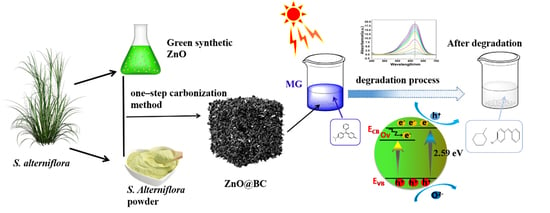
Synthesis of ZnO Nanoparticles Loaded on Biochar Derived from Spartina alterniflora with Superior Photocatalytic Degradation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO Nanoparticles
2.3. Synthesis of ZnO@BC Photocatalyst
2.4. Characterization of ZnO@BC Photocatalyst
2.5. Photodegradation of MG Using ZnO@BC Photocatalyst
2.6. Active Species Detection
2.7. Photocatalytic Degradation Products of MG Analysis
3. Results
3.1. Morphology and Microstructure Characterization
3.1.1. SEM and TEM Analysis
3.1.2. Porous Structure
3.2. Chemical Compositions Characterization
3.2.1. XRD Analysis
3.2.2. Raman Analysis
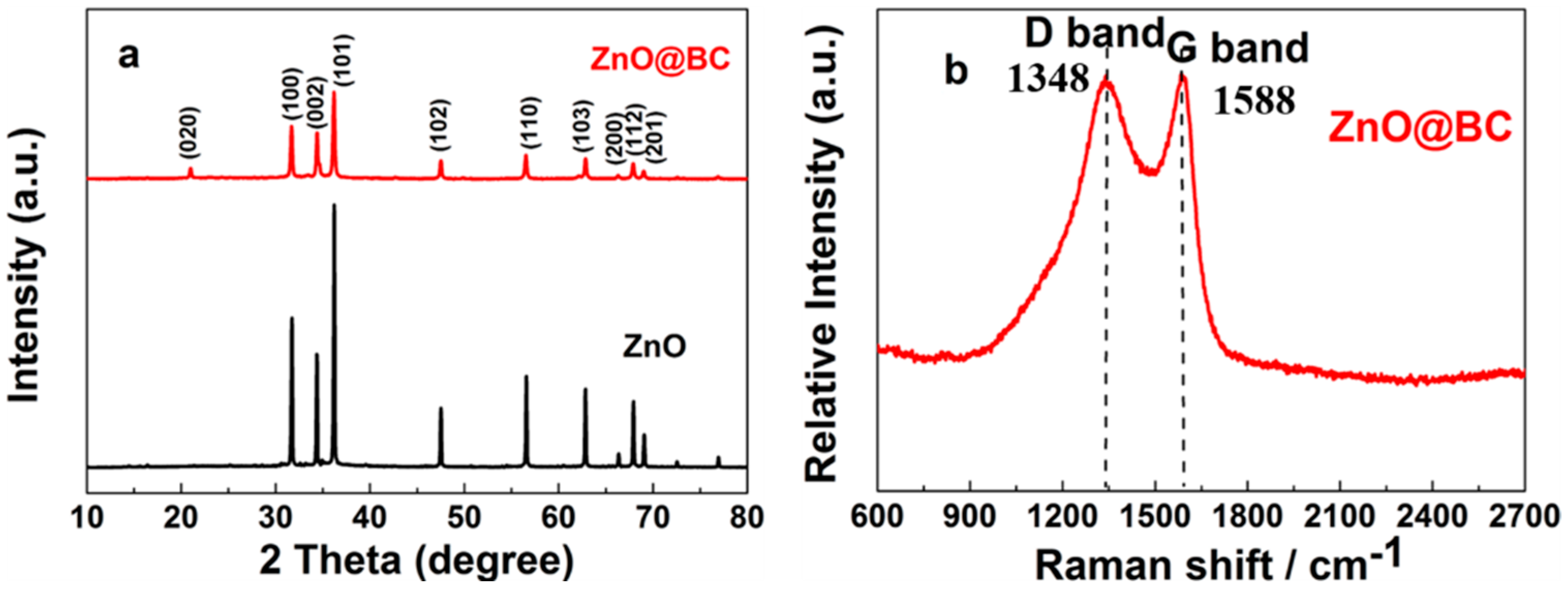
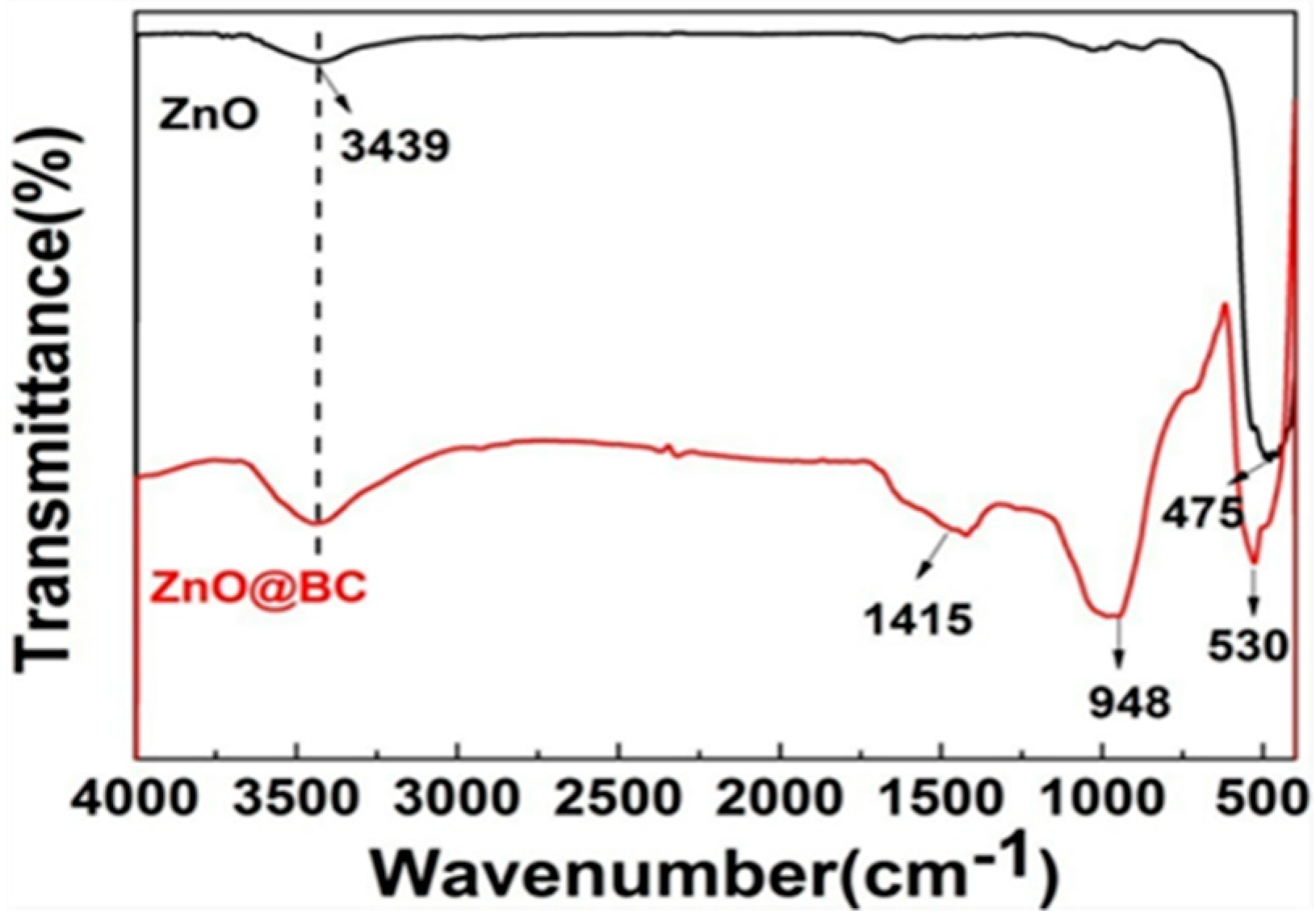
3.2.3. FTIR Analysis
3.2.4. XPS Analysis
3.3. Semiconductor Performance Characterization
3.3.1. UV–Vis DRS and Energy Band Gap Analysis
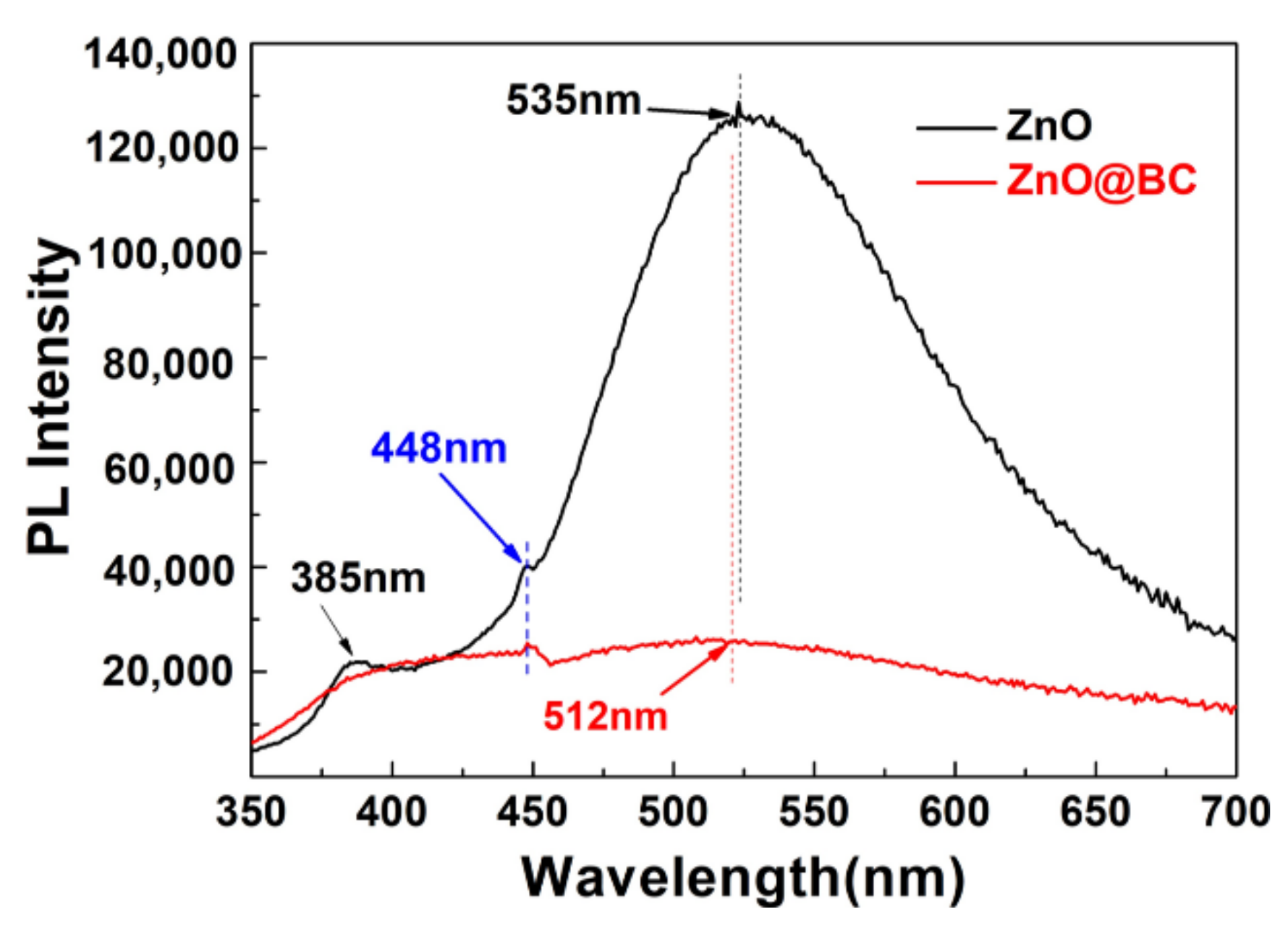
3.3.2. Photoluminescence Analysis
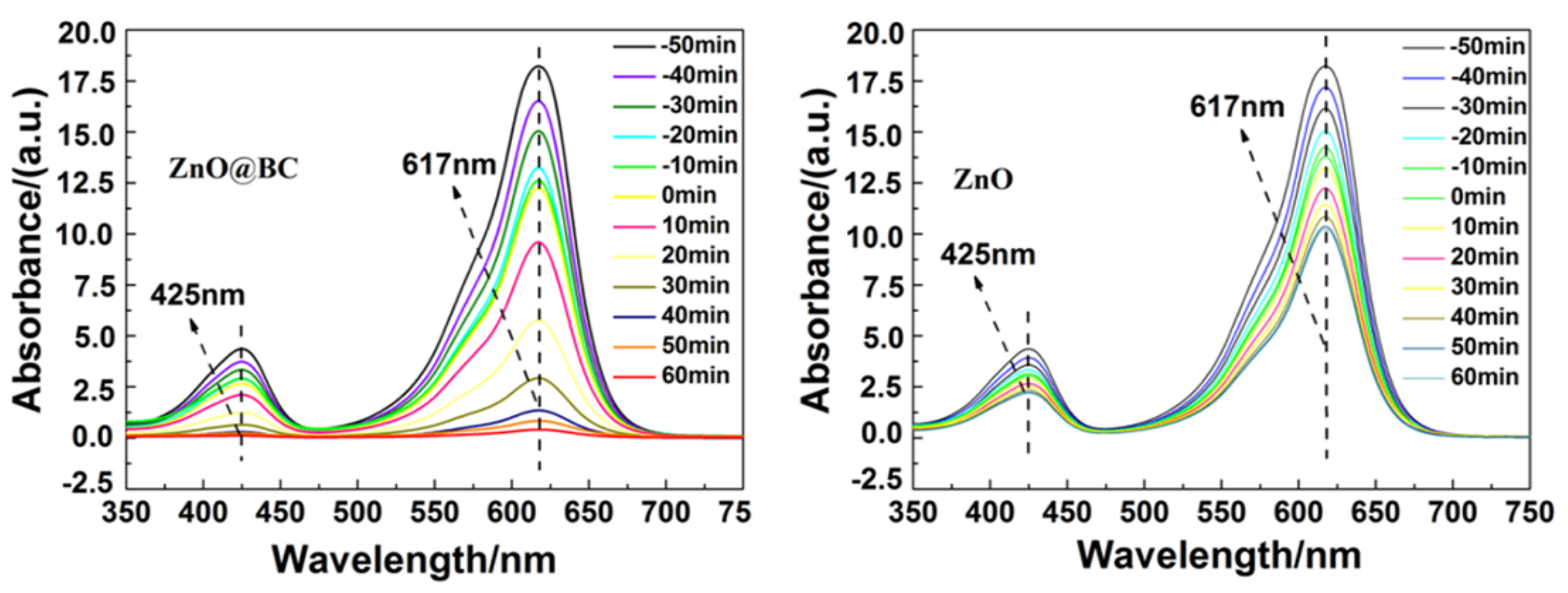
3.4. Photocatalysis Performance of MG Using ZnO@BC
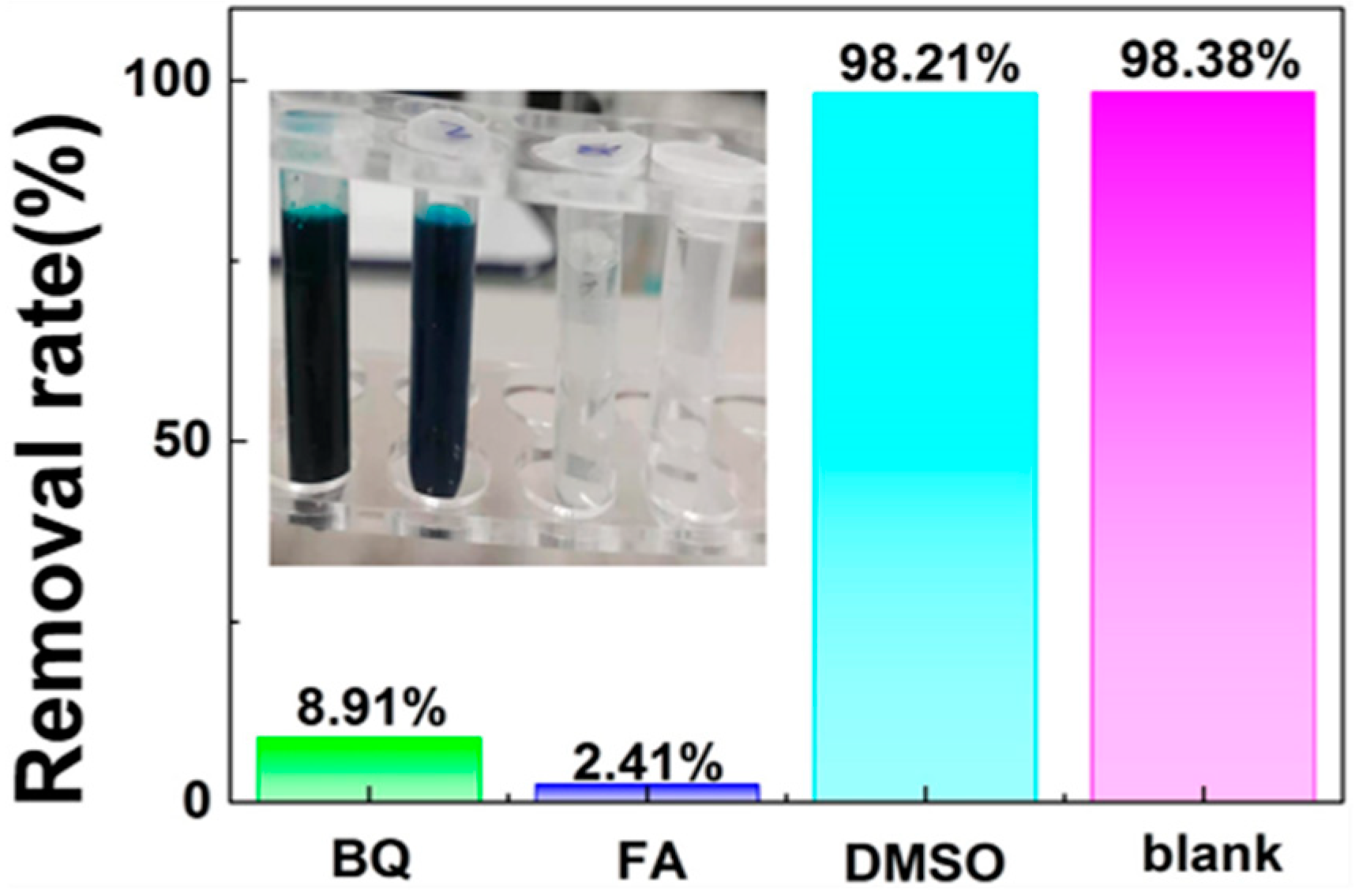
3.5. Active Species Analysis
3.6. Photocatalytic Degradation Products of MG Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anju, R.P.P.; Sunil, J.T.; Dinoop, L. Chitosan stabilized Fe/Ni bimetallic nanoparticles for the removal of cationic and anionic triphenylmethane dyes from water. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100295. [Google Scholar]
- Demirbas, A. Agricultural based activated carbons for the removal of dyes from aqueous solutions A review. J. Hazard. Mater. 2009, 167, 1–9. [Google Scholar]
- Xiao, W.; Jiang, X.; Liu, X.; Zhou, W.; Garba, Z.N.; Lawan, I.; Wang, L.; Yuan, Z. Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J. Clean. Prod. 2021, 284, 124773. [Google Scholar]
- Ren, Q.; Kong, C.; Chen, Z.; Zhou, J.; Li, W.; Li, D.; Cui, Z.; Xue, Y.; Lu, Y. Ultrasonic assisted electrochemical degradation of malachite green in wastewater. Microchem. J. 2021, 164, 106059. [Google Scholar]
- Kooravand, M.; Asadpour, S.; Haddadi, H.; Farhadian, S. An insight into the interaction between malachite green oxalate with human serum albumin: Molecular dynamic simulation and spectroscopic approaches. J. Hazard. Mater. 2021, 407, 124878. [Google Scholar]
- Liu, J.; Zhao, Q.; Cao, W.; Zhao, H.; Cheng, J.; Li, B.; Yang, X. Simple synthesis of magnetic porous organic cages for adsorption of triphenylmethane dyes in aquatic products. Microchem. J. 2020, 158, 105275. [Google Scholar]
- Wen, X.; Du, C.; Wan, J.; Zeng, G.; Huang, D.; Yin, L.; Deng, R.; Tan, S.; Zhang, J. Immobilizing laccase on kaolinite and its application in treatment of malachite green effluent with the coexistence of Cd (II). Chemosphere 2019, 217, 843–850. [Google Scholar]
- Loo, W.W.; Pang, Y.L.; Lim, S.; Wong, K.H.; Lai, C.W.; Abdullah, A.Z. Enhancement of photocatalytic degradation of Malachite Green using iron doped titanium dioxide loaded on oil palm empty fruit bunch-derived activated carbon. Chemosphere 2021, 272, 129588. [Google Scholar]
- Ganiyu, S.O.; Brito, L.R.D.; Costa, E.C.T.A.; Santos, E.V.; Huitle, C.A.M. Solar photovoltaic-battery system as a green energy for driven electrochemical wastewater treatment technologies: Application to elimination of Brilliant Blue FCF dye solution. J. Environ. Chem. Eng. 2019, 7, 102924. [Google Scholar]
- Silva, L.G.M.; Moreira, F.C.; Cechinel, M.A.P.; Mazur, L.P.; Souza, A.A.U.; Souza, S.M.A.G.; Boaventura, R.A.R.; Vilar, V.J.P. Integration of Fenton’s reaction based processes and cation exchange processes in textile wastewater treatment as a strategy for water reuse. J. Environ. Manag. 2020, 272, 111082. [Google Scholar]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [PubMed]
- Yin, X.; Zhang, Z.; Ma, H.; Venkateswaran, S.; Hsiao, B.S. Ultra-fine electrospun nanofibrous membranes for multicomponent wastewater treatment: Filtration and adsorption. Sep. Purif. Technol. 2020, 242, 116794. [Google Scholar]
- Herrmann, J.M. Fundamentals and misconceptions in photocatalysis. J. Photochem. Photobiol. A Chem. 2010, 216, 85–93. [Google Scholar]
- Bahadur, N.; Bhargava, N. Novel pilot scale photocatalytic treatment of textile & dyeing industry wastewater to achieve process water quality and enabling zero liquid discharge. J. Water Process Eng. 2019, 32, 100934. [Google Scholar]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar]
- Saravanan, M.; Dhivakar, S.; Jayanthi, S.S. An eco friendly and solvent free method for the synthesis of Zinc oxide nano particles using glycerol as organic dispersant. Mater. Lett. 2012, 67, 128–130. [Google Scholar]
- Dindar, B.; Içli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J. Photochem. Photobiol. A 2001, 140, 263–268. [Google Scholar]
- Li, W.; Wang, G.; Feng, Y.; Li, Z. Efficient photocatalytic performance enhancement in Co-doped ZnO nanowires coupled with CuS nanoparticles. Appl. Surf. Sci. 2018, 428, 154–164. [Google Scholar]
- Liu, Y.C.; Li, J.F.; Ahn, J.C.; Pu, J.Y.; Rupa, E.J.; Huo, Y.; Yang, D.C. Biosynthesis of zinc oxide nanoparticles by one-pot green synthesis using fruit extract of Amomum longiligulare and its activity as a photocatalyst. Optik 2020, 218, 165245. [Google Scholar]
- Tian, H.; Fan, H.; Ma, J.; Ma, L.; Dong, G. Noble metal-free modified electrode of exfoliated graphitic carbon nitride/ZnO nanosheets for highly efficient hydrogen peroxide sensing. Electrochim. Acta 2017, 247, 787–794. [Google Scholar]
- Li, Z.; Sun, Y.; Xing, J.; Xing, Y.; Meng, A. One step synthesis of Co/Cr-codoped ZnO nanoparticle with superb adsorption properties for various anionic organic pollutants and its regeneration. J. Hazard. Mater. 2018, 352, 204–214. [Google Scholar]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphoslogies. Nanomaterials 2020, 10, 1086. [Google Scholar]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 24, 102495. [Google Scholar]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; Crespo, J.S. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar]
- Król, A.; Pomastowski, P.; Rafinska, K.; Plugaru, V.R.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [PubMed]
- Zhou, Y.; Wu, W.; Hu, G.; Wu, H.; Cui, S. Hydrothermal synthesis of ZnO nanorod arrays with the addition of polyethyleneimine. Mater. Res. Bull. 2008, 43, 2113–2118. [Google Scholar]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [PubMed]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar]
- Choudhary, D.; Kumar, M.; Prasad, R.; Kumar, V. In Silico Modulation Techniques for Upgrading Sustainability and Competitiveness in Agri-food Sector. In In Silico Approach for Sustainable Agriculture; Springer: Singapore, 2018; pp. 161–167. [Google Scholar]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar]
- Alrajhi, A.H.; Ahmed, N.M.; Shafouri, M.A.; Almessiere, M.A.; Ghamdi, A.M.A. Green synthesis of zinc oxide nanoparticles using Salvia officials extract. Mater. Sci. Semicond. Process. 2021, 125, 105641. [Google Scholar]
- Ekennia, A.; Uduagwu, D.; Olowu, O.; Nwanji, O.; Oje, O.; Daniel, B.; Mgbii, S.; Uba, C.E. Biosynthesis of zinc oxide nanoparticles using leaf extracts of Alchornea laxiflora and its tyrosinase inhibition and catalytic studies. Micron 2021, 141, 102964. [Google Scholar]
- Sathishkumar, G.; Rajkuberan, C.; Manikandan, K.; Prabukumar, S.; Danieljohn, J.; Sivaramakrishnan, S. Facile biosynthesis of antimicrobial zinc oxide (ZnO) nanoflakes using leaf extract of Couroupita guianensis. Aubl. Mater. Lett. 2017, 188, 383–386. [Google Scholar]
- Jackson, M.V.; Fuller, R.A.; Gan, X.J.; Li, J.; Mao, D.H.; Melville, D.S.; Murray, N.J.; Wang, Z.M.; Choi, C. Dual threat of tidal flat loss and invasive Spartina alterniflora endanger important shorebird habitat in coastal mainland China. J. Environ. Manag. 2021, 278, 111549. [Google Scholar]
- Zhang, G.; Bai, J.; Tebbe, C.C.; Huang, L.; Jia, J.; Wang, W.; Wang, X.; Yu, L.; Zhao, Q. Spartina alterniflora invasions reduce soil fungal diversity and simplify co-occurrence networks in a salt marsh ecosystem. Sci. Total Environ. 2021, 758, 143667. [Google Scholar] [PubMed]
- Feng, Q.; Wang, B.; Chen, M.; Wu, P.; Lee, X.; Xing, Y. Invasive plants as potential sustainable feedstocks for biochar production and multiple applications: A review. Resour. Conserv. Recycl. 2021, 164, 105204. [Google Scholar]
- Cai, J.F.; Zhang, L.; Zhang, Y.; Zhang, M.X.; Li, H.L.; Xia, H.J.; Kong, W.J.; Yu, F.H. Remediation of cadmium-contaminated coastal saline-alkaline soil by Spartina alterniflora derived biochar. Ecotoxicol. Environ. Saf. 2020, 205, 111172. [Google Scholar] [PubMed]
- Xia, H.; Kong, W.; Liu, L.; Lin, K.; Li, H. Effects of harvest time and desalination of feedstock on Spartina alterniflora biochar and its efficiency for Cd2+ removal from aqueous solution. Ecotoxicol. Environ. Saf. 2020, 207, 111309. [Google Scholar]
- Li, M.; Liu, Q.; Guo, L.; Zhang, Y.; Lou, Z.; Wang, Y.; Qian, G. Cu (Ⅱ) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresour. Technol. 2012, 141, 83–88. [Google Scholar]
- Xu, C.; Ge, Z.; Li, C.; Wan, F.; Xiao, X. Inhibition of harmful algae Phaeocystis globosa and Prorocentrum donghaiense by extracts of coastal invasive plant Spartina alterniflora. Sci. Total Environ. 2019, 696, 133930. [Google Scholar]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar]
- Shen, J.; Liu, A.; Tu, Y.; Foo, G.; Yeo, C.; Chan-Park, M.B.; Jiang, R.; Chen, Y. How carboxylic groups improve the performance of single-walled carbon nanotube electrochemical capacitors? Energy Environ. Sci. 2011, 4, 4220–4229. [Google Scholar]
- Zhou, J.; Zhao, F.; Wang, Y.; Zhang, Y.; Yang, L. Size-controlled synthesis of ZnO nanoparticles and their photoluminescence properties. J. Lumin. 2007, 122, 195–197. [Google Scholar]
- Zhao, G.; Ding, C.; Pan, M.; Zhai, S. Fabrication of NCC-SiO2 hybrid colloids and its application on waterborne poly (acrylic acid) coatings. Prog. Org. Coat. 2018, 122, 88–95. [Google Scholar]
- Jiang, Q.; Zhang, Z.; Yin, S.; Guo, Z.; Wang, S.; Feng, C. Biomass carbon micro/nano-structures derived from ramie fibers and corncobs as anode materials for lithium-ion and sodium-ion batteries. Appl. Surf. Sci. 2016, 379, 73–82. [Google Scholar]
- Cheng, J.; Gu, J.J.; Tao, W.; Wang, P.; Liu, L.; Wang, C.Y.; Li, Y.K.; Feng, X.H.; Qiu, G.H.; Cao, F.F. Edible fungus slag derived nitrogen-doped hierarchical porous carbon as a high-performance adsorbent for rapid removal of organic pollutants from water. Bioresour. Technol. 2019, 294, 122149. [Google Scholar] [PubMed]
- Fazlzadeh, M.; Khosravi, R.; Zarei, A. Green synthesis of zinc oxide nanoparticles using Peganum harmala seed extract and loaded on Peganum harmala seed powdered activated carbon as new adsorbent for removal of Cr (VI) from aqueous solution. Ecol. Eng. 2017, 103, 180–190. [Google Scholar]
- Zheng, Q.F.; Wang, Y.H.; Sun, Y.G.; Niu, H.H.; Zhou, J.R.; Wang, Z.M.; Zhao, J. Study on structural properties of biochar under different materials and carbonized by FTIR. Spectrosc. Spectr. Anal. 2014, 34, 962–966. [Google Scholar]
- Manjula, N.; Chen, S.M. One-pot synthesis of rod-shaped gadolinia doped zinc oxide decorated on graphene oxide composite as an efficient electrode material for isoprenaline sensor. Compos. Part B 2021, 211, 108631. [Google Scholar]
- Mohammed, N.; Lian, H.; Islam, M.S.; Strong, M.; Shi, Z.; Berry, R.M.; Yu, H.Y.; Tam, K.C. Selective adsorption and separation of organic dyes using functionalized cellulose nanocrystals. Chem. Eng. J. 2021, 417, 129237. [Google Scholar]
- Khan, M.; Saadah, N.H.; Khan, M.E.; Harunsani, M.H.; Tan, A.L.; Cho, M.H. Potentials of Costus woodsonii leaf extract in producing narrow band gap ZnO nanoparticles. Mater. Sci. Semicond. Process. 2019, 91, 194–200. [Google Scholar]
- Sun, Y.; Wang, H.; Xing, Q.; Cui, W.; Li, J.; Wu, S.; Sun, L. The pivotal effects of oxygen vacancy on Bi2MoO6: Promoted visible light photocatalytic activity and reaction mechanism. Chin. J. Catal. 2019, 40, 647–655. [Google Scholar]
- Yu, H.; Chen, G.; Wang, Y.; Yao, J. A facile one-pot route for preparing cellulose nanocrystal/zinc oxide nanohybrids with high antibacterial and photocatalytic activity. Cellulose 2015, 22, 261–273. [Google Scholar]
- Ahmed, G.; Hanif, M.; Zhao, L.; Hussain, M.; Khan, J.; Liu, Z. Defect engineering of ZnO nanoparticles by graphene oxide leading to enhanced visible light photocatalysis. J. Mol. Catal. A Chem. 2016, 425, 310–321. [Google Scholar]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Łomot, D.; Chernyayeva, O.; Lisovytskiy, D. Novel biomass-derived hybrid TiO2/carbon material using tar-derived secondary char to improve TiO2 bonding to carbon maatrix. J. Anal. Appl. Pyrolysis 2018, 131, 35–41. [Google Scholar]
- Fazal, T.; Razzaq, A.; Javed, F.; Hafeez, A.; Rashid, N.; Amjad, U.S.; Rehman, M.S.U.; Faisal, A.; Rehman, F. Integrating adsorption and photocatalysis: A cost effective strategy for textile wastewater treatment using hybrid biochar-TiO2 composite. J. Hazard. Mater. 2019, 390, 121623. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Graphene Transforms Wide Band Gap ZnS to a Visible Light Photocatalyst. The New Role of Graphene as a Macromolecular Photosensitizer. ACS Nano 2012, 6, 9777–9789. [Google Scholar]
- Bai, X.; Wang, L.; Zong, R.; Lv, Y.; Sun, Y.; Zhu, Y. Performance enhancement of ZnO photocatalyst via synergic effect of surface oxygen defect and graphene hybridization. Langmuir 2013, 29, 3097–3105. [Google Scholar]
- Son, D.I.; Yang, H.Y.; Kim, T.W.; Park, W.I. Photoresponse mechanisms of ultraviolet photodetectors based on colloidal ZnO quantum dot-graphene nanocomposites. Appl. Phys. Lett. 2013, 102, 021105. [Google Scholar]
- Dhandapani, K.V.; Anbumani, D.; Gandhi, A.D.; Annamalai, P.; Muthuvenkatachalam, B.S.; Kavitha, P.; Ranganathan, B. Green route for the synthesis of zinc oxide nanoparticles from Melia azedarach leaf extract and evaluation of their antioxidant and antibacterial activities. Biocatal. Agric. Biotechnol. 2020, 24, 101517. [Google Scholar]
- Wang, Y.; Zhu, H.; Tam, N.F.Y. Effect of a polybrominated diphenyl ether congener (BDE-47) on growth and antioxidative enzymes of two mangrove plant species, Kandelia obovate and Avicennia marina, in South China. Mar. Pollut. Bull. 2014, 85, 376–384. [Google Scholar] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, H.; Ji, L.; Wang, Z.; Guo, J.; Lu, S.; Sun, J.; Cai, L.; Wang, Y. Synthesis of ZnO Nanoparticles Loaded on Biochar Derived from Spartina alterniflora with Superior Photocatalytic Degradation Performance. Nanomaterials 2021, 11, 2479. https://doi.org/10.3390/nano11102479
Jing H, Ji L, Wang Z, Guo J, Lu S, Sun J, Cai L, Wang Y. Synthesis of ZnO Nanoparticles Loaded on Biochar Derived from Spartina alterniflora with Superior Photocatalytic Degradation Performance. Nanomaterials. 2021; 11(10):2479. https://doi.org/10.3390/nano11102479
Chicago/Turabian StyleJing, Hua, Lili Ji, Zhen Wang, Jian Guo, Shiyao Lu, Jiaxing Sun, Lu Cai, and Yaning Wang. 2021. "Synthesis of ZnO Nanoparticles Loaded on Biochar Derived from Spartina alterniflora with Superior Photocatalytic Degradation Performance" Nanomaterials 11, no. 10: 2479. https://doi.org/10.3390/nano11102479
APA StyleJing, H., Ji, L., Wang, Z., Guo, J., Lu, S., Sun, J., Cai, L., & Wang, Y. (2021). Synthesis of ZnO Nanoparticles Loaded on Biochar Derived from Spartina alterniflora with Superior Photocatalytic Degradation Performance. Nanomaterials, 11(10), 2479. https://doi.org/10.3390/nano11102479