CuS/rGO-PEG Nanocomposites for Photothermal Bonding of PMMA-Based Plastic Lab-on-a-Chip
Abstract
1. Introduction
2. Materials and Methods
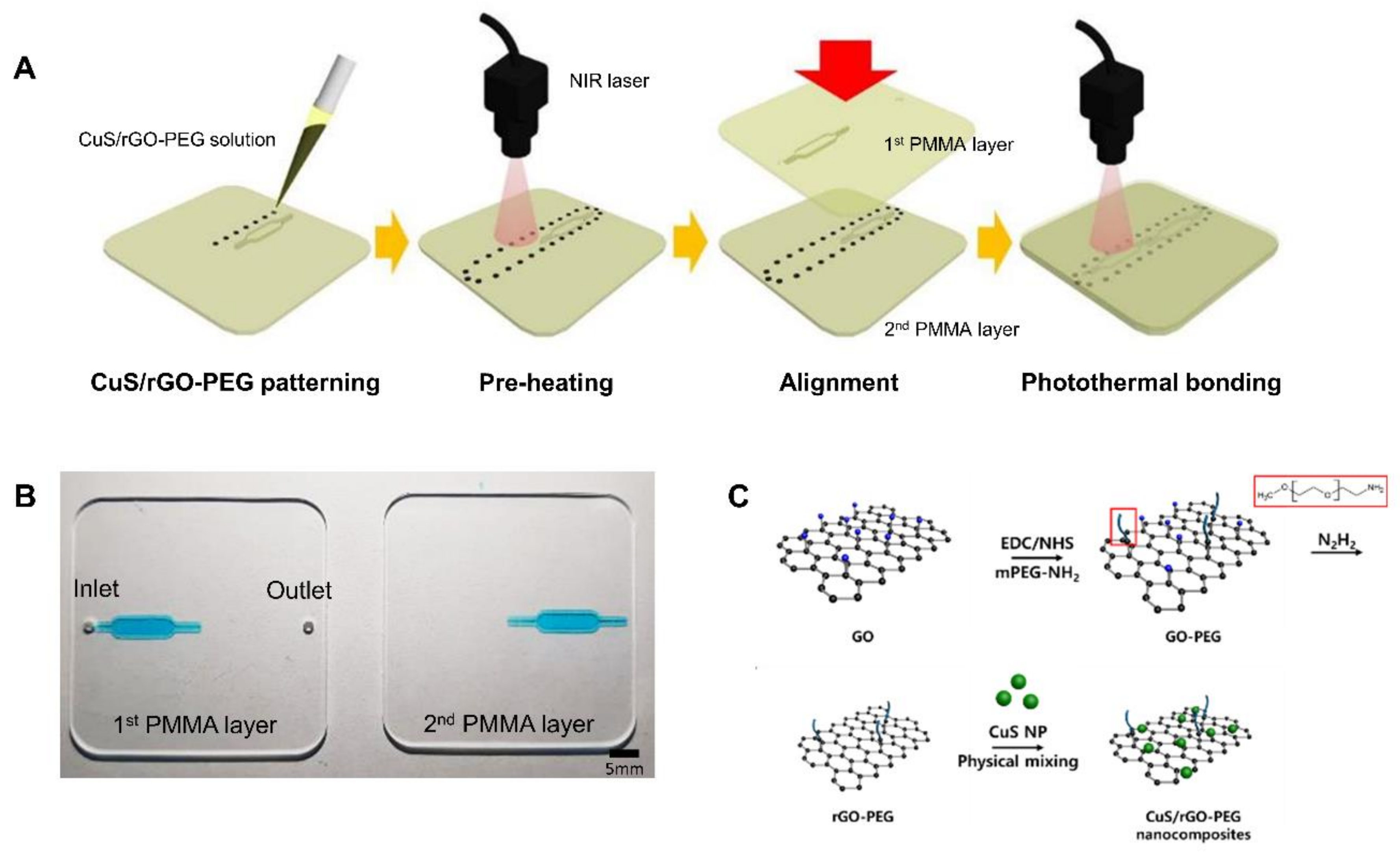
2.1. Fabrication of PMMA-Based Plastic Lab-on-a-Chip
2.2. Synthesis of CuS/rGO-PEG Nanocomposites
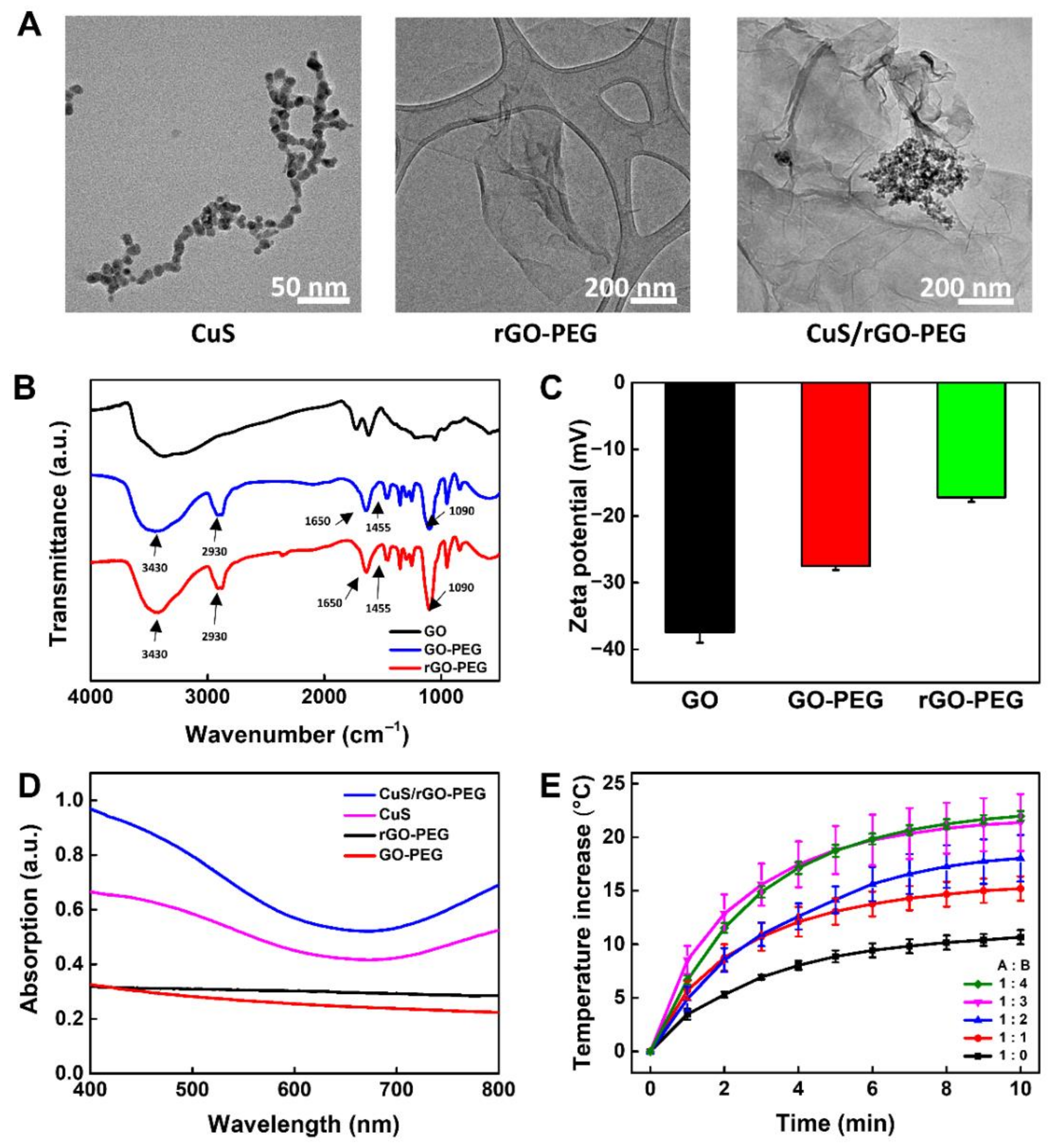
2.3. Characterization of CuS/rGO-PEG Nanocomposites
2.4. Experimental Setup for Photothermal Bonding
2.5. Analysis of Mechanical Properties for Photothermal Bonding
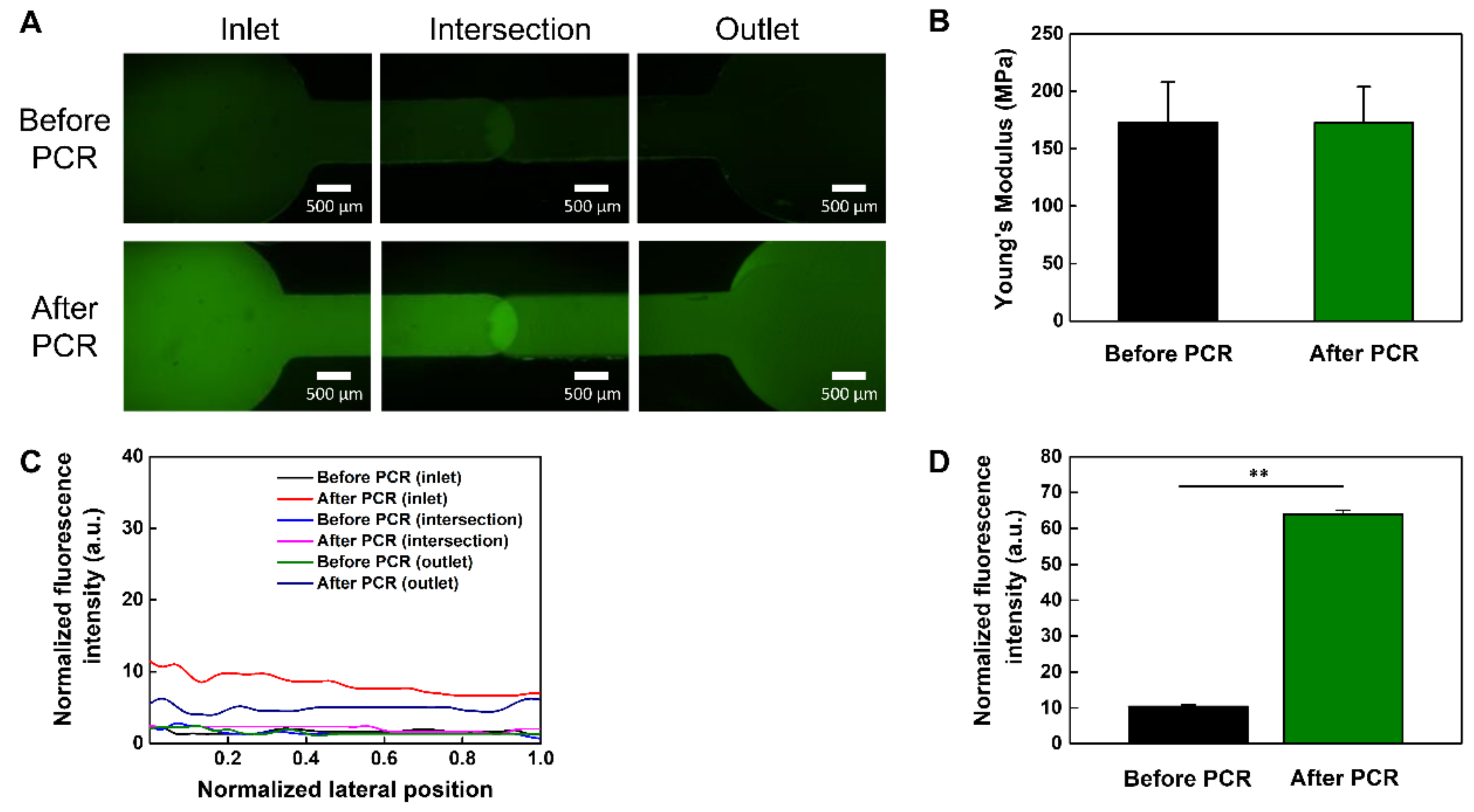
2.6. Polymerase Chain Reaction (PCR) Applications
2.7. Statisitical Analysis
3. Results and Discussion
3.1. Fabrication of PMMA-Based Plastic Lab-on-a-Chip and Analysis CuS/rGO-PEG Nanocomposites
3.2. Analysis of Mechanical Properties of PMMA-Based Plastic Lab-on-a-Chip
3.3. PCR Applications in the PMMA Plastic Lab-on-a-Chip
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coppola, S.; Nasti, G.; Todino, M.; Olivieri, F.; Vespini, V.; Ferraro, P. Direct Writing of Microfluidic Footpaths by Pyro-EHD Printing. ACS Appl. Mater. Interfaces 2017, 9, 16488–16494. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.; Ryoo, J. Analysis of flip chip bonding for performance stability of UHF RFID tags. IEEE Trans. Compon. Packag. Manuf. Technol. 2014, 4, 1714–1721. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on surface wettability of poly (dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Lee, H.G.; Shin, J.-W.; Choi, Y.-W.; Paik, K.-W. Effects of thermocompression bonding parameters on cu pillar/sn-ag microbump solder joint morphology using nonconductive films. IEEE Trans. Compon. Packag. Manuf. Technol. 2017, 7, 450–455. [Google Scholar] [CrossRef]
- Nunna, B.B.; Mandal, D.; Lee, J.U.; Singh, H.; Zhuang, S.; Misra, D.; Bhuyian, M.N.U.; Lee, E.S. Detection of cancer antigens (CA-125) using gold nano particles on interdigitated electrode-based microfluidic biosensor. Nano Converg. 2019, 6, 3. [Google Scholar] [CrossRef]
- Parameswaran, C.; Gupta, D. Large area flexible pressure/strain sensors and arrays using nanomaterials and printing techniques. Nano Converg. 2019, 6, 1–23. [Google Scholar] [CrossRef]
- Chen, C.-F.; Wharton, K. Characterization and failure mode analyses of air plasma oxidized PDMS–PDMS bonding by peel testing. RSC Adv. 2017, 7, 1286–1289. [Google Scholar] [CrossRef]
- Beh, C.W.; Zhou, W.; Wang, T.-H. PDMS–glass bonding using grafted polymeric adhesive–alternative process flow for compatibility with patterned biological molecules. Lab Chip 2012, 12, 4120–4127. [Google Scholar] [CrossRef]
- Liga, A.; Morton, J.A.; Kersaudy-Kerhoas, M. Safe and cost-effective rapid-prototyping of multilayer PMMA microfluidic devices. Microfluid Nanofluidics 2016, 20, 164. [Google Scholar] [CrossRef]
- Chang, Y.; You, H. A hybrid adhesive bonding of PMMA and PCB with an application on microchip electrophoresis. Anal. Methods 2019, 11, 1229–1236. [Google Scholar] [CrossRef]
- Barry, C.P.; Morose, G.J.; Begin, K.; Atwater, M.; Hansen, C.J. The identification and screening of lower toxicity solvents for contact adhesives. Int. J. Adhes. 2017, 78, 174–181. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Microfluidic device fabrication mediated by surface chemical bonding. Analyst 2020, 145, 4096–4110. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.M.; Sadri, A.; Young, E.W. Liquid phase solvent bonding of plastic microfluidic devices assisted by retention grooves. Lab Chip 2015, 15, 3785–3792. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Chakrabarty, D.; Mukherjee, S.; Bhowmik, S. Physicochemical Characteristics of Solvent Vapor Bonded Polycarbonate. J. Polym. Environ. 2018, 26, 1088–1099. [Google Scholar] [CrossRef]
- Ng, S.P.; Wiria, F.E.; Tay, N.B. Low distortion solvent bonding of microfluidic chips. Procedia. Eng. 2016, 141, 130–137. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Ren, Q.; Fu, F.; Zou, R.; Wang, Q.; Xin, G.; Xiao, Z.; Huang, X.; Liu, Q.; Hu, J. CuS@mSiO2-PEG core–shell nanoparticles as a NIR light responsive drug delivery nanoplatform for efficient chemo-photothermal therapy. Dalton Trans. 2015, 44, 10343–10351. [Google Scholar] [CrossRef]
- Han, F.; Lv, S.; Li, Z.; Jin, L.; Fan, B.; Zhang, J.; Zhang, R.; Zhang, X.; Han, L.; Li, J. Triple-synergistic 2D material-based dual-delivery antibiotic platform. NPG Asia Mater. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Gholampour, A.; Kiamahalleh, M.V.; Tran, D.N.; Ozbakkaloglu, T.; Losic, D. From graphene oxide to reduced graphene oxide: Impact on the physiochemical and mechanical properties of graphene–cement composites. ACS Appl. Mater. Interfaces 2017, 9, 43275–43286. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, K.; Fan, Y. Application of a new UV curable adhesive for rapid bonding in thermoplastic-based microfluidics. Micro Nano Lett. 2019, 14, 211–214. [Google Scholar] [CrossRef]
- Yin, Z. Rapid prototyping of PET microfluidic chips by laser ablation and water-soaking bonding method. Micro Nano Lett. 2018, 13, 1302–1305. [Google Scholar] [CrossRef]
- Al-Hamaoy, I.R.; Jawad, A. Through Transmission Laser Spot Welding for PMMA. Int. J. Res. Eng. Technol. 2016, 3, 234–236. [Google Scholar]
- Bardin, F.; Kloss, S.; Wang, C.; Moore, A.J.; Jourdain, A.; de Wolf, I.; Hand, D.P. Laser bonding of glass to silicon using polymer for microsystems packaging. J. Microelectromech. Syst. 2007, 16, 571–580. [Google Scholar] [CrossRef]
- Lo, P.-Y.; Lee, G.-Y.; Zheng, J.-H.; Huang, J.-H.; Cho, E.-C.; Lee, K.-C. GFP plasmid and chemoreagent conjugated with graphene quantum dots as a novel gene delivery platform for colon cancer inhibition in vitro and in vivo. ACS. Appl. Bio Mater. 2020, 3, 5948–5956. [Google Scholar] [CrossRef]
- Mun, S.G.; Choi, H.W.; Lee, J.M.; Lim, J.H.; Ha, J.H.; Kang, M.-J.; Kim, E.-J.; Kang, L.; Chung, B.G. rGO nanomaterial-mediated cancer targeting and photothermal therapy in a microfluidic co-culture platform. Nano Converg. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.J. Photothermally controlled gene delivery by reduced graphene oxide–polyethylenimine nanocomposite. Small 2014, 10, 117–126. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Zhu, M.; Chen, Y.; Xiao, Y.; Shen, Y.; Xie, A. RGO/AuNR/HA-5FU nanocomposite with multi-stage release behavior and efficient antitumor activity for synergistic therapy. Biomater. Sci. 2017, 5, 990–1000. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.; Huang, M.; Lu, W.; Song, S.; Melancon, M.P.; Tian, M.; Liang, D.; Li, C. A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J. Am. Chem. Soc. 2010, 132, 15351–15358. [Google Scholar] [CrossRef]
- Qi, X.; Pu, K.Y.; Zhou, X.; Li, H.; Liu, B.; Boey, F.; Huang, W.; Zhang, H. Conjugated-polyelectrolyte-functionalized reduced graphene oxide with excellent solubility and stability in polar solvents. Small 2010, 6, 663–669. [Google Scholar] [CrossRef]
- Lu, F.; Wang, J.; Yang, L.; Zhu, J.-J. A facile one-pot synthesis of colloidal stable, monodisperse, highly PEGylated CuS@mSiO2 nanocomposites for the combination of photothermal therapy and chemotherapy. Chem. Commun. 2015, 51, 9447–9450. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Zhu, W.; Hu, Z.T.; Lim, T.T.; Yan, X. Facile room-temperature synthesis of carboxylated graphene oxide-copper sulfide nanocomposite with high photodegradation and disinfection activities under solar light irradiation. Sci. Rep. 2015, 5, 16369. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, P. Biocompatible graphene oxide as a folate receptor-targeting drug delivery system for the controlled release of anti-cancer drugs. RSC Adv. 2014, 4, 24232–24239. [Google Scholar] [CrossRef]
- Schwaighofer, A.; Montemurro, M.; Freitag, S.; Kristament, C.; Culzoni, M.J.; Lendl, B. Beyond Fourier Transform Infrared Spectroscopy: External cavity quantum cascade laser-based mid-infrared transmission spectroscopy of proteins in the Amide I and Amide II Region. Anal Chem. 2018, 90, 7072–7079. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.; Baek, Y.M.; Kim, H.-M.; Jun, B.-H.; Kim, D.-E. Polyethylene glycol-engrafted graphene oxide as biocompatible materials for peptide nucleic acid delivery into cells. Bioconjug. Chem. 2018, 29, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Hao, Y.-N.; Wei, X.; Chen, X.-W.; Shu, Y.; Wang, J.-H. Hollow copper sulfide nanosphere–doxorubicin/graphene oxide core–shell nanocomposite for photothermo-chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 3230–3235. [Google Scholar] [CrossRef]
- Perween, M.; Parmar, D.B.; Bhadu, G.R.; Srivastava, D.N. Polymer–graphite composite: A versatile use and throw plastic chip electrode. Analyst 2014, 139, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, O.C.A.; Romero, O.J. Influence of oil leakage in the pressure and flow rate behaviors in pipeline. Lat. Am. J. Energy Res. 2017, 4, 17–29. [Google Scholar] [CrossRef]
- Patel, J.N.; Kaminska, B.; Gray, B.L.; Gates, B.D. PDMS as a sacrificial substrate for SU-8-based biomedical and microfluidic applications. J. Micromech. Microeng. 2008, 18, 095028. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, D.H.; Lee, S.; Kim, S.-J. Water-head pumps provide precise and fast microfluidic pumping and switching versus syringe pumps. Microfluid Nanofluidics 2016, 20, 4. [Google Scholar] [CrossRef]
- Yeung, A.T.; Holloway, B.P.; Adams, P.S.; Shipley, G.L. Evaluation of dual-labeled fluorescent DNA probe purity versus performance in real-time PCR. Biotechniques 2004, 36, 266–275. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Lim, J.H.; Lee, J.M.; Choi, J.W.; Choi, H.W.; Seo, W.H.; Lee, K.G.; Lee, S.J.; Chung, B.G. CuS/rGO-PEG Nanocomposites for Photothermal Bonding of PMMA-Based Plastic Lab-on-a-Chip. Nanomaterials 2021, 11, 176. https://doi.org/10.3390/nano11010176
Kim YJ, Lim JH, Lee JM, Choi JW, Choi HW, Seo WH, Lee KG, Lee SJ, Chung BG. CuS/rGO-PEG Nanocomposites for Photothermal Bonding of PMMA-Based Plastic Lab-on-a-Chip. Nanomaterials. 2021; 11(1):176. https://doi.org/10.3390/nano11010176
Chicago/Turabian StyleKim, Young Jae, Jae Hyun Lim, Jong Min Lee, Ji Wook Choi, Hyung Woo Choi, Won Ho Seo, Kyoung G. Lee, Seok Jae Lee, and Bong Geun Chung. 2021. "CuS/rGO-PEG Nanocomposites for Photothermal Bonding of PMMA-Based Plastic Lab-on-a-Chip" Nanomaterials 11, no. 1: 176. https://doi.org/10.3390/nano11010176
APA StyleKim, Y. J., Lim, J. H., Lee, J. M., Choi, J. W., Choi, H. W., Seo, W. H., Lee, K. G., Lee, S. J., & Chung, B. G. (2021). CuS/rGO-PEG Nanocomposites for Photothermal Bonding of PMMA-Based Plastic Lab-on-a-Chip. Nanomaterials, 11(1), 176. https://doi.org/10.3390/nano11010176




