Hydration Characteristics of Tricalcium Aluminate in the Presence of Nano-Silica
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods
3. Results and Discussion
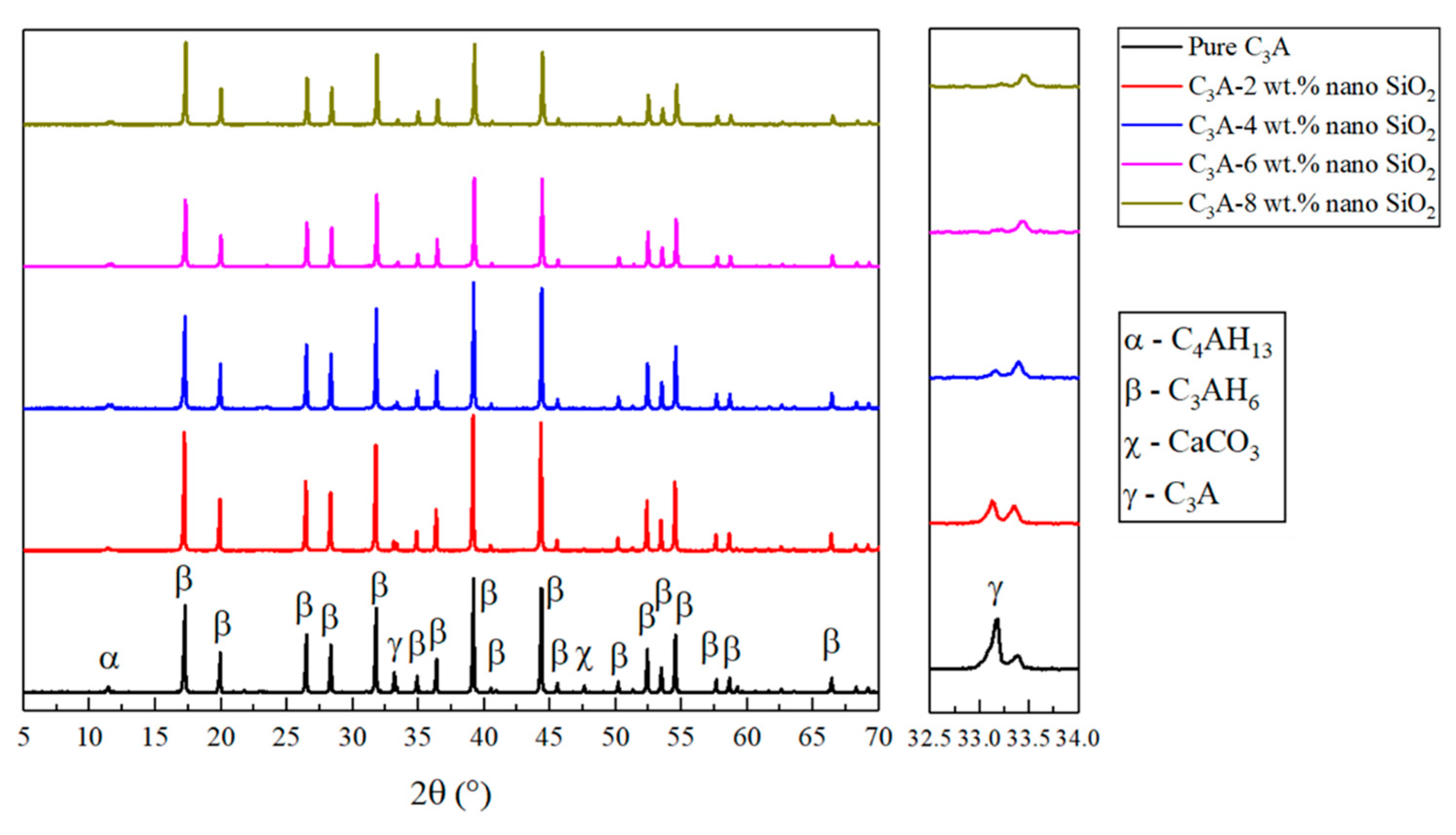
3.1. Mineral Composition Analysis
3.2. Hydration Exothermic Analysis
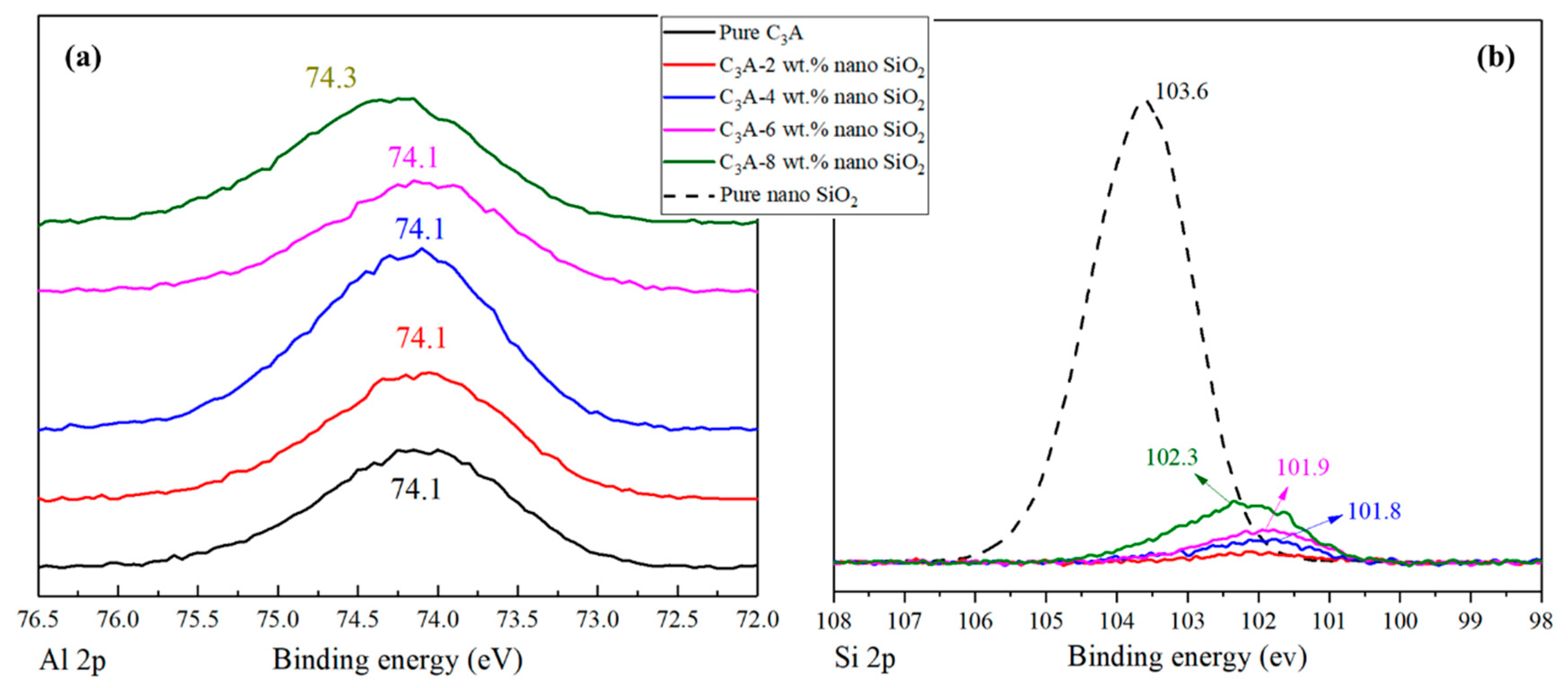
3.3. X-ray Photoelectron Spectroscopy Results
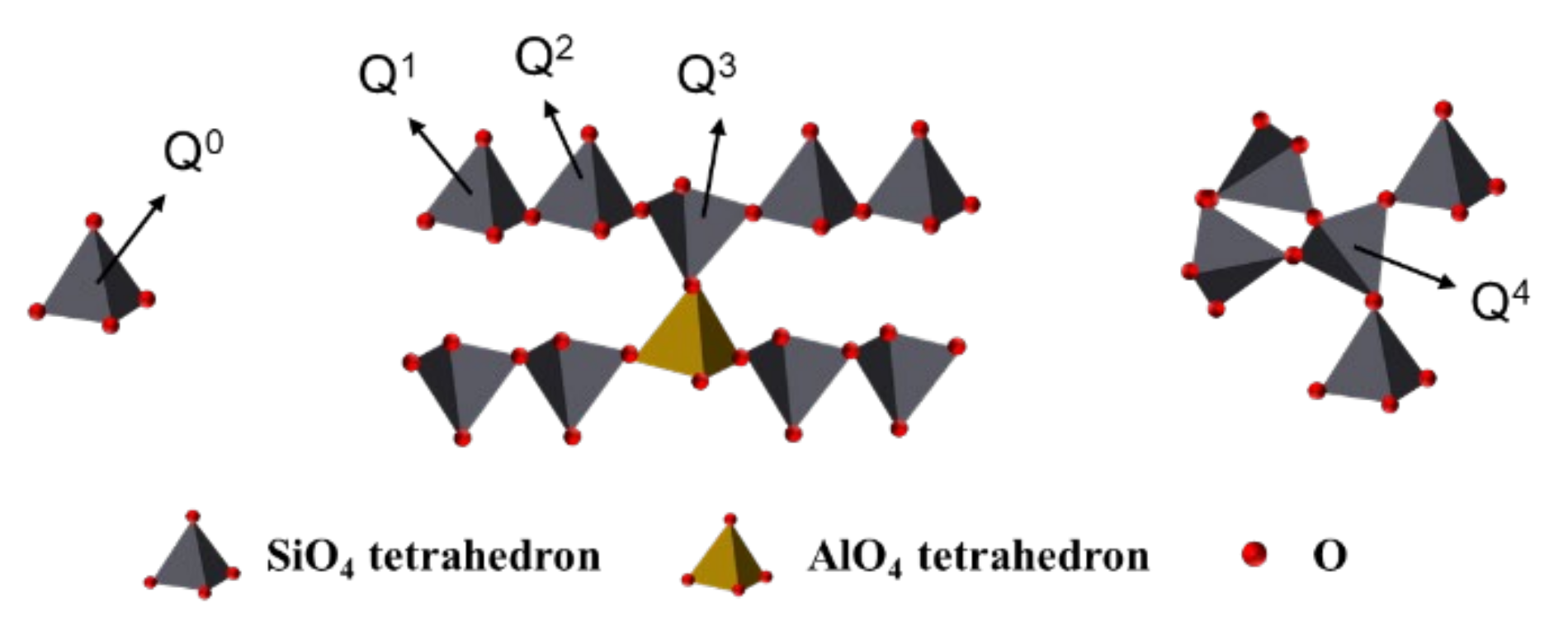
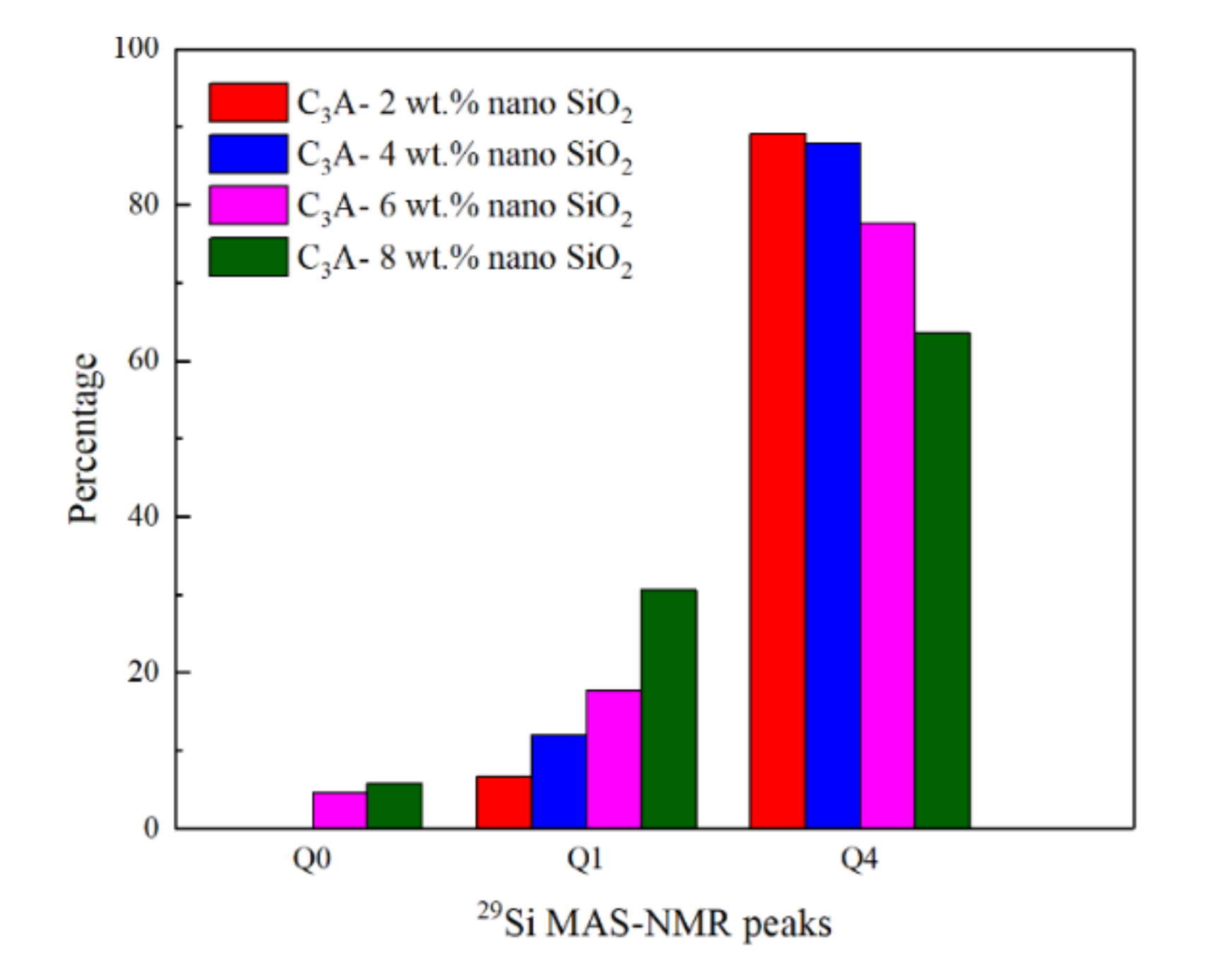
3.4. Structural Changes Observed by Nuclear Magnetic Resonance
4. Conclusions and Recommendations
- (1)
- The addition of nano-SiO2 can promote the hydration degree of C3A while significantly reducing the heat release rate of C3A hydration from 0.34 to less than 0.1 mW/g, and the occurrence time of the hydration exothermic peak was delayed by more than 2 min. The main reasons are probably the surface of C3A being adsorbed by nano-SiO2 and/or covered by the C-A-S-H gel (formed by the pozzolanic hydration reaction of nano-SiO2 and C3A) at an early age, thereby reducing the contact area of C3A with water.
- (2)
- The reaction between nano-SiO2 and C3A can establish Si-O-Al bonds and generate C-A-S-H gels. The chemical shifts in Al 2p and Si 2p both confirm this conclusion. In addition, 29Si MAS-NMR results showed that Q1 appeared after the nano-SiO2 was added to the C3A hydration system. With the nano-SiO2 content increased, the proportion of Q1 in the hydration product increased from 6.74% to 30.6%, while the proportion of Q4 gradually decreased from 89.1% to 63.6%.
- (3)
- The addition of nano-silica can promote the hydration reaction rate of C3S while delaying the hydration of C3A. For the two hydration systems of C3A and C3S, the addition of nano-SiO2 has shown completely different effects, and the influence mechanism of nano-SiO2 in the two different hydration processes needs further exploration.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C3A | Tricalcium aluminate |
| C3S | Tricalcium silicate, alite |
| C2S | Dicalcium silicate, belite |
| C4AF | Tetracalcium aluminate, ferrite |
| CS | Calcium sulfate, CaSO4 |
| C-S-H | Calcium silicate hydrate |
| C-A-S-H | Calcium silicoaluminate hydrate |
| C6AS3H32, Aft | Ettringite |
| 3C4ASH12, AFm | Monosulfate |
| Ca3Al2(OH)12, or C3AH6 | Calcium aluminium hydrate |
| C4AH13/C2AH8 | Calcium aluminium hydrate |
References
- Li, H.; Du, T.; Xiao, H.; Zhang, Q. Crystallization of calcium silicate hydrates on the surface of nanomaterials. J. Am. Ceram. Soc. 2017, 100, 3227–3238. [Google Scholar] [CrossRef]
- Borrmann, T.; Johnston, J.H.; McFarlane, A.J.; Richardson, M.J.; O’Connor, S.J. Nano-structured calcium silicate hydrate functionalised with iodine. J. Colloid Interface Sci. 2009, 339, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, Z.; Du, P.; Cheng, X. Effects of nano-silica on hydration properties of tricalcium silicate. Constr. Build. Mater. 2016, 125, 1169–1177. [Google Scholar] [CrossRef]
- Hewlett, P.; Liska, M. Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Quennoz, A.; Scrivener, K.L. Hydration of C3A–gypsum systems. Cem. Concr. Res. 2012, 42, 1032–1041. [Google Scholar] [CrossRef]
- Maier, A.-K.; Dezmirean, L.; Will, J.; Greil, P. Three-dimensional printing of flash-setting calcium aluminate cement. J. Mater. Sci. 2011, 46, 2947–2954. [Google Scholar] [CrossRef]
- Manzano, H.; Dolado, J.S.; Ayuela, A. Structural, mechanical, and reactivity properties of tricalcium aluminate using first-principles calculations. J. Am. Ceram. Soc. 2009, 92, 897–902. [Google Scholar] [CrossRef]
- Skibsted, J.; Henderson, E.; Jakobsen, H.J. Characterization of calcium aluminate phases in cements by aluminum-27 MAS NMR spectroscopy. Inorg. Chem. 1993, 32, 1013–1027. [Google Scholar] [CrossRef]
- Gismera-Diez, S.; Manchobas-Pantoja, B.; Carmona-Quiroga, P.M.; Blanco-Varela, M. Effect of BaCO3 on C3A hydration. Cem. Concr. Res. 2015, 73, 70–78. [Google Scholar] [CrossRef]
- Nunes, C.; Slížková, Z.; Stefanidou, M.; Němeček, J. Microstructure of lime and lime-pozzolana pastes with nanosilica. Cem. Concr. Res. 2016, 83, 152–163. [Google Scholar] [CrossRef]
- Singh, L.; Ali, D.; Sharma, U. Studies on optimization of silica nanoparticles dosage in cementitious system. Cem. Concr. Compos. 2016, 70, 60–68. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.-F.; Li, M.-Q. Influence of nano-SiO2 on the consistency, setting time, early-age strength, and shrinkage of composite cement pastes. Adv. Mater. Sci. Eng. 2016, 1-8. [Google Scholar]
- Shih, J.-Y.; Chang, T.-P.; Hsiao, T.-C. Effect of nanosilica on characterization of Portland cement composite. Mater. Sci. Eng. A 2006, 424, 266–274. [Google Scholar] [CrossRef]
- Gu, Y.; Ran, Q.; Shu, X.; Yu, C.; Chang, H.; Liu, J. Synthesis of nanoSiO2@ PCE core-shell nanoparticles and its effect on cement hydration at early age. Constr. Build. Mater. 2016, 114, 673–680. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; Khayat, K.H.; Wan, S. Effects of different nanomaterials on hardening and performance of ultra-high strength concrete (UHSC). Cem. Concr. Compos. 2016, 70, 24–34. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Young, J. The hydration of tricalcium silicate in the presence of colloidal silica. J. Mater. Sci. 1984, 19, 3477–3486. [Google Scholar] [CrossRef]
- Singh, L.; Bhattacharyya, S.; Shah, S.P.; Mishra, G.; Ahalawat, S.; Sharma, U. Studies on early stage hydration of tricalcium silicate incorporating silica nanoparticles: Part I. Constr. Build. Mater. 2015, 74, 278–286. [Google Scholar] [CrossRef]
- Bentz, D.P.; Hansen, A.S.; Guynn, J.M. Optimization of cement and fly ash particle sizes to produce sustainable concretes. Cem. Concr. Compos. 2011, 33, 824–831. [Google Scholar] [CrossRef]
- Kong, D.; Corr, D.J.; Hou, P.; Yang, Y.; Shah, S.P. Influence of colloidal silica sol on fresh properties of cement paste as compared to nano-silica powder with agglomerates in micron-scale. Cem. Concr. Compos. 2015, 63, 30–41. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.; Rico, A.; Rodríguez, J. A model for the CASH gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D. Compatibility studies between NASH and CASH gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Pardal, X.; Pochard, I.; Nonat, A. Experimental study of Si–Al substitution in calcium-silicate-hydrate (CSH) prepared under equilibrium conditions. Cem. Concr. Res. 2009, 39, 637–643. [Google Scholar] [CrossRef]
- Singh, L.; Bhattacharyya, S.; Mishra, G.; Ahalawat, S. Reduction of calcium leaching in cement hydration process using nanomaterials. Mater. Technol. 2012, 27, 233–238. [Google Scholar] [CrossRef]
- Lin, T.T.; Lin, C.F.; Wei, W.C.J. Mechanisms of metal stabilization in cementitious matrix: Interaction of dicalcium silicate (C2S) paste and copper oxide. Toxicol. Environ. Chem. 1994, 43, 51–62. [Google Scholar] [CrossRef]
- Myers, R.J.; Geng, G.; Rodriguez, E.D.; da Rosa, P.; Kirchheim, A.P.; Monteiro, P.J. Solution chemistry of cubic and orthorhombic tricalcium aluminate hydration. Cem. Concr. Res. 2017, 100, 176–185. [Google Scholar] [CrossRef]
- De Jong, J.; Stein, H.; Stevels, J. Influence of amorphous silica on the hydration of tricalcium aluminate. J. Appl. Chem. 1969, 19, 25–28. [Google Scholar] [CrossRef]
- Hou, P.; Wang, X.; Cheng, X. Effects of nanosilica on C3A hydration. In Proceedings of the 73rd RILEM Annual Week & the International Conference on Innovative Materials for Sustainable Civil Engineering, Nanjing, China, 25–30 August 2019; p. 119. [Google Scholar]
- Hou, P.; Wang, X.; Zhao, P.; Wang, K.; Kawashima, S.; Li, Q.; Xie, N.; Cheng, X.; Shah, S.P. Physicochemical effects of nanosilica on C3A/C3S hydration. J. Am. Ceram. Soc. 2020, 103, 6505–6518. [Google Scholar] [CrossRef]
- Barr, T. ESCA studies of the coordination state of aluminium in oxide environments. J. Chem. Soc. Faraday Trans. 1997, 93, 181–186. [Google Scholar] [CrossRef]
- Dubina, E.; Plank, J.; Black, L. Impact of water vapour and carbon dioxide on surface composition of C3A polymorphs studied by X-ray photoelectron spectroscopy. Cem. Concr. Res. 2015, 73, 36–41. [Google Scholar] [CrossRef]
- Dubina, E.; Black, L.; Sieber, R.; Plank, J. Interaction of water vapour with anhydrous cement minerals. Adv. Appl. Ceram. 2010, 109, 260–268. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, Y.; Wang, W.; Wang, D.; Qi, T. Hydrous alumina/silica double-layer surface coating of TiO2 pigment. Colloids Surf. A Physicochem. Eng. Asp. 2012, 407, 77–84. [Google Scholar] [CrossRef]
- Barr, T.; Hoppe, E.; Hardcastle, S.; Seal, S. X-ray photoelectron spectroscopy investigations of the chemistries of soils. J. Vac. Sci. Technol. A Vac. Surf. Film. 1999, 17, 1079–1085. [Google Scholar] [CrossRef]
- Seyama, H.; Soma, M. Bonding-state characterization of the constitutent elements of silicate minerals by X-ray photoelectron spectroscopy. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1985, 81, 485–495. [Google Scholar] [CrossRef]
- Pardal, X.; Brunet, F.; Charpentier, T.; Pochard, I.; Nonat, A. 27Al and 29Si solid-state NMR characterization of calcium-aluminosilicate-hydrate. Inorg. Chem. 2012, 51, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Woessner, D.E. Characterization of clay minerals by 27Al nuclear magnetic resonance spectroscopy. Am. Mineral. 1989, 74, 203–215. [Google Scholar]
- Slade, R.; Southern, J.; Thompson, I. 27Al Nuclear magnetic resonance spectroscopy investigation of thermal transformation sequences of alumina hydrate. PT. 1. GIBBSITE, GAMMA-Al (OH)3. J. Mater. Chem. 1991, 1, 563–568. [Google Scholar] [CrossRef]
- Monasterio, M.; Gaitero, J.J.; Erkizia, E.; Bustos, A.M.G.; Miccio, L.A.; Dolado, J.S.; Cerveny, S. Effect of addition of silica-and amine functionalized silica-nanoparticles on the microstructure of calcium silicate hydrate (C–S–H) gel. J. Colloid Interface Sci. 2015, 450, 109–118. [Google Scholar] [CrossRef]
- Yang, H.; Monasterio, M.; Cui, H.; Han, N. Experimental study of the effects of graphene oxide on microstructure and properties of cement paste composite. Compos. Part A Appl. Sci. Manuf. 2017, 102, 263–272. [Google Scholar] [CrossRef]
- Klur, I.; Pollet, B.; Virlet, J.; Nonat, A. CSH structure evolution with calcium content by multinuclear NMR. In Nuclear Magnetic Resonance Spectroscopy of Cement-Based Materials; Springer: Berlin, Germany, 1998; pp. 119–141. [Google Scholar]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Richardson, I.; Brough, A.; Groves, G.; Dobson, C. The characterization of hardened alkali-activated blast-furnace slag pastes and the nature of the calcium silicate hydrate (CSH) phase. Cem. Concr. Res. 1994, 24, 813–829. [Google Scholar] [CrossRef]
- Schilling, P.J.; Butler, L.G.; Roy, A.; Eaton, H.C. 29Si and 27Al MAS-NMR of NaOH-activated blast-furnace slag. J. Am. Ceram. Soc. 1994, 77, 2363–2368. [Google Scholar] [CrossRef]
- Škvára, F.; Jílek, T.; Kopecký, L. Geopolymer materials based on fly ash. Ceram. Silik. 2005, 49, 195–204. [Google Scholar]
- Justnes, H.; Meland, I.; Bjoergum, J.; Krane, J.; Skjetne, T. Nuclear magnetic resonance (NMR)—A powerful tool in cement and concrete research. Adv. Cem. Res. 1990, 3, 105–110. [Google Scholar] [CrossRef]
- Gaitero, J.J.; Campillo, I.; Guerrero, A. Reduction of the calcium leaching rate of cement paste by addition of silica nanoparticles. Cem. Concr. Res. 2008, 38, 1112–1118. [Google Scholar] [CrossRef]



| Sample Name | C3A (g) | Nano-SiO2 (g) | Water (g) | L/S |
|---|---|---|---|---|
| Pure C3A | 1 | 0 | 50 | 50 |
| C3A-2 wt.% nano SiO2 | 1 | 0.02 | 51 | 50 |
| C3A-4 wt.% nano SiO2 | 1 | 0.04 | 52 | 50 |
| C3A-6 wt.% nano SiO2 | 1 | 0.06 | 53 | 50 |
| C3A-8 wt.% nano SiO2 | 1 | 0.08 | 54 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Monasterio, M.; Feng, W.; Tang, W.; Cui, H.; Dong, Z. Hydration Characteristics of Tricalcium Aluminate in the Presence of Nano-Silica. Nanomaterials 2021, 11, 199. https://doi.org/10.3390/nano11010199
Zheng D, Monasterio M, Feng W, Tang W, Cui H, Dong Z. Hydration Characteristics of Tricalcium Aluminate in the Presence of Nano-Silica. Nanomaterials. 2021; 11(1):199. https://doi.org/10.3390/nano11010199
Chicago/Turabian StyleZheng, Dapeng, Manuel Monasterio, Weipeng Feng, Waiching Tang, Hongzhi Cui, and Zhijun Dong. 2021. "Hydration Characteristics of Tricalcium Aluminate in the Presence of Nano-Silica" Nanomaterials 11, no. 1: 199. https://doi.org/10.3390/nano11010199
APA StyleZheng, D., Monasterio, M., Feng, W., Tang, W., Cui, H., & Dong, Z. (2021). Hydration Characteristics of Tricalcium Aluminate in the Presence of Nano-Silica. Nanomaterials, 11(1), 199. https://doi.org/10.3390/nano11010199







