Effects of Few-Layer Graphene on the Sexual Reproduction of Seed Plants: An In Vivo Study with Cucurbita pepo L.
Abstract
1. Introduction
2. Materials and Methods
2.1. NMs’ Preparation
2.2. NMs’ Characterization
2.3. Cultivation and Growth of Cucurbita Pepo L.
2.4. Effects of NMs on C. Pepo Reproduction
2.5. Pollen Viability
2.6. Stigma Exposure to NMs
2.7. NMs’ Effect on the Stigmatic Surface
2.8. Pollen Detachment from the Stigmatic Surface
2.9. In Vivo Pollen Germination on Stigma
2.10. Statistical Analysis
3. Results and Discussion
3.1. NMs’ Characterization
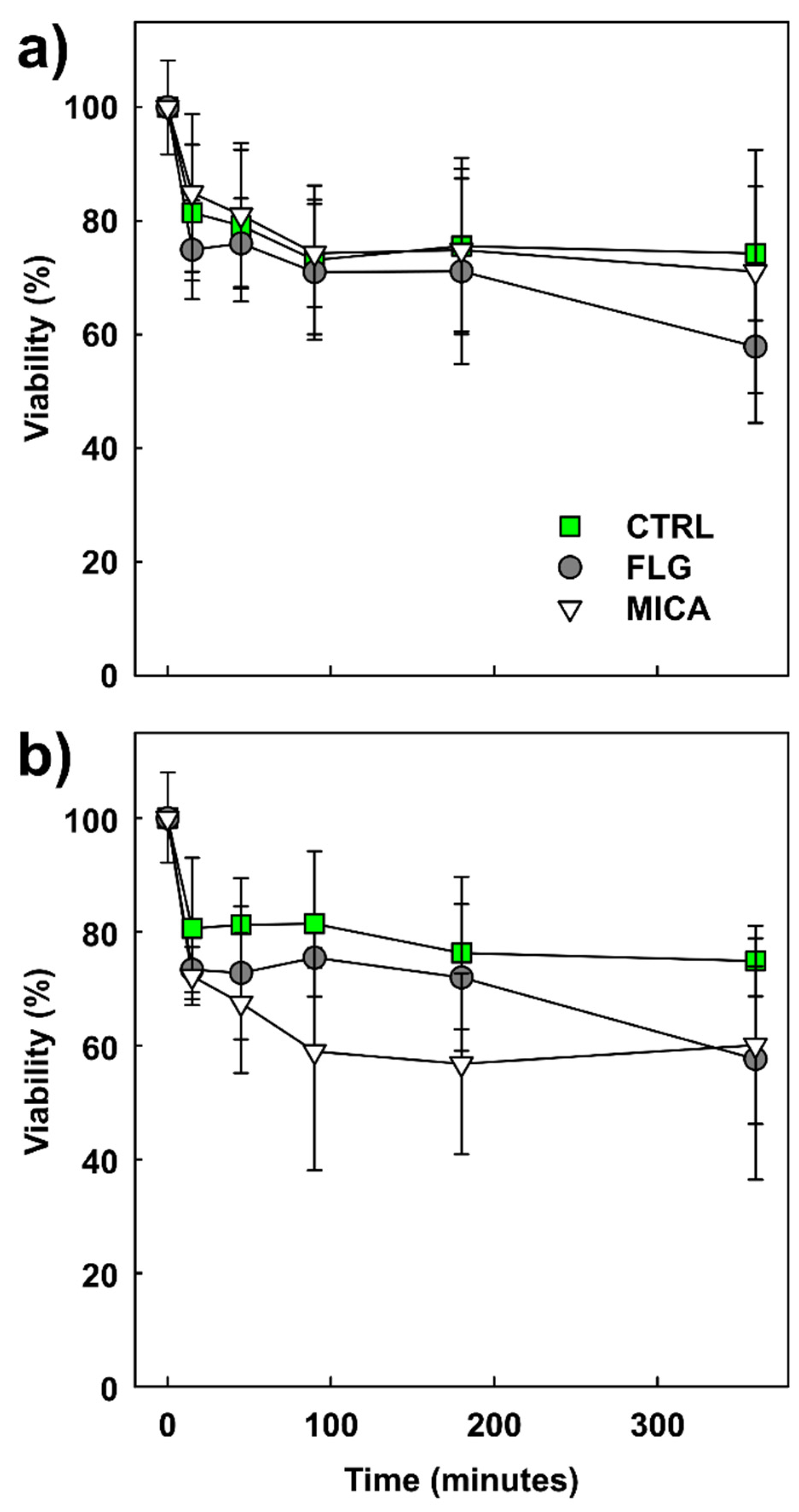
3.2. Effects of Planar Materials on Pollen Viability
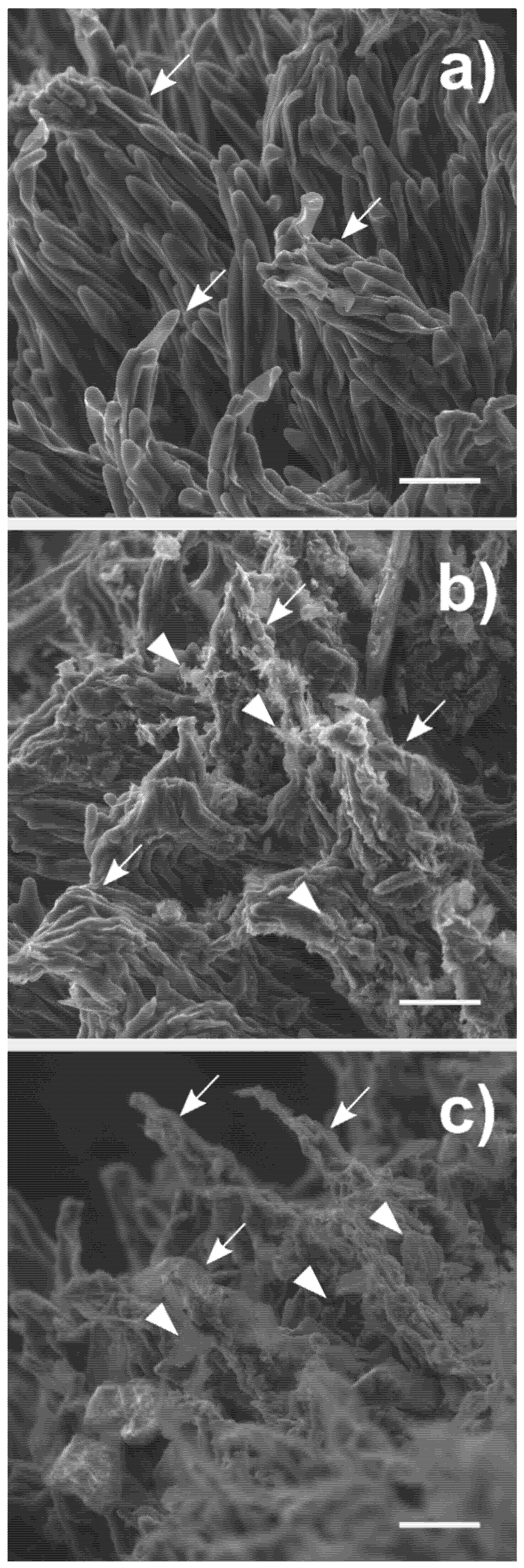
3.3. Interaction of MICA and FLG with the Stigmatic Surface
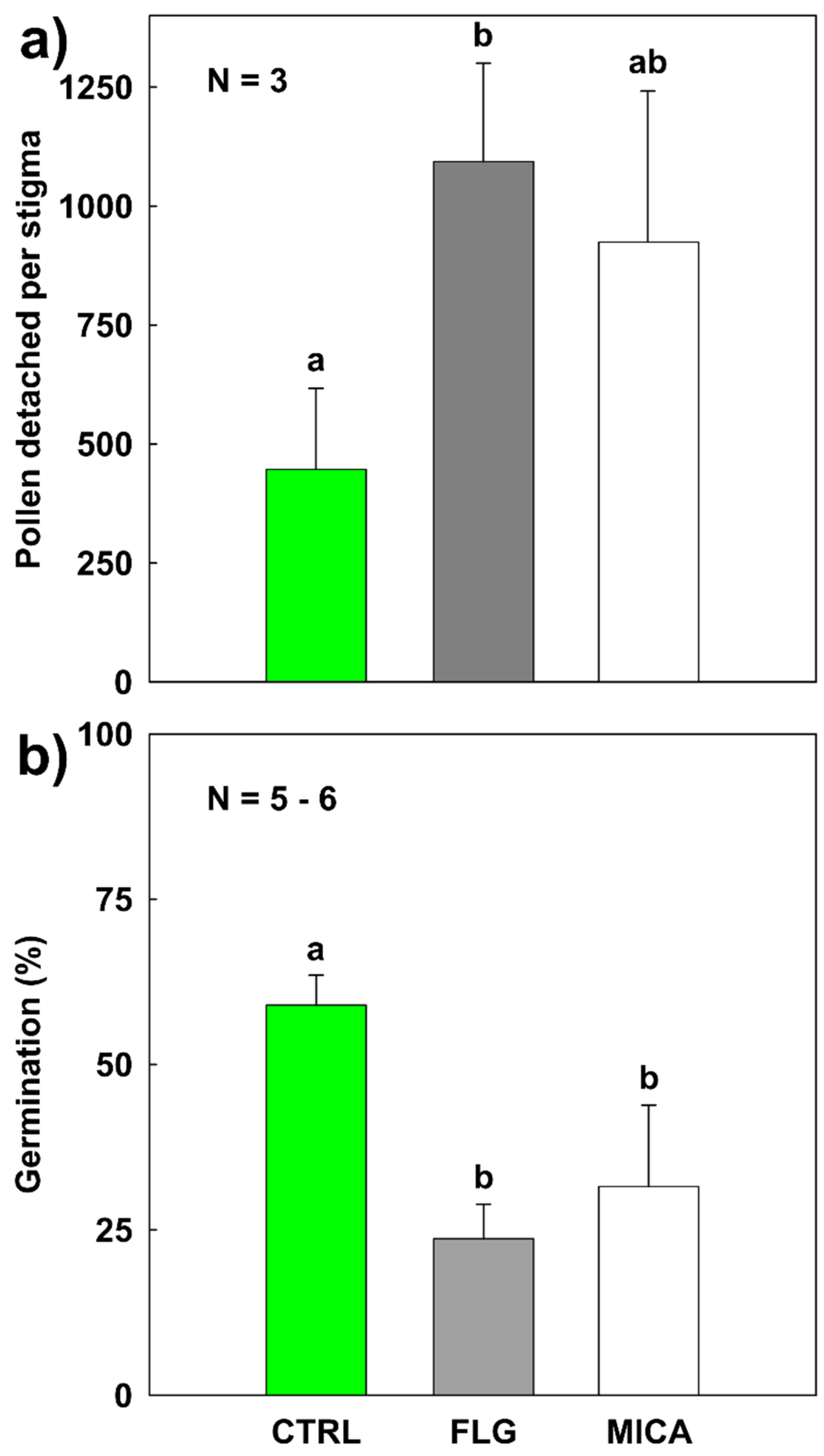
3.4. Pollen–Stigma Adhesion in the Presence of FLG and MICA
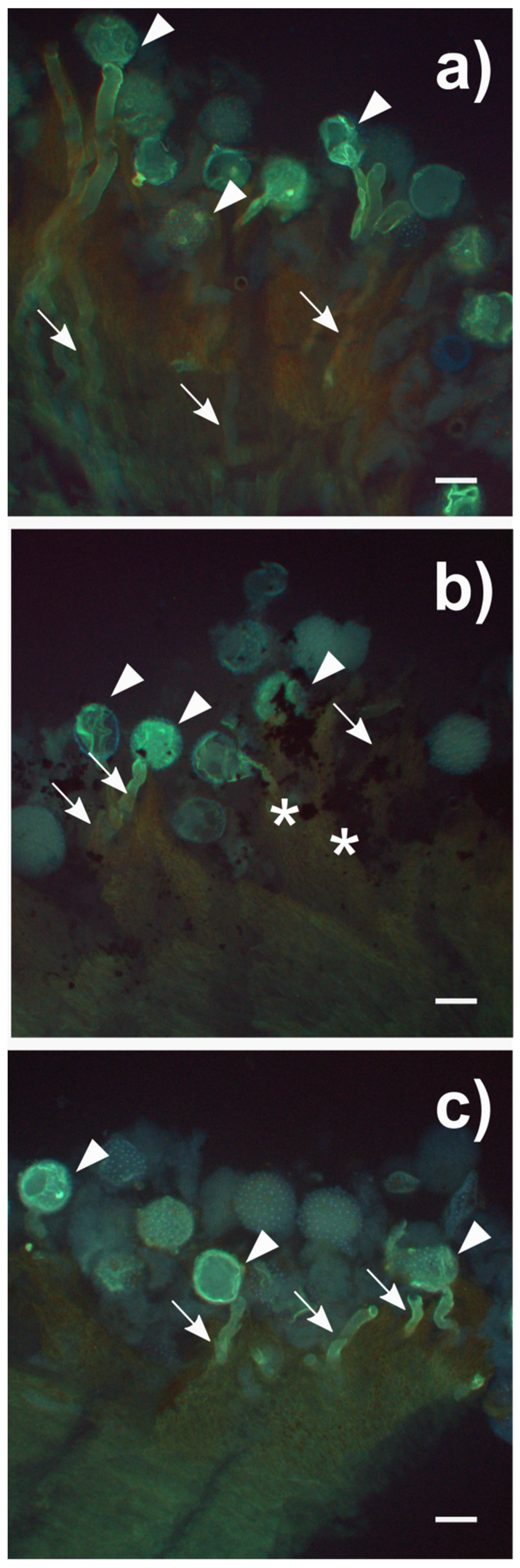
3.5. In Vivo Pollen Germination on Stigma Exposed to NMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Fal’Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Mazumder, B.; Chattopadhyay, P.; Bora, N.S.; Goyary, D.; Karmakar, S. Graphene family of nanomaterials: Reviewing advanced applications in drug delivery and medicine. Curr. Drug Deliv. 2019, 16, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tosi, M.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, H.; Ang, H.; Tade, M. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, Z.; He, K.; Wang, Z. Graphene oxide as a pesticide delivery vector for enhancing acaricidal activity against spider mites. Colloids Surfaces B Biointerfaces 2019, 173, 632–638. [Google Scholar] [CrossRef]
- Kabiri, S.; Degryse, F.; Tran, D.N.; Da Silva, R.; McLaughlin, M.; Losic, D. Graphene Oxide: A new carrier for slow release of plant micronutrients. ACS Appl. Mater. Interfaces 2017, 9, 43325–43335. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Sreeprasad, T.S.; Krishnan, D.; Kouser, S.; Mishra, A.K.; Waghmare, U.; Pradeep, T. Graphene: A reusable substrate for unprecedented adsorption of pesticides. Small 2012, 9, 273–283. [Google Scholar] [CrossRef]
- Qi, L.; Wang, S. Sources of black carbon in the atmosphere and in snow in the Arctic. Sci. Total. Environ. 2019, 691, 442–454. [Google Scholar] [CrossRef]
- Wang, H.; Maher, B.A.; Ahmed, I.A.; Davison, B. Efficient removal of ultrafine particles from diesel exhaust by selected tree species: Implications for roadside planting for improving the quality of urban air. Environ. Sci. Technol. 2019, 53, 6906–6916. [Google Scholar] [CrossRef]
- Tretiach, M.; Pittao, E.; Crisafulli, P.; Adamo, P. Influence of exposure sites on trace element enrichment in moss-bags and characterization of particles deposited on the biomonitor surface. Sci. Total. Environ. 2011, 409, 822–830. [Google Scholar] [CrossRef]
- Montagner, A.; Bosi, S.; Tenori, E.; Bidussi, M.; Alshatwi, A.A.; Tretiach, M.; Prato, M.; Syrgiannis, Z. Ecotoxicological effects of graphene-based materials. 2D Mater. 2016, 4, 12001. [Google Scholar] [CrossRef]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.B.; et al. Safety assessment of graphene-based materials: Focus on human health and the environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, P. Pollen germination In Vitro. In Pollination in Plants; Mokwala, P.W., Ed.; InTech: London, UK, 2018. [Google Scholar]
- Carniel, F.C.; Gorelli, D.; Flahaut, E.; Fortuna, L.; Del Casino, C.; Cai, G.; Nepi, M.; Prato, M.; Tretiach, M. Graphene oxide impairs the pollen performance of Nicotiana tabacum and Corylus avellana suggesting potential negative effects on the sexual reproduction of seed plants. Environ. Sci. Nano 2018, 5, 1608–1617. [Google Scholar] [CrossRef]
- Carniel, F.C.; Fortuna, L.; Nepi, M.; Cai, G.; Del Casino, C.; Adami, G.; Bramini, M.; Bosi, S.; Flahaut, E.; Martín, C.; et al. Beyond graphene oxide acidity: Novel insights into graphene related materials effects on the sexual reproduction of seed plants. J. Hazard. Mater. 2020, 393, 122380. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Law, S.E.; McCoy, D.; Wetzstein, H.Y. Stigma development and receptivity in almond (Prunus dulcis). Ann. Bot. 2005, 97, 57–63. [Google Scholar] [CrossRef]
- Shivanna, K.R. Pollen Biology and Biotechnology; Science Publisher Inc.: Enfield, NH, USA, 2003. [Google Scholar]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Influence of genotype-temperature interaction on pollen performance. J. Evol. Boil. 2005, 18, 1494–1502. [Google Scholar] [CrossRef]
- Ortega, E.; Dicenta, F.; Egea, J. Rain effect on pollen–stigma adhesion and fertilization in almond. Sci. Hortic. 2007, 112, 345–348. [Google Scholar] [CrossRef]
- Yi, W.; Law, S.E.; Wetzstein, H.Y. Fungicide sprays can injure the stigmatic surface during receptivity in almond flowers. Ann. Bot. 2003, 91, 335–341. [Google Scholar] [CrossRef]
- Gillespie, S.; Long, R.; Seitz, N.; Williams, N. Insecticide use in hybrid onion seed production affects pre- and postpollination processes. J. Econ. Èntomol. 2014, 107, 29–37. [Google Scholar] [CrossRef]
- Cox, R.M. Sensitivity of forest plant reproduction to long-range transported air pollutants: The effects of wet deposited acidity and copper on reproduction of Populus tremuloides. N. Phytol. 1988, 110, 33–38. [Google Scholar] [CrossRef]
- Zhang, L.; Beede, R.H.; Banuelos, G.; Wallis, C.M.; Ferguson, L. Dust interferes with pollen–stigma interaction and fruit set in pistachio pistacia vera cv. Kerman. HortScience 2019, 54, 1967–1971. [Google Scholar] [CrossRef]
- Mandal, B.K.; Galib, A.J.; Sultana, N. Relationship of urban dust precipitation on pollination and fruit falling of Mangifera indica and Litchi chinensis in Dhaka District, Bangladesh. J. Entomol. Zool. Stud. 2016, 4, 1185–1191. [Google Scholar]
- Von Reichenbach, H.G.; Rich, C.I.; Gieseking, J.E. Fine-grained micas in soils. In Soil Components; Springer: Berlin/Heidelberg, Germany, 1975; pp. 59–95. [Google Scholar]
- Vernon, P.D.; Reville, W.J. The dust fall of November 1979. J. Earth Sci. 1983, 5, 135–143. [Google Scholar]
- Holopainen, M.; Vallyathan, V.; Hedenborg, M.; Klockars, M. Toxicity of phlogopite and muscovite In Vitro. In Health Related Effects of Phyllosilicates; Springer: Berlin/Heidelberg, Germany, 1990; pp. 349–360. [Google Scholar]
- Christenson, H.K.; Thomson, N.H. The nature of the air-cleaved mica surface. Surf. Sci. Rep. 2016, 71, 367–390. [Google Scholar] [CrossRef]
- Leon, V.; Rodríguez, A.M.; Prieto, P.; Prato, M.; Vázquez, E. Exfoliation of graphite with triazine derivatives under ball-milling conditions: Preparation of few-layer graphene via selective noncovalent interactions. ACS Nano 2014, 8, 563–571. [Google Scholar] [CrossRef]
- Sahu, A.P. Biological effects of mica dust in experimental animals. In Health Related Effects of Phyllosilicates; Springer: Berlin/Heidelberg, Germany, 1990; pp. 387–393. [Google Scholar]
- Knapp, J.L.; Osborne, J.L. Cucurbits as a model system for crop pollination management. J. Pollinat. Ecol. 2019, 25, 89–102. [Google Scholar]
- Radoslovich, E.W. The structure of muscovite, KAl2(Si3 Al)O10(OH)2. Acta Crystallogr. 1960, 13, 919–932. [Google Scholar] [CrossRef]
- Winsor, J.A.; Stephenson, A.G. Demographics of pollen tube growth in Cucurbita pepo. Can. J. Bot. 1995, 73, 583–589. [Google Scholar] [CrossRef]
- Nepi, M.; Pacini, E. Pollination, Pollen Viability and Pistil Receptivity in Cucurbita pepo. Ann. Bot. 1993, 72, 527–536. [Google Scholar] [CrossRef]
- Nepi, M.; Franchi, G.G.; Padni, E. Pollen hydration status at dispersal: Cytophysiological features and strategies. Protoplasma 2001, 216, 171–180. [Google Scholar] [CrossRef]
- Nepi, M.; Cresti, L.; Guarnieri, M.; Pacini, E. Effect of relative humidity on water content, viability and carbohydrate profile of Petunia hybrida and Cucurbita pepo pollen. Plant Syst. Evol. 2009, 284, 57–64. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain. Technol. 1970, 45, 115–120. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Kwack, B.H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Herburger, K.; Holzinger, A. Aniline blue and calcofluor white staining of callose and cellulose in the Streptophyte Green Algae Zygnema and Klebsormidium. Bio-Protocol 2016, 6, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Ferrari, A.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Mogera, U.; Dhanya, R.; Pujar, R.; Narayana, C.; Kulkarni, G.U. Highly decoupled graphene multilayers: Turbostraticity at its best. J. Phys. Chem. Lett. 2015, 6, 4437–4443. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, F.; Hasan, T.; Wu, W.; Sun, Z.; Lombardo, A.; Kulmala, T.S.; Hsieh, G.-W.; Jung, S.; Bonaccorso, F.; Paul, P.J.; et al. Inkjet-printed graphene electronics. ACS Nano 2012, 6, 2992–3006. [Google Scholar] [CrossRef]
- Shtein, M.; Pri-Bar, I.; Varenik, M.; Regev, O. Characterization of graphene-nanoplatelets structure via thermogravimetry. Anal. Chem. 2015, 87, 4076–4080. [Google Scholar] [CrossRef]
- Novoselov, K.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Gupta, A.; Chen, G.; Joshi, P.; Tadigadapa, S. Eklund raman scattering from high-frequency phonons in supportedn-graphene layer films. Nano Lett. 2006, 6, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K.S.; Basko, D.M.; Ferrari, A.C. Raman spectroscopy of graphene edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, J.M.; León, V.; Lucío, M.I.; Prato, M.; Vázquez, E. Production of ready-to-use few-layer graphene in aqueous suspensions. Nat. Protoc. 2018, 13, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.S.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Duarte, A.H.; Laurenço, P.M.; Heine, T.; Guimares, L. Clay mineral nanotubes: Stability, structure and properties. In Stoichiometry and Materials Science—When Numbers Matter; Innocenti, A., Ed.; IntechOpen Limited: London, UK, 2012; pp. 3–24. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. 1. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; DE GRUYTER: Berlin, Germany; München, Germany; Boston, MA, USA, 2016; pp. 1–30. [Google Scholar]
- Bian, Z.; Kawi, S. Preparation, characterization and catalytic application of phyllosilicate: A review. Catal. Today 2020, 339, 3–23. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Piasentin, S.; Furini, A. Nutrient metal elements in plants. Metallomics 2014, 6, 1770–1788. [Google Scholar] [CrossRef]
- Kim, S.S.; Van Khai, T.; Kulish, V.; Kim, Y.-H.; Gil Na, H.; Katoch, A.; Osada, M.; Wu, P.; Kim, H.W. Tunable bandgap narrowing induced by controlled molecular thickness in 2D mica nanosheets. Chem. Mater. 2015, 27, 4222–4228. [Google Scholar] [CrossRef]
- Nepi, M.; Franchi, G.G. Cytochemistry of mature angiosperm pollen. Plant Syst. Evol. 2000, 222, 45–62. [Google Scholar] [CrossRef]
- Proctor, H.C. Effect of pollen age on fruit set, fruit weight, and seed set in three orchid species. Can. J. Bot. 1998, 76, 420–427. [Google Scholar]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, X.; Wang, Z.; Dai, Y.; Xing, B. Mechanistic understanding toward the toxicity of graphene-family materials to freshwater algae. Water Res. 2017, 111, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Piffanelli, P.; Ross, J.H.; Murphy, D.J. Intra- and extracellular lipid composition and associated gene expression patterns during pollen development in Brassica napus. Plant J. 1997, 11, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y. Pollen-pistil interactions during pollen-tube growth. Trends Plant Sci. 1996, 1, 45–51. [Google Scholar] [CrossRef]
- Hiscock, S.J.; Allen, A.M. Diverse cell signalling pathways regulate pollen–stigma interactions: The search for consensus. N. Phytol. 2008, 179, 286–317. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Ghorbannezhad, F.; Sajadi, S.M.; Varma, R.S. Pd nanocatalyst adorning coral reef nanocomposite for the synthesis of nitriles: Utility of Cucurbita pepo leaf extract as a stabilizing and reducing agent. Nanomaterials 2019, 9, 565. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y.; Shivanna, K.R. The receptive surface of the angiosperm stigma. Ann. Bot. 1977, 41, 1233–1258. [Google Scholar] [CrossRef]
- Pacini, E.; Hesse, M. Pollenkitt-its composition, forms and functions. Flora Morphol. Distrib. Funct. Ecol. Plants 2005, 200, 399–415. [Google Scholar] [CrossRef]
- Zinkl, G.M.; Wilhelmi, L.K.; Preuss, D. Sticking together: Cell adhesion interactions in Arabidopsis reproduction. J. Plant Res. 1998, 111, 299–305. [Google Scholar] [CrossRef]
- McCrea, P. An Assessment of the Effects of Road Dust on Agricultural Production Systems; Unit., Lincoln College, Agricultural Economics Research: Lincoln, UK, 1984. [Google Scholar]
- Ahmed, S.S.; Jabeen, R.; Johar, S.; Hameed, M.; Irfan, S. Effects of roadside dust pollution on fruit trees of miyyaghundi (Quetta) and ghanjdori (mastung), Pakistan. Int. J. Basic Appl. Sci. 2015, 5, 38–44. [Google Scholar] [CrossRef]
- Primack, R.B. Longevity of individual flowers. Annu. Rev. Ecol. Syst. 1985, 16, 15–37. [Google Scholar] [CrossRef]
- Holm, S.O. Pollination density-effects on pollen germination and pollen tube growth in Betula pubescens Ehrh. in northern Sweden. N. Phytol. 1994, 126, 541–547. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M. Effect of pistil age on pollen tube growth, fruit development and seed set in Cucurbita pepo L. Acta Soc. Bot. Pol. 2014, 70, 165–172. [Google Scholar] [CrossRef][Green Version]
- Speranza, A.; Leopold, K.; Maier, M.; Taddei, A.-R.; Scoccianti, V. Pd-nanoparticles cause increased toxicity to kiwifruit pollen compared to soluble Pd(II). Environ. Pollut. 2010, 158, 873–882. [Google Scholar] [CrossRef]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Taddei, A.-R.; Iacobucci, M.; Bhattacharya, P.; Ke, P.C. In Vitro toxicity of silver nanoparticles to kiwifruit pollen exhibits peculiar traits beyond the cause of silver ion release. Environ. Pollut. 2013, 179, 258–267. [Google Scholar] [CrossRef]
- Bombin, S.; Lefebvre, M.; Sherwood, J.; Xu, Y.; Bao, Y.; Ramonell, K.M. Developmental and reproductive effects of iron oxide nanoparticles in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 24174. [Google Scholar] [CrossRef]
- Zinkl, G.M.; Zwiebel, B.I.; Grier, D.G.; Preuss, D. Pollen-stigma adhesion in Arabidopsis: A species-specific interaction mediated by lipophilic molecules in the pollen exine. Development 1999, 126, 5431–5440. [Google Scholar]
- Goldman, M.H.S.; Goldberg, R.; Mariani, C. Female sterile tobacco plants are produced by stigma-specific cell ablation. EMBO J. 1994, 13, 2976–2984. [Google Scholar] [CrossRef]
- Huang, C.; Xia, T.; Niu, J.; Yang, Y.; Lin, S.; Wang, X.; Yang, G.; Mao, L.; Xing, B. Transformation of 14 C-labeled graphene to 14 CO2 in the shoots of a rice plant. Angew. Chem. 2018, 130, 9907–9911. [Google Scholar] [CrossRef]
- Petersen, E.J.; Mortimer, M.; Burgess, R.M.; Handy, R.D.; Hanna, S.K.; Ho, K.T.; Johnson, M.; Loureiro, S.; Selck, H.; Scott-Fordsmand, J.J.; et al. Strategies for robust and accurate experimental approaches to quantify nanomaterial bioaccumulation across a broad range of organisms. Environ. Sci. Nano 2019, 6, 1619–1656. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanelli, D.; Candotto Carniel, F.; Garrido, M.; Fortuna, L.; Nepi, M.; Cai, G.; Del Casino, C.; Vázquez, E.; Prato, M.; Tretiach, M. Effects of Few-Layer Graphene on the Sexual Reproduction of Seed Plants: An In Vivo Study with Cucurbita pepo L. Nanomaterials 2020, 10, 1877. https://doi.org/10.3390/nano10091877
Zanelli D, Candotto Carniel F, Garrido M, Fortuna L, Nepi M, Cai G, Del Casino C, Vázquez E, Prato M, Tretiach M. Effects of Few-Layer Graphene on the Sexual Reproduction of Seed Plants: An In Vivo Study with Cucurbita pepo L. Nanomaterials. 2020; 10(9):1877. https://doi.org/10.3390/nano10091877
Chicago/Turabian StyleZanelli, Davide, Fabio Candotto Carniel, Marina Garrido, Lorenzo Fortuna, Massimo Nepi, Giampiero Cai, Cecilia Del Casino, Ester Vázquez, Maurizio Prato, and Mauro Tretiach. 2020. "Effects of Few-Layer Graphene on the Sexual Reproduction of Seed Plants: An In Vivo Study with Cucurbita pepo L." Nanomaterials 10, no. 9: 1877. https://doi.org/10.3390/nano10091877
APA StyleZanelli, D., Candotto Carniel, F., Garrido, M., Fortuna, L., Nepi, M., Cai, G., Del Casino, C., Vázquez, E., Prato, M., & Tretiach, M. (2020). Effects of Few-Layer Graphene on the Sexual Reproduction of Seed Plants: An In Vivo Study with Cucurbita pepo L. Nanomaterials, 10(9), 1877. https://doi.org/10.3390/nano10091877






