Magnetic and Magneto-Optical Oroperties of Iron Oxides Nanoparticles Synthesized under Atmospheric Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetite Nanoparticles
2.2. Characterizations
3. Results and Discussions
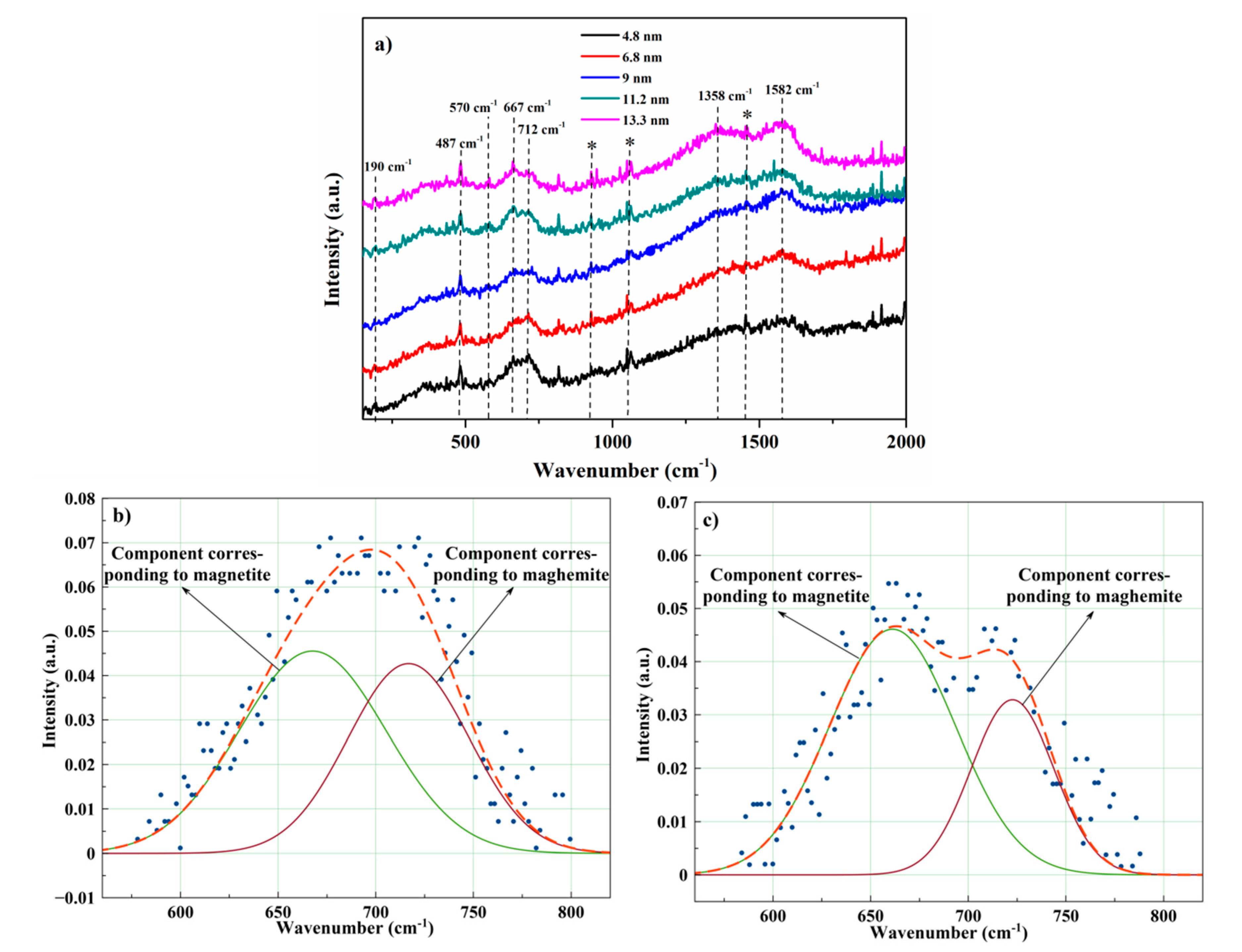
3.1. Structural Characterization
3.2. Magnetic Measurements
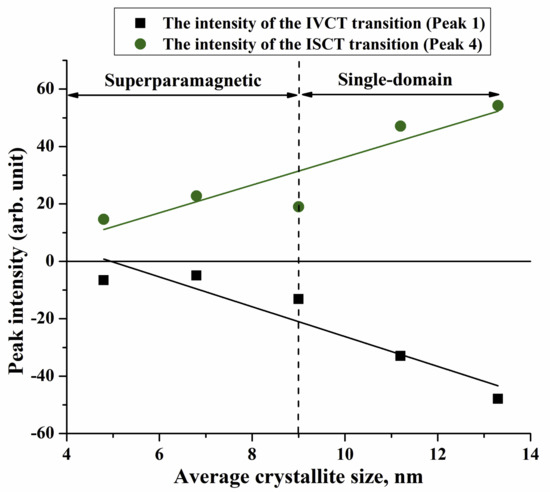
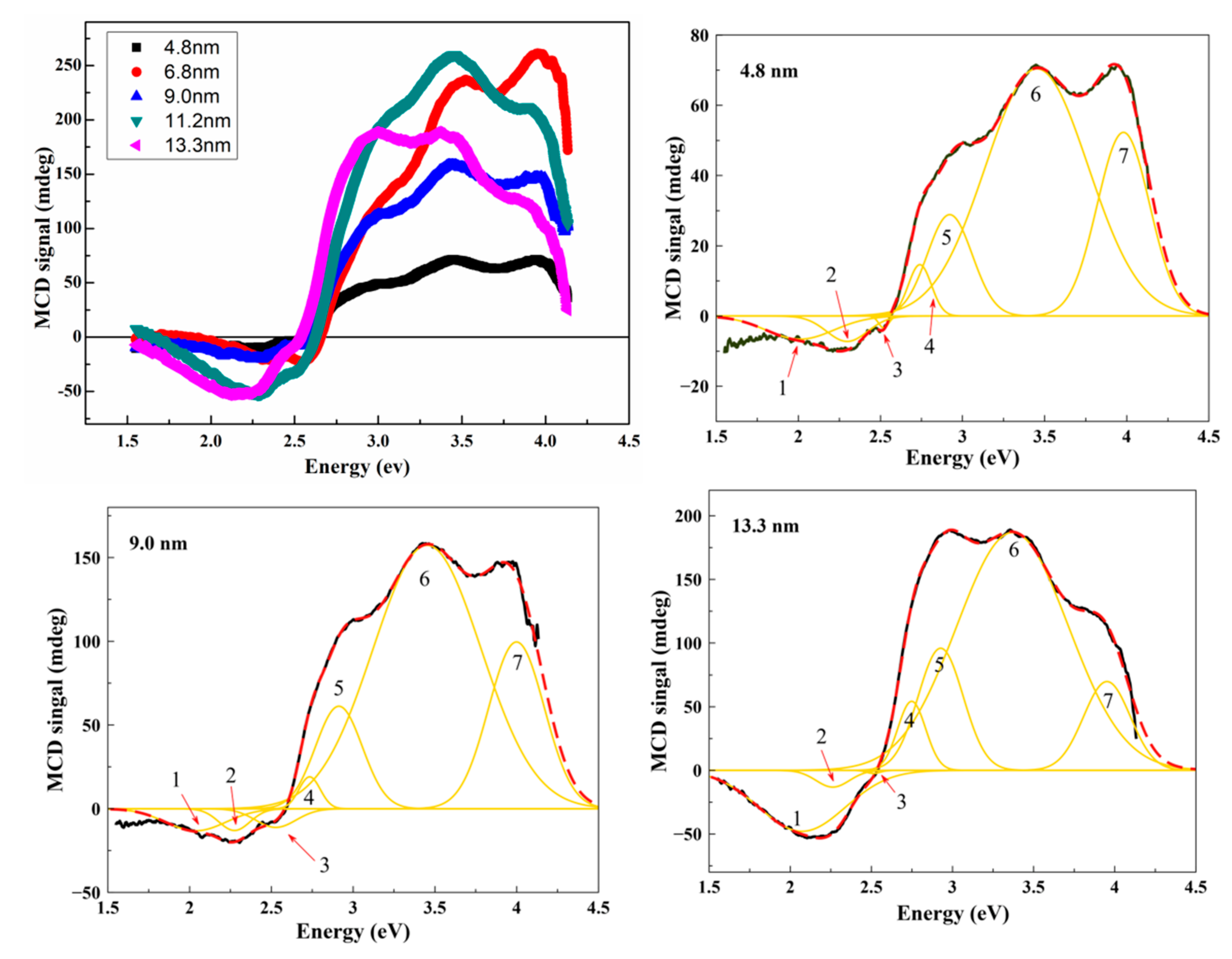
MCD Spectroscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dalpozzo, R. Magnetic nanoparticle supports for asymmetric catalysts. Green Chem. 2015, 17, 3671–3686. [Google Scholar] [CrossRef]
- Battiato, M.; Minar, J.; Wang, W.; Ndiaye, W.; Richter, M.C.; Heckmann, O.; Mariot, J.-M.; Parmigiani, F.; Hricovini, K.; Cacho, C. Distinctive Picosecond Spin Polarization Dynamics in Bulk Half Metals. Phys. Rev. Lett. 2018, 121, 077205. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, Y.; Zeng, X.; Cui, T.; Zhao, Y.; Li, Y.; Tong, G. Facile Hydrothermal Synthesis of Fe3O4/C Core–Shell Nanorings for Efficient Low-Frequency Microwave Absorption. ACS Appl. Mater. Interfaces 2016, 8, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.R.; Arjmand, H.; Hoseini, S.J.; Nasrabadi, H. Surface modified magnetic nanoparticles as efficient and green sorbents: Synthesis, characterization, and application for the removal of anionic dye. J. Magn. Magn. Mater. 2015, 394, 7–13. [Google Scholar] [CrossRef]
- Molina, L.; Gaete, J.; Alfaro, I.; Ide, V.; Valenzuela, F.; Parada, J.; Basualto, C. Synthesis and characterization of magnetite nanoparticles functionalized with organophosphorus compounds and its application as an adsorbent for La (III), Nd (III) and Pr (III) ions from aqueous solutions. J. Mol. Liq. 2019, 275, 178–191. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Wang, X.; Pang, X.; Wu, D.; Du, B.; Wei, Q. Novel signal amplification strategy for ultrasensitive sandwich-type electrochemical immunosensor employing Pd-Fe3O4-GS as the matrix and SiO2 as the label. Biosens. Bioelectron. 2015, 74, 59–65. [Google Scholar] [CrossRef]
- Patra, M.; Manzoor, K.; Manoth, M.; Negi, S.; Vadera, S.; Kumar, N. Nanotechnology Applications for Chemical and Biological Sensors. Def. Sci. J. 2008, 58, 636–649. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.-J. Preparation and application of iron oxide/graphene based composites for electrochemical energy storage and energy conversion devices: Current status and perspective. Nano Energy 2015, 11, 277–293. [Google Scholar] [CrossRef]
- Hussein, A.K. Applications of nanotechnology in renewable energies—A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015, 42, 460–476. [Google Scholar] [CrossRef]
- Magro, M.; Baratella, D.; Bonaiuto, E.; Roger, J.D.A.; Vianello, F. New Perspectives on Biomedical Applications of Iron Oxide Nanoparticles. Curr. Med. Chem. 2018, 25, 540–555. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Sun, W.; Xiao, Y.; Shi, X. Ultrasmall iron oxide nanoparticles: Synthesis, surface modification, assembly, and biomedical applications. Drug Discov. Today 2019, 24, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic Phase Syntheses of Magnetic Nanoparticles and Their Applications. Chem. Rev. 2016, 116, 10473–10512. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Nairan, A.; Irfan, M.; Ali, H.; Han, X. Temperature mediated morphological and magnetic phase transitions of iron/iron oxide Core/Shell nanostructures. J. Alloys Compd. 2017, 696, 362–368. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Huie, M.M.; Choi, D.; Chang, M.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S.; Reichmanis, E. Toward Uniformly Dispersed Battery Electrode Composite Materials: Characteristics and Performance. ACS Appl. Mater. Interfaces 2016, 8, 3452–3463. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Pirmoradian, M.; Riahi, F. Electrochemical Detection of Pb and Cu by Using DTPA Functionalized Magnetic Nanoparticles. Electrochim. Acta 2014, 115, 573–580. [Google Scholar] [CrossRef]
- Reddy, K.R.; Lee, K.-P.; Saianand, G. Novel electrically conductive and ferromagnetic composites of poly(aniline-co-aminonaphthalenesulfonic acid) with iron oxide nanoparticles: Synthesis and characterization. J. Appl. Polym. Sci. 2007, 106, 1181–1191. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. Optimal size for heating efficiency of superparamagnetic dextran-coated magnetite nanoparticles for application in magnetic fluid hyperthermia. Phys. C Supercond. 2018, 549, 84–87. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Masoumi, H.R.F.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid Adsorption of Heavy Metals by Fe3O4/Talc Nanocomposite and Optimization Study Using Response Surface Methodology. Int. J. Mol. Sci. 2014, 15, 12913–12927. [Google Scholar] [CrossRef]
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef]
- Rezayan, A.H.; Mousavi, M.; Kheirjou, S.; Amoabediny, G.; Ardestani, M.S.; Mohammadnejad, J. Monodisperse magnetite (Fe3O4) nanoparticles modified with water soluble polymers for the diagnosis of breast cancer by MRI method. J. Magn. Magn. Mater. 2016, 420, 210–217. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Dukenbayev, K.; Korolkov, I.; Tishkevich, D.I.; Kozlovskiy, A.; Trukhanov, S.; Gorin, Y.G.; Shumskaya, A.; Kaniukov, E.Y.; Vinnik, D.; Zdorovets, M.V.; et al. Fe3O4 Nanoparticles for Complex Targeted Delivery and Boron Neutron Capture Therapy. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Prout, J.; Seifalian, A.M. Magnetic Nanoparticles: New Perspectives in Drug Delivery. Curr. Pharm. Des. 2017, 23, 2908–2917. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.Y.; Eko, A.S.; Candra, K.; Hasibuan, D.P.; Ginting, M.; Sebayang, P.; Simamora, P. Synthesis, Properties and Application of Glucose Coated Fe3O4Nanoparticles Prepared by Co-precipitation Method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 214, 12021. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Rani, S.; Varma, G. Superparamagnetism and metamagnetic transition in Fe3O4 nanoparticles synthesized via co-precipitation method at different pH. Phys. B Condens. Matter 2015, 472, 66–77. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, B.; Feng, L. Preparation and magnetic properties of magnetite nanoparticles. Mater. Lett. 2012, 68, 112–114. [Google Scholar] [CrossRef]
- Ahmadi, S.; Chia, C.H.; Zakaria, S.; Saeedfar, K.; Asim, N. Synthesis of Fe3O4 nanocrystals using hydrothermal approach. J. Magn. Magn. Mater. 2012, 324, 4147–4150. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G. One-step hydrothermal synthesis of magnetic Fe3O4 nanoparticles immobilized on polyamide fabric. Appl. Surf. Sci. 2012, 258, 4952–4959. [Google Scholar] [CrossRef]
- Lemine, O.; Omri, K.; Zhang, B.; El Mir, L.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol–gel synthesis of 8nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattices Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Chomchoey, N.; Bhongsuwan, D.; Bhongsuwan, T. Magnetic Properties of Magnetite Nanoparticles Synthesized by Oxidative Alkaline Hydrolysis of Iron Powder. Nat. Sci. 2010, 44, 963–971. [Google Scholar]
- Worawong, A.; Jutarosaga, T.; Onreabroy, W. Influence of Calcination Temperature on Synthesis of Magnetite (Fe3O4) Nanoparticles by Sol-Gel Method. Adv. Mater. Res. 2014, 979, 208–211. [Google Scholar] [CrossRef]
- Belaïd, S.; Laurent, S.; Vermeersch, M.; Elst, L.V.; Pérez-Morga, D.; Muller, R.N. A new approach to follow the formation of iron oxide nanoparticles synthesized by thermal decomposition. Nanotechnology 2013, 24, 55705. [Google Scholar] [CrossRef]
- Amara, D.; Felner, I.; Nowik, I.; Margel, S. Synthesis and characterization of Fe and Fe3O4 nanoparticles by thermal decomposition of triiron dodecacarbonyl. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 106–110. [Google Scholar] [CrossRef]
- Toyos-Rodríguez, C.; Calleja-García, J.; Torres-Sánchez, L.; López, A.; Abu-Dief, A.M.; Costa, A.; Elbaile, L.; Crespo, R.D.; Garitaonandia, J.S.; Lastra, E.; et al. A Simple and Reliable Synthesis of Superparamagnetic Magnetite Nanoparticles by Thermal Decomposition of Fe(acac)3. J. Nanomater. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Roca, A.G.; Morales, M.P.; O’Grady, K.; Serna, C.J. Structural and magnetic properties of uniform magnetite nanoparticles prepared by high temperature decomposition of organic precursors. Nanotechnology 2006, 17, 2783–2788. [Google Scholar] [CrossRef]
- Li, D.; Jiang, D.; Jiang, D.; Xie, J.; Wu, Y.; Dang, S.; Zhang, J. An easy fabrication of monodisperse oleic acid-coated Fe3O4 nanoparticles. Mater. Lett. 2010, 64, 2462–2464. [Google Scholar] [CrossRef]
- Majewski, P.; Thierry, B. Functionalized Magnetite Nanoparticles—Synthesis, Properties, and Bio-Applications. Crit. Rev. Solid State Mater. Sci. 2007, 32, 203–215. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Muhammed, M.; Zagorodni, A.A. Novel flow injection synthesis of iron oxide nanoparticles with narrow size distribution. Chem. Eng. Sci. 2006, 61, 4625–4633. [Google Scholar] [CrossRef]
- Abbas, M.; Rao, B.P.; Naga, S.; Takahashi, M.; Kim, C. Synthesis of high magnetization hydrophilic magnetite (Fe3O4) nanoparticles in single reaction—Surfactantless polyol process. Ceram. Int. 2013, 39, 7605–7611. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Chen, X.; Gui, Z.; Liu, L.; Chen, Z. A new simple hydrothermal preparation of nanocrystalline magnetite Fe3O4. Mater. Res. Bull. 2001, 36, 497–502. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, B.; Wang, L.; Wang, M.; Gao, H. One-pot synthesis of water-soluble superparamagnetic iron oxide nanoparticles and their MRI contrast effects in the mouse brains. Mater. Sci. Eng. C 2015, 48, 416–423. [Google Scholar] [CrossRef]
- Naidek, K.P.; Bianconi, F.; Rocha, T.C.R.; Zanchet, D.; Bonacin, J.A.; Novak, M.A.; Vaz, M.G.F.; Winnischofer, H. Structure and morphology of spinel MFe2O4 (M = Fe, Co, Ni) nanoparticles chemically synthesized from heterometallic complexes. J. Colloid Interface Sci. 2011, 358, 39–46. [Google Scholar] [CrossRef]
- Kwon, S.G.; Piao, Y.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N.-M.; Park, J.-G.; Hyeon, T. Kinetics of Monodisperse Iron Oxide Nanocrystal Formation by “Heating-Up” Process. J. Am. Chem. Soc. 2007, 129, 12571–12584. [Google Scholar] [CrossRef]
- Riaz, N.; Faheem, M.; Riaz, A. Surfactant-modified silver nanoparticle ink for high-resolution ink-jet printed narrow-gaped organic electrodes. Mater. Express 2017, 7, 113–122. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Chen, D. Monodispersed Nickel Nanoparticles with Tunable Phase and Size: Synthesis, Characterization, and Magnetic Properties. J. Phys. Chem. C 2008, 112, 18793–18797. [Google Scholar] [CrossRef]
- Winter, H.; Christopher-Allison, E.; Brown, A.L.; Goforth, A.M. Aerobic method for the synthesis of nearly size-monodisperse bismuth nanoparticles from a redox non-innocent precursor. Nanotechnology 2018, 29, 155603. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, J.; Gao, S. Solvothermal reduction synthesis and characterization of superparamagnetic magnetite nanoparticlesElectronic supplementary information (ESI) available: Size distributions of samples modified with TOPO + PVP, HDA + PVP, and PVP only. J. Mater. Chem. 2003, 13, 1983. [Google Scholar] [CrossRef]
- He, H.; Zhong, Y.; Liang, X.; Tan, W.; Zhu, J.; Wang, C.Y. Natural Magnetite: An efficient catalyst for the degradation of organic contaminant. Sci. Rep. 2015, 5, 10139. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Goss, C. Saturation magnetisation, coercivity and lattice parameter changes in the system Fe3O4-Fe2O3, and their relationship to structure. Phys. Chem. Miner. 1988, 16, 164–171. [Google Scholar] [CrossRef]
- Cabe, W.M.; Smith, J.; Harriott, P. Unit Operations of Chemical Engineering, 5th ed.; McGraw-Hill: New York, NY, USA, 1993; Volume 1130, p. 895. [Google Scholar]
- White, W.; De Angelis, B. Interpretation of the vibrational spectra of spinels. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 985–995. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Silva, S.V.; de Oliveira, M.T. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectr. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Jacintho, G.V.M.; Brolo, A.G.; Corio, P.; Suarez, P.A.Z.; Rubim, J.C. Structural Investigation of MFe2O4(M=Fe, Co) Magnetic Fluids. J. Phys. Chem. C 2009, 113, 7684–7691. [Google Scholar] [CrossRef]
- Verble, J. Temperature-dependent light-scattering studies of the Verwey transition and electronic disorder in magnetite. Phys. Rev. B 1974, 9, 5236–5248. [Google Scholar] [CrossRef]
- Gasparov, L.V.; Romero, D.; Tanner, D.B.; Berger, H.; Margaritondo, G.; Forró, L. Infrared and Raman studies of the Verwey transition in magnetite. Phys. Rev. B 2000, 62, 7939–7944. [Google Scholar] [CrossRef]
- Letti, C.; Paterno, L.G.; Pereira-Da-Silva, M.A.A.; Morais, P.; Soler, M. The role of polymer films on the oxidation of magnetite nanoparticles. J. Solid State Chem. 2017, 246, 57–64. [Google Scholar] [CrossRef]
- Graves, P.; Johnston, C.; Campaniello, J. Raman scattering in spinel structure ferrites. Mater. Res. Bull. 1988, 23, 1651–1660. [Google Scholar] [CrossRef]
- Legodi, M.; DeWaal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigments 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Dubois, F.; Mendibide, C.; Pagnier, T.; Perrard, F.; Duret, C. Raman mapping of corrosion products formed onto spring steels during salt spray experiments. A correlation between the scale composition and the corrosion resistance. Corros. Sci. 2008, 50, 3401–3409. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Stoner, E.C.; Wohlfarth, E.P. A mechanism of magnetic hysteresis in heterogeneous alloys. IEEE Trans. Magn. 1991, 27, 3475–3518. [Google Scholar] [CrossRef]
- Bean, C.P.; Livingston, J.D. Superparamagnetism. J. Appl. Phys. 1959, 30, S120–S129. [Google Scholar] [CrossRef]
- Kneller, E.F.; Luborsky, F.E. Particle Size Dependence of Coercivity and Remanence of Single-Domain Particles. J. Appl. Phys. 1963, 34, 656. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; Wiley-IEEE Press: Hoboken, NJ, USA, 2008; p. 568. ISBN 978-0-471-47741-9. [Google Scholar]
- Caruntu, D.; Caruntu, G.; O’Connor, C.J. Magnetic properties of variable-sized Fe3O4 nanoparticles synthesized from non-aqueous homogeneous solutions of polyols. J. Phys. D Appl. Phys. 2007, 40, 5801–5809. [Google Scholar] [CrossRef]
- Goya, G.; Berquoó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520. [Google Scholar] [CrossRef]
- Coey, J.M.D. Noncollinear Spin Arrangement in Ultrafine Ferrimagnetic Crystallites. Phys. Rev. Lett. 1971, 27, 1140–1142. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Lahut, J.A.; Jacobs, I.S.; Levinson, L.M.; Forester, D.W. Spin Pinning at Ferrite-Organic Interfaces. Phys. Rev. Lett. 1975, 34, 594–597. [Google Scholar] [CrossRef]
- Cao, D.; Li, H.; Pan, L.; Li, J.; Wang, X.; Jing, P.; Cheng, X.; Wang, W.; Wang, J.; Liu, Q. High saturation magnetization of γ-Fe2O3 nano-particles by a facile one-step synthesis approach. Sci. Rep. 2016, 6, 32360. [Google Scholar] [CrossRef]
- Vega-Chacón, J.; Picasso, G.; Félix, L.A.; Jafelicci, M., Jr. Influence of synthesis experimental parameters on the formation of magnetite nanoparticles prepared by polyol method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 15014. [Google Scholar] [CrossRef]
- Gee, S.H.; Hong, Y.-K.; Erickson, D.W.; Park, M.H.; Sur, J.C. Synthesis and aging effect of spherical magnetite (Fe3O4) nanoparticles for biosensor applications. J. Appl. Phys. 2003, 93, 7560. [Google Scholar] [CrossRef]
- Ou, P.; Xu, G.; Xu, C.; Zhang, Y.; Hou, X.; Han, G. Synthesis and characterization of magnetite nanoparticles by a simple solvothermal method. Mater. Sci. 2010, 28, 817–822. [Google Scholar]
- Lyubutin, I.; Lin, C.-R.; Tseng, Y.-T.; Spivakov, A.; Baskakov, A.; Starchikov, S.; Funtov, K.; Jhang, C.-J.; Tsai, Y.-J.; Hsu, H.-S. Structural and magnetic evolution of FexOy@carbon core-shell nanoparticles synthesized by a one-step thermal pyrolysis. Mater. Charact. 2019, 150, 213–219. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, M.; Ou, P.; Zhang, Y.; Han, G.R. Synthesis of Monodispersed Fe3O4 Magnetite Nanoparticles by Ethylene Glycol Solvothermal Method. Adv. Mater. Res. 2013, 634, 2276–2279. [Google Scholar] [CrossRef]
- Fantechi, E.; Campo, G.; Carta, D.; Corrias, A.; Fernández, C.D.J.; Gatteschi, D.; Innocenti, C.; Pineider, F.; Rugi, F.; Sangregorio, C. Exploring the Effect of Co Doping in Fine Maghemite Nanoparticles. J. Phys. Chem. C 2012, 116, 8261–8270. [Google Scholar] [CrossRef]
- Antonov, V.N.; Harmon, B.N.; Antropov, V.P.; Perlov, A.Y.; Yaresko, A.N. Electronic structure and magneto-optical Kerr effect of Fe3O4 and Mg2+- orAl3+-substituted Fe3O4. Phys. Rev. B 2001, 64. [Google Scholar] [CrossRef]
- Fontijn, W.F.J.; Van Der Zaag, P.; Feiner, L.F.; Metselaar, R.; Devillers, M.A.C. A consistent interpretation of the magneto-optical spectra of spinel type ferrites (invited). J. Appl. Phys. 1999, 85, 5100–5105. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, H.-S.; Lee, M.H.; Lee, S.H. Comparative magneto-optical investigation of d–d charge–transfer transitions in Fe3O4, CoFe2O4, and NiFe2O4. J. Appl. Phys. 2002, 91, 9974. [Google Scholar] [CrossRef]
- Fontijn, W.F.J.; Van Der Zaag, P.J.; Devillers, M.A.C.; Brabers, V.A.M.; Metselaar, R. Optical and magneto-optical polar Kerr spectra of Fe3O4 and Mg2+- or Al3+-substituted Fe3O4. Phys. Rev. B 1997, 56, 5432–5442. [Google Scholar] [CrossRef]
- Chen, J.; Hsu, H.; Huang, Y.-H.; Huang, D.-J. Spin-dependent optical charge transfer in magnetite from transmitting optical magnetic circular dichroism. Phys. Rev. B 2018, 98, 085141. [Google Scholar] [CrossRef]
- Jolivet, J.-P.; Tronc, E. Interfacial electron transfer in colloidal spinel iron oxide. Conversion of Fe3O4-γFe2O3 in aqueous medium. J. Colloid Interface Sci. 1988, 125, 688–701. [Google Scholar] [CrossRef]
- Rebodos, R.L.; Vikesland, P.J. Effects of Oxidation on the Magnetization of Nanoparticulate Magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef]
- Joos, A.; Rümenapp, C.; Wagner, F.E.; Gleich, B. Characterisation of iron oxide nanoparticles by Mössbauer spectroscopy at ambient temperature. J. Magn. Magn. Mater. 2016, 399, 123–129. [Google Scholar] [CrossRef]
- Lengyel, A.; Tolnai, G.; Klencsar, Z.; Garg, V.K.; De Oliveira, A.C.; Singh, L.H.; Homonnay, Z.; Szalay, R.; Nemeth, P.; Szabolcs, B.; et al. The effect of carboxylic acids on the oxidation of coated iron oxide nanoparticles. J. Nanopart. Res. 2018, 20, 137. [Google Scholar] [CrossRef]
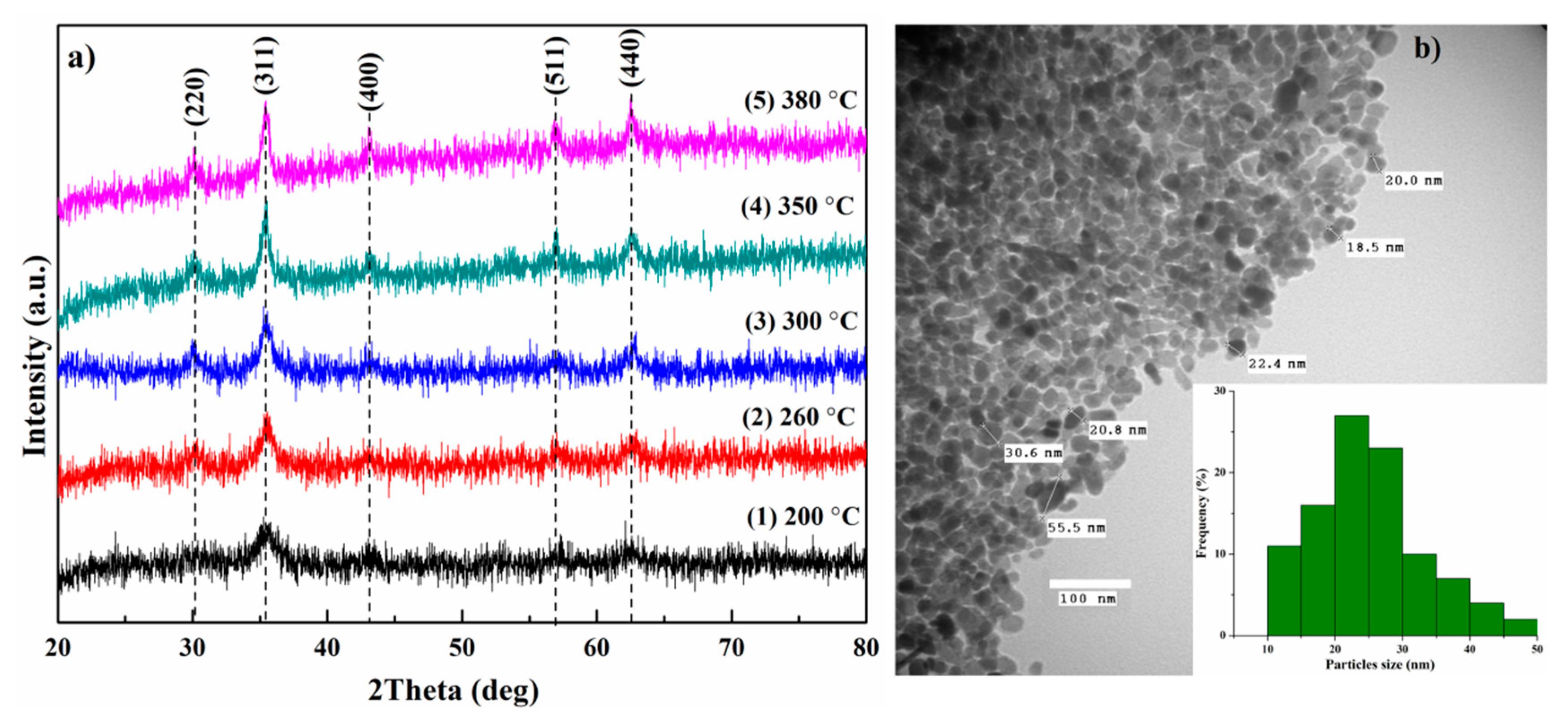

| TR, °C | 200 | 260 | 300 | 350 | 380 |
|---|---|---|---|---|---|
| dXRD, nm | 4.8 | 6.8 | 9 | 11.2 | 13.3 |
| a, Å | 8.36 | 8.379 | 8.373 | 8.386 | 8.392 |
| Method of Synthesis | dXRD, nm | Reference | MS, emu/g | MR, emu/g | HC, Oe |
|---|---|---|---|---|---|
| Thermal decomposition of iron (III) nitrate nonahydrate using HDA | 4.8 | 36 | 0 | 0.6 | |
| 6.8 | 43 | 0 | 1 | ||
| 9 | This work | 46 | 0.3 | 2 | |
| 11.2 | 56 | 1.5 | 14 | ||
| 13.3 | 55 | 3.9 | 47 | ||
| Co-precipitation | 14.7 | [27] | 35.41 | 4.62 | 83.58 |
| 15.1 | [28] | 58.722 | - | 27.076 | |
| 20.3 | [29] | 53 | - | 0 | |
| Hydrothermal | 9.3 | [30] | 45 | 0 | 0 |
| 13.4 | [31] | 27.2 | - | 58.4 | |
| 25.5 | [32] | 50 | 8 | 95 | |
| Sol–gel | 8 | [33] | 47 | - | 0.655 |
| 12.23 | [34] | 52.2 | 1.3 | 21.1 | |
| 13 | [35] | 35 | - | 17 | |
| Thermal decomposition using various reagents | 5.5 | [36] | 43.7 | - | - |
| 7.4 | [37] | 41.7 | 0 | 0 | |
| 9 | [38] | 65 | 0 | 1 | |
| 13.6 | [39] | 72 | - | 0 | |
| 15 | [38] | 70 | - | 12 | |
| 24.2 | [40] | 78.68 | - | 0 | |
| Various methods using iron (III) nitrate nonahydrate as a precursor | 4.4 * | [78] | 39.2 | - | 0 |
| 6 * | [78] | 52 | - | 0 | |
| 7 * | [79] | 49 | - | 0 | |
| 12 * | [80] | 43.6 | 0 | 0 | |
| 13 | [35] | 35 | - | 17 | |
| ~45 * | [81] | 90 | 9 | 44 | |
| ~75 * | [82] | 68.8 | 12.9 | 138.5 |
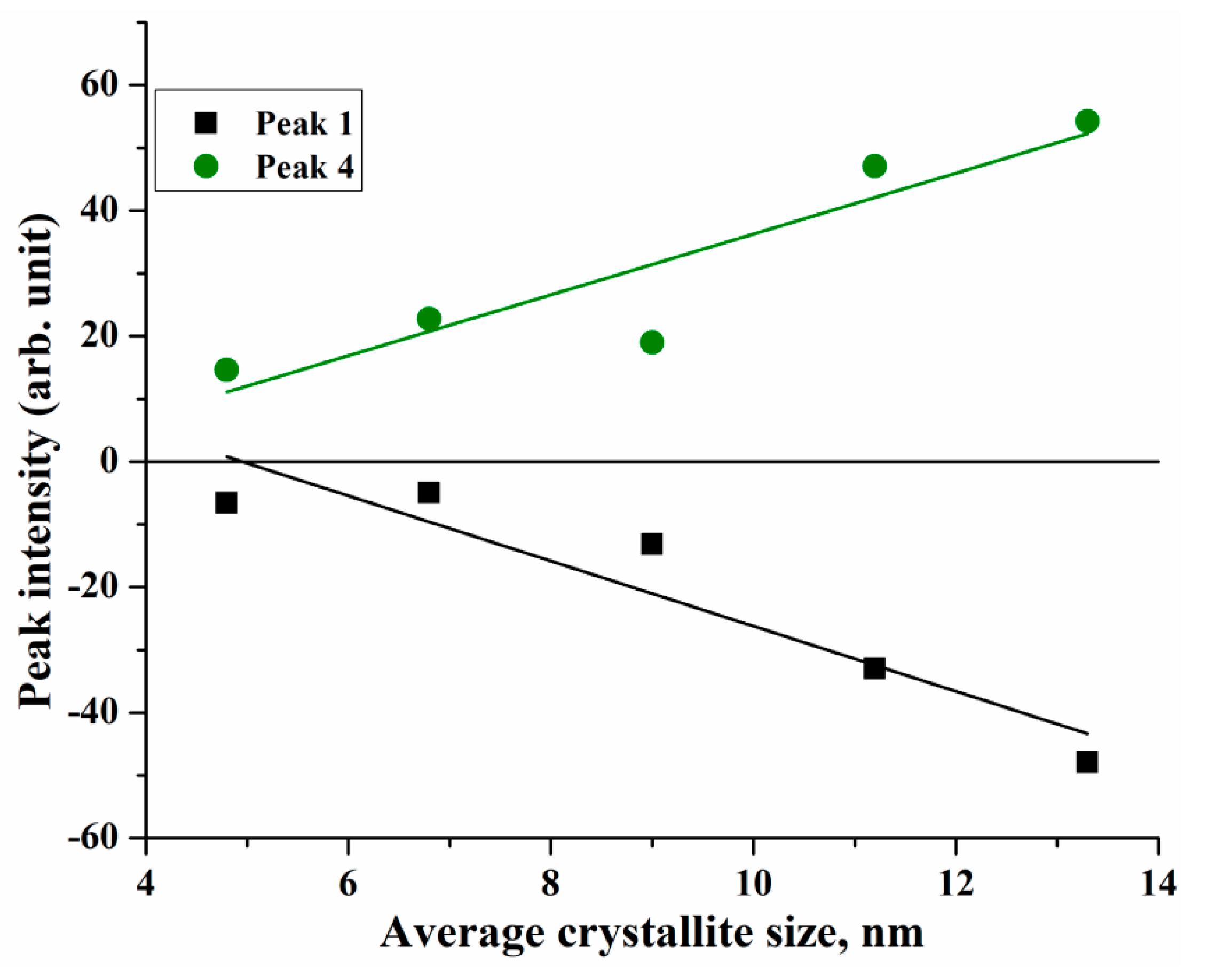
| Component | Type | Transition | d = 4.8 nm | d = 6.8 nm | d = 9 nm | |||||
| E | I | E | I | E | I | |||||
| 1 | IVCT | [Fe2+]t2g→[Fe3+]eg | 2.01 | −6.59 | 2.07 | −4.95 | 2.04 | −13.15 | ||
| 2 | ISCT | [Fe2+]t2g→(Fe2+)e | 2.30 | −7.23 | 2.34 | −20.47 | 2.27 | −12.96 | ||
| 3 | LMCT | O(2p)→Fe(3d) | 2.52 | −3.4 | 2.56 | −18.67 | 2.53 | −11.17 | ||
| 4 | ISCT | (Fe3+)t2→[Fe3+]t2g | 2.74 | 14.62 | 2.8 | 22.79 | 2.73 | 19.02 | ||
| 5 | LMCT | O(2p)→Fe(3d) | 2.92 | 28.86 | 2.98 | 75.64 | 2.91 | 61.0 | ||
| 6 | ISCT | [Fe3+]eg→(Fe2+)t2 | 3.45 | 70.4 | 3.49 | 230.19 | 3.45 | 157.44 | ||
| 7 | LMCT | O(2p)→Fe(3d) | 3.98 | 52.29 | 4.02 | 219.85 | 4.0 | 99.16 | ||
| Component | Type | Transition | d = 11.2 nm | d = 13.3 nm | ||||||
| E | I | E | I | |||||||
| 1 | IVCT | [Fe2+]t2g→[Fe3+]eg | 2.04 | −33.02 | 2.07 | −47.91 | ||||
| 2 | ISCT | [Fe2+]t2g→Fe2+)e | 2.32 | −43.28 | 2.26 | −13.16 | ||||
| 3 | LMCT | O(2p)→Fe(3d) | 2.54 | −19.63 | 2.51 | −2.42 | ||||
| 4 | ISCT | (Fe3+)t2→[Fe3+]t2g | 2.8 | 47.07 | 2.75 | 54.24 | ||||
| 5 | LMCT | O(2p)→Fe(3d) | 2.99 | 113.51 | 2.93 | 95.95 | ||||
| 6 | ISCT | [Fe3+]eg→(Fe2+)t2 | 3.45 | 258.06 | 3.37 | 186.92 | ||||
| 7 | LMCT | O(2p)→Fe(3d) | 3.97 | 148.43 | 3.95 | 69.77 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spivakov, A.; Lin, C.-R.; Chang, Y.-C.; Wang, C.-C.; Sarychev, D. Magnetic and Magneto-Optical Oroperties of Iron Oxides Nanoparticles Synthesized under Atmospheric Pressure. Nanomaterials 2020, 10, 1888. https://doi.org/10.3390/nano10091888
Spivakov A, Lin C-R, Chang Y-C, Wang C-C, Sarychev D. Magnetic and Magneto-Optical Oroperties of Iron Oxides Nanoparticles Synthesized under Atmospheric Pressure. Nanomaterials. 2020; 10(9):1888. https://doi.org/10.3390/nano10091888
Chicago/Turabian StyleSpivakov, Aleksandr, Chun-Rong Lin, Yu-Chuan Chang, Cheng-Chien Wang, and Dmitriy Sarychev. 2020. "Magnetic and Magneto-Optical Oroperties of Iron Oxides Nanoparticles Synthesized under Atmospheric Pressure" Nanomaterials 10, no. 9: 1888. https://doi.org/10.3390/nano10091888
APA StyleSpivakov, A., Lin, C.-R., Chang, Y.-C., Wang, C.-C., & Sarychev, D. (2020). Magnetic and Magneto-Optical Oroperties of Iron Oxides Nanoparticles Synthesized under Atmospheric Pressure. Nanomaterials, 10(9), 1888. https://doi.org/10.3390/nano10091888