Rationally Designed CdS-Based Ternary Heterojunctions: A Case of 1T-MoS2 in CdS/TiO2 Photocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalysts
2.2. Characterization
2.3. Photocatalysis
2.4. Photoelectrochemical Measurements
3. Results and Discussion
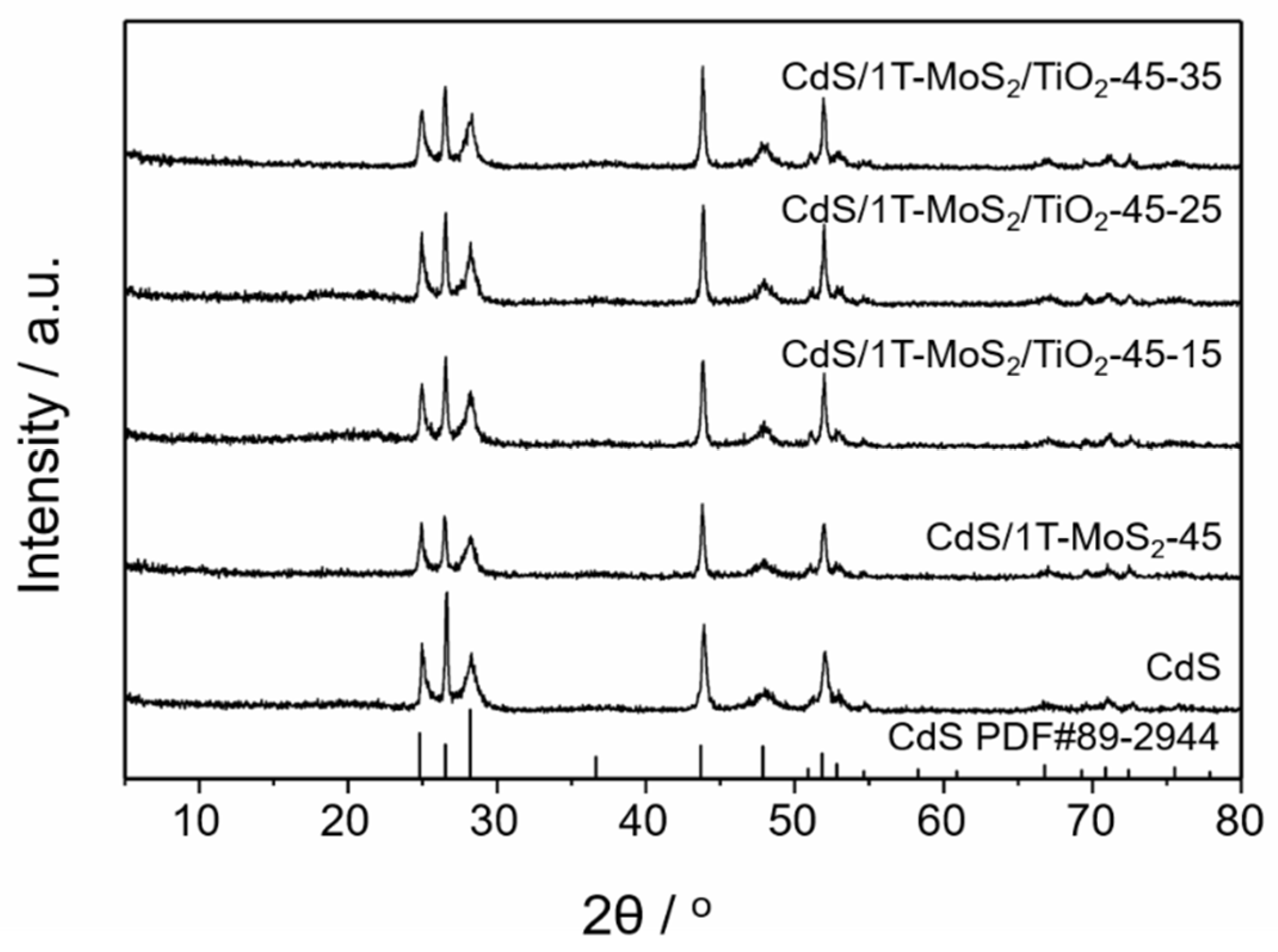
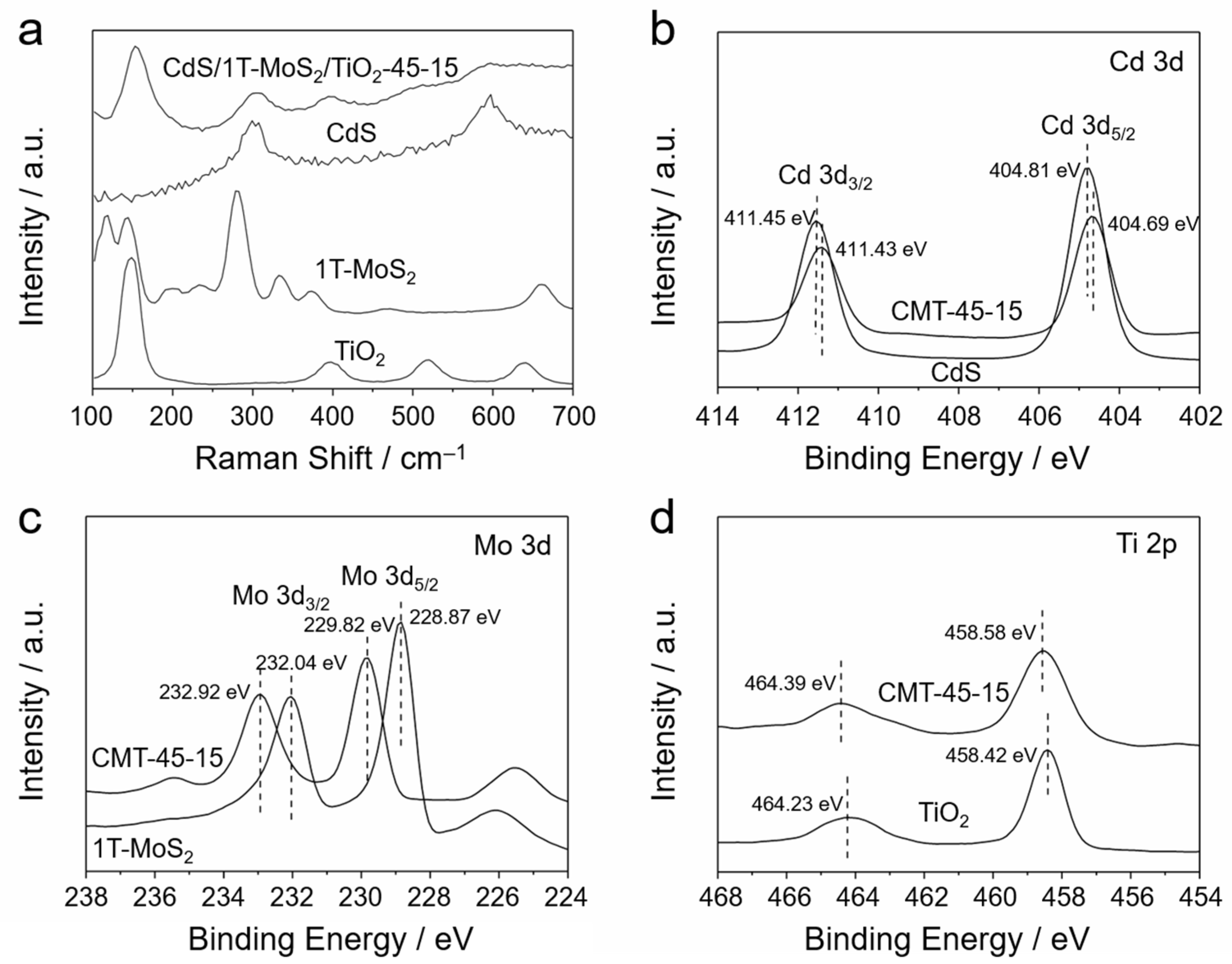
3.1. Structural Analysis
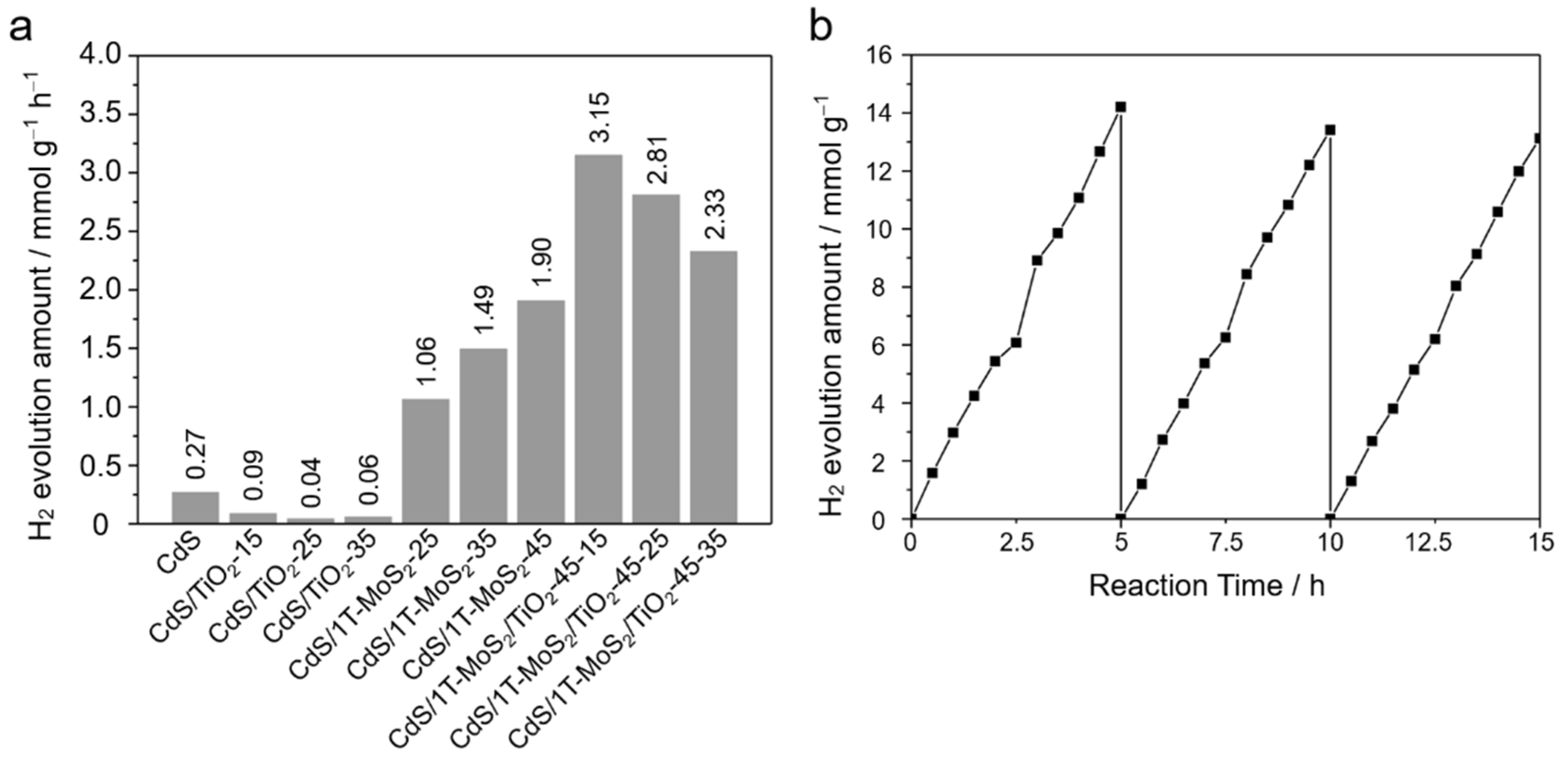
3.2. Photocatalysis
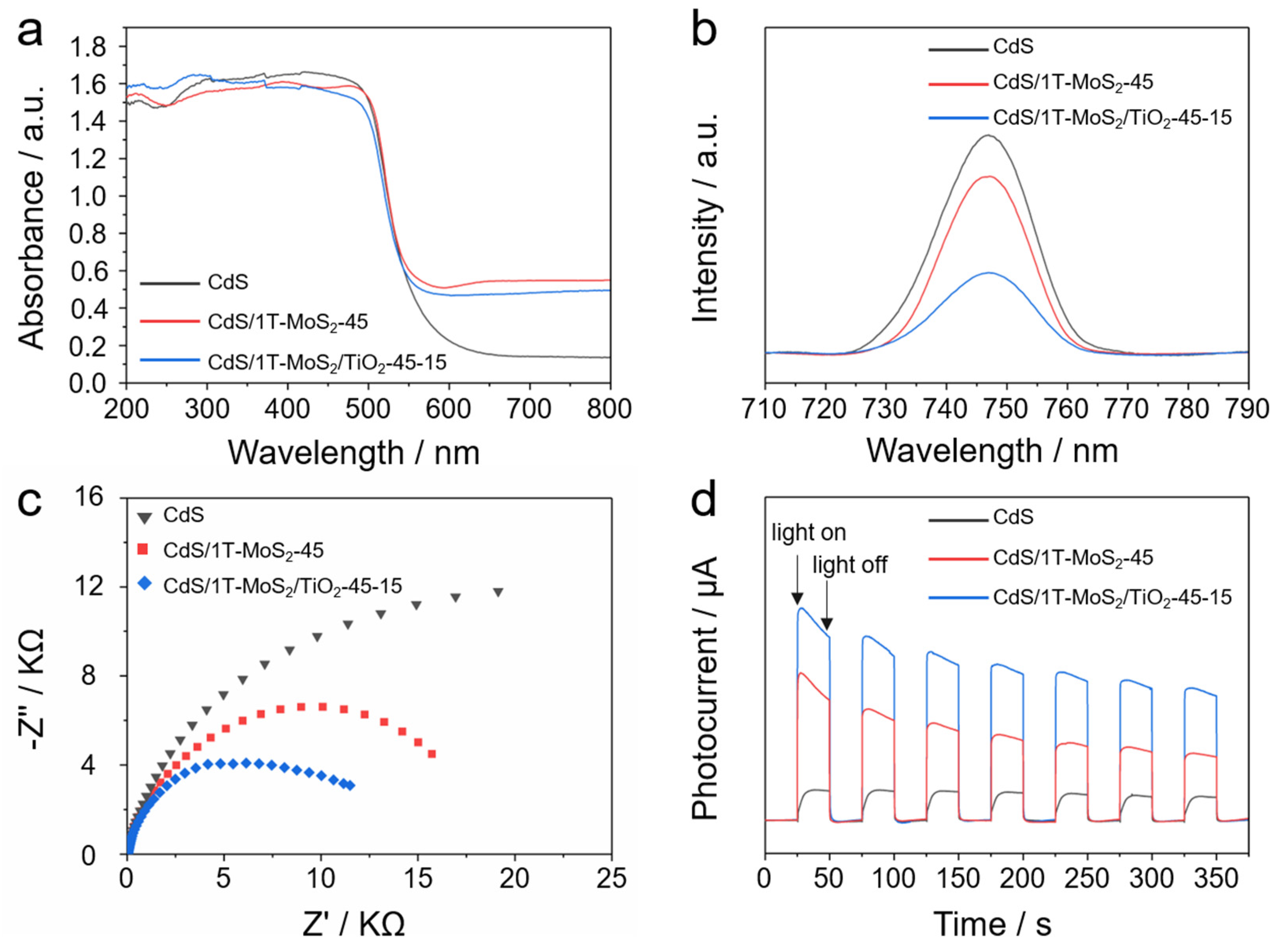
3.3. Light Absorption Properties
3.4. Charge Carrier Dynamics
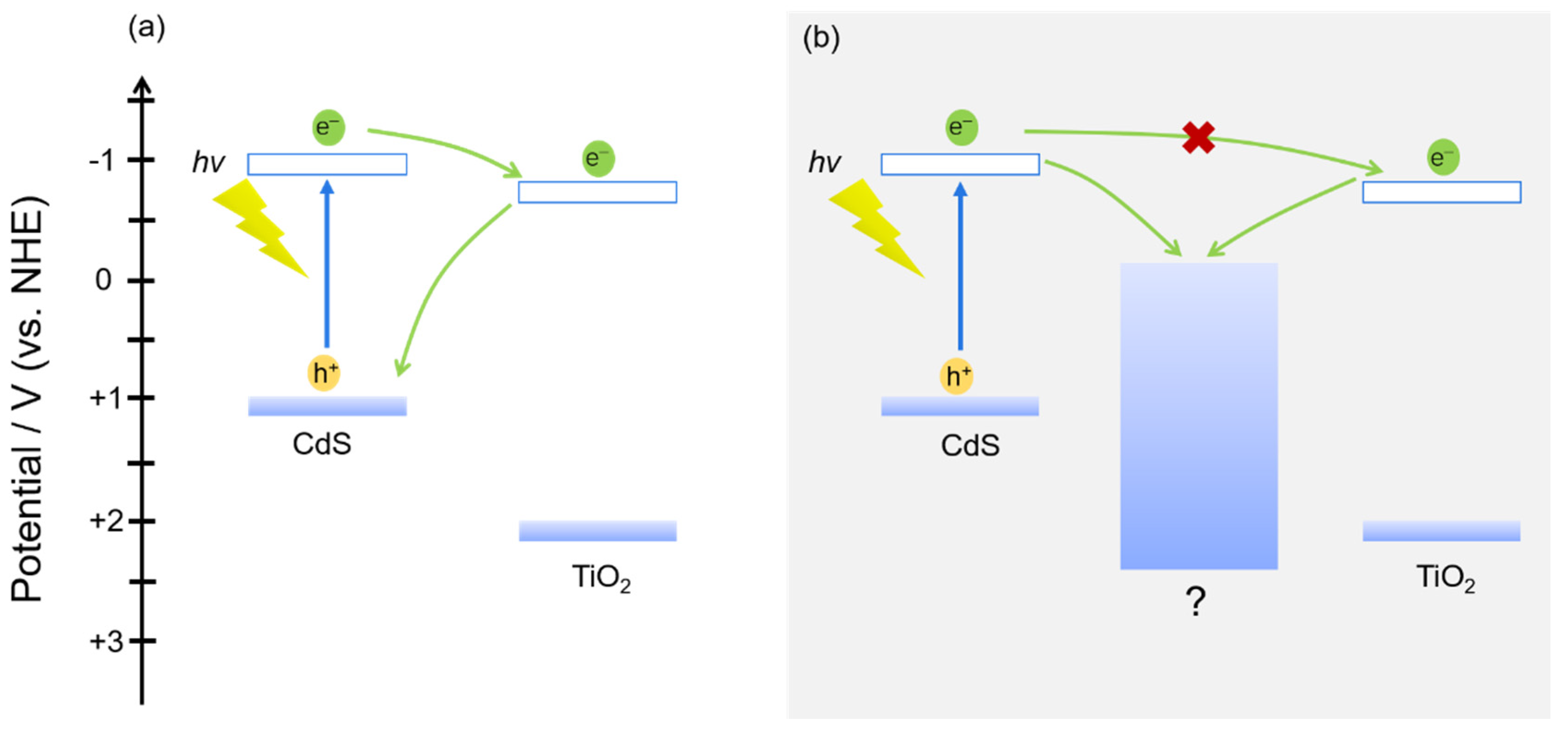
3.5. Band Potential Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.; Sellappa, K. Ni-doped ZnO nanocrystalline material for electrocatalytic oxygen reduction reaction. Energy Source Part A 2019, 42, 719–729. [Google Scholar] [CrossRef]
- Oladeji, I.O.; Chow, L.; Ferekides, C.S.; Viswanathan, V.; Zhao, Z. Metal/CdTe/CdS/Cd1− xZnxS/TCO/glass: A new CdTe thin film solar cell structure. Sol. Energy Mater. Sol. Cells 2000, 61, 203–211. [Google Scholar] [CrossRef]
- Zhong, W.; Tu, W.; Feng, S.; Xu, A. Photocatalytic H2 evolution on CdS nanoparticles by loading FeSe nanorods as co-catalyst under visible light irradiation. J. Alloys Compd. 2019, 772, 669–674. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Choi, M.; Baek, M.; Hsieh, P.-Y.; Yong, K.; Hsu, Y.-J. CdS/CdSe co-sensitized brookite H: TiO2 nanostructures: Charge carrier dynamics and photoelectrochemical hydrogen generation. Appl. Catal. B 2018, 225, 379–385. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Kouser, S.; Ingampalli, S.R.L.; Chithaiah, P.; Roy, A.; Saha, S.; Waghmare, U.V.; Rao, C.N. Extraordinary changes in the electronic structure and properties of CdS and ZnS by anionic substitution: Cosubstitution of P and Cl in place of S. Angew. Chem. Int. Ed. 2015, 54, 8149–8153. [Google Scholar] [CrossRef]
- Roy, A.; Shenoy, U.S.; Manjunath, K.; Vishnoi, P.; Waghmare, U.V.; Rao, C.N.R. Structure and Properties of Cd4P2Cl3, an Analogue of CdS. J. Phys. Chem. C 2016, 120, 15063–15069. [Google Scholar] [CrossRef]
- Xu, M.; Ye, T.; Dai, F.; Yang, J.; Shen, J.; He, Q.; Chen, W.; Liang, N.; Zai, J.; Qian, X. Rationally designed n–n heterojunction with highly efficient solar hydrogen evolution. ChemSusChem 2015, 8, 1218–1225. [Google Scholar] [CrossRef]
- Sant, P.A.; Kamat, P.V. Interparticle electron transfer between size-quantized CdS and TiO2 semiconductor nanoclusters. Phys. Chem. Chem. Phys. 2002, 4, 198–203. [Google Scholar] [CrossRef]
- Kim, H.-I.; Kim, J.; Kim, W.; Choi, W. Enhanced Photocatalytic and Photoelectrochemical Activity in the Ternary Hybrid of CdS/TiO2/WO3 through the Cascadal Electron Transfer. J. Phys. Chem. C 2011, 115, 9797–9805. [Google Scholar] [CrossRef]
- Mahadik, M.A.; Shinde, P.S.; Cho, M.; Jang, J.S. Fabrication of a ternary CdS/ZnIn2S4/TiO2 heterojunction for enhancing photoelectrochemical performance: Effect of cascading electron–hole transfer. J. Mater. Chem. A 2015, 3, 23597–23606. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Ding, H.; Li, W.; Wang, X. Well-Designed CdS/TiO2/MS-SiO2 Z-Scheme Photocatalyst for Combating Poison with Poison. Ind. Eng. Chem. Res. 2020, 59, 7659–7669. [Google Scholar] [CrossRef]
- Bian, X.; Hong, K.; Liu, L.; Xu, M. Magnetically separable hybrid CdS-TiO2-Fe3O4 nanomaterial: Enhanced photocatalystic activity under UV and visible irradiation. Appl. Surf. Sci. 2013, 280, 349–353. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Li, L.; Xue, Y.-C.; Xu, G.; Tang, L.; Liu, N.; Huang, W.-Y. Fabrication of ternary GO/g-C3N4/MoS2 flower-like heterojunctions with enhanced photocatalytic activity for water remediation. Appl. Catal. B 2018, 228, 103–112. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D.-E. Porous MS2/MO2 (M = W, Mo) Nanorods as Efficient Hydrogen Evolution Reaction Catalysts. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- Lei, Z.; Zhan, J.; Tang, L.; Zhang, Y.; Wang, Y. Recent Development of Metallic (1T) Phase of Molybdenum Disulfide for Energy Conversion and Storage. Adv. Energy Mater. 2018, 8, 1703482. [Google Scholar] [CrossRef]
- Xiong, F.; Wang, H.; Liu, X.; Sun, J.; Brongersma, M.; Pop, E.; Cui, Y. Li Intercalation in MoS2: In Situ Observation of Its Dynamics and Tuning Optical and Electrical Properties. Nano Lett. 2015, 15, 6777–6784. [Google Scholar] [CrossRef]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Kim, B.; Kim, Y.K. Highly Enhanced Photoactivity of Anatase TiO2 Nanocrystals by Controlled Hydrogenation-Induced Surface Defects. ACS Catal. 2013, 3, 2479–2486. [Google Scholar] [CrossRef]
- Ai, Z.; Shao, Y.; Chang, B.; Huang, B.; Wu, Y.; Hao, X. Effective orientation control of photogenerated carrier separation via rational design of a Ti3C2 (TiO2)@ CdS/MoS2 photocatalytic system. Appl. Catal. B 2019, 242, 202–208. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; He, Q.; Khalil, A.; Liu, D.; Xiang, T.; Wu, X.; Song, L. Gram-Scale Aqueous Synthesis of Stable Few-Layered 1T-MoS2: Applications for Visible-Light-Driven Photocatalytic Hydrogen Evolution. Small 2015, 11, 5556–5564. [Google Scholar] [CrossRef]
- Wu, M.; Ke, S.; Chen, W.; Zhang, S.; Zhu, M.; Zhang, Y.; Foo, M.L.; Tang, L. Optimization of the facet structure of cobalt oxide catalysts for enhanced hydrogen evolution reaction. Catal. Sci. Technol. 2020, 10, 1040–1047. [Google Scholar] [CrossRef]
- Xu, J.; Cao, X. Characterization and mechanism of MoS2/CdS composite photocatalyst used for hydrogen production from water splitting under visible light. Chem. Eng. J. 2015, 260, 642–648. [Google Scholar] [CrossRef]
- Yin, X.L.; Li, L.L.; Jiang, W.J.; Zhang, Y.; Zhang, X.; Wan, L.J.; Hu, J.S. MoS2/CdS Nanosheets-on-Nanorod Heterostructure for Highly Efficient Photocatalytic H2 Generation under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2016, 8, 15258–15266. [Google Scholar] [CrossRef]
- Li, Z.; Sun, P.; Zhan, X.; Zheng, Q.; Feng, T.; Braun, P.V.; Qi, S. Metallic 1T phase MoS2/MnO composites with improved cyclability for lithium-ion battery anodes. J. Alloys Compd. 2019, 796, 25–32. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Wei, Y.; Zhang, X.; Zhang, Q.; Gu, L.; Zhang, L.; Yang, N.; Yu, R. High Phase-Purity 1T-MoS2 Ultrathin Nanosheets by a Spatially Confined Template. Angew. Chem. Int. Ed. 2019, 58, 17621–17624. [Google Scholar] [CrossRef]
- Pi, Y.; Li, Z.; Xu, D.; Liu, J.; Li, Y.; Zhang, F.; Zhang, G.; Peng, W.; Fan, X. High Yield Exfoliation of WS2 Crystals into 1–2 Layer Semiconducting Nanosheets and Efficient Photocatalytic Hydrogen Evolution from WS2/CdS Nanorod Composites. ACS Sustain. Chem. Eng. 2017, 5, 5175–5182. [Google Scholar] [CrossRef]
- Dong, N.; Li, Y.; Zhang, S.; Zhang, X.; Wang, J. Optically Induced Transparency and Extinction in Dispersed MoS2, MoSe2, and Graphene Nanosheets. Adv. Opt. Mater. 2017, 5, 1700543. [Google Scholar] [CrossRef]
- Matsumoto, Y. Energy positions of oxide semiconductors and photocatalysis with iron complex oxides. J. Solid State Chem. 1996, 126, 227–234. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, S.; Wang, G.; Huang, G.; Yu, Z.; Li, Y.; Tang, L. Rationally Designed CdS-Based Ternary Heterojunctions: A Case of 1T-MoS2 in CdS/TiO2 Photocatalyst. Nanomaterials 2021, 11, 38. https://doi.org/10.3390/nano11010038
Chen W, Zhang S, Wang G, Huang G, Yu Z, Li Y, Tang L. Rationally Designed CdS-Based Ternary Heterojunctions: A Case of 1T-MoS2 in CdS/TiO2 Photocatalyst. Nanomaterials. 2021; 11(1):38. https://doi.org/10.3390/nano11010038
Chicago/Turabian StyleChen, Wenqian, Shaomei Zhang, Ganyu Wang, Gang Huang, Zhichong Yu, Yirui Li, and Liang Tang. 2021. "Rationally Designed CdS-Based Ternary Heterojunctions: A Case of 1T-MoS2 in CdS/TiO2 Photocatalyst" Nanomaterials 11, no. 1: 38. https://doi.org/10.3390/nano11010038
APA StyleChen, W., Zhang, S., Wang, G., Huang, G., Yu, Z., Li, Y., & Tang, L. (2021). Rationally Designed CdS-Based Ternary Heterojunctions: A Case of 1T-MoS2 in CdS/TiO2 Photocatalyst. Nanomaterials, 11(1), 38. https://doi.org/10.3390/nano11010038





