Single Crystalline Higher Manganese Silicide Nanowire Arrays with Outstanding Physical Properties through Double Tube Chemical Vapor Deposition
Abstract
1. Introduction
2. Experimental Section
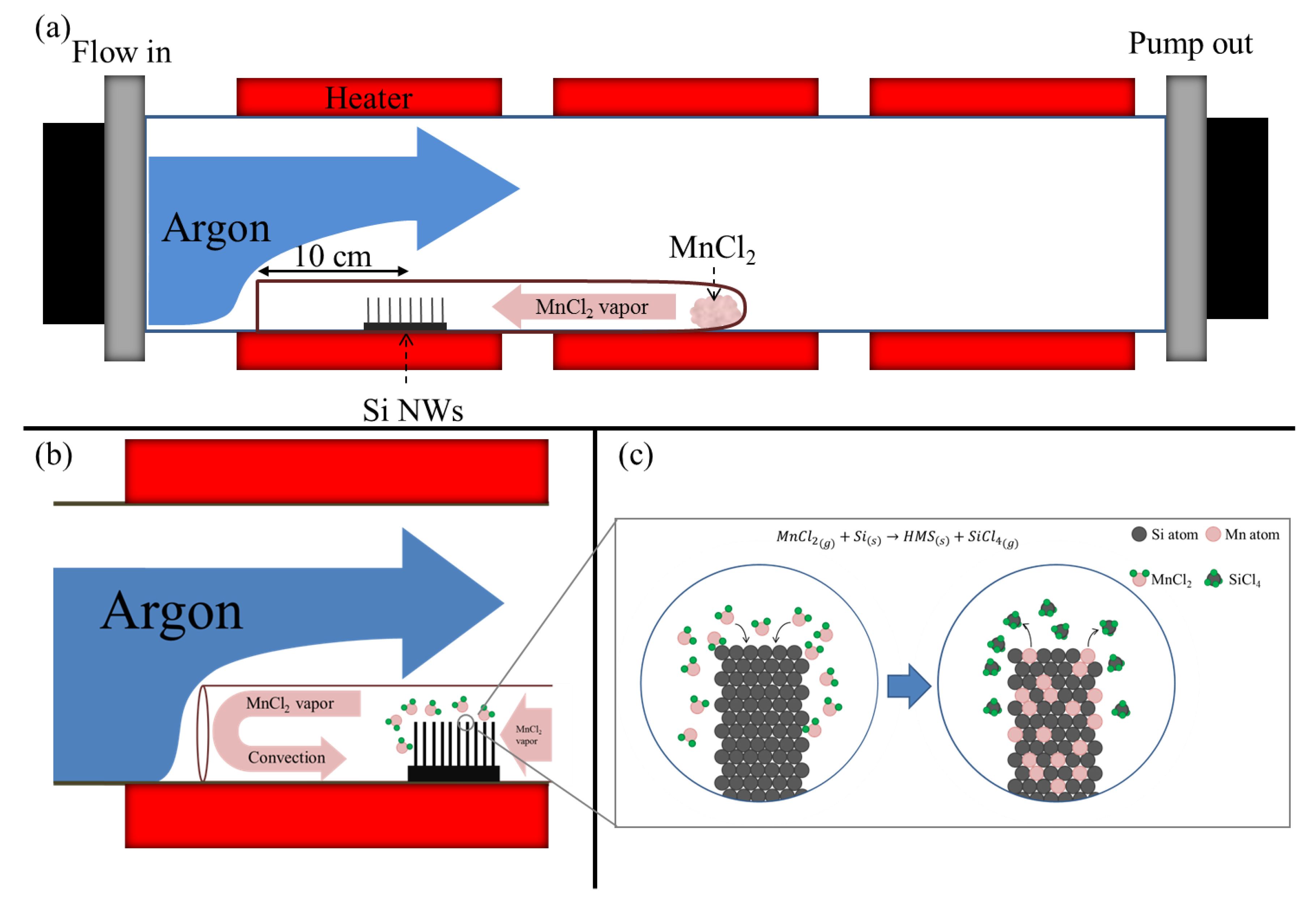
2.1. Preparation of Si Nanowire Arrays
2.2. Chemical Reaction Synthesis of HMS Nanowire Arrays
2.3. Characterization and Physical Properties of HMS NW Arrays
3. Results and Discussion
3.1. Sealing Effect of the Double Tube System
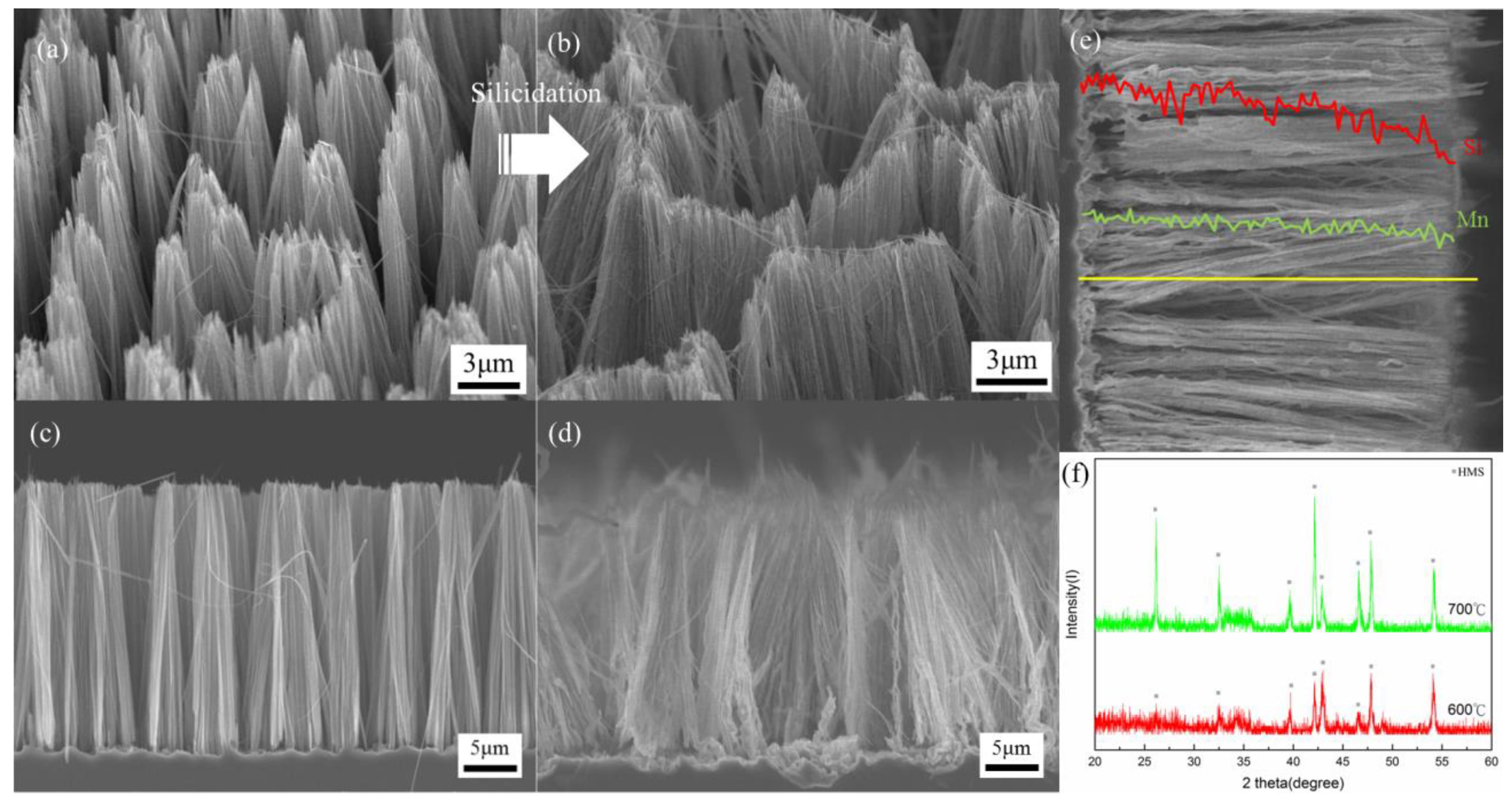
3.2. Dependence on Reaction Time at 600 °C
3.3. Dependence on Reaction Temperature
3.4. High Resolution Transmission Electron Microscopy
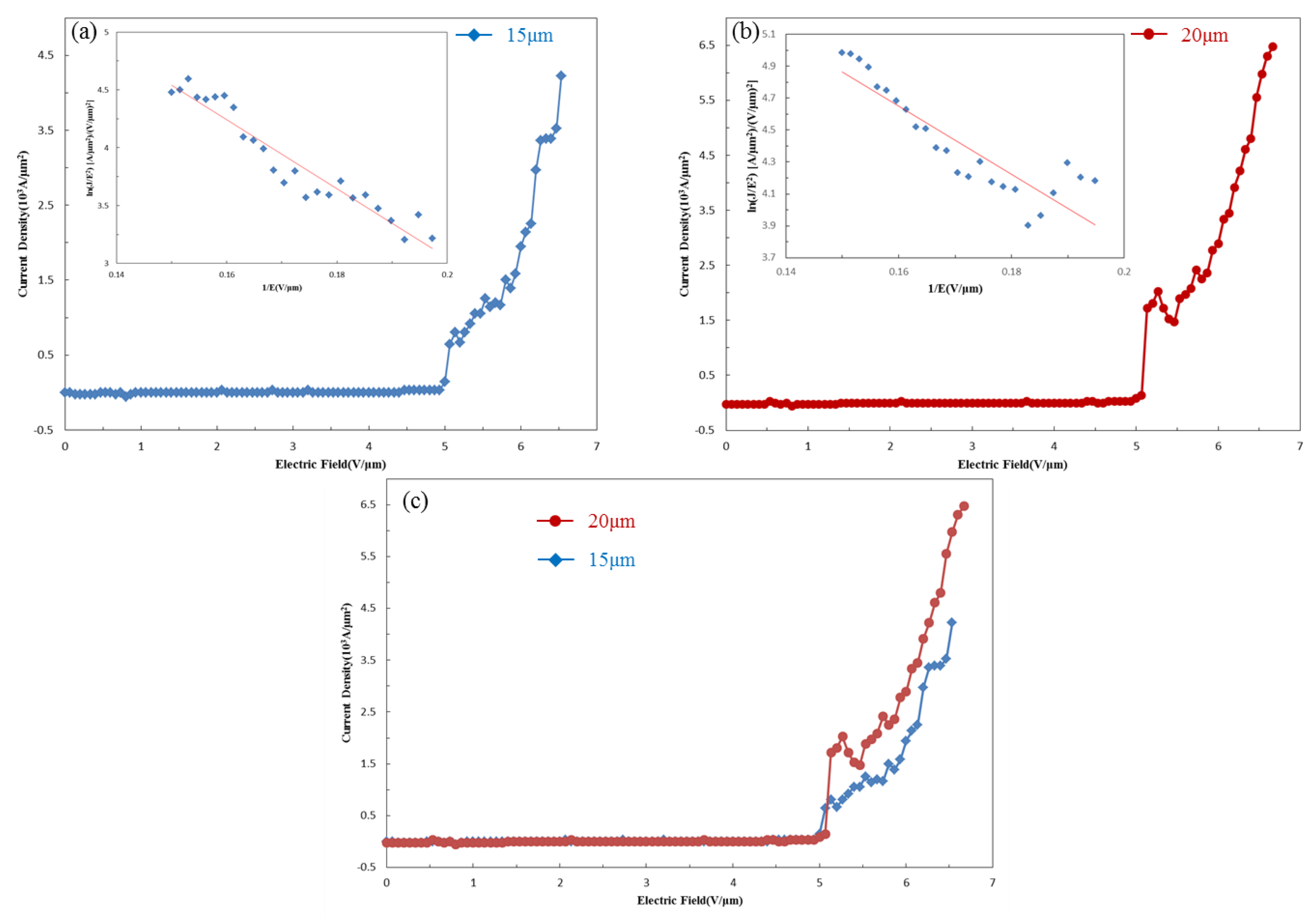
3.5. Field Emission Measurements of HMS Nanowires
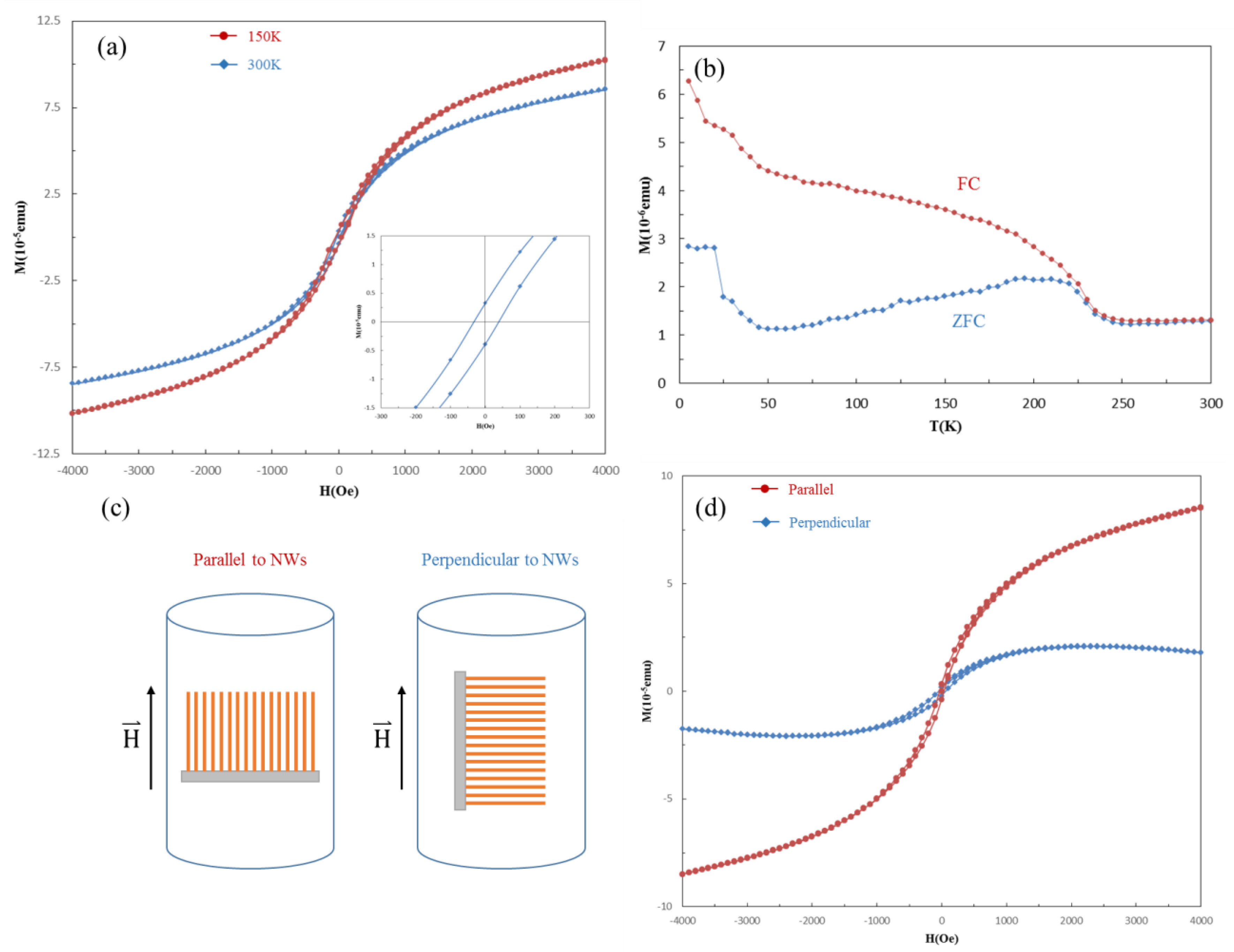
3.6. Magnetic Properties of HMS Nanowires
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.Y.; Zhang, X.D.; Li, D.Z.; Liu, C.; Ma, H.; Ying, C.H.; Wang, F. Insight into the vacancy effects on mechanical and electronic properties of Tantalum Silicide. Ceram. Int. 2020, 46, 4595–4601. [Google Scholar] [CrossRef]
- Ramly, M.M.; Omar, F.S.; Rohaizad, A.; Aspanut, Z.; Rahman, S.A.; Goh, B.T. Solid-phase diffusion controlled growth of nickel silicide nanowires for supercapacitor electrode. Appl. Surf. Sci. 2018, 456, 515–525. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, C.Y.; Lee, Y.A.; Park, S.H.; Cho, Y.; Jun, J.H.; Kim, W.Y.; Kim, B.; Yoon, H. Single-crystalline Co2Si nanowires directly synthesized on silicon substrate for high-performance micro-supercapacitor. Chem. Eng. J. 2019, 370, 973–979. [Google Scholar] [CrossRef]
- Chen, X.; Liang, C. Transition metal silicides: Fundamentals, preparation and catalytic applications. Catal. Sci. Technol. 2019, 9, 4785–4820. [Google Scholar] [CrossRef]
- Lin, J.Y.; Hsu, H.M.; Lu, K.C. Growth of single-crystalline nickel silicide nanowires with excellent physical properties. CrystEngComm 2015, 17, 1911–1916. [Google Scholar] [CrossRef]
- Chiu, C.H.; Huang, C.W.; Chen, J.Y.; Huang, Y.T.; Hu, J.C.; Chen, L.T.; Wu, W.W. Copper silicide/silicon nanowire heterostructures: In situ TEM observation of growth behaviors and electron transport properties. Nanoscale 2013, 5, 5086. [Google Scholar] [CrossRef]
- Hsu, H.F.; Tsai, P.C.; Lu, K.C. Single-crystalline chromium silicide nanowires and their physical properties. Nanoscale Res. Lett. 2015, 10, 50. [Google Scholar] [CrossRef]
- Chen, L.J.; Wu, W.W. Metal silicide nanowires. Jpn. J. Appl. Phys. 2015, 54, 07JA04. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, Y.Y.; Hsu, H.M.; Lu, K.C. Single Crystalline Iron Silicide and Beta-Iron Disilicide Nanowires Formed through Chemical Vapor Deposition. Materials 2018, 11, 2384. [Google Scholar] [CrossRef]
- Lavoie, C.; d’Heurle, F.; Detavernier, C.; Cabral, C. Towards implementation of a nickel silicide process for CMOS technologies. Microelectron. Eng. 2003, 70, 144–157. [Google Scholar] [CrossRef]
- Wu, Y.; Xiang, J.; Yang, C.; Lu, W.; Lieber, C.M. Single-crystal metallic nanowires and metal/semiconductor nanowire heterostructures. Nature 2004, 430, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Bush, J.; Chirayos, V.; Solanki, R.; Jiao, J.; Ono, Y.; Conley, J.F.; Ulrich, B.D. Dielectrophoretically controlled fabrication of single-crystal nickel silicide nanowire interconnects. Nano Lett. 2005, 5, 2112–2115. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Schmitt, A.L.; Jin, S. Ultralong single-crystal metallic Ni2Si nanowires with low resistivity. Nano Lett. 2007, 7, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Bierman, M.J.; Jin, S. Potential applications of hierarchical branching nanowires in solar energy conversion. Energy Environ. Sci. 2009, 2, 1050–1059. [Google Scholar] [CrossRef]
- Garnett, E.C.; Brongersma, M.L.; Cui, Y.; McGehee, M.D. Nanowire solar cells. Ann. Rev. Mater. Res. 2011, 41, 269–295. [Google Scholar] [CrossRef]
- Yu, X.Z.; Onose, Y.; Kanazawa, N.; Park, J.H.; Han, J.H.; Matsui, Y.; Nagaosa, N.; Tokura, Y. Real-space observation of a two-dimensional skyrmion crystal. Nature 2010, 465, 901–904. [Google Scholar] [CrossRef]
- Schmitt, A.L.; Higgins, J.M.; Jin, S. Chemical Synthesis and Magnetotransport of Magnetic Semiconducting Fe1− x Co x Si Alloy Nanowires. Nano Lett. 2008, 8, 810–815. [Google Scholar] [CrossRef]
- Kim, J.; Lee, E.-S.; Han, C.-S.; Kang, Y.; Kim, D.; Anderson, W.A. Observation of Ni silicide formations and field emission properties of Ni silicide nanowires. Microelectron. Eng. 2008, 85, 1709–1712. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Wang, L.; Yang, D. Controlling the growth and field emission properties of silicide nanowire arrays by direct silicification of Ni foil. Nanotechnology 2008, 19, 375602. [Google Scholar] [CrossRef]
- Xiang, B.; Wang, Q.X.; Wang, Z.; Zhang, X.Z.; Liu, L.Q.; Xu, J.; Yu, D.P. Synthesis and field emission properties of TiSi2 nanowires. Appl. Phys. Lett. 2005, 86, 243103. [Google Scholar] [CrossRef]
- Higgins, J.M.; Schmitt, A.L.; Guzei, I.A.; Jin, S. Higher manganese silicide nanowires of nowotny chimney ladder phase. J. Am. Chem. Soc. 2008, 130, 16086–16094. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, A.; Degregorio, Z.P.; Higgins, J.M.; Girard, S.N.; Jin, S. Vapor Phase Conversion Synthesis of Higher Manganese Silicide (MnSi1. 75) Nanowire Arrays for Thermoelectric Applications. Chem. Mater. 2013, 25, 632–638. [Google Scholar] [CrossRef]
- Szczech, J.R.; Schmitt, A.L.; Bierman, M.J.; Jin, S. Single-crystal semiconducting chromium disilicide nanowires synthesized via chemical vapor transport. Chem. Mater. 2007, 19, 3238–3243. [Google Scholar] [CrossRef]
- Zaitsev, V.; Fedorov, M. Chapter 6: Thermal Conductivity of Semiconductors with Complex Crystal Structures. In Thermoelectrics Handbook: Macro to Nano; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 6.1–6.7. [Google Scholar]
- Chen, X.; Weathers, A.; Moore, A.; Zhou, J.; Shi, L. Thermoelectric properties of cold-pressed higher manganese silicides for waste heat recovery. J. Electron. Mater. 2012, 41, 1564–1572. [Google Scholar] [CrossRef]
- Zhou, A.J.; Zhao, X.B.; Zhu, T.J.; Yang, S.H.; Dasgupta, T.; Stiewe, C.; Hassdorf, R.; Mueller, E. Microstructure and thermoelectric properties of SiGe-added higher manganese silicides. Mater. Chem. Phys. 2010, 124, 1001–1005. [Google Scholar] [CrossRef]
- Bux, S.K.; Yeung, M.T.; Toberer, E.S.; Snyder, G.J.; Kaner, R.B.; Fleurial, J.-P. Mechanochemical synthesis and thermoelectric properties of high quality magnesium silicide. J. Mater. Chem. 2011, 21, 12259–12266. [Google Scholar] [CrossRef]
- Mahan, J.E. The potential of higher manganese silicide as an optoelectronic thin film material. Thin Solid Films 2004, 461, 152–159. [Google Scholar] [CrossRef]
- Aoyama, I.; Fedorov, M.I.; Zaitsev, V.K.; Solomkin, Y.F.; Eremin, I.S.; Samunin, A.Y.; Mukoujima, M.; Sano, S.; Tsuji, T. Effects of Ge Doping on Micromorphology of MnSi in MnSi∼ 1.7 and on Their Thermoelectric Transport Properties. Jpn. J. Appl. Phys. 2005, 44, 8562. [Google Scholar] [CrossRef]
- Aoyama, I.; Kaibe, H.; Rauscher, L.; Kanda, T.; Mukoujima, M.; Sano, S.; Tsuji, T. Doping effects on thermoelectric properties of higher manganese silicides (HMSs, MnSi1. 74) and characterization of thermoelectric generating module using p-type (Al, Ge and Mo)-doped HMSs and n-type Mg2Si0.4Sn0.6 legs. Jpn. J. Appl. Phys. 2005, 44, 4275. [Google Scholar] [CrossRef]
- Liu, H.; She, G.; Huang, X.; Qi, X.; Mu, L.; Meng, X.; Shi, W. Synthesis and Magnetic Properties of Mn4Si7 and Si–Mn4Si7 Axial Heterostructure Nanowire Arrays. J. Phys. Chem. C 2013, 117, 2377–2381. [Google Scholar] [CrossRef]
- Liu, H.; She, G.; Ling, S.; Mu, L.; Shi, W. Ferromagnetic Si/Mn27Si47 core/shell nanowire arrays. Jpn. J. Appl. Phys. 2011, 109, 044305–044305-4. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Kumar, D.; Schmitt, S.; Sood, K.; Christiansen, S.; Singh, P. Large area fabrication of vertical silicon nanowire arrays by silver-assisted single-step chemical etching and their formation kinetics. Nanotechnology 2014, 25, 175601. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, B.; Kulakci, M.; Turan, R.; Unalan, H.E. Effect of electroless etching parameters on the growth and reflection properties of silicon nanowires. Nanotechnology 2011, 22, 155606. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Yan, Y.; Gao, S.; Zhu, J. Dendrite-Assisted Growth of Silicon Nanowires in Electroless Metal Deposition. Adv. Funct. Mater. 2003, 13, 127–132. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-L.; Yang, S.-M.; Lu, K.-C. Single Crystalline Higher Manganese Silicide Nanowire Arrays with Outstanding Physical Properties through Double Tube Chemical Vapor Deposition. Nanomaterials 2020, 10, 1880. https://doi.org/10.3390/nano10091880
Shen C-L, Yang S-M, Lu K-C. Single Crystalline Higher Manganese Silicide Nanowire Arrays with Outstanding Physical Properties through Double Tube Chemical Vapor Deposition. Nanomaterials. 2020; 10(9):1880. https://doi.org/10.3390/nano10091880
Chicago/Turabian StyleShen, Chin-Li, Shu-Meng Yang, and Kuo-Chang Lu. 2020. "Single Crystalline Higher Manganese Silicide Nanowire Arrays with Outstanding Physical Properties through Double Tube Chemical Vapor Deposition" Nanomaterials 10, no. 9: 1880. https://doi.org/10.3390/nano10091880
APA StyleShen, C.-L., Yang, S.-M., & Lu, K.-C. (2020). Single Crystalline Higher Manganese Silicide Nanowire Arrays with Outstanding Physical Properties through Double Tube Chemical Vapor Deposition. Nanomaterials, 10(9), 1880. https://doi.org/10.3390/nano10091880




