MoS2@ZnO Nanoheterostructures Prepared by Electrospark Erosion for Photocatalytic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Nanostructured MoS2 by Self-Propagating High-Temperature Synthesis
2.2. Synthesis of MoS2-ZnO Heterostructures
2.3. Characterization Techniques
3. Results and Discussion
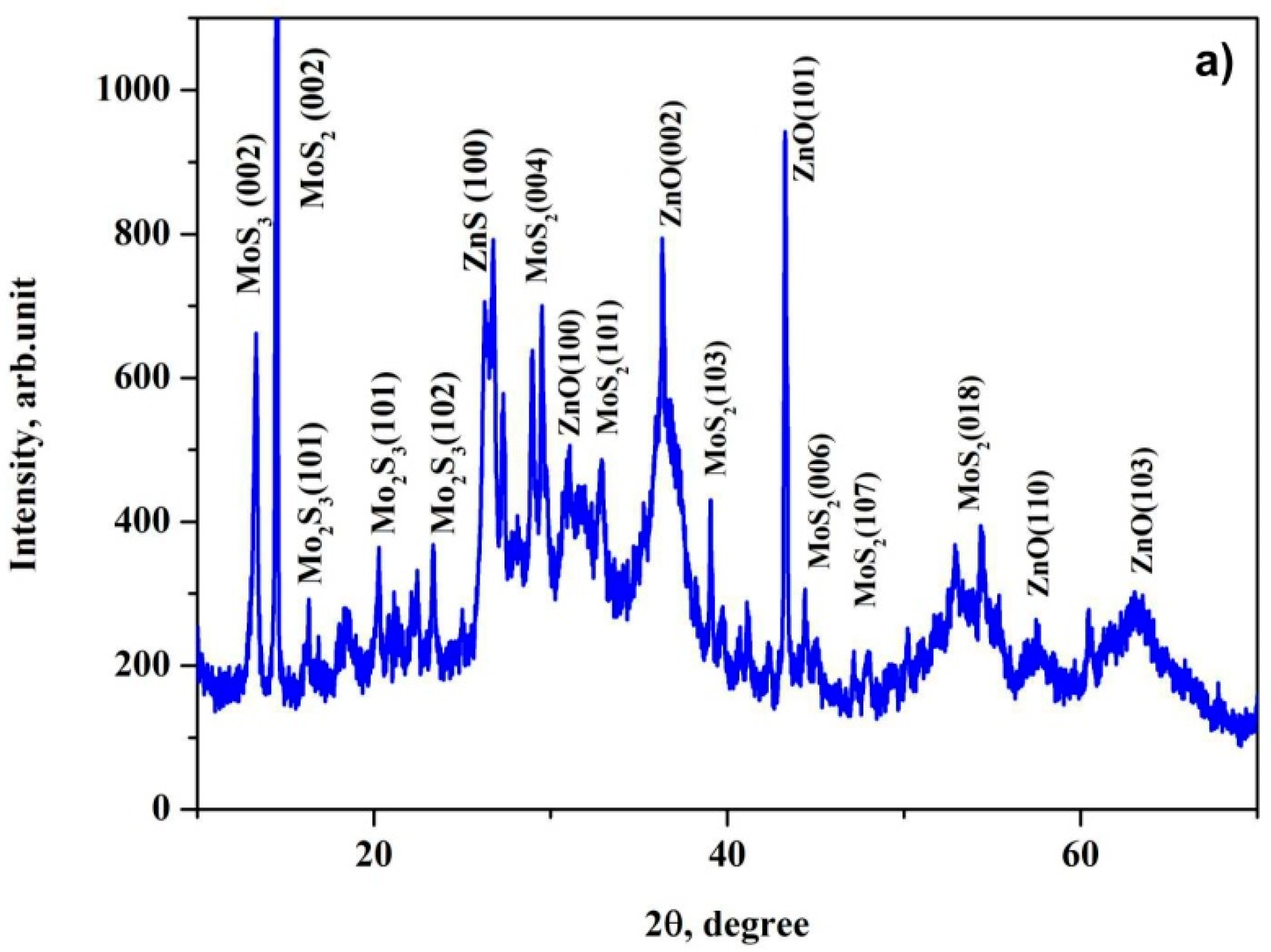
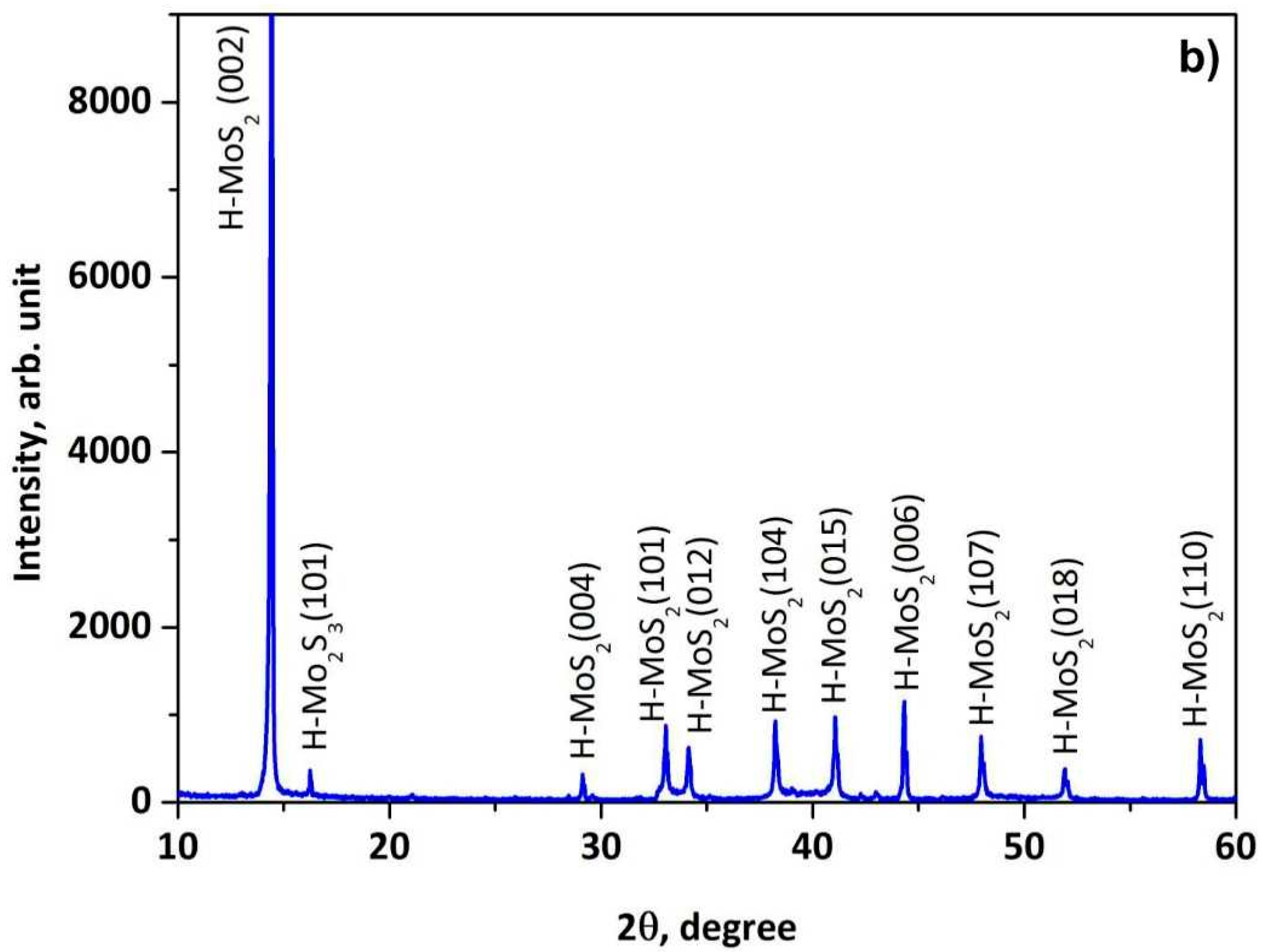
3.1. X-ray Phase and Structural Analysis of the Prepared MoS2@ZnO Heterostructures
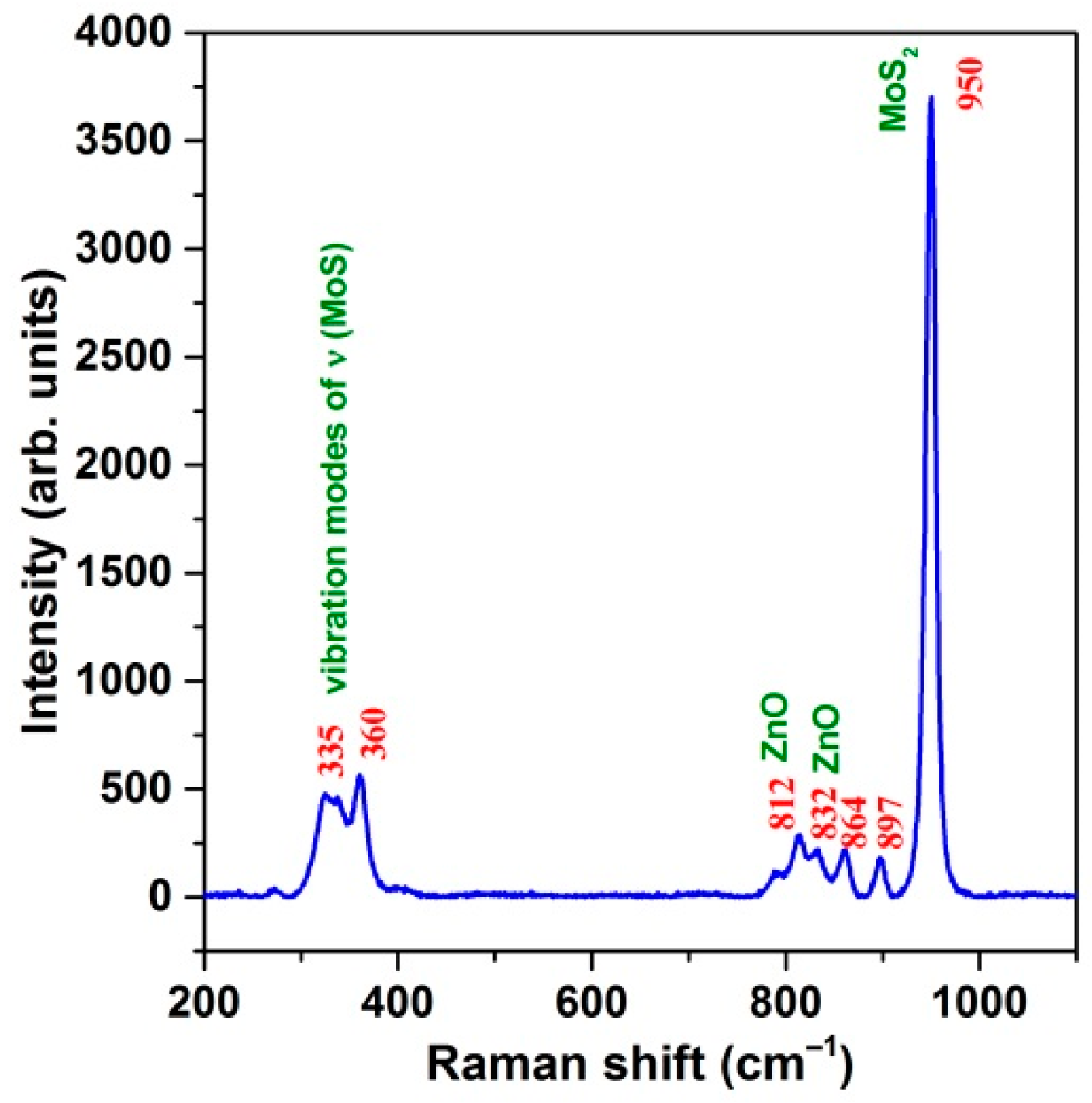
3.2. Raman Analysis of the Prepared MoS2@ZnO Heterostructures
3.3. Structural and Compositional Analysis
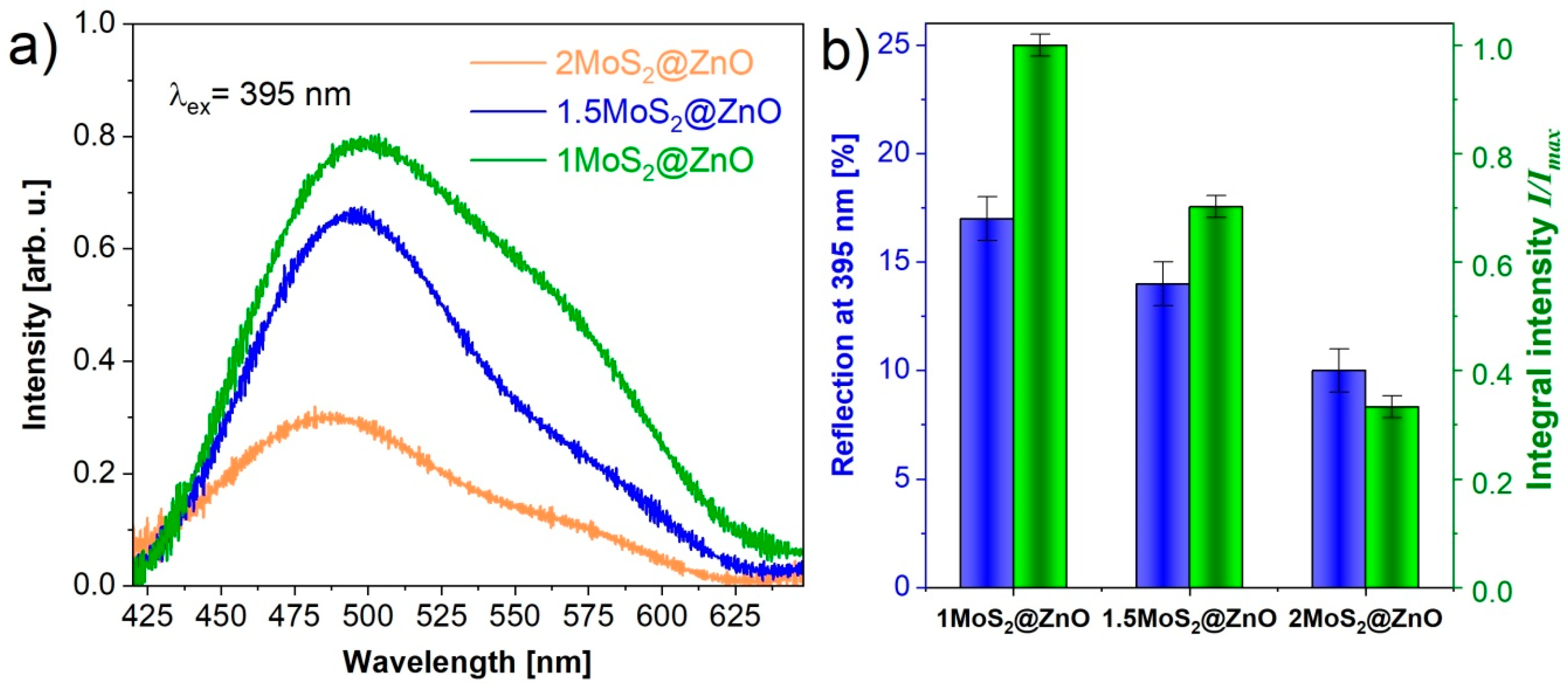
3.4. Photoluminescent Measurements
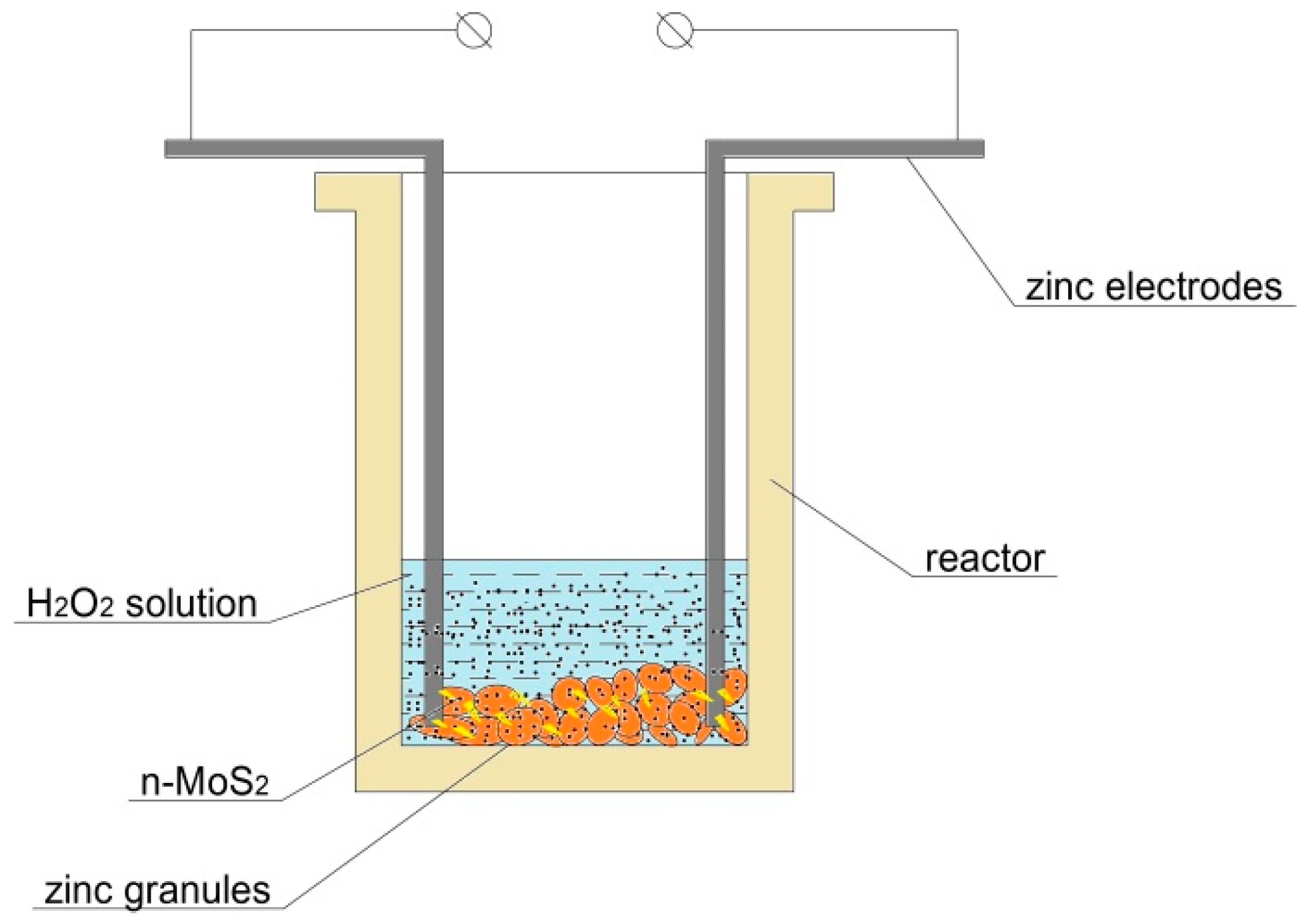
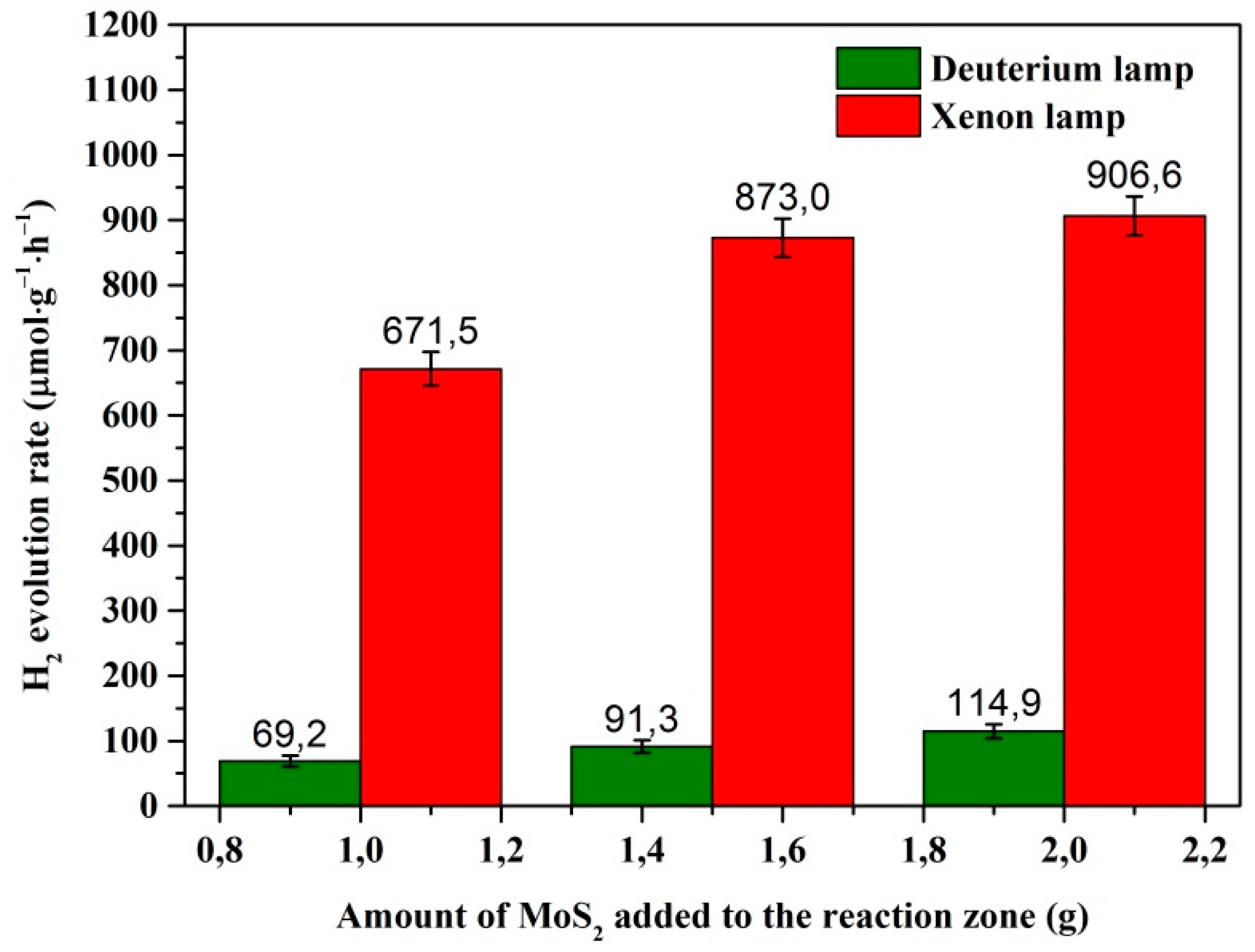
3.5. Hydrogen Evolution Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, H.; Li, J.; Chen, M.; Wang, H.; Jiang, X.; Xiao, Y.; Zhang, X. High-throughput production of ZnO-MoS2-graphene heterostructures for highly efficient photocatalytic hydrogen evolution. Materials 2019, 12, 2233. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Wang, F.; Hu, B.; Lu, H.W.; Yu, Z.T.; Zou, Z.G. Significant enhancement in photocatalytic hydrogen evolution from water using a MoS2 nanosheet-coated ZnO heterostructure photocatalyst. Dalton Trans. 2015, 44, 10997–11003. [Google Scholar] [CrossRef] [PubMed]
- Sumesh, C.K. Zinc oxide functionalized molybdenum disulfide heterostructures as efficient electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 619–628. [Google Scholar] [CrossRef]
- Wang, S.; Ren, C.; Tian, H.; Yu, J.; Sun, M. MoS2/ZnO van der Waals heterostructure as a high-efficiency water splitting photocatalyst: A first-principles study. Phys. Chem. Chem. Phys. 2018, 19, 13394–13399. [Google Scholar] [CrossRef] [PubMed]
- Kotov, Y.A. Electric explosion of wires as a method for preparation of nanopowders. J. Nanoparticle Res. 2003, 5, 539–550. [Google Scholar] [CrossRef]
- Pustovalov, A.; An, V.; Kim, J.C. Optimal modes for the fabrication of aluminum nanopowders by the electrical explosion of wires. Adv. Mater. Sci. Eng. 2017, 2017. [Google Scholar] [CrossRef]
- An, V.; Liu, B. Synthesis of multilevel ZnS/ZnO nanostructures by electrospark erosion. Chalcogenide Letters 2015, 12, 639–644. [Google Scholar]
- Kumar, S.; Reddy, N.L.; Kushwaha, H.S.; Kumar, A.; Shankar, M.V.; Bhattacharyya, K.; Halder, A.; Krishnan, V. Efficient electron transfer across a ZnO–MoS2–Reduced graphene oxide heterojunction for enhanced sunlight-driven photocatalytic hydrogen evolution. Chem. Sus. Chem. 2017. [Google Scholar] [CrossRef]
- Skromme, B.J.; Sujan, G.K. Semiconductor Heterojunctions. In Reference Module in Materials Science and Materials Engineering; Beddows, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–11. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Gupta, U.; Rao, C.N.R. Hydrogen generation by water splitting using MoS2 and other transition metal dichalcogenides. Nano Energy 2017, 41, 49–65. [Google Scholar] [CrossRef]
- Ding, Q.; Song, B.; Xu, P.; Jin, S. Efficient electrocatalytic and photoelectrochemical hydrogen generation using MoS2 and related compounds. Chem 2016, 1, 699–726. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Kegel, J.; Povey, I.M.; Pemble, M.E. Zinc oxide for solar water splitting: A brief review of the material’s challenges and associated opportunities. Nano Energy 2018, 54, 409–428. [Google Scholar] [CrossRef]
- Rahimi, K.; Moradi, M.; Dehghan, R.; Yazdani, A. Enhancement of sunlight-induced photocatalytic activity of ZnO nanorods by few-layer MoS2 nanosheets. Mater. Lett. 2019, 234, 134–137. [Google Scholar] [CrossRef]
- Selvaraj, R.; Kalimuthu, K.R.; Kalimuthu, V. A type-II MoS2/ZnO heterostructure with enhanced photocatalytic activity. Mater. Lett. 2019, 243, 183–186. [Google Scholar] [CrossRef]
- Krishnan, U.; Kaur, M.; Kaur, G.; Singha, K.; Dogra, A.R.; Kumar, M.; Kumar, A. MoS2/ZnO nanocomposites for efficient photocatalytic degradation of industrial pollutants. Mater. Res. Bull. 2019, 111, 212–221. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Liu, Q.; He, J.; Chen, L.; Li, K.; Jia, F.; Zeng, Y.; Lu, Y.; Yu, W.; et al. Band alignment of ZnO/multilayer MoS2 interface determined by x-ray photoelectron spectroscopy. Appl. Phys. Lett. 2016, 109, 071602. [Google Scholar] [CrossRef]
- Wang, S.; Tian, H.; Ren, C.; Yu, J.; Sun, M. Electronic and optical properties of heterostructures based on transition metal dichalcogenides and graphene-like zinc oxide. Sci. Rep. 2018, 8, 12009. [Google Scholar] [CrossRef]
- Lamouchi, A.; Assaker, I.B.; Chtourou, R. Enhanced photoelectrochemical activity of MoS2-decorated ZnO nanowires electrodeposited onto stainless steel mesh for hydrogen production. Appl. Surf. Sci. 2019, 478, 937–945. [Google Scholar] [CrossRef]
- Quan, Y.; Yao, J.; Yang, S.; Chen, L.; Li, J.; Liu, Y.; Lang, J.; Shen, H.; Wang, Y.; Wang, Y.; et al. ZnO nanoparticles on MoS2 microflowers for ultrasensitive SERS detection of bisphenol A. Microchim. Acta 2019, 186, 593. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Zhang, Y.; Wang, P. Nano-photo-thermal energy drive MoS2/ZnO nanoheterojunctions growing. Opt. Mater. Express 2016, 6, 876–883. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Li, J.; Song, Z.; Zhang, H.; Zeng, W. Low working temperature of ZnO-MoS2 nanocomposites for delaying aging with good acetylene gas-sensing properties. Nanomaterials 2020, 10, 1902. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, X.F.; Yi, Z.; Yi, Y. Fabrication of p-n heterostructure ZnO/Si moth-eye structures: Antireflection, enhanced charge separation and photocatalytic properties. Appl. Surf. Sci. 2018, 441, 40–48. [Google Scholar] [CrossRef]
- Wu, H.; Jile, H.; Chen, Z.; Xu, D.; Yi, Z.; Chen, X.; Chen, J.; Yao, W.; Wu, P.; Yi, Y. Fabrication of ZnO@MoS2 Nanocomposite Heterojunction Arrays and Their Photoelectric Properties. Micromachines 2020, 11, 189. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, T.; Zulfiqar; Khan, M. Enhanced photoluminescence performance of MoS2 nanostructures after amalgamation with ZnO NPs. Optik Int. J. Light Electron Optics 2020, 220, 165201. [Google Scholar] [CrossRef]
- Tan, Y.-H.; Yu, K.; Li, J.-Z.; Fu, H.; Zhu, Z.-Q. MoS2@ZnO nano-heterojunctions with enhanced photocatalysis and field emission properties. J. Appl. Phys. 2014, 116, 064305. [Google Scholar] [CrossRef]
- Mehmet, S.; Nurdan Demirci, S. (Eds.) Hydrogen Production Technologies; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Li, H.; Dong, W.; Zhang, J.; Xi, J.; Du, G.; Ji, Z. MoS2 nanosheet/ZnO nanowire hybrid nanostructures for photoelectrochemical water splitting. J. Am. Ceram. Soc. 2018, 101, 3989–3996. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; Li, H.; Wang, X. A highly efficient sunlight driven ZnO nanosheet photocatalyst: Synergetic effect of p-doping and MoS2 atomic layer loading. ChemCatChem 2014, 6, 2522–2526. [Google Scholar] [CrossRef]
- Zhuravkov, S.P.; Lobanova, G.L.; Pustovalov, A.V.; Slyadnikov, P.E.; Nadeina, L.V. Composition of Powders Produced by Electrospark Dispersion of Metal Granules in Water. IOP Conf. Ser. Mater. Sci. Eng. 2016, 110, 012082. [Google Scholar] [CrossRef]
- Bozheyev, F.; Valiev, D.; Nemkayeva, R.; An, V.; Tikhonov, A.; Sugurbekova, G. Pulsed cathodoluminescence of WS2 nanocrystals at various electron excitation energy densities: Defect induced sub-band gap emission. J. Lumin. 2017, 192, 1308–1312. [Google Scholar] [CrossRef]
- Yu, H.; Liu, C.M.; Huang, X.Y.; Lei, M.Y. The microstructure and photoluminescence of ZnO–MoS2 core shell nano-materials. Mater. Res. Express 2017, 4, 015024. [Google Scholar] [CrossRef]
- Rauwel, P.; Salumaa, M.; Aasna, A.; Galeckas, A.; Rauwel, E. A review of the synthesis and photoluminescence properties of hybrid ZnO and carbon nanomaterials. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Tang, C.-M.; Zhang, H.-Y.; Zhang, J. Study on photocatalytic activity of MoS2/ZnO composite in visible light. Optoelectron. Lett. 2020, 16, 446–450. [Google Scholar] [CrossRef]
- Tahir, M.B.; Sohaib, M.; Rafique, M.; Sagir, M.; Rehman, N.U. Shabbir Muhammad, Visible light responsive photocatalytic hydrogen evolution using MoS2 incorporated ZnO. Appl. Nanosc. 2020, 10, 3925–3931. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef]
- Bozheyev, F.; An, V.V.; Irtegov, Y. Properties of copper and molybdenum sulfide powders produced by self-propagating high-temperature synthesis. Adv. Mater. Res. 2014, 872, 191–196. [Google Scholar] [CrossRef]
- Pham, K.C.; McPhail, D.S.; Wee, A.T.; Chua, D.H. Amorphous molybdenum sulfide on graphene–carbon nanotube hybrids as supercapacitor electrode materials. RSC Adv. 2017, 7, 6856–6864. [Google Scholar] [CrossRef]
- Goldenblum, A.; Marian, A.B.; Teodorescu, V. Optical properties of ZnO nanocrystallites embedded in a gold-oxide matrix. J. Optoelectron. Adv. Mater. 2006, 8, 2129–2132. [Google Scholar]
- Thandavan, T.M.K.; Gani, S.M.A.; San Wong, C.; Nor, R.M. Enhanced photoluminescence and Raman properties of Al-doped ZnO nanostructures prepared using thermal chemical vapor deposition of methanol assisted with heated brass. PLoS ONE 2015, 10, e0121756. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Choy, W.C.; Roy, V.A.L.; Leung, Y.H.; Kwong, C.Y.; Cheah, K.W.; Gundu Rao, T.; Chan, W.; Fei Lui, H.; Surya, C. Photoluminescence and electron paramagnetic resonance of ZnO tetrapod structures. Adv. Funct. Mater. 2004, 14, 856–864. [Google Scholar] [CrossRef]
- Lv, J.; Li, C.; Chai, Z. Defect luminescence and its mediated physical properties in ZnO. J. Lumin. 2019, 208, 225–237. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO–nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Cheng, C.; Fan, H.J. Branched nanowires: Synthesis and energy applications. Nano Today 2012, 7, 327–343. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Corolewski, C.D.; Lv, J.; Tarun, M.C.; Teklemichael, S.T.; Walter, E.D.; Norton, G.M.; Harrison, K.W.; Ha, S. Acceptors in ZnO. J. Appl. Phys. 2015, 117, 112802. [Google Scholar] [CrossRef]
- Bozheyev, F.; Valiev, D.; Nemkayeva, R. Pulsed cathodoluminescence and Raman spectra of MoS2 nanocrystals at different excitation electron energy densities and laser wavelengths. J. Lumi. 2017, 188, 529–532. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, F.; Liu, J.; Che, W.; Su, H.; Liu, W.; Huang, Y.Y.; Jiang, Y.; Yao, T.; Liu, Q.H.; et al. MoS2-coated ZnO nanocomposite as an active heterostructure photocatalyst for hydrogen evolution. Radiation Phys. Chem. 2017, 137, 104–107. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, V.; Potgieter, H.; Usoltseva, N.; Valiev, D.; Stepanov, S.; Pustovalov, A.; Baryshnikov, A.; Titov, M.; Dolinina, A. MoS2@ZnO Nanoheterostructures Prepared by Electrospark Erosion for Photocatalytic Applications. Nanomaterials 2021, 11, 157. https://doi.org/10.3390/nano11010157
An V, Potgieter H, Usoltseva N, Valiev D, Stepanov S, Pustovalov A, Baryshnikov A, Titov M, Dolinina A. MoS2@ZnO Nanoheterostructures Prepared by Electrospark Erosion for Photocatalytic Applications. Nanomaterials. 2021; 11(1):157. https://doi.org/10.3390/nano11010157
Chicago/Turabian StyleAn, Vladimir, Herman Potgieter, Natalia Usoltseva, Damir Valiev, Sergei Stepanov, Alexey Pustovalov, Arsenii Baryshnikov, Maksim Titov, and Alesya Dolinina. 2021. "MoS2@ZnO Nanoheterostructures Prepared by Electrospark Erosion for Photocatalytic Applications" Nanomaterials 11, no. 1: 157. https://doi.org/10.3390/nano11010157
APA StyleAn, V., Potgieter, H., Usoltseva, N., Valiev, D., Stepanov, S., Pustovalov, A., Baryshnikov, A., Titov, M., & Dolinina, A. (2021). MoS2@ZnO Nanoheterostructures Prepared by Electrospark Erosion for Photocatalytic Applications. Nanomaterials, 11(1), 157. https://doi.org/10.3390/nano11010157







