Electrospinning Synthesis of Carbon-Supported Pt3Mn Intermetallic Nanocrystals and Electrocatalytic Performance towards Oxygen Reduction Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
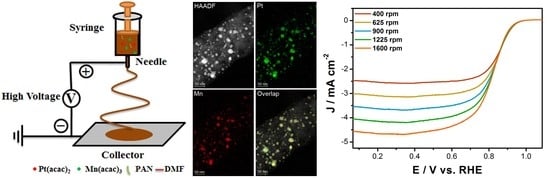
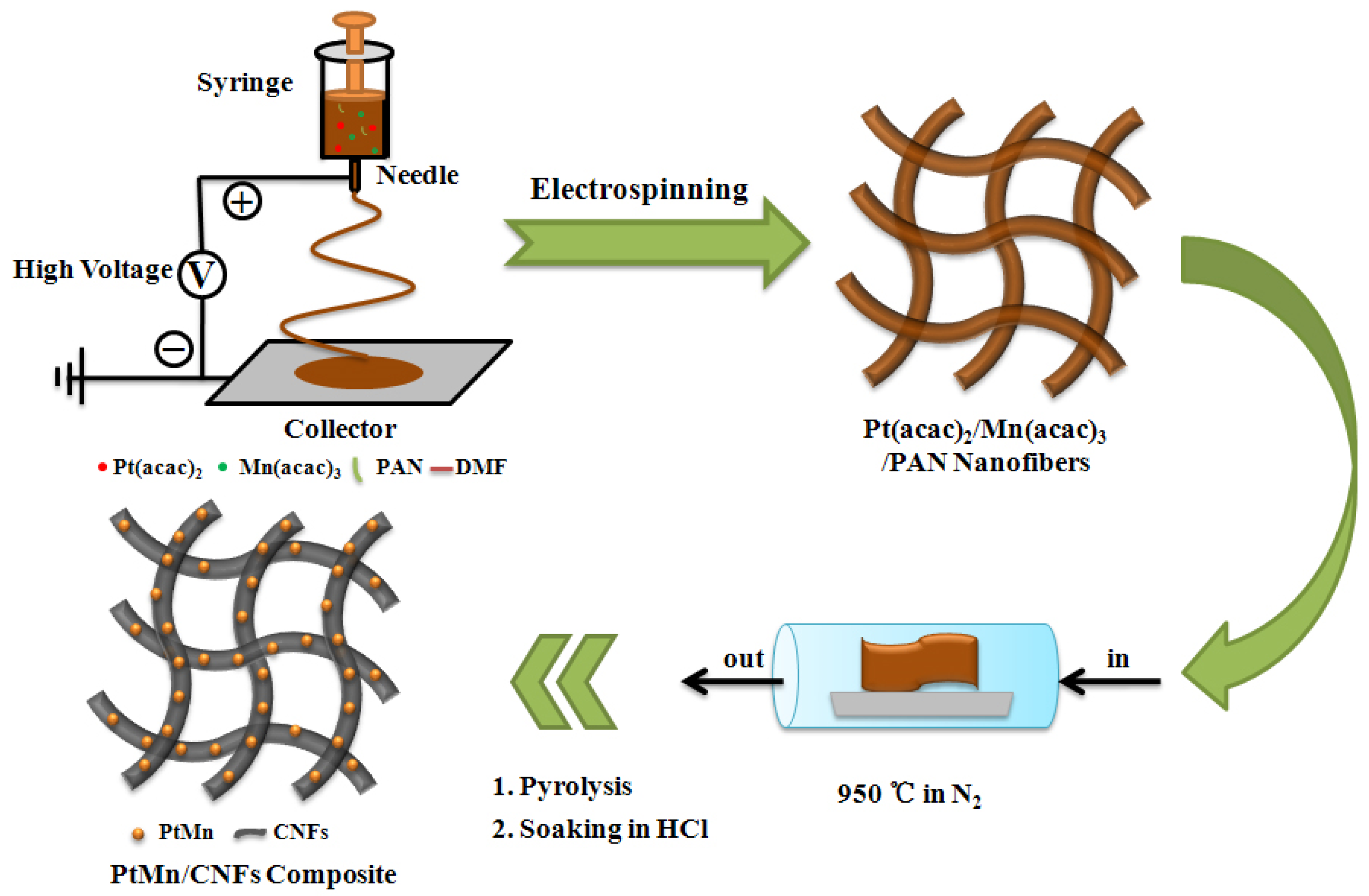
2.2. Preparation of Pt(acac)2/Mn(acac)3/PAN Nanofibers
2.3. Preparation of PtMn/CNFs Catalysts
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
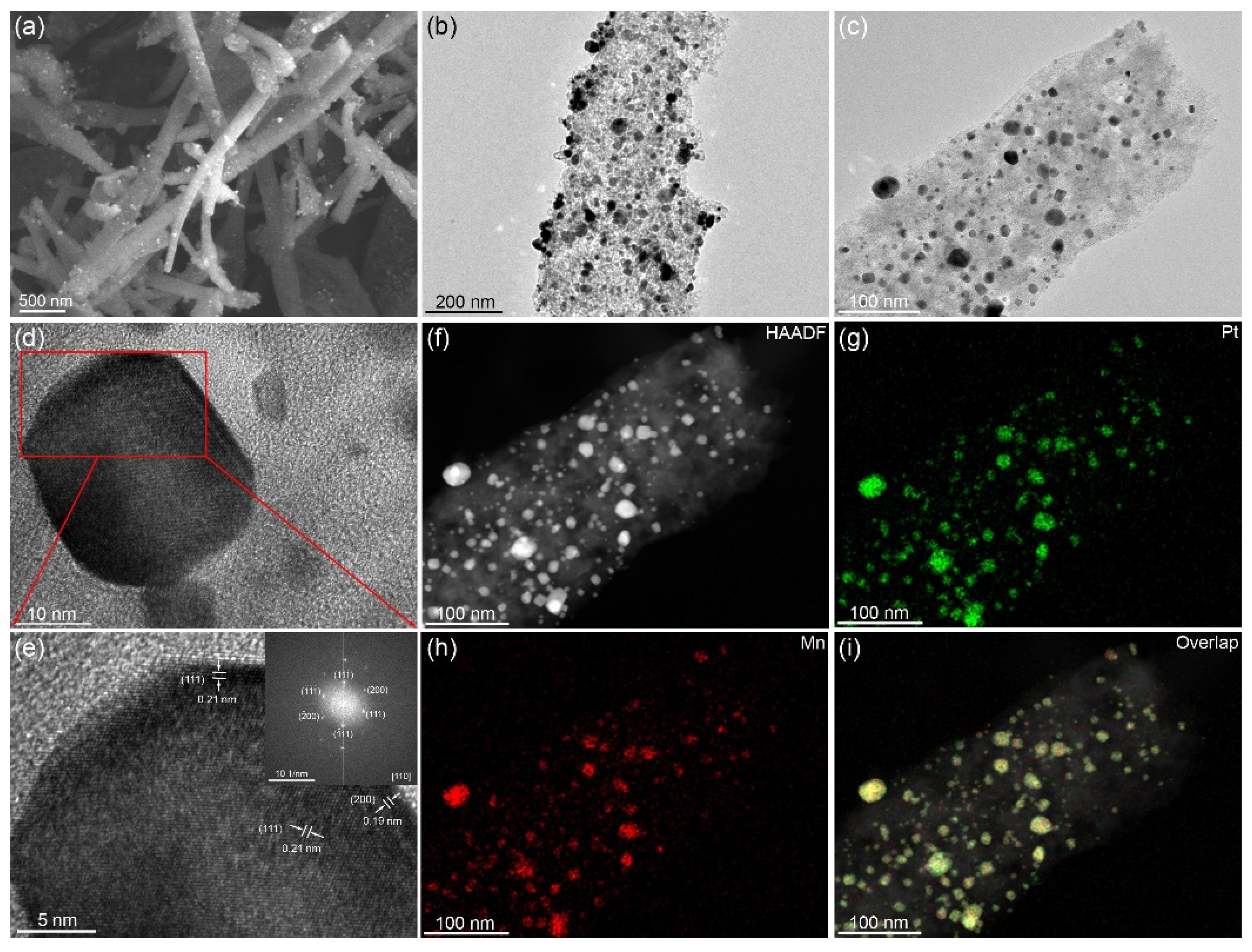
3.1. Structure and Morphology Characterization
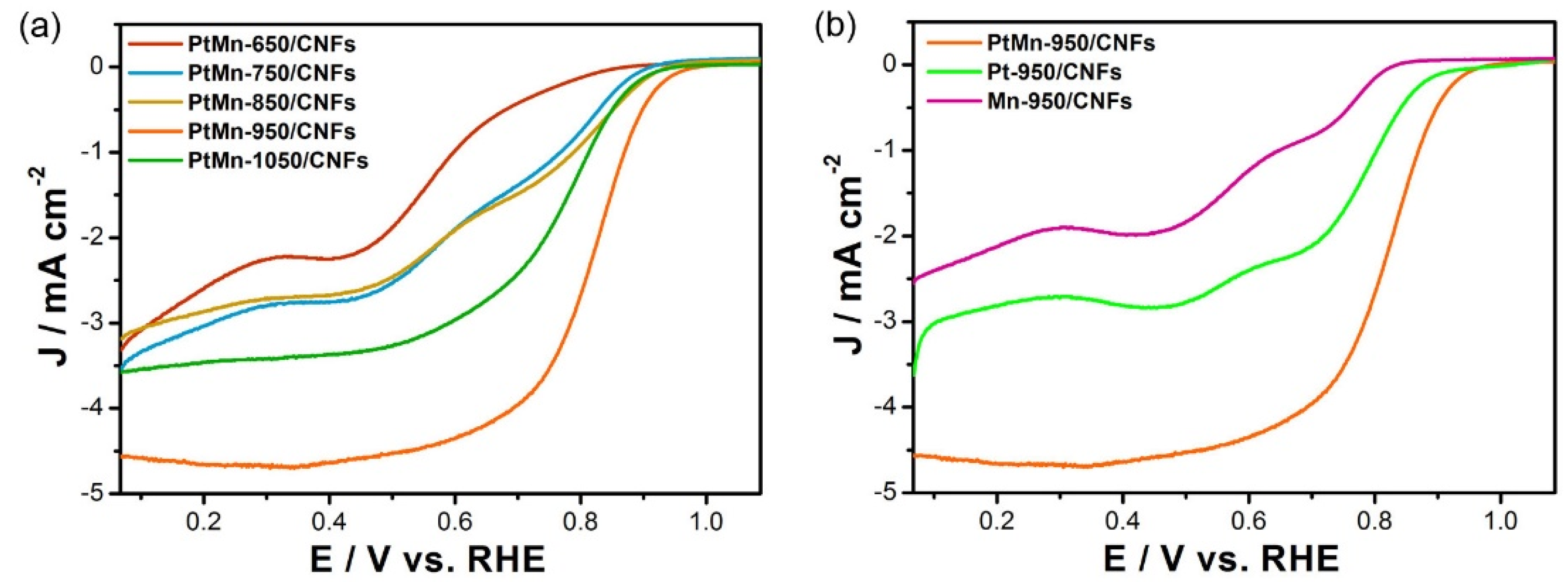
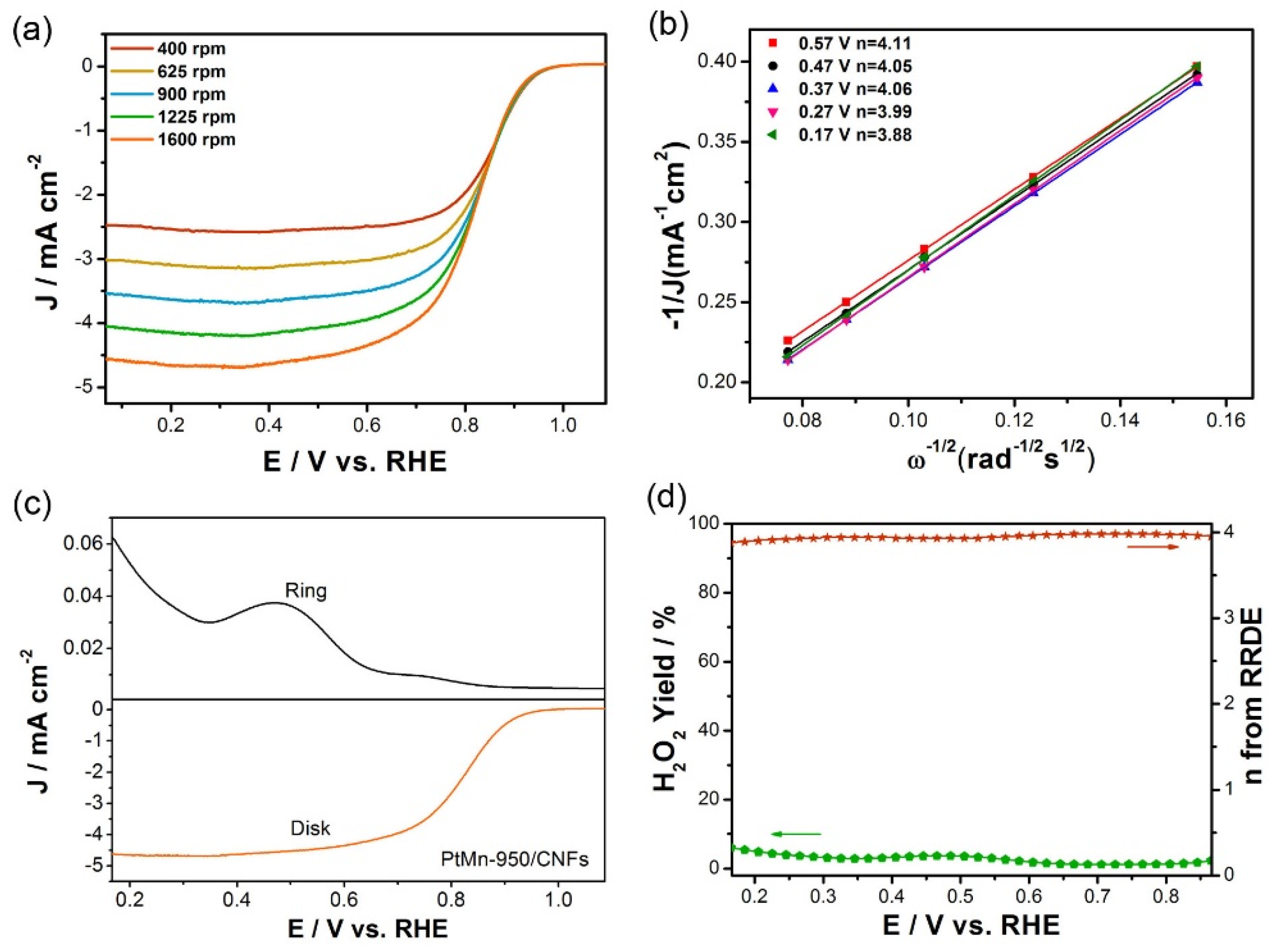
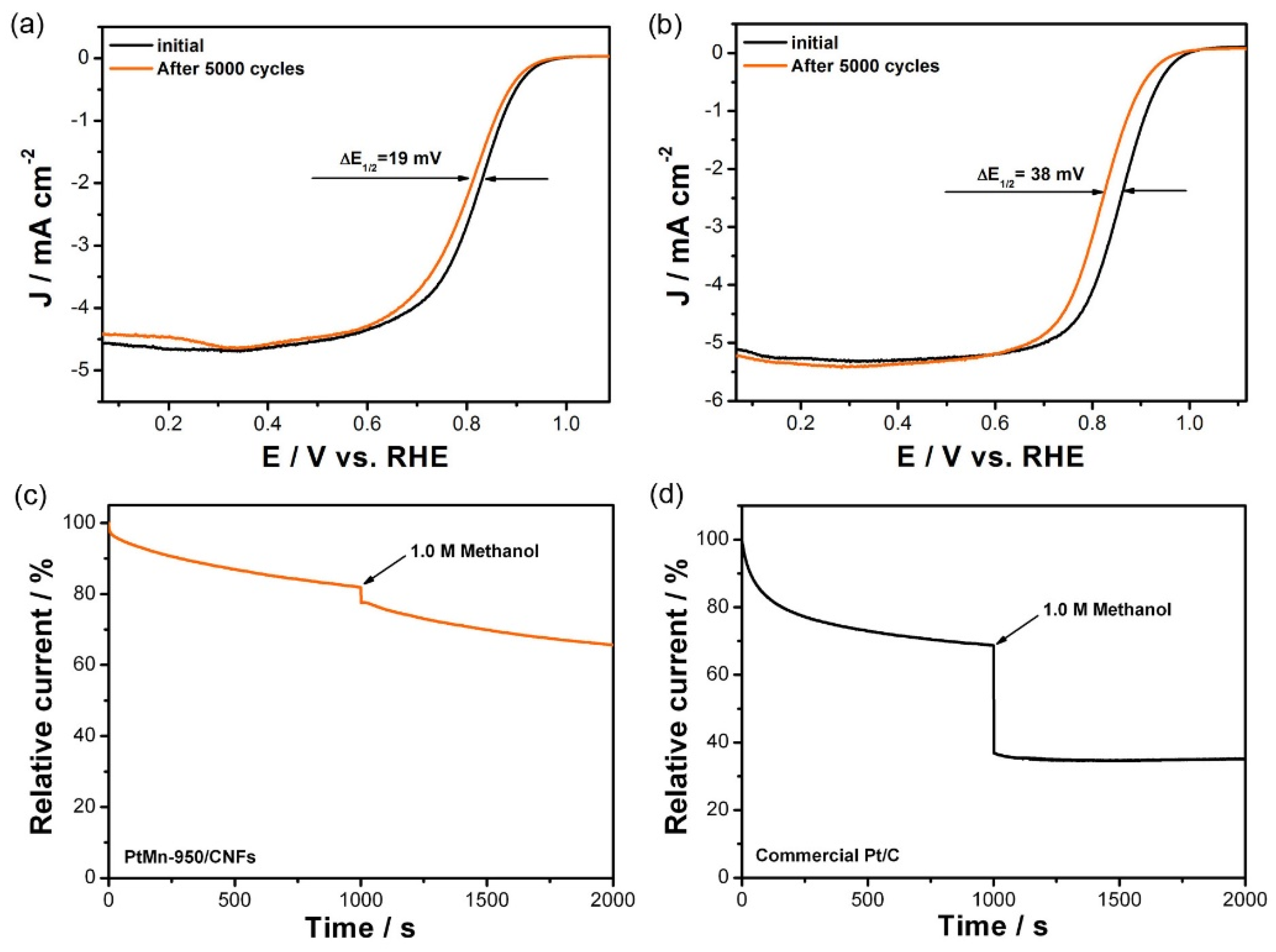
3.2. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.Y.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef]
- Wang, X.Y.; Feng, J.J.; Zhang, L.; Luo, X.; Zhang, Q.L.; Wang, A.J. Bioinspired one-pot fabrication of triple-layered Rh@Co@Pt-skin core-shell nanodendrites: A highly active and durable electrocatalyst towards oxygen reduction reaction. Electrochim. Acta 2019, 321, 134660. [Google Scholar] [CrossRef]
- Chao, T.; Zhang, Y.; Hu, Y.; Zheng, X.; Qu, Y.; Xu, Q.; Hong, X. Atomically dispersed Pt on screw-like Pd/Au core-shell nanowires for enhanced electrocatalysis. Chem. Eur. J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yue, Q.; Chen, G.; Zhai, Y.; Wang, L.; Wang, H.; Zhao, J.; Liu, J.; Jia, J.; Li, H. Effects of acid treatment of Pt-Ni alloy nanoparticles@graphene on the kinetics of the oxygen reduction reaction in acidic and alkaline solutions. J. Phys. Chem. C 2011, 115, 379–389. [Google Scholar] [CrossRef]
- Meng, Z.; Xiao, F.; Wei, Z.; Guo, X.; Zhu, Y.; Liu, Y.; Li, G.; Yu, Z.Q.; Shao, M.; Wong, W.Y. Direct synthesis of L10-FePt nanoparticles from single-source bimetallic complex and their electrocatalytic applications in oxygen reduction and hydrogen evolution reactions. Nano Res. 2019, 12, 2954–2959. [Google Scholar] [CrossRef]
- Xue, Y.; Li, H.; Ye, X.; Yang, S.; Zheng, Z.; Han, X.; Zhang, X.; Chen, L.; Xie, Z.; Kuang, Q.; et al. N-doped carbon shell encapsulated PtZn intermetallic nanoparticles as highly efficient catalysts for fuel cells. Nano Res. 2019, 12, 2490–2497. [Google Scholar] [CrossRef]
- Dionigi, F.; Weber, C.C.; Primbs, M.; Gocyla, M.; Bonastre, A.M.; Spöri, C.; Schmies, H.; Hornberger, E.; Kühl, S.; Drnec, J.; et al. Controlling near-surface Ni composition in octahedral PtNi(Mo) nanoparticles by Mo doping for a highly active oxygen reduction reaction catalyst. Nano Lett. 2019, 19, 6876–6885. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhao, X.; Su, Y.Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.M.; Lou, X.W.; et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lei, B.; Guo, S. Engineering multimetallic nanocrystals for highly efficient oxygen reduction catalysts. Adv. Energy Mater. 2016, 6, 1600236. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiao, L.; Yang, Y.; DiSalvo, F.J.; Abruña, H.D. High-loading intermetallic Pt3Co/C core–shell nanoparticles as enhanced activity electrocatalysts toward the oxygen reduction reaction (ORR). Chem. Mater. 2018, 30, 1532–1539. [Google Scholar] [CrossRef]
- Liang, J.; Li, N.; Zhao, Z.; Ma, L.; Wang, X.; Li, S.; Liu, X.; Wang, T.; Du, Y.; Lu, G.; et al. Tungsten-doped L10-PtCo ultrasmall nanoparticles as a high-performance fuel cell cathode. Angew. Chem. Int. Ed. 2019, 58, 15471–15477. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, Q.; Zhu, Y.; Tang, S.; Du, Y. Facile synthesis of quaternary structurally ordered L12-Pt(Fe, Co, Ni)3 nanoparticles with low content of platinum as efficient oxygen reduction reaction electrocatalysts. ACS Omega 2019, 4, 17894–17902. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, S. Intermetallic nanoparticles: Synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 2019, 52, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.L.; Zhu, X.X.; Wang, J.N. Remarkable improvement of the catalytic performance of PtFe nanoparticles by structural ordering and doping. ACS Appl. Mater. Interfaces 2019, 11, 11527–11536. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, J.; Wang, J.; Xiao, W.; Lei, W.; Zhao, T.; Huang, T.; Zhu, Y.; Wang, D. Phase conversion of Pt3Ni2/C from disordered alloy to ordered intermetallic with strained lattice for oxygen reduction reaction. Electrochim. Acta 2018, 283, 1253–1260. [Google Scholar] [CrossRef]
- Qin, Y.; Luo, M.; Sun, Y.; Li, C.; Huang, B.; Yang, Y.; Li, Y.; Wang, L.; Guo, S. Intermetallic hcp-PtBi/fcc-Pt core/shell nanoplates enable efficient bifunctional oxygen reduction and methanol oxidation electrocatalysis. ACS Catal. 2018, 8, 5581–5590. [Google Scholar] [CrossRef]
- Gamler, J.T.L.; Ashberry, H.M.; Skrabalak, S.E.; Koczkur, K.M. Random alloyed versus intermetallic nanoparticles: A comparison of electrocatalytic performance. Adv. Mater. 2018, 30, 1801563. [Google Scholar] [CrossRef]
- Ghavidel, M.R.Z.; Easton, E.B. Thermally induced changes in the structure and ethanol oxidation activity of Pt0.25Mn0.75/C. Appl. Catal. B Environ. 2015, 176–177, 150–159. [Google Scholar] [CrossRef]
- Ghosh, T.; Leonard, B.M.; Zhou, Q.; DiSalvo, F.J. Pt alloy and intermetallic phases with V, Cr, Mn, Ni, and Cu: Synthesis as nanomaterials and possible applications as fuel cell catalysts. Chem. Mater. 2010, 22, 2190–2202. [Google Scholar] [CrossRef]
- Xu, C.; Su, Y.; Tan, L.; Liu, Z.; Zhang, J.; Chen, S.; Jiang, S.P. Electrodeposited PtCo and PtMn electrocatalysts for methanol and ethanol electrooxidation of direct alcohol fuel cells. Electrochim. Acta 2009, 54, 6322–6326. [Google Scholar] [CrossRef]
- Ammam, M.; Easton, E.B. Oxygen reduction activity of binary PtMn/C, ternary PtMnX/C (X = Fe, Co, Ni, Cu, Mo and, Sn) and quaternary PtMnCuX/C (X = Fe, Co, Ni, and Sn) and PtMnMoX/C (X = Fe, Co, Ni, Cu and Sn) alloy catalysts. J. Power Sources 2013, 236, 311–320. [Google Scholar] [CrossRef]
- Wu, Z.; Bukowski, B.C.; Li, Z.; Milligan, C.; Zhou, L.; Ma, T.; Wu, Y.; Ren, Y.; Ribeiro, F.H.; Delgass, W.N.; et al. Changes in catalytic and adsorptive properties of 2 nm Pt3Mn nanoparticles by subsurface atoms. J. Am. Chem. Soc. 2018, 140, 14870–14877. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Murray, C.B. Synthesis and electrocatalytic properties of cubic Mn-Pt nanocrystals (nanocubes). J. Am. Chem. Soc. 2010, 132, 7568–7569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, H.; Sun, H.; Zhang, X.; Dai, X.; Luan, C.; Qin, C.; Zhao, H.; Li, J.; Wang, M.; et al. Implanting Mo atoms into surface lattice of Pt3Mn alloys enclosed by high-indexed facets: Promoting highly active sites for ethylene glycol oxidation. ACS Catal. 2019, 9, 442–455. [Google Scholar] [CrossRef]
- Pan, Y.; Hwang, S.Y.; Shen, X.; Yang, J.; Zeng, J.; Wu, M.; Peng, Z. Computation-guided development of platinum alloy catalyst for carbon monoxide preferential oxidation. ACS Catal. 2018, 8, 5777–5786. [Google Scholar] [CrossRef]
- Jin, X.; Zeng, C.; Yan, W.; Zhao, M.; Bobba, P.; Shi, H.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R.V. Lattice distortion induced electronic coupling results in exceptional enhancement in the activity of bimetallic PtMn nanocatalysts. Appl. Catal. A Gen. 2017, 534, 46–57. [Google Scholar] [CrossRef]
- Wang, X.X.; Hwang, S.; Pan, Y.T.; Chen, K.; He, Y.; Karakalos, S.; Zhang, H.; Spendelow, J.S.; Su, D.; Wu, G. Ordered Pt3Co intermetallic nanoparticles derived from metal–organic frameworks for oxygen reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Liu, J.; Wang, F. PDA-assisted formation of ordered intermetallic CoPt3 catalysts with enhanced oxygen reduction activity and stability. Nanoscale 2018, 10, 9038–9043. [Google Scholar] [CrossRef]
- Lee, D.C.; Ghezelbash, A.; Stowell, C.A.; Korgel, B.A. Synthesis and magnetic properties of colloidal MnPt3 nanocrystals. J. Phys. Chem. B 2006, 110, 20906–20911. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Lee, C.; Bang, K.; Lim, J.; Lee, H.; Ryu, H.J.; Cho, E.; Lee, H.M. Synthesis of chemically ordered Pt3Fe/C intermetallic electrocatalysts for oxygen reduction reaction with enhanced activity and durability via a removable carbon coating. ACS Appl. Mater. Interfaces 2017, 9, 31806–31815. [Google Scholar] [CrossRef]
- Ammam, M.; Easton, E.B. A simple method to reduce the particle size and amount of oxide phase growth in Pt-Mn catalysts for ethanol oxidation. J. Electrochem. Soc. 2013, 160, F212–F217. [Google Scholar] [CrossRef]
- Ammam, M.; Prest, L.E.; Pauric, A.D.; Easton, E.B. Synthesis, characterization and catalytic activity of binary PtMn/C alloy catalysts towards ethanol oxidation. J. Electrochem. Soc. 2012, 159, B195–B200. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, X.; Peng, L.; Kang, W.; Li, H. The enhanced lithium-storage performance for MnO nanoparticles anchored on electrospun nitrogen-doped carbon fibers. Nanomaterials 2018, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Siu, A.C.; Leung, K.T. Anomalous electrodeposition of metallic Mn nanostructured films on H-terminated Si(100) at anodic potential. Chem. Mater. 2007, 19, 6414–6420. [Google Scholar] [CrossRef]
- Rößner, L.; Armbrüster, M. Electrochemical energy conversion on intermetallic compounds: A review. ACS Catal. 2019, 9, 2018–2062. [Google Scholar] [CrossRef]
- Xiao, W.; Lei, W.; Gong, M.; Xin, H.L.; Wang, D. Recent advances of structurally ordered intermetallic nanoparticles for electrocatalysis. ACS Catal. 2018, 8, 3237–3256. [Google Scholar] [CrossRef]
- Feng, Y.; Shao, Q.; Lv, F.; Bu, L.; Guo, J.; Guo, S.; Huang, X. Intermetallic PtBi nanoplates boost oxygen reduction catalysis with superior tolerance over chemical fuels. Adv. Sci. 2018, 1800178. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Zhou, L.; Kang, W.; Li, R.; Qu, K.; Wang, L.; Li, H. Electrospinning Synthesis of Carbon-Supported Pt3Mn Intermetallic Nanocrystals and Electrocatalytic Performance towards Oxygen Reduction Reaction. Nanomaterials 2020, 10, 1893. https://doi.org/10.3390/nano10091893
Peng L, Zhou L, Kang W, Li R, Qu K, Wang L, Li H. Electrospinning Synthesis of Carbon-Supported Pt3Mn Intermetallic Nanocrystals and Electrocatalytic Performance towards Oxygen Reduction Reaction. Nanomaterials. 2020; 10(9):1893. https://doi.org/10.3390/nano10091893
Chicago/Turabian StylePeng, Lechao, Lan Zhou, Wenjun Kang, Rui Li, Konggang Qu, Lei Wang, and Haibo Li. 2020. "Electrospinning Synthesis of Carbon-Supported Pt3Mn Intermetallic Nanocrystals and Electrocatalytic Performance towards Oxygen Reduction Reaction" Nanomaterials 10, no. 9: 1893. https://doi.org/10.3390/nano10091893
APA StylePeng, L., Zhou, L., Kang, W., Li, R., Qu, K., Wang, L., & Li, H. (2020). Electrospinning Synthesis of Carbon-Supported Pt3Mn Intermetallic Nanocrystals and Electrocatalytic Performance towards Oxygen Reduction Reaction. Nanomaterials, 10(9), 1893. https://doi.org/10.3390/nano10091893