl. Mater. Interfaces 2019, 11, 20425–20436. [Google Scholar] [CrossRef]
- Yan, K.; Li, J.; Pan, L.; Shi, Y. Inkjet printing for flexible and wearable electronics. APL Mater. 2020, 8, 120705. [Google Scholar] [CrossRef]
- Chaudhary, V.; Chavali, M. Novel methyl-orange assisted core-shell polyaniline-silver nanosheets for highly sensitive ammonia chemiresistors. J. Appl. Polym. Sci. 2021, 138, 51288. [Google Scholar] [CrossRef]
- Chaudhary, V.; Singh, H.; Kaur, A. Effect of charge carrier transport on sulfur dioxide monitoring performance of highly porous polyaniline nanofibres. Polym. Int. 2017, 66, 699–704. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaur, A. Solitary surfactant assisted morphology dependent chemiresistive polyaniline sensors for room temperature monitoring of low parts per million sulfur dioxide. Polym. Int. 2015, 64, 1475–1481. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaur, A. Surfactant directed polyaniline nanostructures for high performance sulphur dioxide chemiresistors: Effect of morphologies, chemical structure and porosity. RSC Adv. 2016, 6, 95349–95357. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhang, X.; Li, Y.; Wang, J. Ti3C2Tx Filler Effect on the Proton Conduction Property of Polymer Electrolyte Membrane. ACS Appl. Mater. Interfaces 2016, 8, 20352–20363. [Google Scholar] [CrossRef]
- Fei, M.; Lin, R.; Deng, Y.; Xian, H.; Bian, R.; Zhang, X.; Cheng, J.; Xu, C.; Cai, D. Polybenzimidazole/Mxene composite membranes for intermediate temperature polymer electrolyte membrane fuel cells. Nanotechnology 2018, 29, 035403. [Google Scholar] [CrossRef]
- Cao, Y.; Deng, Q.; Liu, Z.; Shen, D.; Wang, T.; Huang, Q.; Du, S.; Jiang, N.; Lin, C.-T.; Yu, J. Enhanced thermal properties of poly (vinylidene fluoride) composites with ultrathin nanosheets of MXene. RSC Adv. 2017, 7, 20494–20501. [Google Scholar] [CrossRef] [Green Version]
- Kang, R.; Zhang, Z.; Guo, L.; Cui, J.; Chen, Y.; Hou, X.; Wang, B.; Lin, C.-T.; Jiang, N.; Yu, J. Enhanced Thermal Conductivity of Epoxy Composites Filled with 2D Transition Metal Carbides (MXenes) with Ultralow Loading. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Li, W. High-Thermal-Stability and High-Thermal-Conductivity Ti3C2Tx MXene/Poly (vinyl alcohol) (PVA) Composites. ACS Omega 2018, 3, 2609–2617. [Google Scholar] [CrossRef] [Green Version]
- Yi, N.; Cheng, Z.; Li, H.; Yang, L.; Zhu, J.; Zheng, X.; Chen, Y.; Liu, Z.; Zhu, H.; Cheng, H. Stretchable, ultrasensitive, and low-temperature NO2 sensors based on MoS2@rGO nanocomposites. Mater. Today Phys. 2020, 15, 100265. [Google Scholar] [CrossRef]
- Chaudhary, V. One-dimensional variable range charge carrier hopping in polyaniline–tungsten oxide nanocomposite-based hydrazine chemiresistor. Appl. Phys. A 2021, 127, 1–7. [Google Scholar] [CrossRef]
- Debbarma, R.; Nguyen, N.H.L.; Berry, V. Defect guided conduction in graphene-derivatives and MoS2: Two-Dimensional nanomaterial models. Appl. Mater. Today 2021, 23, 101072. [Google Scholar] [CrossRef]
- Hajian, S.; Khakbaz, P.; Moshayedi, M.; Maddipatla, D.; Narakathu, B.B.; Turkani, V.S.; Bazuin, B.J.; Pourfath, M.; Atashbar, M.Z. Impact of Different Ratios of Fluorine, Oxygen, and Hydroxyl Surface Terminations on Ti3C2Tx MXene as Ammonia Sensor: A First-Principles Study. In Proceedings of the 2018 IEEE Sensors, New Delhi, India, 28–31 October 2018; Volume 1, pp. 1–4. [Google Scholar]
- Zhu, C.; Cheng, X.; Dong, X.; Xu, Y.M. Enhanced Sub-ppm NH3 Gas Sensing Performance of PANI/TiO2 Nanocomposites at Room Temperature. Front. Chem. 2018, 6, 493. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B Chem. 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Šetka, M.; Calavia, R.; Vojkůvka, L.; Polčák, J.; Llobet, E.; Drbohlavová, J.; Vallejos, S. Electrochemically deposited polypyrrole nanorods and study of their ammonia sensing properties. Mater. Today Proc. 2020, 20, 305–310. [Google Scholar] [CrossRef]
- Gund, G.; Park, J.H.; Harpalsinh, R.; Kota, M.; Shin, J.H.; Kim, T.-I.; Gogotsi, Y.; Park, H.S. MXene/Polymer Hybrid Materials for Flexible AC-Filtering Electrochemical Capacitors. Joule 2019, 3, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Thirumalairajan, S.; Girija, K.; Mastelaro, V.; Ponpandian, N. Surface Morphology-Dependent Room-Temperature LaFeO3 Nanostructure Thin Films as Selective NO2 Gas Sensor Prepared by Radio Frequency Magnetron Sputtering. ACS Appl. Mater. Interfaces 2014, 6, 13917–13927. [Google Scholar] [CrossRef]
- Gao, J.; Qin, J.; Chang, J.; Liu, H.; Wu, Z.-S.; Feng, L. NH3 Sensor Based on 2D Wormlike Polypyrrole/Graphene Heterostructures for a Self-Powered Integrated System. ACS Appl. Mater. Interfaces 2020, 12, 38674–38681. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B Chem. 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Patil, R.; Gaikwad, S.; Karanjekar, A.; Khanna, P.; Jain, G.; Gaikwad, V.; More, P.; Bisht, N. Optimization of strontium-doping concentration in BaTiO3 nanostructures for room temperature NH3 and NO2 gas sensing. Mater. Today Chem. 2020, 16, 100240. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Two-Dimensional Vanadium Carbide MXene for Gas Sensors with Ultrahigh Sensitivity Toward Nonpolar Gases. ACS Sens. 2019, 4, 1603–1611. [Google Scholar] [CrossRef]
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-cost and flexible printed graphene–PEDOT:PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, A.; Wang, C.; Liu, F.; He, J.; Li, S.; Wang, J.; You, R.; Yan, X.; Sun, P.; et al. Improvement of Gas and Humidity Sensing Properties of Organ-like MXene by Alkaline Treatment. ACS Sens. 2019, 4, 1261–1269. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Yang, Z.; He, J.; Wang, J.; Zhao, L.; Lu, H.; Tian, T.; Liu, F.; Sun, P.; et al. Room temperature high performance NH3 sensor based on GO-rambutan-like polyaniline hollow nanosphere hybrid assembled to flexible PET substrate. Sens. Actuators B Chem. 2018, 273, 726–734. [Google Scholar] [CrossRef]
- Wang, S.; Xie, G.; Tai, H.; Su, Y.; Yang, B.; Zhang, Q.; Du, X.; Jiang, Y. Ultrasensitive flexible self-powered ammonia sensor based on triboelectric nanogenerator at room temperature. Nano Energy 2018, 51, 231–240. [Google Scholar] [CrossRef]
- Du, C.-F.; Zhao, X.; Wang, Z.; Yu, H.; Ye, Q. Recent Advanced on the MXene–Organic Hybrids: Design, Synthesis, and Their Applications. Nanomaterials 2021, 11, 166. [Google Scholar] [CrossRef]
- Yu, X.-F.; Li, Y.-C.; Cheng, J.-B.; Liu, Z.-B.; Li, Q.-Z.; Li, W.-Z.; Yang, X.; Xiao, B. Monolayer Ti2CO2: A Promising Candidate for NH3 Sensor or Capturer with High Sensitivity and Selectivity. ACS Appl. Mater. Interfaces 2015, 7, 13707–13713. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.B.; Navale, Y.H.; Navale, S.T.; Stadler, F.; Patil, V.B. Room temperature ammonia gas sensing properties of polyaniline nanofibers. J. Mater. Sci. Mater. Electron. 2019, 30, 8371–8380. [Google Scholar] [CrossRef]
- Pandey, S. Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. J. Sci. Adv. Mater. Devices 2016, 1, 431–453. [Google Scholar] [CrossRef] [Green Version]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Mogera, U.; Sagade, A.; George, S.J.; Kulkarni, G.U. Ultrafast response humidity sensor using supramolecular nanofibre and its application in monitoring breath humidity and flow. Sci. Rep. 2015, 4, 4103. [Google Scholar] [CrossRef] [Green Version]
- Tanguy, N.R.; Thompson, M.; Yan, N. A review on advances in application of polyaniline for ammonia detection. Sensors Actuators B Chem. 2018, 257, 1044–1064. [Google Scholar] [CrossRef]
- Jha, R.K.; Wan, M.; Jacob, C.; Guha, P.K. Ammonia vapour sensing properties of in situ polymerized conducting PANI-nanofiber/WS2 nanosheet composites. New J. Chem. 2018, 42, 735–745. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Io, J.; Sugiyama, G.; Sakai, Y. Effect of NH3 gas on the electrical conductivity of polyaniline blend films. Synth. Met. 2002, 128, 15–19. [Google Scholar] [CrossRef]
- Ly, T.N.; Park, S. Highly sensitive ammonia sensor for diagnostic purpose using reduced graphene oxide and conductive polymer. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Lee, C.-S.; Kim, D.H.; Lee, J.-H. Flexible Room-Temperature NH3 Sensor for Ultrasensitive, Selective, and Humidity-Independent Gas Detection. ACS Appl. Mater. Interfaces 2018, 10, 27858–27867. [Google Scholar] [CrossRef]
- Ma, Z.; Yue, Y.; Feng, M.; Li, Y.; Ma, X.; Zhao, X.; Wang, S. Mitigation of ammonia volatilization and nitrate leaching via loss control urea triggered H-bond forces. Sci. Rep. 2019, 9, 15140. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Bai, Z.; Chadwick, D.; Hou, Y.; Qin, W.; Zhao, Z.; Jiang, R.; Ma, L. Greenhouse gas and ammonia emissions and mitigation options from livestock production in peri-urban agriculture: Beijing—A case study. J. Clean. Prod. 2018, 178, 515–525. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, M.; Shi, Q.; Wen, F.; Liu, L.; Dong, B.; Haroun, A.; Yang, Y.; Vachon, P.; Guo, X.; et al. Progress in TENG technology—A journey from energy harvesting to nanoenergy and nanosystem. EcoMat 2020, 2, 12058. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Wu, Z.; Zhang, L.; Sun, W.; Li, T.; Wang, D.; Zhou, F. Conductive elastic sponge-based triboelectric nanogenerator (TENG) for effective random mechanical energy harvesting and ammonia sensing. Nano Energy 2021, 79, 105422. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, J.H.; Kim, J.K.; Jeong, U. Material aspects of triboelectric energy generation and sensors. NPG Asia Mater. 2020, 12, 6. [Google Scholar] [CrossRef]
- Sutton, M.A.; Erisman, J.W.; Dentener, F.; Möller, D. Ammonia in the environment: From ancient times to the present. Environ. Pollut. 2008, 156, 583–604. [Google Scholar] [CrossRef]
- Wang, S.; Nan, J.; Shi, C.; Fu, Q.; Gao, S.; Wang, D.; Cui, H.; Saiz-Lopez, A.; Zhou, B. Atmospheric ammonia and its impacts on regional air quality over the megacity of Shanghai, China. Sci. Rep. 2015, 5, 15842. [Google Scholar] [CrossRef] [Green Version]
- Updyke, K.M.; Nguyen, T.; Nizkorodov, S.A. Formation of brown carbon via reactions of ammonia with secondary organic aerosols from biogenic and anthropogenic precursors. Atmos. Environ. 2012, 63, 22–31. [Google Scholar] [CrossRef]
- Park, R.S.; Lee, S.; Shin, S.-K.; Song, C.H. Contribution of ammonium nitrate to aerosol optical depth and direct radiative forcing by aerosols over East Asia. Atmos. Chem. Phys. Discuss. 2014, 14, 2185–2201. [Google Scholar] [CrossRef] [Green Version]
- Nor, N.S.M.; Yip, C.W.; Ibrahim, N.; Jaafar, M.H.; Rashid, Z.Z.; Mustafa, N.; Hamid, H.H.A.; Chandru, K.; Latif, M.T.; Saw, P.E.; et al. Particulate matter (PM2.5) as a potential SARS-CoV-2 carrier. Sci. Rep. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Mendy, A.; Wu, X.; Keller, J.L.; Fassler, C.S.; Apewokin, S.; Mersha, T.B.; Xie, C.; Pinney, S.M. Long-term exposure to fine particulate matter and hospitalization in COVID-19 patients. Respir. Med. 2021, 178, 106313. [Google Scholar] [CrossRef] [PubMed]
- Khunger, A.; Kaur, N.; Mishra, Y.K.; Chaudhary, G.R.; Kaushik, A. Perspective and prospects of 2D MXenes for smart biosensing. Mater. Lett. 2021, 304, 130656. [Google Scholar] [CrossRef]
- Kaur, N.; Khunger, A.; Wallen, S.L.; Kaushik, A.; Chaudhary, G.R.; Varma, R.S. Advanced green analytical chemistry for environmental pesticide detection. Curr. Opin. Green Sustain. Chem. 2021, 30, 100488. [Google Scholar] [CrossRef]
- Chaudhary, V. Charge Carrier Dynamics of Electrochemically Synthesized Poly (Aniline Co-Pyrrole) Nanospheres Based Sulfur Dioxide Chemiresistor. Polym. Plast. Technol. Mater. 2021, 1–9. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, X.; Zhao, F.; Song, Q.; Su, G.; Tan, Y.; Tao, Q.; Zhou, T.; Yu, Y.; Zhou, Z.; et al. Protein-Inspired Self-Healable Ti3C2 MXenes/Rubber-Based Supramolecular Elastomer for Intelligent Sensing. ACS Nano 2020, 14, 2788–2797. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, J.; Song, J.; Gao, C.; Wang, Z.; Song, C.; Wu, Y.; Liu, Y. Self-Healing Ti3C2 MXene/PDMS Supramolecular Elastomers Based on Small Biomolecules Modification for Wearable Sensors. ACS Appl. Mater. Interfaces 2020, 12, 45306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Wang, Y.; Li, X. Humidity-Enabled Ionic Conductive Trace Carbon Dioxide Sensing of Nitrogen-Doped Ti3C2Tx MXene/Polyethyleneimine Composite Films Decorated with Reduced Graphene Oxide Nanosheets. Anal. Chem. 2020, 92, 16033–16042. [Google Scholar] [CrossRef] [PubMed]
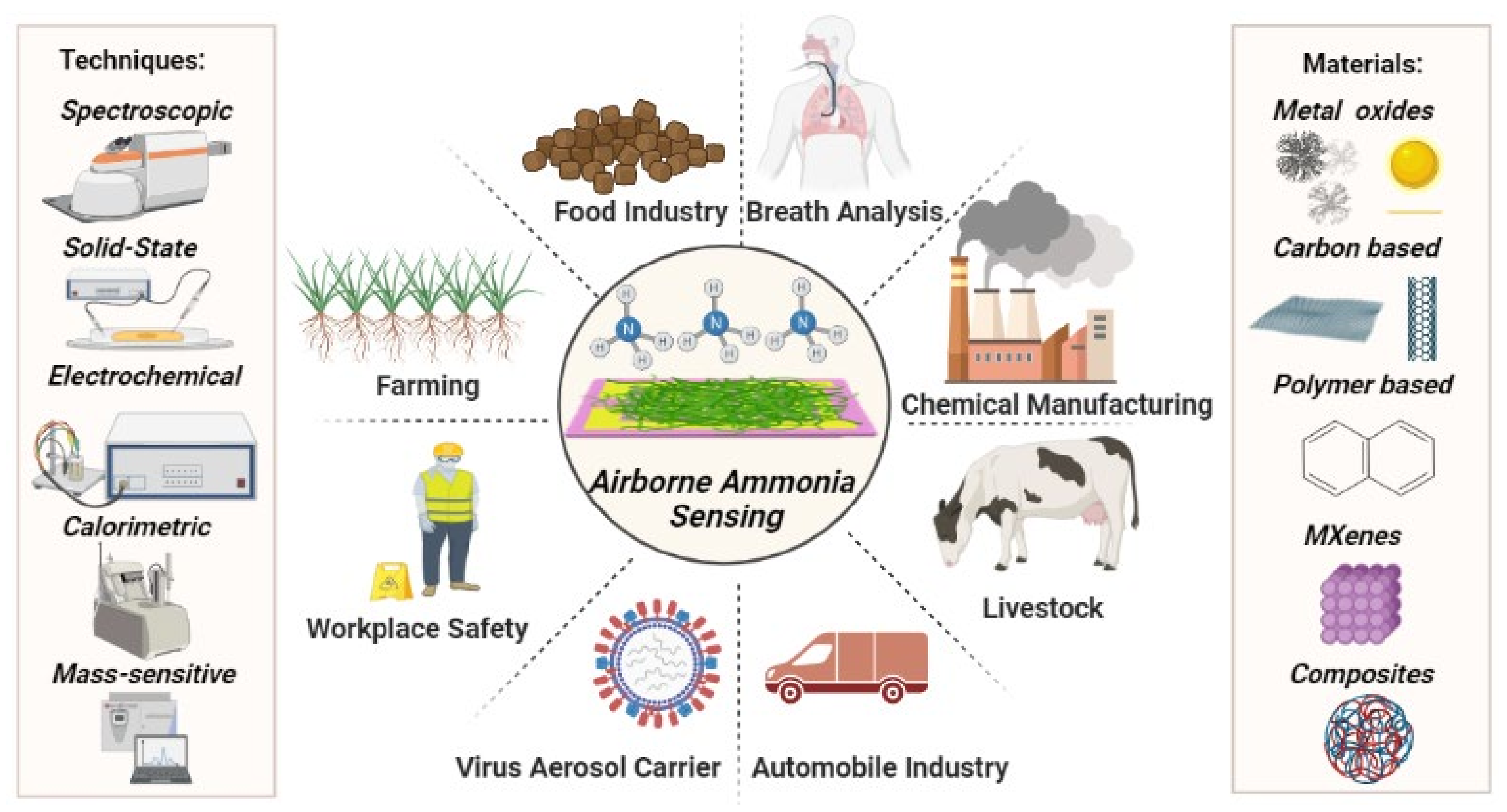
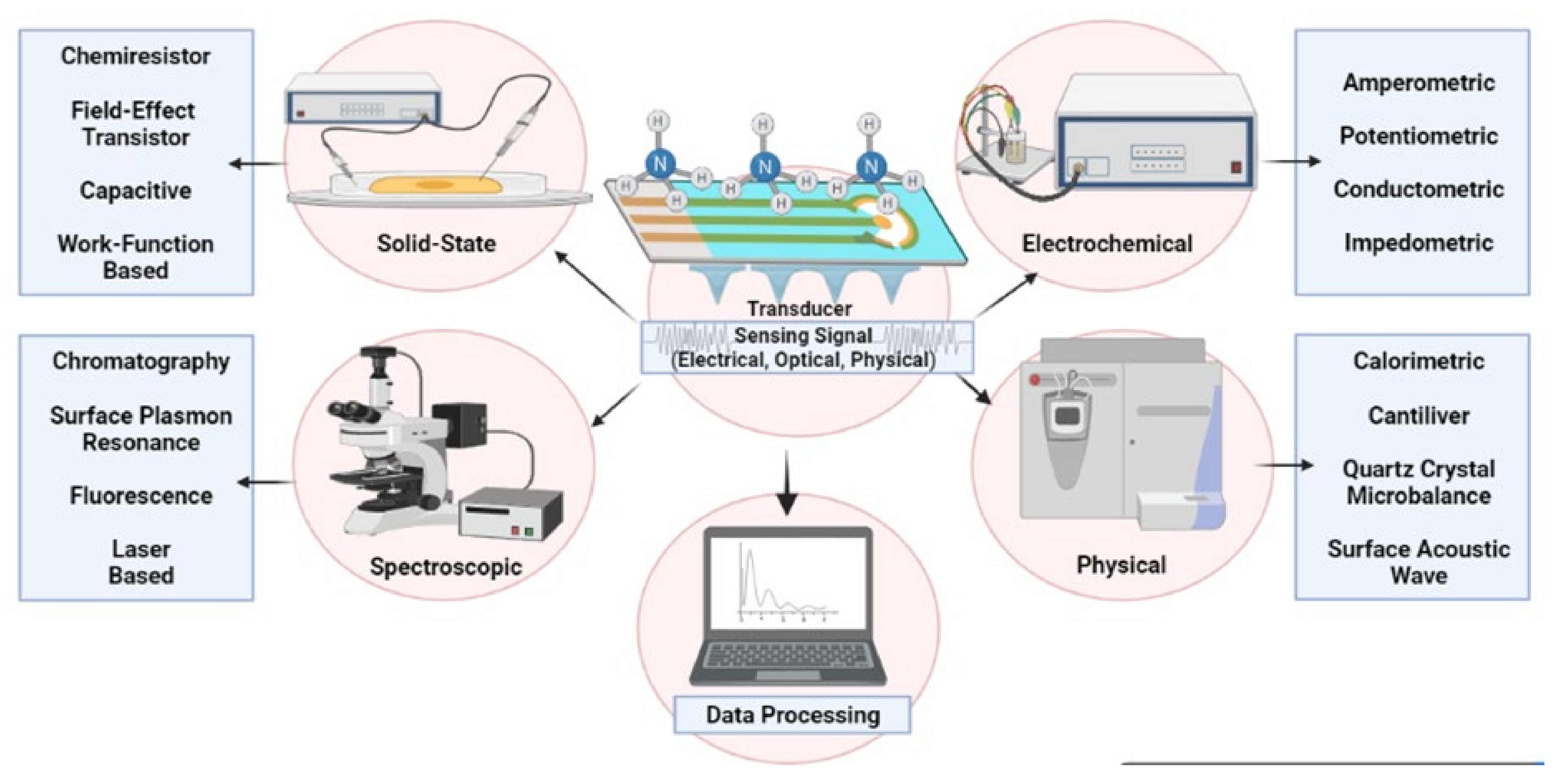
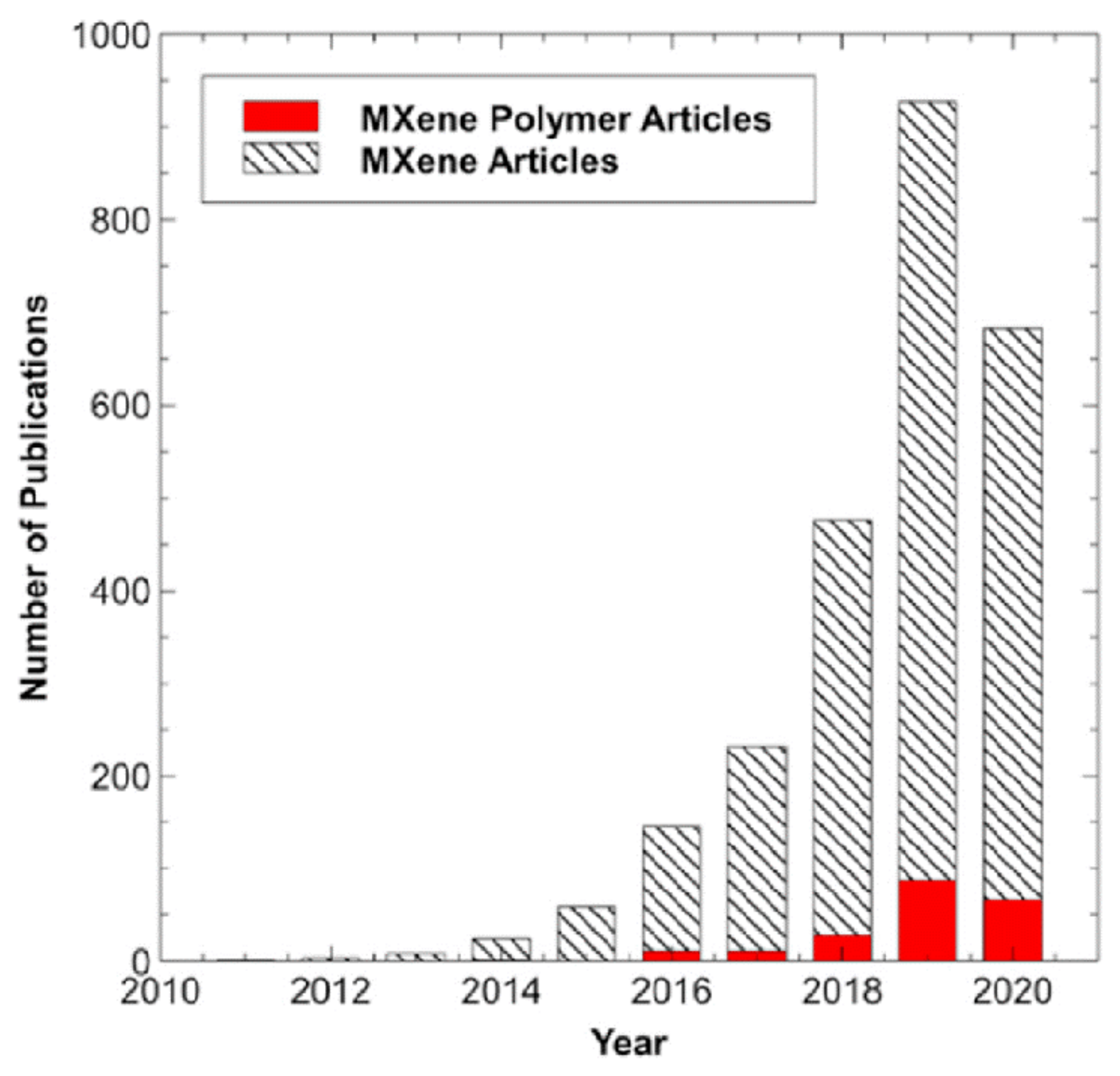
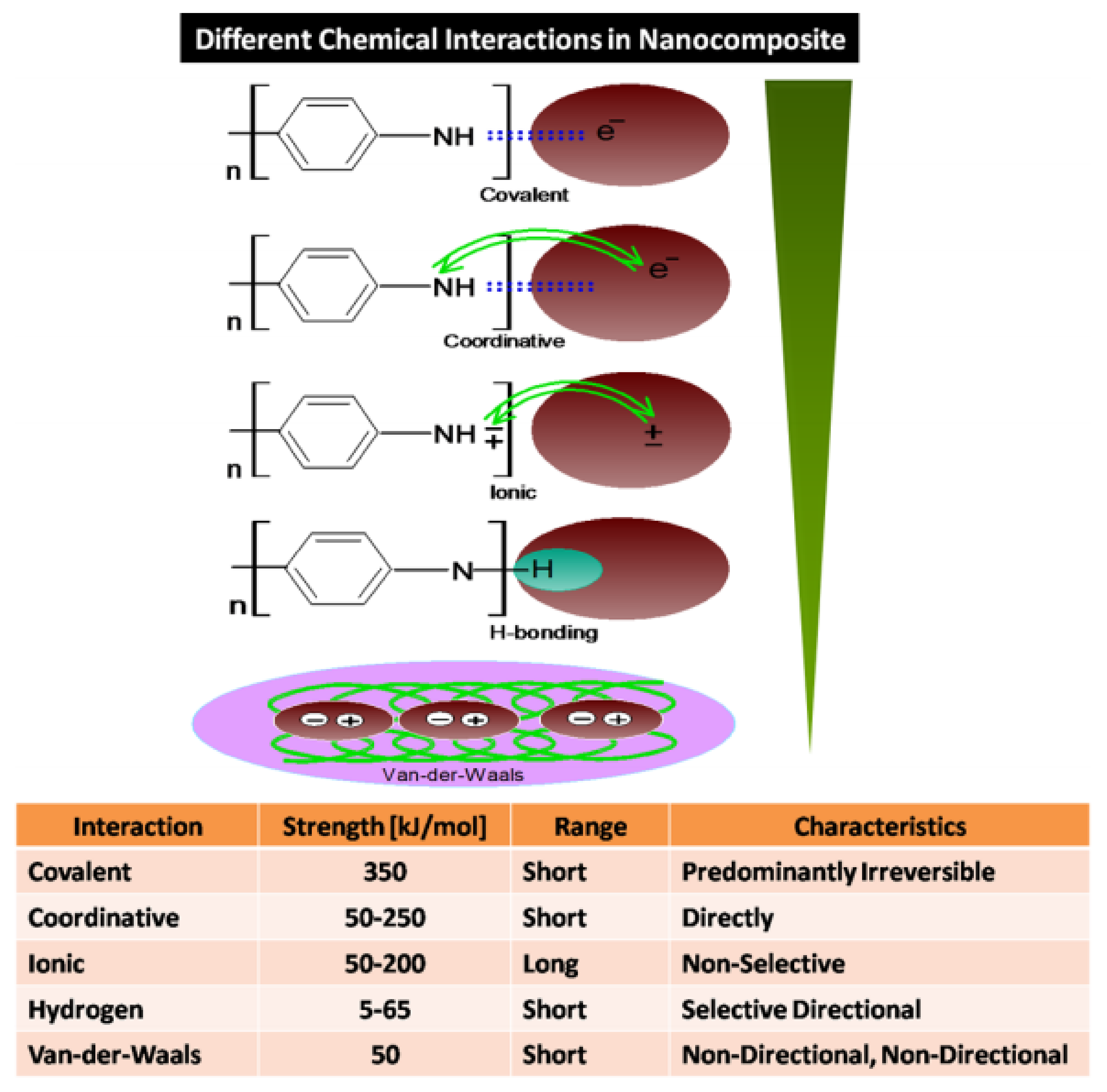

| Type of Sensors | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Electrochemical Sensors [10,11,17] | Low detection limit | Short lives | Typical laboratory analysis |
| Insensitive to environment change | Malfunction of Electrodes | ||
| High and accurate sensitivity | Requirement of special design for selective detection | Workplaces such as chemical industries | |
| Spectroscopic Sensors [10,11,17] | Long lifetime | Difficulty in miniaturization to be installed at every emission site | Remote air quality monitoring |
| Expensive | |||
| Insensitive to environment change | Complicated design | Gas leak detection systems with accuracy and safety | |
| High sensitivity, selectivity, and stability | Time consuming | ||
| Calorimetric Sensors [10,11,17] | Cost effective | Selectivity | Most combustible gases under industrial environment |
| Adequate sensitivity for industrial detection | Low versatility | ||
| Stable at ambient temperature | Risk of catalyst poisoning and explosion | Petrochemical plants | |
| Mass sensitive sensors [10,11,17] | Long lifetime | Low sensitivity | Components of Wireless Sensor Networks. |
| Sensitive to environmental change | |||
| Avoiding secondary pollution | Selectivity | ||
| Sophisticated equipment | |||
| FET and Diode based Sensors [10,11,17] | Adequate sensitivity | Fabrication complexity | Industrial applications and civil use |
| Cost effective | Miniaturization | ||
| Detailed Analysis | Characterization | ||
| Chemiresistors [10,11,17,18] | Cost effective | Sensitiveness to surrounding environment | Humidity monitoring |
| Long lifetime | |||
| Eco-friendly | Industrial applications | ||
| Energy efficient |
| Sensing Material | Sensing Parameter | Sensitivity @ Lowest Detection Range | Temperature of Detection | Reference |
|---|---|---|---|---|
| Ti3C2Tx/PEDOT:PSS | Resistance | 4.94% @ 10 ppm | 27 °C | [55] |
| Ti3C2Tx/PAN | Resistance | 0.05% @ 25 ppb | RT | [39] |
| Nb2CTx/PAN-TENG | Voltage | 9.33% @ 1 ppm | RT | [45] |
| Nb2CTx/PAN | Resistance | 1.19% @ 20 ppb | 25 °C | [46] |
| Ti3C2Tx/CPAM | Current | 1.5% @ 50 ppm | RT | [51] |
| Nb2CTx | Voltage | 8.15% @ 100 ppm | 25 °C | [45] |
| PAN | Resistance | 11% @ 1 ppm | RT | [77] |
| V2CTx | Resistance | 1.7% @100 ppm | RT | [78] |
| PEDOT:PSS: Graphene | Resistance | 0.9% @ 5 ppm | RT | [79] |
| PAP | Resistance | 9% @ 10 ppm | 25 °C | [2] |
| Alkalise-Ti3C2Tx | Resistance | 11% @ 10 ppm | 25 °C | [80] |
| PAN-Ag | Resistance | 47.1% @ 1 ppm | 27 °C | [58] |
| PAN-GO | Resistance | 21.8% @ 0.5 ppm | 20 °C | [81] |
| PAN-MWCNT | Voltage | 10% @ 0.01 ppm | RT | [82] |
| MXP-NC | Response Time | Recovery Time | Linear Regression Value | Repeatability | Reference |
|---|---|---|---|---|---|
| PEDOT:PSS/Ti3C2Tx | 116 s for 100 ppm | 40 s for 100 ppm | 0.957 for 10–100 ppm range | 3 cycles | [55] |
| 0.983 for 100–1000 ppm range | |||||
| PAN/Ti3C2Tx | ~600 s for 25 ppb | ~1400 s for 25 ppb | 0.997 for 2–10 ppm | 4 cycles | [39] |
| CPAM/Ti3C2Tx | 12.7 s for 150 ppm | 14.6 s for 150 ppm | 0–2000 ppm | 10 cycles | [51] |
| Nb2CTx/PAN-TENG | 105 s for 100 ppm | 143 s for 100 ppm | 0.9655 for 1–100 ppm | 3 cycles | [45] |
| Nb2CTx/PAN | 218 s for 10 ppm | 300 for 10 ppm | 0.9951 for 0.1–10 ppm | 3 cycles | [46] |
| V2CTx | 105 s for 100 ppm | 120 s for 100 ppm | Not mentioned | 3 cycles | [78] |
| PAN/MWCNT-TENG | 120 s for 100 ppm | 127 s for 100 ppm | 0.9928 for 20–100 ppm | 3 cycles | [82] |
| Alkaised-Ti3C2Tx | 1 s for 100 ppm | 201 s for 100 ppm | Not mentioned | 5 Cycles | [80] |
| PAN-Ag | 271 s for 5 ppm | 575 s for 5 ppm | 1–100 ppm | 4 cycles | [58] |
| Analytes | PEDOT:PSS/Ti3C2Tx [55] | PAN/Ti3C2Tx [39] | CPAM/Ti3C2Tx [51] | Nb2CTx/PAN-TENG [45] | Nb2CTx/PAN [46] |
|---|---|---|---|---|---|
| Ammonia | 36.6% @100 ppm | ~1.7% @10 ppm | 4.7% @200 ppm | ~59% @10 ppm | 74.46% @10 ppm |
| Toluene | 1.2% @100 ppm | - | - | - | - |
| Methanol | 14% @100 ppm | - | ~15% @2000 ppm | - | - |
| Ethanol | 4.6% @100 ppm | - | ~10% @2000 ppm | ~3% @10 ppm | ~2% @10 ppm |
| Acetone | 3.4% @100 ppm | - | ~10% @2000 ppm | ~2% @10 ppm | ~2% @10 ppm |
| Sulfur dioxide | - | ~0.02% @25 ppm | - | ~6% @10 ppm | ~9% @10 ppm |
| Hydrogen Sulfide | - | ~1% @25 ppm | - | ~2% @10 ppm | ~1% @10 ppm |
| Formaldehyde | - | ~0.02% @25 ppm | - | ~0.5% @10 ppm | ~0.5% @10 ppm |
| Carbon Monoxide | - | ~0.05% @25 ppm | - | ~1% @10 ppm | Negligible @10 ppm |
| Methane | - | - | ~2.5% @10 ppm |
| MXP-NC | Bending Angle/Number of Folds | ||||
|---|---|---|---|---|---|
| PEDOT: PSS/Ti3C2Tx over PI substrate: [55] | |||||
| Bending Angle (in degrees) | 0 | 60 | 120 | 180 | 240 |
| Sensitivity @100 ppm | 36.4% | ~35–37% | |||
| PAN/Ti3C2Tx over PI substrate: [39] | |||||
| Bending Angle (in degrees) | 0 | 20 | 30 | 40 | |
| Bending Cycles: (in number) | 0 | 100 | 300 | 500 | |
| Sensitivity @10 ppm | ~1.6% | ||||
| CPAM/Ti3C2Tx over PET substrate: [51] | |||||
| Bending Angle (in degrees) | 0 | 40 | 60 | 80 | 100 |
| Bending Cycles: (in number) | 0 | 3 | |||
| Sensitivity @2000 ppm | ~45% | ~43–45% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, V.; Gautam, A.; Mishra, Y.K.; Kaushik, A. Emerging MXene–Polymer Hybrid Nanocomposites for High-Performance Ammonia Sensing and Monitoring. Nanomaterials 2021, 11, 2496. https://doi.org/10.3390/nano11102496
Chaudhary V, Gautam A, Mishra YK, Kaushik A. Emerging MXene–Polymer Hybrid Nanocomposites for High-Performance Ammonia Sensing and Monitoring. Nanomaterials. 2021; 11(10):2496. https://doi.org/10.3390/nano11102496
Chicago/Turabian StyleChaudhary, Vishal, Akash Gautam, Yogendra K. Mishra, and Ajeet Kaushik. 2021. "Emerging MXene–Polymer Hybrid Nanocomposites for High-Performance Ammonia Sensing and Monitoring" Nanomaterials 11, no. 10: 2496. https://doi.org/10.3390/nano11102496
APA StyleChaudhary, V., Gautam, A., Mishra, Y. K., & Kaushik, A. (2021). Emerging MXene–Polymer Hybrid Nanocomposites for High-Performance Ammonia Sensing and Monitoring. Nanomaterials, 11(10), 2496. https://doi.org/10.3390/nano11102496








