Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape
Abstract
1. Introduction
2. Enhancing ION Internalization
2.1. An Overview of the Endocytic Mechanisms
2.1.1. Clathrin-Dependent Endocytosis
2.1.2. Caveolin-Dependent Endocytosis
2.1.3. Clathrin- and Caveolin-Independent Endocytosis
2.2. Tuning IONs for Internalization
2.2.1. Nonspecific Adsorptive Interactions
Cationic Coatings
Anionic Coatings
Effects of Serum Protein Adsorption on Coated Surfaces
2.2.2. Receptor-Mediated Interactions: Targeted Internalization of IONs
Uptake in Carcinogenic Cells
Uptake by the BBB: Delivery to the Brain
3. Enhancing ION Endosomal Escape
3.1. Proton-Sponge Effect and Osmotic Lysis
3.2. Membrane Translocation Mechanisms
3.3. Membrane Fusion
3.4. pH-Triggered Endosomal Escape
3.5. Enhanced Photoinduced Endosomal Escape via Near-Infrared Irradiation
3.5.1. Photochemical Internalization
Direct PS activation via NIR Irradiation
Upconverted Nanoparticles
3.5.2. Photothermal Therapy
4. An Overview of ION-Mediated Transfection in Gene Editing
4.1. Cationic Peptides and Polymers
4.2. Cationic Lipids
4.3. Dendrimers
4.4. Enhancing the Transfection Process with Magnetic Fields
5. Concluding Remarks
Funding
Conflicts of Interest
References
- Tong, S.; Zhu, H.; Bao, G. Magnetic Iron Oxide Nanoparticles for Disease Detection and Therapy. Mater. Today 2019, 31, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Zoppellaro, G. Iron Oxide Magnetic Nanoparticles (NPs) Tailored for Biomedical Applications. In Magnetic Nanoheterostructures; Springer International Publishing: Cham, Switzerland, 2020; pp. 57–102. [Google Scholar] [CrossRef]
- Bohara, R.A.; Singh, P. Multiple Myeloma: Role of Magnetic Nanoparticles. In Magnetic Nanoheterostructures; Springer International Publishing: Cham, Switzerland, 2020; pp. 479–494. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Arias, L.; Pessan, J.; Vieira, A.; Lima, T.; Delbem, A.; Monteiro, D. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Wan, R.; Mo, Y.; Zhang, Z.; Jiang, M.; Tang, S.; Zhang, Q. Cobalt Nanoparticles Induce Lung Injury DNA Damage and Mutations in Mice. Part. Fibre Toxicol. 2017, 14. [Google Scholar] [CrossRef]
- Ahamed, M. Toxic Response of Nickel Nanoparticles in Human Lung Epithelial A549 Cells. Toxicol. Vitr. 2011, 25, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.C.; Pieber, M.; Boehm-Sturm, P.; Ramberger, E.; Karampelas, V.; Möller, K.; Schleicher, M.; Wiekhorst, F.; Löwa, N.; Wagner, S.; et al. Very Small Superparamagnetic Iron Oxide Nanoparticles: Long-Term Fate and Metabolic Processing in Atherosclerotic Mice. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2575–2586. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Chertow, G.M.; Rosner, M. Ferumoxytol for the Treatment of Iron Deficiency Anemia. Expert Rev. Hematol. 2018, 11, 829–834. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and Fabrication of Magnetic Nanoparticles for Targeted Drug Delivery and Imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Janko, C.; Ratschker, T.; Nguyen, K.; Zschiesche, L.; Tietze, R.; Lyer, S.; Alexiou, C. Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as Platform for the Targeted Multimodal Tumor Therapy. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Pöttler, M.; Cicha, I.; Unterweger, H.; Janko, C.; Friedrich, R.P.; Alexiou, C. Nanoparticles for Regenerative Medicine. Nanomedicine 2019, 14, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Ma, C.; Zhu, M.-Q.; Ju, W.-N.; Yang, Y.; Wang, X. Application of Iron Oxide Nanoparticles in the Diagnosis and Treatment of Neurodegenerative Diseases with Emphasis on Alzheimer’s Disease. Front. Cell. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Zuvin, M.; Kuruoglu, E.; Kaya, V.O.; Unal, O.; Kutlu, O.; Acar, H.Y.; Gozuacik, D.; Koşar, A. Magnetofection of Green Fluorescent Protein Encoding DNA-Bearing Polyethyleneimine-Coated Superparamagnetic Iron Oxide Nanoparticles to Human Breast Cancer Cells. ACS Omega 2019, 4, 12366–12374. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.L.M.; Jelicks, L.; De Carvalho, A.C.C.; Spray, D.C.; Mendez-Otero, R. Labeling Stem Cells with Superparamagnetic Iron Oxide Nanoparticles: Analysis of the Labeling Efficacy by Microscopy and Magnetic Resonance Imaging. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2012; pp. 239–252. [Google Scholar] [CrossRef]
- Kim, S.J.; Lewis, B.; Steiner, M.-S.; Bissa, U.V.; Dose, C.; Frank, J.A. Superparamagnetic Iron Oxide Nanoparticles for Direct Labeling of Stem Cells Andin VivoMRI Tracking. Contrast Media Mol. Imaging 2015, 11, 55–64. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite Nanoparticles for Medical MR Imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- D’Souza, M.S.; Sarkar, A.B. Radiological Contrast Agents and Radiopharmaceuticals. In Side Effects of Drugs Annual 40; Elsevier: Amsterdam, The Netherlands, 2018; pp. 579–594. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumar, B.S.H.; Khushu, S.; Tripathi, R.P.; Gangenahalli, G. Increased Transverse Relaxivity in Ultrasmall Superparamagnetic Iron Oxide Nanoparticles Used as MRI Contrast Agent for Biomedical Imaging. Contrast Media Mol. Imaging 2016, 11, 350–361. [Google Scholar] [CrossRef]
- Leong, S.S.; Yeap, S.P.; Lim, J.K. Working Principle and Application of Magnetic Separation for Biomedical Diagnostic at High- and Low-Field Gradients. Interface Focus 2016, 6, 20160048. [Google Scholar] [CrossRef]
- Dai, Y.-T.; Zhu, L.-L.; Zhang, Z.; Jiang, H.-S.; Chen, H.; Chen, Y. Superparamagnetic Iron Oxide Nanoparticle Targeting of Adipose Tissue-Derived Stem Cells in Diabetes-Associated Erectile Dysfunction. Asian J. 2017, 19, 425. [Google Scholar] [CrossRef]
- Xu, H.; Aguilar, Z.P.; Yang, L.; Kuang, M.; Duan, H.; Xiong, Y.; Wei, H.; Wang, A. Antibody Conjugated Magnetic Iron Oxide Nanoparticles for Cancer Cell Separation in Fresh Whole Blood. Biomaterials 2011, 32, 9758–9765. [Google Scholar] [CrossRef]
- Usov, N.A. Iron Oxide Nanoparticles for Magnetic Hyperthermia. SPIN 2019, 9, 1940001. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Achouri, S.; Yoon, Y.-Z.; Herre, J.; Bryant, C.E.; Cicuta, P. Phagocytosis Dynamics Depends on Target Shape. Biophys. J. 2013, 105, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A Fundamental Process in Immunity. Biomed. Res. Int. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Stillwell, W. Membrane Transport. In An Introduction to Biological Membranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–451. [Google Scholar] [CrossRef]
- Rabinovitch, M. Professional and Non-Professional Phagocytes: An Introduction. Trends Cell Biol. 1995, 5, 85–87. [Google Scholar] [CrossRef]
- Wu, X.-S.; Elias, S.; Liu, H.; Heureaux, J.; Wen, P.J.; Liu, A.P.; Kozlov, M.M.; Wu, L.-G. Membrane Tension Inhibits Rapid and Slow Endocytosis in Secretory Cells. Biophys. J. 2017, 113, 2406–2414. [Google Scholar] [CrossRef]
- Boulant, S.; Kural, C.; Zeeh, J.-C.; Ubelmann, F.; Kirchhausen, T. Actin Dynamics Counteract Membrane Tension during Clathrin-Mediated Endocytosis. Nat. Cell Biol. 2011, 13, 1124–1131. [Google Scholar] [CrossRef]
- Royle, S.J. The Cellular Functions of Clathrin. Cell. Mol. Life Sci. 2006, 63, 1823–1832. [Google Scholar] [CrossRef]
- Fotin, A.; Cheng, Y.; Sliz, P.; Grigorieff, N.; Harrison, S.C.; Kirchhausen, T.; Walz, T. Molecular Model for a Complete Clathrin Lattice from Electron Cryomicroscopy. Nature 2004, 432, 573–579. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of Clathrin-Mediated Endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Kelly, B.T.; Graham, S.C.; Liska, N.; Dannhauser, P.N.; Honing, S.; Ungewickell, E.J.; Owen, D.J. AP2 Controls Clathrin Polymerization with a Membrane-Activated Switch. Science 2014, 345, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Warrington, A.; Taylor, K.A.; Svitkina, T. Structural Organization of the Actin Cytoskeleton at Sites of Clathrin-Mediated Endocytosis. Curr. Biol. 2011, 21, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Antonny, B.; Burd, C.; Camilli, P.D.; Chen, E.; Daumke, O.; Faelber, K.; Ford, M.; Frolov, V.A.; Frost, A.; Hinshaw, J.E.; et al. Membrane Fission by Dynamin: What We Know and What We Need to Know. EMBO J. 2016, 35, 2270–2284. [Google Scholar] [CrossRef]
- Mettlen, M.; Chen, P.-H.; Srinivasan, S.; Danuser, G.; Schmid, S.L. Regulation of Clathrin-Mediated Endocytosis. Annu. Rev. Biochem. 2018, 87, 871–896. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.G.; Hislop, J.N.; Grove, J.; Thorn, K.; Marsh, M.; Von Zastrow, M. Regulation of Endocytic Clathrin Dynamics by Cargo Ubiquitination. Dev. Cell 2012, 23, 519–532. [Google Scholar] [CrossRef]
- Traub, L.M. Tickets to Ride: Selecting Cargo for Clathrin-Regulated Internalization. Nat. Rev. Mol. Cell Biol. 2009, 10, 583–596. [Google Scholar] [CrossRef]
- Goh, L.K.; Sorkin, A. Endocytosis of Receptor Tyrosine Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a017459. [Google Scholar] [CrossRef]
- Tian, X.; Kang, D.S.; Benovic, J.L. B-Arrestins and G Protein-Coupled Receptor Trafficking. In Arrestins—Pharmacology and Therapeutic Potential; Springer: Berlin/Heidelberg, Germany, 2013; pp. 173–186. [Google Scholar] [CrossRef]
- Bitsikas, V.; Corrêa, I.R.; Nichols, B.J. Clathrin-Independent Pathways Do Not Contribute Significantly to Endocytic Flux. eLife 2014, 3. [Google Scholar] [CrossRef]
- Lajoie, P.; Nabi, I.R. Lipid Rafts Caveolae, and Their Endocytosis. In International Review of Cell and Molecular Biology 282; Elsevier: Amsterdam, The Netherlands, 2010; pp. 135–163. [Google Scholar] [CrossRef]
- Parton, R.G.; Simons, K. The Multiple Faces of Caveolae. Nat. Rev. Mol. Cell Biol. 2007, 8, 185–194. [Google Scholar] [CrossRef]
- Krishna, A.; Sengupta, D. Interplay between Membrane Curvature and Cholesterol: Role of Palmitoylated Caveolin-1. Biophys. J. 2019, 116, 69–78. [Google Scholar] [CrossRef]
- Pelkmans, L.; Helenius, A. Endocytosis Via Caveolae. Traffic 2002, 3, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Stan, R.V. Structure of Caveolae. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2005, 1746, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Del Pozo, M.A. Caveolae as Plasma Membrane Sensors Protectors and Organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.L. Caveolae and the Regulation of Endocytosis. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; pp. 14–28. [Google Scholar] [CrossRef]
- Han, B.; Copeland, C.A.; Tiwari, A.; Kenworthy, A.K. Assembly and Turnover of Caveolae: What Do We Really Know? Front. Cell Dev. Biol. 2016, 4. [Google Scholar] [CrossRef]
- Pelkmans, L.; Zerial, M. Kinase-Regulated Quantal Assemblies and Kiss-and-Run Recycling of Caveolae. Nature 2005, 436, 128–133. [Google Scholar] [CrossRef]
- Zimnicka, A.M.; Husain, Y.S.; Shajahan, A.N.; Sverdlov, M.; Chaga, O.; Chen, Z.; Toth, P.T.; Klomp, J.; Karginov, A.V.; Tiruppathi, C.; et al. Src-Dependent Phosphorylation of Caveolin-1 Tyr-14 Promotes Swelling and Release of Caveolae. Mol. Biol. Cell 2016, 27, 2090–2106. [Google Scholar] [CrossRef]
- Lee, H.; Volonte’, D.; Galbiati, F.; Iyengar, P.; Lublin, D.M.; Bregman, D.B.; Wilson, M.T.; Campos-Gonzalez, R.; Bouzahzah, B.; Pestell, R.G.; et al. Constitutive and Growth Factor-Regulated Phosphorylation of Caveolin-1 Occurs at the Same Site (Tyr-14) in Vivo: Identification of a c-Src/Cav-1/Grb7 Signaling Cassette. Mol. Endocrinol. 2000, 14, 1750–1775. [Google Scholar] [CrossRef]
- Minshall, R.D.; Tiruppathi, C.; Vogel, S.M.; Niles, W.D.; Gilchrist, A.; Hamm, H.E.; Malik, A.B. Endothelial Cell-Surface Gp60 Activates Vesicle Formation and Trafficking via Gi-Coupled Src Kinase Signaling Pathway. J. Cell Biol. 2000, 150, 1057–1070. [Google Scholar] [CrossRef]
- Marjomäki, V.; Pietiäinen, V.; Matilainen, H.; Upla, P.; Ivaska, J.; Nissinen, L.; Reunanen, H.; Huttunen, P.; Hyypiä, T.; Heino, J. Internalization of Echovirus 1 in Caveolae. J. Virol. 2002, 76, 1856–1865. [Google Scholar] [CrossRef]
- Engel, S.; Heger, T.; Mancini, R.; Herzog, F.; Kartenbeck, J.; Hayer, A.; Helenius, A. Role of Endosomes in Simian Virus 40 Entry and Infection. J. Virol. 2011, 85, 4198–4211. [Google Scholar] [CrossRef]
- Pelkmans, L. Local Actin Polymerization and Dynamin Recruitment in SV40-Induced Internalization of Caveolae. Science 2002, 296, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Kumar, A.; Dasmahapatra, A.K. Multi-Scale Molecular Dynamics Study of Cholera Pentamer Binding to a GM1-Phospholipid Membrane. J. Mol. Graph. Model. 2016, 68, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Solovyeva, V.; Johannes, L.; Simonsen, A.C. Shiga Toxin Induces Membrane Reorganization and Formation of Long Range Lipid Order. Soft Matter 2015, 11, 186–192. [Google Scholar] [CrossRef]
- Cendrowski, J.; Mamińska, A.; Miaczynska, M. Endocytic Regulation of Cytokine Receptor Signaling. Cytokine Growth Factor Rev. 2016, 32, 63–73. [Google Scholar] [CrossRef]
- Gesbert, F.; Sauvonnet, N.; Dautry-Varsat, A. Clathrin-Independent Endocytosis and Signalling of Interleukin 2 Receptors. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2004; pp. 119–148. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Boucrot, E.; Ferreira, A.P.A.; Almeida-Souza, L.; Debard, S.; Vallis, Y.; Howard, G.; Bertot, L.; Sauvonnet, N.; McMahon, H.T. Endophilin Marks and Controls a Clathrin-Independent Endocytic Pathway. Nature 2014, 517, 460–465. [Google Scholar] [CrossRef]
- Basquin, C.; Malarde, V.; Mellor, P.; Anderson, D.H.; Meas-Yedid, V.; Olivo-Marin, J.-C.; Dautry-Varsat, A.; Sauvonnet, N. The Signalling Factor PI3K Is a Specific Regulator of the Clathrin-Independent Dynamin-Dependent Endocytosis of IL-2 Receptors. J. Cell Sci. 2013, 126, 1099–1108. [Google Scholar] [CrossRef]
- Renard, H.-F.; Simunovic, M.; Lemière, J.; Boucrot, E.; Garcia-Castillo, M.D.; Arumugam, S.; Chambon, V.; Lamaze, C.; Wunder, C.; Kenworthy, A.K.; et al. Endophilin-A2 Functions in Membrane Scission in Clathrin-Independent Endocytosis. Nature 2014, 517, 493–496. [Google Scholar] [CrossRef]
- Boucrot, E.; Pick, A.; Çamdere, G.; Liska, N.; Evergren, E.; McMahon, H.T.; Kozlov, M.M. Membrane Fission Is Promoted by Insertion of Amphipathic Helices and Is Restricted by Crescent BAR Domains. Cell 2012, 149, 124–136. [Google Scholar] [CrossRef]
- Mayor, S.; Parton, R.G.; Donaldson, J.G. Clathrin-Independent Pathways of Endocytosis. Cold Spring Harb. Perspect. Biol. 2014, 6, a016758. [Google Scholar] [CrossRef]
- Zurzolo, C.; Simons, K. Glycosylphosphatidylinositol-Anchored Proteins: Membrane Organization and Transport. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 632–639. [Google Scholar] [CrossRef]
- Chadda, R.; Howes, M.T.; Plowman, S.J.; Hancock, J.F.; Parton, R.G.; Mayor, S. Cholesterol-Sensitive Cdc42 Activation Regulates Actin Polymerization for Endocytosis via the GEEC Pathway. Traffic 2007, 8, 702–717. [Google Scholar] [CrossRef] [PubMed]
- Lundmark, R.; Doherty, G.J.; Howes, M.T.; Cortese, K.; Vallis, Y.; Parton, R.G.; McMahon, H.T. The GTPase-Activating Protein GRAF1 Regulates the CLIC/GEEC Endocytic Pathway. Curr. Biol. 2008, 18, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.; Fujita, A.; Chadda, R.; Nixon, S.J.; Kurzchalia, T.V.; Sharma, D.K.; Pagano, R.E.; Hancock, J.F.; Mayor, S.; Parton, R.G. Ultrastructural Identification of Uncoated Caveolin-Independent Early Endocytic Vehicles. J. Cell Biol. 2005, 168, 465–476. [Google Scholar] [CrossRef]
- Howes, M.T.; Kirkham, M.; Riches, J.; Cortese, K.; Walser, P.J.; Simpson, F.; Hill, M.M.; Jones, A.; Lundmark, R.; Lindsay, M.R.; et al. Clathrin-Independent Carriers Form a High Capacity Endocytic Sorting System at the Leading Edge of Migrating Cells. J. Cell Biol. 2010, 190, 675–691. [Google Scholar] [CrossRef]
- Eyster, C.A.; Higginson, J.D.; Huebner, R.; Porat-Shliom, N.; Weigert, R.; Wu, W.W.; Shen, R.-F.; Donaldson, J.G. Discovery of New Cargo Proteins That Enter Cells through Clathrin-Independent Endocytosis. Traffic 2009, 10, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Sabharanjak, S.; De, A. Endocytosis of Glycosylphosphatidylinositol-Anchored Proteins. J. Biomed. Sci. 2009, 16, 93. [Google Scholar] [CrossRef]
- Grant, B.D.; Donaldson, J.G. Pathways and Mechanisms of Endocytic Recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef]
- Humphreys, D.; Davidson, A.C.; Hume, P.J.; Makin, L.E.; Koronakis, V. Arf6 Coordinates Actin Assembly through the WAVE Complex a Mechanism Usurped by Salmonella to Invade Host Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 16880–16885. [Google Scholar] [CrossRef]
- DSouza-Schorey, C.; Van Donselaar, E.; Hsu, V.W.; Yang, C.; Stahl, P.D.; Peters, P.J. ARF6 Targets Recycling Vesicles to the Plasma Membrane: Insights from an Ultrastructural Investigation. J. Cell Biol. 1998, 140, 603–616. [Google Scholar] [CrossRef]
- Schweitzer, J.K.; Sedgwick, A.E.; DSouza-Schorey, C. ARF6-Mediated Endocytic Recycling Impacts Cell Movement Cell Division and Lipid Homeostasis. Semin. Cell Dev. Biol. 2011, 22, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Zhang, Z.; Degeest, G.; Mortier, E.; Leenaerts, I.; Coomans, C.; Schulz, J.; N’Kuli, F.; Courtoy, P.J.; David, G. Syndecan Recyling Is Controlled by Syntenin-PIP2 Interaction and Arf6. Dev. Cell 2005, 9, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Weide, M.; Huntsman, C.; Xu, Z.; Jan, L.Y.; Ma, D. Identification and Characterization of a New Class of Trafficking Motifs for Controlling Clathrin-Independent Internalization and Recycling. J. Biol. Chem. 2007, 282, 13087–13097. [Google Scholar] [CrossRef] [PubMed]
- Montealegre, S.; Van Endert, P.M. Endocytic Recycling of MHC Class I Molecules in Non-Professional Antigen Presenting and Dendritic Cells. Front. Immunol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Barral, D.C.; Cavallari, M.; McCormick, P.J.; Garg, S.; Magee, A.I.; Bonifacino, J.S.; Libero, G.D.; Brenner, M.B. CD1a and MHC Class I Follow a Similar Endocytic Recycling Pathway. Traffic 2008, 9, 1446–1457. [Google Scholar] [CrossRef]
- Cai, B.; Katafiasz, D.; Horejsi, V.; Naslavsky, N. Pre-Sorting Endosomal Transport of the GPI-Anchored Protein CD59, Is Regulated by EHD1. Traffic 2010, 12, 102–120. [Google Scholar] [CrossRef]
- Karacsonyi, C.; Miguel, A.S.; Puertollano, R. Mucolipin-2 Localizes to the Arf6-Associated Pathway and Regulates Recycling of GPI-APs. Traffic 2007, 8, 1404–1414. [Google Scholar] [CrossRef]
- Cardarelli, F.; Pozzi, D.; Bifone, A.; Marchini, C.; Caracciolo, G. Cholesterol-Dependent Macropinocytosis and Endosomal Escape Control the Transfection Efficiency of Lipoplexes in CHO Living Cells. Mol. Pharm. 2012, 9, 334–340. [Google Scholar] [CrossRef]
- Garrett, W.S.; Mellman, I. Studies of Endocytosis. In Dendritic Cells; Elsevier: London, UK, 2001; pp. 213–230. [Google Scholar] [CrossRef]
- Halder, C.V.F.; Fonseca, E.M.B.; Faria, A.V.d.S.; Clerici, S.P. Extracellular Vesicles as a Recipe for Design Smart Drug Delivery Systems for Cancer Therapy. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 411–445. [Google Scholar] [CrossRef]
- Xiang, S.; Tong, H.; Shi, Q.; Fernandes, J.C.; Jin, T.; Dai, K.; Zhang, X. Uptake Mechanisms of Non-Viral Gene Delivery. J. Control. Release 2012, 158, 371–378. [Google Scholar] [CrossRef]
- Chiasson-MacKenzie, C.; Morris, Z.S.; Liu, C.-H.; Bradford, W.B.; Koorman, T.; McClatchey, A.I. Merlin/ERM Proteins Regulate Growth Factor-Induced Macropinocytosis and Receptor Recycling by Organizing the Plasma Membrane: Cytoskeleton Interface. Genes Dev. 2018, 32, 1201–1214. [Google Scholar] [CrossRef]
- Salloum, G.; Jakubik, C.T.; Erami, Z.; Heitz, S.D.; Bresnick, A.R.; Backer, J.M. PI3K-B Is Selectively Required for Growth Factor-Stimulated Macropinocytosis. J. Cell Sci. 2019, 132, jcs231639. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.; Park, I.-K.; Cho, C.-S. Surface Modification of Iron Oxide Nanoparticles by Biocompatible Polymers for Tissue Imaging and Targeting. Biotechnol. Adv. 2013, 31, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Capek, I. Stabilizers-Mediated Nanoparticles Syntheses. In Noble Metal Nanoparticles; Springer: Tokyo, Japan, 2017; pp. 211–316. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Bakhru, S.H.; Altiok, E.; Highley, C.; Delubac, D.; Suhan, J.; Hitchens, T.K.; Ho, C.; Zappe, S. Enhanced Cellular Uptake and Long-Term Retention of Chitosan-Modified Iron-Oxide Nanoparticles for MRI-Based Cell Tracking. Int. J. Nanomed. 2012, 4613. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Degirmenci, V.; Xin, H.; Li, Y.; Wang, L.; Chen, J.; Hu, X.; Zhang, D. PEI-Coated Fe3O4 Nanoparticles Enable Efficient Delivery of Therapeutic SiRNA Targeting REST into Glioblastoma Cells. Int. J. Mol. Sci. 2018, 19, 2230. [Google Scholar] [CrossRef]
- Torres, A.L.M.; Nunes, H.M.P.; Passipieri, J.A.; Jelicks, L.A.; Gasparetto, E.L.; Spray, D.C.; De Carvalho, A.C.C.; Mendez-Otero, R. Optimized Labeling of Bone Marrow Mesenchymal Cells with Superparamagnetic Iron Oxide Nanoparticles and in Vivo Visualization by Magnetic Resonance Imaging. J. Nanobiotechnol. 2011, 9, 4. [Google Scholar] [CrossRef]
- Sharkey, J.; Lewis, P.J.S.; Barrow, M.; Alwahsh, S.M.; Noble, J.; Livingstone, E.; Lennen, R.J.; Jansen, M.A.; Carrion, J.G.; Liptrott, N.; et al. Functionalized Superparamagnetic Iron Oxide Nanoparticles Provide Highly Efficient Iron-Labeling in Macrophages for Magnetic ResonanceBased Detection In Vivo. Cytotherapy 2017, 19, 555–569. [Google Scholar] [CrossRef]
- Laurent, S.; Boutry, S.; Mahieu, I.; Elst, L.; Muller, R. Iron Oxide Based MR Contrast Agents: From Chemistry to Cell Labeling. Curr. Med. Chem. 2009, 16, 4712–4727. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic Iron Oxide Nanoparticles as Drug Carriers: Clinical Relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef]
- Mislick, K.A.; Baldeschwieler, J.D. Evidence for the Role of Proteoglycans in Cation-Mediated Gene Transfer. Proc. Natl. Acad. Sci. USA 1996, 93, 12349–12354. [Google Scholar] [CrossRef] [PubMed]
- Siow, W.X.; Chang, Y.-T.; Babič, M.; Lu, Y.-C.; Horák, D.; Ma, Y.-H. Interaction of Poly-L-Lysine Coating and Heparan Sulfate Proteoglycan on Magnetic Nanoparticle Uptake by Tumor Cells. Int. J. Nanomed. 2018, 13, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Poon, G.M.K.; Gariépy, J. Cell-Surface Proteoglycans as Molecular Portals for Cationic Peptide and Polymer Entry into Cells. Biochem. Soc. Trans. 2007, 35, 788–793. [Google Scholar] [CrossRef]
- Payne, C.K.; Jones, S.A.; Chen, C.; Zhuang, X. Internalization and Trafficking of Cell Surface Proteoglycans and Proteoglycan-Binding Ligands. Traffic 2007, 8, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Pöyry, S.; Vattulainen, I. Role of Charged Lipids in Membrane Structures Insight given by Simulations. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, B.; Yang, K.; Zhang, X.; Yan, B.; Cao, D. Counterintuitive Cooperative Endocytosis of like-Charged Nanoparticles in Cellular Internalization: Computer Simulation and Experiment. Nanotechnology 2017, 28, 085102. [Google Scholar] [CrossRef]
- Singh, A.K. Principles of Nanotoxicology. In Engineered Nanoparticles; Elsevier: London, UK, 2016; pp. 171–227. [Google Scholar] [CrossRef]
- Lin, J.; Alexander-Katz, A. Cell Membranes Open Doors for Cationic Nanoparticles/Biomolecules: Insights into Uptake Kinetics. ACS Nano 2013, 7, 10799–10808. [Google Scholar] [CrossRef]
- Leventis, P.A.; Grinstein, S. The Distribution and Function of Phosphatidylserine in Cellular Membranes. Annu. Rev. Biophys. 2010, 39, 407–427. [Google Scholar] [CrossRef]
- Rezvani, A.Z.; Rahimizadeh, M.; Eshghi, H.; Dehshahri, A.; Ramezani, M. The Effect of Cationic Charge Density Change on Transfection Efficiency of Polyethylenimine. Iran J. Basic Med. Sci. 2013, 16, 150–156. [Google Scholar]
- Kadlecova, Z.; Baldi, L.; Hacker, D.; Wurm, F.M.; Klok, H.-A. Comparative Study on the In Vitro Cytotoxicity of Linear Dendritic, and Hyperbranched Polylysine Analogues. Biomacromolecules 2012, 13, 3127–3137. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A Two-Stage Poly(Ethylenimine)-Mediated Cytotoxicity: Implications for Gene Transfer/Therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The Role of Phosphatidylserine in Recognition of Apoptotic Cells by Phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.M.; McNerny, D.Q.; Baker, J.R.; Orr, B.G.; Holl, M.M.B. Wide Varieties of Cationic Nanoparticles Induce Defects in Supported Lipid Bilayers. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.-M.; Islam, M.T.; Orr, B.G.; Baker, J.R.; Holl, M.M.B. Interaction of Polycationic Polymers with Supported Lipid Bilayers and Cells: Nanoscale Hole Formation and Enhanced Membrane Permeability. Bioconj. Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.-D.; Lee, H.-J.; Lee, H.-J.; Jung, S.-H.; Choi, N.-R.; Vo, M.-C.; Nguyen-Pham, T.-N.; Kim, H.-J.; Park, I.-K.; Lee, J.-J. Branched Polyethylenimine-Superparamagnetic Iron Oxide Nanoparticles (BPEI-SPIONs) Improve the Immunogenicity of Tumor Antigens and Enhance Th1 Polarization of Dendritic Cells. J. Immunol. Res. 2015, 2015, 706379. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef]
- Chertok, B.; David, A.E.; Yang, V.C. Polyethyleneimine-Modified Iron Oxide Nanoparticles for Brain Tumor Drug Delivery Using Magnetic Targeting and Intra-Carotid Administration. Biomaterials 2010, 31, 6317–6324. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Lu, J.; Xia, C.; Gao, F.; Gong, Q.; Song, B.; Zhao, X.; Shuai, X.; Chen, X.; et al. Low Molecular Weight Alkyl-Polycation Wrapped Magnetite Nanoparticle Clusters as MRI Probes for Stem Cell Labeling and in Vivo Imaging. Biomaterials 2011, 32, 528–537. [Google Scholar] [CrossRef]
- Du, J.; Zhu, W.; Yang, L.; Wu, C.; Lin, B.; Wu, J.; Jin, R.; Shen, T.; Ai, H. Reduction of Polyethylenimine-Coated Iron Oxide Nanoparticles Induced Autophagy and Cytotoxicity by Lactosylation. Regen. Biomater. 2016, 3, 223–229. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, B.; Wang, J.; Xie, S.; Li, X. Preparation of Polylysine-Modified Superparamagnetic Iron Oxide Nanoparticles. J. Magn. Magn. Mater. 2015, 374, 205–208. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, G.; Liao, C.; Wang, W.; Zeng, L.; Qian, C.; Huang, K.; Shuai, X. Characterization of Polyethylene Glycol-Grafted Polyethylenimine and Superparamagnetic Iron Oxide Nanoparticles (PEG-g-PEI-SPION) as an MRI-Visible Vector for SiRNA Delivery in Gastric Cancer in Vitro and in Vivo. J. Gastroenterol. 2012, 48, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Song, X.; Gu, X.; Sun, H.; Fu, C.; Meng, F. Synthesis of Superparamagnetic Iron Oxide Nanoparticles Modified with MPEG-PEI via Photochemistry as New MRI Contrast Agent. J. Nanomater. 2015, 2015, 417389. [Google Scholar] [CrossRef]
- Guo, R.M.; Cao, N.; Zhang, F.; Wang, Y.R.; Wen, X.H.; Shen, J.; Shuai, X.T. Controllable Labelling of Stem Cells with a Novel Superparamagnetic Iron OxideLoaded Cationic Nanovesicle for MR Imaging. Eur. Radiol. 2012, 22, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Shahnaz, G.; Kremser, C.; Reinisch, A.; Vetter, A.; Laffleur, F.; Rahmat, D.; Iqbal, J.; Dünnhaupt, S.; Salvenmoser, W.; Tessadri, R.; et al. Efficient MRI Labeling of Endothelial Progenitor Cells: Design of Thiolated Surface Stabilized Superparamagnetic Iron Oxide Nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Cengelli, F.; Voinesco, F.; Juillerat-Jeanneret, L. Interaction of Cationic Ultrasmall Superparamagnetic Iron Oxide Nanoparticles with Human Melanoma Cells. Nanomedicine 2010, 5, 1075–1087. [Google Scholar] [CrossRef]
- Petri-Fink, A.; Chastellain, M.; Juillerat-Jeanneret, L.; Ferrari, A.; Hofmann, H. Development of Functionalized Superparamagnetic Iron Oxide Nanoparticles for Interaction with Human Cancer Cells. Biomaterials 2005, 26, 2685–2694. [Google Scholar] [CrossRef]
- Cañete, M.; Soriano, J.; Villanueva, A.; Roca, A.G.; Veintemillas, S.; Serna, C.J.; Miranda, R.; Morales, M.D.P. The Endocytic Penetration Mechanism of Iron Oxide Magnetic Nanoparticles with Positively Charged Cover: A Morphological Approach. Int. J. Mol. Med. 2010, 26. [Google Scholar] [CrossRef][Green Version]
- Mulens-Arias, V.; Rojas, J.M.; Pérez-Yagüe, S.; Del Puerto Morales, M.; Barber, D.F. Polyethylenimine-Coated SPION Exhibits Potential Intrinsic Anti-Metastatic Properties Inhibiting Migration and Invasion of Pancreatic Tumor Cells. J. Control. Release 2015, 216, 78–92. [Google Scholar] [CrossRef]
- Evans, C.W.; Fitzgerald, M.; Clemons, T.D.; House, M.J.; Padman, B.S.; Shaw, J.A.; Saunders, M.; Harvey, A.R.; Zdyrko, B.; Luzinov, I.; et al. Multimodal Analysis of PEI-Mediated Endocytosis of Nanoparticles in Neural Cells. ACS Nano 2011, 5, 8640–8648. [Google Scholar] [CrossRef]
- Gong, M.; Liu, H.; Sun, N.; Xie, Y.; Yan, F.; Cai, L. Polyethylenimine-Dextran-Coated Magnetic Nanoparticles Loaded with MiR-302b Suppress Osteosarcoma In Vitro and In Vivo. Nanomedicine 2020, 15, 711–723. [Google Scholar] [CrossRef]
- Veiseh, O.; Kievit, F.M.; Fang, C.; Mu, N.; Jana, S.; Leung, M.C.; Mok, H.; Ellenbogen, R.G.; Park, J.O.; Zhang, M. Chlorotoxin Bound Magnetic Nanovector Tailored for Cancer Cell Targeting Imaging, and SiRNA Delivery. Biomaterials 2010, 31, 8032–8042. [Google Scholar] [CrossRef]
- Soenen, S.J.; Smedt, S.C.D.; Braeckmans, K. Limitations and Caveats of Magnetic Cell Labeling Using Transfection Agent Complexed Iron Oxide Nanoparticles. Contrast Media Mol. Imaging 2012, 7, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Albukhaty, S.; Naderi-Manesh, H.; Tiraihi, T.; Jabir, M.S. Poly-l-Lysine-Coated Superparamagnetic Nanoparticles: A Novel Method for the Transfection of pro-BDNF into Neural Stem Cells. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S125–S132. [Google Scholar] [CrossRef]
- Wang, X.-H.; Peng, H.-S.; Yang, L.; You, F.-T.; Teng, F.; Tang, A.-W.; Zhang, F.-J.; Li, X.-H. Poly-l-Lysine Assisted Synthesis of Core-Shell Nanoparticles and Conjugation with Triphenylphosphonium to Target Mitochondria. J. Mater. Chem. B 2013, 1, 5143. [Google Scholar] [CrossRef] [PubMed]
- Dombu, C.Y.; Kroubi, M.; Zibouche, R.; Matran, R.; Betbeder, D. Characterization of Endocytosis and Exocytosis of Cationic Nanoparticles in Airway Epithelium Cells. Nanotechnology 2010, 21, 355102. [Google Scholar] [CrossRef] [PubMed]
- Namiki, Y.; Namiki, T.; Yoshida, H.; Ishii, Y.; Tsubota, A.; Koido, S.; Nariai, K.; Mitsunaga, M.; Yanagisawa, S.; Kashiwagi, H.; et al. A Novel Magnetic CrystalLipid Nanostructure for Magnetically Guided in Vivo Gene Delivery. Nat. Nanotechnol. 2009, 4, 598–606. [Google Scholar] [CrossRef]
- Perillo, E.; Hervé-Aubert, K.; Allard-Vannier, E.; Falanga, A.; Galdiero, S.; Chourpa, I. Synthesis and in Vitro Evaluation of Fluorescent and Magnetic Nanoparticles Functionalized with a Cell Penetrating Peptide for Cancer Theranosis. J. Colloid Interface Sci. 2017, 499, 209–217. [Google Scholar] [CrossRef]
- Dowaidar, M.; Abdelhamid, H.N.; Hällbrink, M.; Langel, Ü.; Zou, X. Chitosan Enhances Gene Delivery of Oligonucleotide Complexes with Magnetic NanoparticlesCell-Penetrating Peptide. J. Biomater. Appl. 2018, 33, 392–401. [Google Scholar] [CrossRef]
- Qi, L.; Wu, L.; Zheng, S.; Wang, Y.; Fu, H.; Cui, D. Cell-Penetrating Magnetic Nanoparticles for Highly Efficient Delivery and Intracellular Imaging of SiRNA. Biomacromolecules 2012, 13, 2723–2730. [Google Scholar] [CrossRef]
- Chen, G.-J.; Hsu, C.; Ke, J.-H.; Wang, L.-F. Imaging and Chemotherapeutic Comparisons of Iron Oxide Nanoparticles Chemically and Physically Coated with Poly(Ethylene Glycol)-b-Poly(e-Caprolactone)-g-Poly(Acrylic Acid). J. Biomed. Nanotechnol. 2015, 11, 951–963. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, X.Y.; Chan, C.K.W.; Bai, Q.; Cheng, C.K.; Chen, F.M.; Cheung, M.S.H.; Yin, B.; Yang, H.; Yung, W.-Y.; et al. Promoting the Delivery of Nanoparticles to Atherosclerotic Plaques by DNA Coating. ACS Appl. Mater. Interfaces 2018, 11, 13888–13904. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Zablotskii, V.; Syrovets, T.; Röcker, C.; Tron, K.; Nienhaus, G.U.; Simmet, T. Modeling Receptor-Mediated Endocytosis of Polymer-Functionalized Iron Oxide Nanoparticles by Human Macrophages. Biomaterials 2011, 32, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Ayala, V.; Herrera, A.P.; Latorre-Esteves, M.; Torres-Lugo, M.; Rinaldi, C. Effect of Surface Charge on the Colloidal Stability and in Vitro Uptake of Carboxymethyl Dextran-Coated Iron Oxide Nanoparticles. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Valenzuela, A.; Petit, F.; Chow, S.; Leung, K.; Gorin, F.; Louie, A.Y.; Dhenain, M. In Vivo MRI of Functionalized Iron Oxide Nanoparticles for Brain Inflammation. Contrast Media Mol. Imaging 2018, 2018, 3476476. [Google Scholar] [CrossRef]
- Bohmer, N.; Jordan, A. Caveolin-1 and CDC42 Mediated Endocytosis of Silica-Coated Iron Oxide Nanoparticles in HeLa Cells. Beilstein J. Nanotechnol. 2015, 6, 167–176. [Google Scholar] [CrossRef]
- Luther, E.M.; Petters, C.; Bulcke, F.; Kaltz, A.; Thiel, K.; Bickmeyer, U.; Dringen, R. Endocytotic Uptake of Iron Oxide Nanoparticles by Cultured Brain Microglial Cells. Acta Biomater. 2013, 9, 8454–8465. [Google Scholar] [CrossRef] [PubMed]
- Petters, C.; Dringen, R. Accumulation of Iron Oxide Nanoparticles by Cultured Primary Neurons. Neurochem. Int. 2015, 81, 1–9. [Google Scholar] [CrossRef]
- Gu, J.L.; Xu, H.F.; Han, Y.H.; Dai, W.; Hao, W.; Wang, C.Y.; Gu, N.; Xu, H.Y.; Cao, J.M. The Internalization Pathway Metabolic Fate and Biological Effect of Superparamagnetic Iron Oxide Nanoparticles in the Macrophage-like RAW264.7 Cell. Sci. China Life Sci. 2011, 54, 793–805. [Google Scholar] [CrossRef]
- Moros, M.; Hernáez, B.; Garet, E.; Dias, J.T.; Sáez, B.; Grazú, V.; González-Fernández, Á.; Alonso, C.; De la Fuente, J.M. Monosaccharides versus PEG-Functionalized NPs: Influence in the Cellular Uptake. ACS Nano 2012, 6, 1565–1577. [Google Scholar] [CrossRef]
- Sun, Z.; Worden, M.; Wroczynskyj, Y.; Manna, P.K.; Thliveris, J.A.; Van Lierop, J.; Hegmann, T.; Miller, D.W. Differential Internalization of Brick Shaped Iron Oxide Nanoparticles by Endothelial Cells. J. Mater. Chem. B 2016, 4, 5913–5920. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, J.; Pan, C.; Liu, N.; Wang, Z.; Zhang, J. Enhanced Uptake of Fe3O4 Nanoparticles by Intestinal Epithelial Cells in a State of Inflammation. Molecules 2017, 22, 1240. [Google Scholar] [CrossRef] [PubMed]
- Peigneux, A.; Glitscher, E.A.; Charbaji, R.; Weise, C.; Wedepohl, S.; Calderón, M.; Jimenez-Lopez, C.; Hedtrich, S. Protein Corona Formation and Its Influence on Biomimetic Magnetite Nanoparticles. J. Mater. Chem. B 2020, 8, 4870–4882. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Estrela-Lopis, I.; Böttner, J.; Lopes, C.A.P.; Guido, B.C.; Souza, A.; Bao, S. Exploring Cellular Uptake of Iron Oxide Nanoparticles Associated with Rhodium Citrate in Breast Cancer Cells. Int. J. Nanomed. 2017, 12, 5511–5523. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.C.; Ramberger, E.; Boehm-Sturm, P.; Mueller, S.; Möller, K.; Löwa, N.; Wiekhorst, F.; Wagner, S.; Taupitz, M.; Schellenberger, E.; et al. Uptake of Citrate-Coated Iron Oxide Nanoparticles into Atherosclerotic Lesions in Mice Occurs via Accelerated Transcytosis through Plaque Endothelial Cells. Nano Res. 2016, 9, 3437–3452. [Google Scholar] [CrossRef]
- Mishra, S.K.; Khushu, S.; Gangenahalli, G. Potential Stem Cell Labeling Ability of Poly-L-Lysine Complexed to Ultrasmall Iron Oxide Contrast Agent: An Optimization and Relaxometry Study. Exp. Cell Res. 2015, 339, 427–436. [Google Scholar] [CrossRef]
- Preiss, M.R.; Cournoyer, E.; Paquin, K.L.; Vuono, E.A.; Belanger, K.; Walsh, E.; Howlett, N.G.; Bothun, G.D. Tuning the Multifunctionality of Iron Oxide Nanoparticles Using Self-Assembled Mixed Lipid Layers. Bioconj. Chem. 2017, 28, 2729–2736. [Google Scholar] [CrossRef]
- Perez, J.; Cifuentes, J.; Cuellar, M.; Suarez-Arnedo, A.; Cruz, J.C.; Muñoz-Camargo, C. Cell-Penetrating and Antibacterial BUF-II Nanobioconjugates: Enhanced Potency Via Immobilization On Polyetheramine-Modified Magnetite Nanoparticles. Int. J. Nanomed. 2019, 14, 8483–8497. [Google Scholar] [CrossRef]
- Ramírez-Acosta, C.M.; Cifuentes, J.; Castellanos, M.C.; Moreno, R.J.; Muñoz-Camargo, C.; Cruz, J.C.; Reyes, L.H. PH-Responsive Cell-Penetrating, Core/Shell Magnetite/Silver Nanoparticles for the Delivery of Plasmids: Preparation, Characterization, and Preliminary In Vitro Evaluation. Pharmaceutics 2020, 12, 561. [Google Scholar] [CrossRef]
- Naik, R.J.; Chatterjee, A.; Ganguli, M. Different Roles of Cell Surface and Exogenous Glycosaminoglycans in Controlling Gene Delivery by Arginine-Rich Peptides with Varied Distribution of Arginines. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 1484–1493. [Google Scholar] [CrossRef]
- Pang, H.-B.; Braun, G.B.; Ruoslahti, E. Neuropilin-1 and Heparan Sulfate Proteoglycans Cooperate in Cellular Uptake of Nanoparticles Functionalized by Cationic Cell-Penetrating Peptides. Sci. Adv. 2015, 1, e1500821. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Y.; Xia, J.; Ma, M.; He, S.; Nie, F.; Gu, N. Effect of Surface Charge and Agglomerate Degree of Magnetic Iron Oxide Nanoparticles on KB Cellular Uptake in Vitro. Colloids Surf. B Biointerfaces 2009, 73, 294–301. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Giri, J.; Banerjee, R.; Bellare, J.; Bahadur, D. Cellular Interactions of Lauric Acid and Dextran-Coated Magnetite Nanoparticles. J. Magn. Magn. Mater. 2007, 311, 282–287. [Google Scholar] [CrossRef]
- Jahn, M.R.; Nawroth, T.; Fütterer, S.; Wolfrum, U.; Kolb, U.; Langguth, P. Iron Oxide/Hydroxide Nanoparticles with Negatively Charged Shells Show Increased Uptake in Caco-2 Cells. Mol. Pharm. 2012, 9, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, L.; Bae, S.C.; Granick, S. Nanoparticle-Induced Surface Reconstruction of Phospholipid Membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 18171–18175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, N. Thermodynamics of Charged Nanoparticle Adsorption on Charge-Neutral Membranes: A Simulation Study. J. Phys. Chem. B 2010, 114, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.L.; Ozment, T.R.; Li, C.; Schweitzer, J.B.; Williams, D.L. Scavenger Receptor-A (CD204): A Two-Edged Sword in Health and Disease. Crit. Rev. Immunol. 2014, 34, 241–261. [Google Scholar] [CrossRef]
- Dowaidar, M.; Abdelhamid, H.N.; Hällbrink, M.; Freimann, K.; Kurrikoff, K.; Zou, X.; Langel, Ü. Magnetic Nanoparticle Assisted Self-Assembly of Cell Penetrating Peptides-Oligonucleotides Complexes for Gene Delivery. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Shannahan, J.; Bai, W.; Brown, J. Implications of Scavenger Receptors in the Safe Development of Nanotherapeutics. Recept. Clin. Investig. 2015. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Secondary Structure of Corona Proteins Determines the Cell Surface Receptors Used by Nanoparticles. J. Phys. Chem. B 2014, 118, 14017–14026. [Google Scholar] [CrossRef]
- Calatayud, M.P.; Sanz, B.; Raffa, V.; Riggio, C.; Ibarra, M.R.; Goya, G.F. The Effect of Surface Charge of Functionalized Fe3O4 Nanoparticles on Protein Adsorption and Cell Uptake. Biomaterials 2014, 35, 6389–6399. [Google Scholar] [CrossRef] [PubMed]
- Stepien, G.; Moros, M.; Pérez-Hernández, M.; Monge, M.; Gutiérrez, L.; Fratila, R.M.; De las Heras, M.; Guillén, S.M.; Lanzarote, J.J.P.; Solans, C.; et al. Effect of Surface Chemistry and Associated Protein Corona on the Long-Term Biodegradation of Iron Oxide Nanoparticles In Vivo. ACS Appl. Mater. Interfaces 2018, 10, 4548–4560. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Caracciolo, G.; Cavaliere, C.; Foglia, P.; Pozzi, D.; Samperi, R.; Laganà, A. Do Plasma Proteins Distinguish between Liposomes of Varying Charge Density? J. Proteom. 2012, 75, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Salaklang, J.; Hofmann, H. Protein Corona Composition of Superparamagnetic Iron Oxide Nanoparticles with Various Physico-Chemical Properties and Coatings. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle Size and Surface Properties Determine the Protein Corona with Possible Implications for Biological Impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Orlando, A.; Colombo, M.; Prosperi, D.; Gregori, M.; Panariti, A.; Rivolta, I.; Masserini, M.; Cazzaniga, E. Iron Oxide Nanoparticles Surface Coating and Cell Uptake Affect Biocompatibility and Inflammatory Responses of Endothelial Cells and Macrophages. J. Nanopart. Res. 2015, 17. [Google Scholar] [CrossRef]
- Adumeau, L.; Delville, M.-H.; Mornet, S. Main Challenges about Surface Biofunctionalization for the In Vivo Targeting of Magnetic Nanoparticles. In Clinical Applications of Magnetic Nanoparticles; CRC Press: Boca Raton, FL, USA, 2018; pp. 77–96. [Google Scholar] [CrossRef]
- Malik, A.; Butt, T.T.; Zahid, S.; Zahid, F.; Waquar, S.; Rasool, M.; Qazi, M.H.; Qazi, A.M. Use of Magnetic Nanoparticles as Targeted Therapy: Theranostic Approach to Treat and Diagnose Cancer. J. Nanotechnol. 2017. [Google Scholar] [CrossRef]
- Ansari, M.O.; Ahmad, M.F.; Shadab, G.G.H.A.; Siddique, H.R. Superparamagnetic Iron Oxide Nanoparticles Based Cancer Theranostics: A Double Edge Sword to Fight against Cancer. J. Drug Deliv. Sci. Technol. 2018, 45, 177–183. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin Receptor 1 in Cancer: A New Sight for Cancer Therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Jiang, W.; Xie, H.; Ghoorah, D.; Shang, Y.; Shi, H.; Liu, F.; Yang, X.; Xu, H. Conjugation of Functionalized SPIONs with Transferrin for Targeting and Imaging Brain Glial Tumors in Rat Model. PLoS ONE 2012, 7, e37376. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, H.; Huang, J.; Qian, W.; Martinson, D.E.; Ji, B.; Li, Y.; Wang, Y.A.; Yang, L.; Mao, H. Probing and Enhancing Ligand-Mediated Active Targeting of Tumors Using Sub-5 Nm Ultrafine Iron Oxide Nanoparticles. Theranostics 2020, 10, 2479–2494. [Google Scholar] [CrossRef]
- Gharib, A.; Faezizadeh, Z.; Mesbah-Namin, S.A.R.; Saravani, R. Experimental Treatment of Breast Cancer-Bearing BALB/c Mice by Artemisinin and Transferrin-Loaded Magnetic Nanoliposomes. Pharmacogn. Mag. 2015, 11, 117. [Google Scholar] [CrossRef]
- Wang, X.; Chang, Y.; Zhang, D.; Tian, B.; Yang, Y.; Wei, F. Transferrin-Conjugated Drug/Dye-Co-Encapsulated Magnetic Nanocarriers for Active-Targeting Fluorescent/Magnetic Resonance Imaging and Anti-Tumor Effects in Human Brain Tumor Cells. RSC Adv. 2016, 6, 105661–105675. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.; Yakovleva, L.; Marchenko, Y.; Mikhrina, A.; Martynova, M.; Bystrova, O.; Dobrodumov, A.; Ischenko, A.; Yakovenko, I.V. Superparamagnetic Iron Oxide Nanoparticles Conjugated with Epidermal Growth Factor (SPION-EGF) for Targeting Brain Tumors. Int. J. Nanomed. 2014, 273. [Google Scholar] [CrossRef]
- Nikolaev, B.P.; Marchenko, Y.Y.; Yakovleva, L.Y.; Zimina, T.M.; Soloviev, A.V.; Luchinin, V.V.; Petrov, A.V.; Scharafutdinova, T.A.; Dobrodumov, A.V. Magnetic Epidermal Growth Factor Conjugate for Targeted Delivery to Grafted Tumor in Mouse Model. IEEE Trans. Magn. 2013, 49, 429–435. [Google Scholar] [CrossRef]
- Chu, I.-M.; Tseng, S.-H.; Chou, M.-Y. Cetuximab-Conjugated Iron Oxide Nanoparticles for Cancer Imaging and Therapy. Int. J. Nanomed. 2015, 3663. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, Y.; Gong, H.; Li, F.; Gu, N. Silica-Coated Magnetite Nanoparticles Labeled by Nimotuzumab A Humanised Monoclonal Antibody to Epidermal Growth Factor Receptor: Preparations, Specific Targeting and Bioimaging. J. Nanosci. Nanotechnol. 2013, 13, 6541–6545. [Google Scholar] [CrossRef]
- Mao, H.; Chen, H.; Wang, L.; Yu, Q.; Qian, W.; Tiwari, D.; Yi, H.; Wang, A.; Huang, J.; Yang, L. Anti-HER2 Antibody and ScFvEGFR-Conjugated Antifouling Magnetic Iron Oxide Nanoparticles for Targeting and Magnetic Resonance Imaging of Breast Cancer. Int. J. Nanomed. 2013, 3781. [Google Scholar] [CrossRef]
- Lin, R.; Huang, J.; Wang, L.; Li, Y.; Lipowska, M.; Wu, H.; Yang, J.; Mao, H. Bevacizumab and near Infrared Probe Conjugated Iron Oxide Nanoparticles for Vascular Endothelial Growth Factor Targeted MR and Optical Imaging. Biomater. Sci. 2018, 6, 1517–1525. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Li, C.; Wang, Y.; Sun, Y.; Wang, J. A Novel Anti-VEGF Targeting and MRI-Visible Smart Drug Delivery System for Specific Diagnosis and Therapy of Liver Cancer. Macromol. Biosci. 2013, 13, 1358–1368. [Google Scholar] [CrossRef]
- Truffi, M.; Colombo, M.; Sorrentino, L.; Pandolfi, L.; Mazzucchelli, S.; Pappalardo, F.; Pacini, C.; Allevi, R.; Bonizzi, A.; Corsi, F.; et al. Multivalent Exposure of Trastuzumab on Iron Oxide Nanoparticles Improves Antitumor Potential and Reduces Resistance in HER2-Positive Breast Cancer Cells. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, M.; Wang, L.; Zielinski, R.; Qian, W.; Lipowska, M.; Capala, J.; Lee, G.Y.; Xu, H.; Wang, Y.A.; Mao, H.; et al. Active Targeting Using HER-2-Affibody-Conjugated Nanoparticles Enabled Sensitive and Specific Imaging of Orthotopic HER-2 Positive Ovarian Tumors. Small 2013, 10, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, V.; Stang, E. The Mysterious Ways of ErbB2/HER2 Trafficking. Membranes 2014, 4, 424–446. [Google Scholar] [CrossRef] [PubMed]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the Folate Receptor for Active Targeting of Cancer Nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11. [Google Scholar] [CrossRef]
- Akal, Z.Ü.; Alpsoy, L.; Baykal, A. Superparamagnetic Iron Oxide Conjugated with Folic Acid and Carboxylated Quercetin for Chemotherapy Applications. Ceram. Int. 2016, 42, 9065–9072. [Google Scholar] [CrossRef]
- Li, L.; Gao, F.; Jiang, W.; Wu, X.; Cai, Y.; Tang, J.; Gao, X.; Gao, F. Folic Acid-Conjugated Superparamagnetic Iron Oxide Nanoparticles for Tumor-Targeting MR Imaging. Drug Deliv. 2015, 1–8. [Google Scholar] [CrossRef]
- Zheng, S.; Han, J.; Jin, Z.; Kim, C.-S.; Park, S.; Kim, K.P.; Park, J.-O.; Choi, E. Dual Tumor-Targeted Multifunctional Magnetic Hyaluronic Acid Micelles for Enhanced MR Imaging and Combined Photothermal-Chemotherapy. Colloids Surf. B Biointerfaces 2018, 164, 424–435. [Google Scholar] [CrossRef]
- Su, Z.; Liu, D.; Chen, L.; Zhang, J.; Ru, L.; Chen, Z.; Gao, Z.; Wang, X. CD44-Targeted Magnetic Nanoparticles Kill Head And Neck Squamous Cell Carcinoma Stem Cells In An Alternating Magnetic Field. Int. J. Nanomed. 2019, 14, 7549–7560. [Google Scholar] [CrossRef]
- Allard-Vannier, E.; Hervé-Aubert, K.; Kaaki, K.; Blondy, T.; Shebanova, A.; Shaitan, K.V.; Ignatova, A.A.; Saboungi, M.-L.; Feofanov, A.V.; Chourpa, I. Folic Acid-Capped PEGylated Magnetic Nanoparticles Enter Cancer Cells Mostly via Clathrin-Dependent Endocytosis. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1578–1586. [Google Scholar] [CrossRef]
- Aires, A.; Ocampo, S.M.; Simões, B.M.; Rodríguez, M.J.; Cadenas, J.F.; Couleaud, P.; Spence, K.; Latorre, A.; Miranda, R.; Somoza, Á.; et al. Multifunctionalized Iron Oxide Nanoparticles for Selective Drug Delivery to CD44-Positive Cancer Cells. Nanotechnology 2016, 27, 065103. [Google Scholar] [CrossRef] [PubMed]
- Dalal, C.; Saha, A.; Jana, N.R. Nanoparticle Multivalency Directed Shifting of Cellular Uptake Mechanism. J. Phys. Chem. C 2016, 120, 6778–6786. [Google Scholar] [CrossRef]
- Mahajan, U.M.; Teller, S.; Sendler, M.; Palankar, R.; Van den Brandt, C.; Schwaiger, T.; Kühn, J.-P.; Ribback, S.; Glöckl, G.; Evert, M.; et al. Tumour-Specific Delivery of SiRNA-Coupled Superparamagnetic Iron Oxide Nanoparticles Targeted against PLK1, Stops Progression of Pancreatic Cancer. Gut 2016, 65, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Khmara, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Antal, I.; Csach, K.; Kopcansky, P.; Vidlickova, I.; Csaderova, L.; Pastorekova, S.; et al. Preparation of Poly-L-Lysine Functionalized Magnetic Nanoparticles and Their Influence on Viability of Cancer Cells. J. Magn. Magn. Mater. 2017, 427, 114–121. [Google Scholar] [CrossRef]
- Satake, N.; Duong, C.; Chen, C.; Barisone, G.A.; Diaz, E.; Tuscano, J.; Rocke, D.M.; Nolta, J.; Nitin, N. Targeted Therapy with MXD3 SiRNA Anti-CD22 Antibody and Nanoparticles for Precursor B-Cell Acute Lymphoblastic Leukaemia. Br. J. Haematol. 2014, 167, 487–499. [Google Scholar] [CrossRef]
- Yang, X.; Hong, H.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Xiao, Y.; Yang, Y.; Zhang, Y.; Nickles, R.J.; et al. CRGD-Functionalized DOX-Conjugated, and 64Cu-Labeled Superparamagnetic Iron Oxide Nanoparticles for Targeted Anticancer Drug Delivery and PET/MR Imaging. Biomaterials 2011, 32, 4151–4160. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting Strategies for Superparamagnetic Iron Oxide Nanoparticles in Cancer Therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef]
- Twigg, M.W.; Freestone, K.; Homer-Vanniasinkam, S.; Ponnambalam, S. The LOX-1 Scavenger Receptor and Its Implications in the Treatment of Vascular Disease. Cardiol. Res. Pract. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Luo, B.; Wen, S.; Chen, Y.-C.; Cui, Y.; Gao, F.-B.; Yao, Y.-Y.; Ju, S.-H.; Teng, G.-J. LOX-1-Targeted Iron Oxide Nanoparticles Detect Early Diabetic Nephropathy in Db/Db Mice. Mol. Imaging Biol. 2015, 17, 652–660. [Google Scholar] [CrossRef]
- Wen, S.; Liu, D.-F.; Liu, Z.; Harris, S.; Yao, Y.-Y.; Ding, Q.; Nie, F.; Lu, T.; Chen, H.-J.; An, Y.-L.; et al. OxLDL-Targeted Iron Oxide Nanoparticles for in Vivo MRI Detection of Perivascular Carotid Collar Induced Atherosclerotic Lesions in ApoE-Deficient Mice. J. Lipid Res. 2012, 53, 829–838. [Google Scholar] [CrossRef]
- Ghadiri, M.; Vasheghani-Farahani, E.; Atyabi, F.; Kobarfard, F.; Mohamadyar-Toupkanlou, F.; Hosseinkhani, H. Transferrin-Conjugated Magnetic Dextran-Spermine Nanoparticles for Targeted Drug Transport across Blood-Brain Barrier. J. Biomed. Mater. Res. Part A 2017, 105, 2851–2864. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shuai, C.; Li, X.; Li, X.; Xiang, J.; Li, G. Mechanism of Poly-l-Lysine-Modified Iron Oxide Nanoparticles Uptake into Cells. J. Biomed. Mater. Res. Part A 2013, 101, 2846–2850. [Google Scholar] [CrossRef]
- Kang, S.; Duan, W.; Zhang, S.; Chen, D.; Feng, J.; Qi, N. Muscone/RI7217 Co-Modified Upward Messenger DTX Liposomes Enhanced Permeability of Blood-Brain Barrier and Targeting Glioma. Theranostics 2020, 10, 4308–4322. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.B.; Linemann, T.; Birkelund, S.; Tarp, G.A.; Moos, T. Evaluation of Targeted Delivery to the Brain Using Magnetic Immunoliposomes and Magnetic Force. Materials 2019, 12, 3576. [Google Scholar] [CrossRef] [PubMed]
- Creixell, M.; Herrera, A.P.; Ayala, V.; Latorre-Esteves, M.; Pérez-Torres, M.; Torres-Lugo, M.; Rinaldi, C. Preparation of Epidermal Growth Factor (EGF) Conjugated Iron Oxide Nanoparticles and Their Internalization into Colon Cancer Cells. J. Magn. Magn. Mater. 2010, 322, 2244–2250. [Google Scholar] [CrossRef]
- Bonvin, D.; Bastiaansen, J.A.M.; Stuber, M.; Hofmann, H.; Ebersold, M.M. Folic Acid on Iron Oxide Nanoparticles: Platform with High Potential for Simultaneous Targeting MRI Detection and Hyperthermia Treatment of Lymph Node Metastases of Prostate Cancer. Dalton Trans. 2017, 46, 12692–12704. [Google Scholar] [CrossRef]
- Cheng, D.; Hong, G.; Wang, W.; Yuan, R.; Ai, H.; Shen, J.; Liang, B.; Gao, J.; Shuai, X. Nonclustered Magnetite Nanoparticle Encapsulated Biodegradable Polymeric Micelles with Enhanced Properties for in Vivo Tumor Imaging. J. Mater. Chem. 2011, 21, 4796. [Google Scholar] [CrossRef]
- Tomitaka, A.; Arami, H.; Gandhi, S.; Krishnan, K.M. Lactoferrin Conjugated Iron Oxide Nanoparticles for Targeting Brain Glioma Cells in Magnetic Particle Imaging. Nanoscale 2015, 7, 16890–16898. [Google Scholar] [CrossRef]
- Chen, G.-J.; Su, Y.-Z.; Hsu, C.; Lo, Y.-L.; Huang, S.-J.; Ke, J.-H.; Kuo, Y.-C.; Wang, L.-F. Angiopep-Pluronic F127-Conjugated Superparamagnetic Iron Oxide Nanoparticles as Nanotheranostic Agents for BBB Targeting. J. Mater. Chem. B 2014, 2, 5666. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, W.; Uckun, F.M.; Wang, L.; Wang, Y.A.; Chen, H.; Kooby, D.; Yu, Q.; Lipowska, M.; Staley, C.A.; et al. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano 2015, 9, 7976–7991. [Google Scholar] [CrossRef]
- Tomanek, B.; Iqbal, U.; Blasiak, B.; Abulrob, A.; Albaghdadi, H.; Matyas, J.R.; Ponjevic, D.; Sutherland, G.R. Evaluation of Brain Tumor Vessels Specific Contrast Agents for Glioblastoma Imaging. Neuro-Oncology 2011, 14, 53–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Itoh, Y. Membrane-Type Matrix Metalloproteinases: Their Functions and Regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef]
- Bourseau-Guilmain, E.; Menard, J.A.; Lindqvist, E.; Chandran, V.I.; Christianson, H.C.; Magaña, M.C.; Lidfeldt, J.; Marko-Varga, G.; Welinder, C.; Belting, M. Hypoxia Regulates Global Membrane Protein Endocytosis through Caveolin-1 in Cancer Cells. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- O’Reilly, M.K.; Tian, H.; Paulson, J.C. CD22 Is a Recycling Receptor That Can Shuttle Cargo between the Cell Surface and Endosomal Compartments of B Cells. J. Immunol. 2010, 186, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Diab, D.E.H.; Connord, V.; Clerc, P.; Meunier, E.; Pipy, B.; Payré, B.; Tan, R.P.; Gougeon, M.; Carrey, J.; et al. Targeting a G-Protein-Coupled Receptor Overexpressed in Endocrine Tumors by Magnetic Nanoparticles to Induce Cell Death. ACS Nano 2014, 8, 1350–1363. [Google Scholar] [CrossRef]
- Paul, N.R.; Jacquemet, G.; Caswell, P.T. Endocytic Trafficking of Integrins in Cell Migration. Curr. Biol. 2015, 25, R1092–R1105. [Google Scholar] [CrossRef]
- Villaseñor, R.; Lampe, J.; Schwaninger, M.; Collin, L. Intracellular Transport and Regulation of Transcytosis across the BloodBrain Barrier. Cell. Mol. Life Sci. 2018, 76, 1081–1092. [Google Scholar] [CrossRef]
- Tian, X.; Nyberg, S.; Sharp, P.S.; Madsen, J.; Daneshpour, N.; Armes, S.P.; Berwick, J.; Azzouz, M.; Shaw, P.; Abbott, N.J.; et al. LRP-1-Mediated Intracellular Antibody Delivery to the Central Nervous System. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Jiang, X.; Gu, J.; Sha, X.; Chen, L.; Law, K.; Chen, Y.; Wang, X.; Jiang, Y.; Fang, X. Angiopep-Conjugated Poly(Ethylene Glycol)-Co-Poly(e-Caprolactone) Nanoparticles as Dual-Targeting Drug Delivery System for Brain Glioma. Biomaterials 2011, 32, 4293–4305. [Google Scholar] [CrossRef]
- Lee, H.J.; Engelhardt, B.; Lesley, J.; Bickel, U.; Pardridge, W.M. Targeting Rat Anti-Mouse Transferrin Receptor Monoclonal Antibodies through Blood-Brain Barrier in Mouse. J. Pharm. Exp. 2000, 292, 1048–1052. [Google Scholar]
- Werner, H.; LeRoith, D. Insulin and Insulin-like Growth Factor Receptors in the Brain: Physiological and Pathological Aspects. Eur. Neuropsychopharmacol. 2014, 24, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Lakkaraju, A. Early Endosome Morphology in Health and Disease. In Retinal Degenerative Diseases; Springer International Publishing: New York, NY, USA, 2018; pp. 335–343. [Google Scholar] [CrossRef]
- Hsu, V.W.; Bai, M.; Li, J. Getting Active: Protein Sorting in Endocytic Recycling. Nat. Rev. Mol. Cell Biol. 2012, 13, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mesaki, K.; Tanabe, K.; Obayashi, M.; Oe, N.; Takei, K. Fission of Tubular Endosomes Triggers Endosomal Acidification and Movement. PLoS ONE 2011, 6, e19764. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Bucci, C. Multiple Roles of the Small GTPase Rab7. Cells 2016, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Ma, D. Enhancing Endosomal Escape for Nanoparticle Mediated SiRNA Delivery—Nanoscale (RSC Publishing). Nanoscale 2014, 6, 6415–6425. [Google Scholar] [CrossRef]
- Akita, H.; Kogure, K.; Moriguchi, R.; Nakamura, Y.; Higashi, T.; Nakamura, T.; Serada, S.; Fujimoto, M.; Naka, T.; Futaki, S. Nanoparticles for Ex Vivo SiRNA Delivery to Dendritic Cells for Cancer Vaccines: Programmed Endosomal Escape and Dissociation. J. Control. Release 2010, 143, 311–317. [Google Scholar] [CrossRef]
- El-Sayed, A.; Khalil, I.A.; Kogure, K.; Futaki, S.; Harashima, H. Octaarginine- and Octalysine-Modified Nanoparticles Have Different Modes of Endosomal Escape. J. Biol. Chem. 2008, 283, 23450–23461. [Google Scholar] [CrossRef]
- Selby, L.I.; Cortez-Jugo, C.M.; Such, G.K.; Johnston, A.P.R. Nanoescapology: Progress toward Understanding the Endosomal Escape of Polymeric Nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1452. [Google Scholar] [CrossRef]
- Vermeulen, L.M.P.; Smedt, S.C.D.; Remaut, K.; Braeckmans, K. The Proton Sponge Hypothesis: Fable or Fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef]
- Freeman, E.C.; Weiland, L.M.; Meng, W.S. Modeling the Proton Sponge Hypothesis: Examining Proton Sponge Effectiveness for Enhancing Intracellular Gene Delivery through Multiscale Modeling. J. Biomater. Sci. Polym. Ed. 2012, 24, 398–416. [Google Scholar] [CrossRef]
- Saadat, M.; Zahednezhad, F.; Zakeri-Milani, P.; Heidari, H.R.; Shahbazi-Mojarrad, J.; Valizadeh, H. Drug Targeting Strategies Based on Charge Dependent Uptake of Nanoparticles into Cancer Cells. J. Pharm. Pharm. Sci. 2019, 22, 191–220. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.M.P.; Brans, T.; Samal, S.K.; Dubruel, P.; Demeester, J.; Smedt, S.C.D.; Remaut, K.; Braeckmans, K. Endosomal Size and Membrane Leakiness Influence Proton Sponge-Based Rupture of Endosomal Vesicles. ACS Nano 2018, 12, 2332–2345. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.; Haag, R. Hyperbranched Polymers in Industry. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 3998–4000. [Google Scholar] [CrossRef]
- Lee, K.; Bae, K.H.; Lee, Y.; Lee, S.H.; Ahn, C.-H.; Park, T.G. Pluronic/Polyethylenimine Shell Crosslinked Nanocapsules with Embedded Magnetite Nanocrystals for Magnetically Triggered Delivery of SiRNA. Macromol. Biosci. 2010, 10, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Namgung, R.; Singha, K.; Yu, M.K.; Jon, S.; Kim, Y.S.; Ahn, Y.; Park, I.-K.; Kim, W.J. Hybrid Superparamagnetic Iron Oxide Nanoparticle-Branched Polyethylenimine Magnetoplexes for Gene Transfection of Vascular Endothelial Cells. Biomaterials 2010, 31, 4204–4213. [Google Scholar] [CrossRef] [PubMed]
- Rohiwal, S.S.; Dvorakova, N.; Klima, J.; Vaskovicova, M.; Senigl, F.; Slouf, M.; Pavlova, E.; Stepanek, P.; Babuka, D.; Benes, H.; et al. Polyethylenimine Based Magnetic Nanoparticles Mediated Non-Viral CRISPR/Cas9 System for Genome Editing. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Steitz, B.; Hofmann, H.; Kamau, S.W.; Hassa, P.O.; Hottiger, M.O.; Von Rechenberg, B.; Hofmann-Amtenbrink, M.; Petri-Fink, A. Characterization of PEI-Coated Superparamagnetic Iron Oxide Nanoparticles for Transfection: Size Distribution Colloidal Properties and DNA Interaction. J. Magn. Magn. Mater. 2007, 311, 300–305. [Google Scholar] [CrossRef]
- Song, H.P.; Yang, J.Y.; Lo, S.L.; Wang, Y.; Fan, W.M.; Tang, X.S.; Xue, J.M.; Wang, S. Gene Transfer Using Self-Assembled Ternary Complexes of Cationic Magnetic Nanoparticles Plasmid DNA and Cell-Penetrating Tat Peptide. Biomaterials 2010, 31, 769–778. [Google Scholar] [CrossRef]
- Cuellar, M.; Cifuentes, J.; Perez, J.; Suarez-Arnedo, A.; Serna, J.; Groot, H.; Muñoz-Camargo, C.; Cruz, J. Novel BUF2-Magnetite Nanobioconjugates with Cell-Penetrating Abilities. Int. J. Nanomed. 2018, 13, 8087–8094. [Google Scholar] [CrossRef]
- Cristofolini, T.; Dalmina, M.; Sierra, J.A.; Silva, A.H.; Pasa, A.A.; Pittella, F.; Creczynski-Pasa, T.B. Multifunctional Hybrid Nanoparticles as Magnetic Delivery Systems for SiRNA Targeting the HER2 Gene in Breast Cancer Cells. Mater. Sci. Eng. C 2020, 109, 110555. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal Escape Pathways for Delivery of Biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Martens, T.F.; Remaut, K.; Demeester, J.; Smedt, S.C.D.; Braeckmans, K. Intracellular Delivery of Nanomaterials: How to Catch Endosomal Escape in the Act. Nano Today 2014, 9, 344–364. [Google Scholar] [CrossRef]
- Duncan, A.L.; Robinson, A.J.; Walker, J.E. Cardiolipin Binds Selectively but Transiently to Conserved Lysine Residues in the Rotor of Metazoan ATP Synthases. Proc. Natl. Acad. Sci. USA 2016, 113, 8687–8692. [Google Scholar] [CrossRef] [PubMed]
- Voegele, A.; Subrini, O.; Sapay, N.; Ladant, D.; Chenal, A. Membrane-Active Properties of an Amphitropic Peptide from the CyaA Toxin Translocation Region. Toxins 2017, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.G.; Fukuda, T.; Mizuki, T.; Hanajiri, T.; Maekawa, T. Intracellular Trafficking of Superparamagnetic Iron Oxide Nanoparticles Conjugated with TAT Peptide: 3-Dimensional Electron Tomography Analysis. Biochem. Biophys. Res. Commun. 2012, 421, 763–767. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, L.; Zhang, Q.; Yan, H.; Liu, K. Enhanced Cell Uptake of Superparamagnetic Iron Oxide Nanoparticles through Direct Chemisorption of FITC-Tat-PEG600-b-Poly(Glycerol Monoacrylate). Int. J. Pharm. 2012, 430, 372–380. [Google Scholar] [CrossRef]
- Lewin, M.; Carlesso, N.; Tung, C.-H.; Tang, X.-W.; Cory, D.; Scadden, D.T.; Weissleder, R. Tat Peptide-Derivatized Magnetic Nanoparticles Allow in Vivo Tracking and Recovery of Progenitor Cells. Nat. Biotechnol. 2000, 18, 410–414. [Google Scholar] [CrossRef]
- Hauser, A.K.; Mitov, M.I.; Daley, E.F.; McGarry, R.C.; Anderson, K.W.; Hilt, J.Z. Targeted Iron Oxide Nanoparticles for the Enhancement of Radiation Therapy. Biomaterials 2016, 105, 127–135. [Google Scholar] [CrossRef]
- Wunderbaldinger, P.; Josephson, L.; Weissleder, R. Tat Peptide Directs Enhanced Clearance and Hepatic Permeability of Magnetic Nanoparticles. Bioconj. Chem. 2002, 13, 264–268. [Google Scholar] [CrossRef]
- Veiseh, O.; Kievit, F.M.; Liu, V.; Fang, C.; Stephen, Z.R.; Ellenbogen, R.G.; Zhang, M. In Vivo Safety Evaluation of Polyarginine Coated Magnetic Nanovectors. Mol. Pharm. 2013, 10, 4099–4106. [Google Scholar] [CrossRef]
- Tudisco, C.; Cambria, M.T.; Giuffrida, A.E.; Sinatra, F.; Anfuso, C.D.; Lupo, G.; Caporarello, N.; Falanga, A.; Galdiero, S.; Oliveri, V.; et al. Comparison Between Folic Acid and GH625 Peptide-Based Functionalization of Fe3O4 Magnetic Nanoparticles for Enhanced Cell Internalization. Nanoscale Res. Lett. 2018, 13. [Google Scholar] [CrossRef]
- Sherwood, J.; Sowell, J.; Beyer, N.; Irvin, J.; Stephen, C.; Antone, A.J.; Bao, Y.; Ciesla, L.M. Cell-Membrane Coated Iron Oxide Nanoparticles for Isolation and Specific Identification of Drug Leads from Complex Matrices. Nanoscale 2019, 11, 6352–6359. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Barbosa, N.; Suárez-Arnedo, A.; Cifuentes, J.; Barrios, A.F.G.; Batista, C.A.S.; Osma, J.F.; Muñoz-Camargo, C.; Cruz, J.C. Magnetite-OmpA Nanobioconjugates as Cell-Penetrating Vehicles with Endosomal Escape Abilities. ACS Biomater. Sci. Eng. 2019, 6, 415–424. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Garcia, J.G.; Cifuentes, J.; Castro, L.M.; Vargas, F.; Ostos, C.; Cardona-Gomez, G.P.; Hernandez, A.M.; Cruz, J.C. Multifunctional Magnetite Nanoparticles to Enable Delivery of SiRNA for the Potential Treatment of Alzheimer’s. Drug Deliv. 2020, 27, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ren, T.; Gao, X. Cationic Transfection Lipids. Curr. Med. Chem. 2003, 10, 1307–1315. [Google Scholar] [CrossRef]
- Alamoudi, K.; Martins, P.; Croissant, J.G.; Patil, S.; Omar, H.; Khashab, N.M. Thermoresponsive Pegylated Bubble Liposome Nanovectors for Efficient SiRNA Delivery via Endosomal Escape. Nanomedicine 2017, 12, 1421–1433. [Google Scholar] [CrossRef]
- Pozzi, D.; Marchini, C.; Cardarelli, F.; Amenitsch, H.; Garulli, C.; Bifone, A.; Caracciolo, G. Transfection Efficiency Boost of Cholesterol-Containing Lipoplexes. Biochim. Biophys. Acta (BBA) Biomembr. 2012, 1818, 2335–2343. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, H.; Lv, R.; Zhao, P.; Sun, X.; Wang, S.; Su, W.; Niu, R.; Chang, J. Polymeric Liposomes-Coated Superparamagnetic Iron Oxide Nanoparticles as Contrast Agent for Targeted Magnetic Resonance Imaging of Cancer Cells. Langmuir 2011, 27, 3100–3105. [Google Scholar] [CrossRef]
- Photos, P.J.; Bacakova, L.; Discher, B.; Bates, F.S.; Discher, D.E. Polymer Vesicles in Vivo: Correlations with PEG Molecular Weight. J. Control. Release 2003, 90, 323–334. [Google Scholar] [CrossRef]
- Carvalho, A.; Martins, M.B.F.; Corvo, M.L.; Feio, G. Enhanced Contrast Efficiency in MRI by PEGylated Magnetoliposomes Loaded with PEGylated SPION: Effect of SPION Coating and Micro-Environment. Mater. Sci. Eng. C 2014, 43, 521–526. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Liang, X.; Song, T.; Gong, X.; Niu, R.; Chang, J. Construction of a Novel Cationic Polymeric Liposomes Formed from PEGylated Octadecyl-Quaternized Lysine Modified Chitosan/Cholesterol for Enhancing Storage Stability and Cellular Uptake Efficiency. Biotechnol. Bioeng. 2010, 106, 952–962. [Google Scholar] [CrossRef]
- MISHRA, S. PEGylation Significantly Affects Cellular Uptake and Intracellular Trafficking of Non-Viral Gene Delivery Particles. Eur. J. Cell Biol. 2004, 83, 97–111. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Yang, M.-C.; Liu, T.-Y.; Kuo, C.-Y.; Huang, L.-Y.; Chan, T.-Y. Hydrophobic Drug-Loaded PEGylated Magnetic Liposomes for Drug-Controlled Release. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered Release from Liposomes through Magnetic Actuation of Iron Oxide Nanoparticle Containing Membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef]
- Cardoso, B.D.; Rio, I.S.R.; Rodrigues, A.R.O.; Fernandes, F.C.T.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetoliposomes Containing Magnesium Ferrite Nanoparticles as Nanocarriers for the Model Drug Curcumin. R. Soc. Open Sci. 2018, 5, 181017. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Lu, X.; Dong, W.; Yuan, Y.; Qian, J.; Li, Y.; Shi, J.; Liu, C. Endosomal PH-Activatable Magnetic Nanoparticle-Capped Mesoporous Silica for Intracellular Controlled Release. J. Mater. Chem. 2012, 22, 15960. [Google Scholar] [CrossRef]
- Rahman, M.A.; Matsumura, Y.; Yano, S.; Ochiai, B. PH-Responsive Charge-Conversional and Hemolytic Activities of Magnetic Nanocomposite Particles for Cell-Targeted Hyperthermia. ACS Omega 2018, 3, 961–972. [Google Scholar] [CrossRef]
- Lee, Y.; Miyata, K.; Oba, M.; Ishii, T.; Fukushima, S.; Han, M.; Koyama, H.; Nishiyama, N.; Kataoka, K. Charge-Conversion Ternary Polyplex with Endosome Disruption Moiety: A Technique for Efficient and Safe Gene Delivery. Angew. Chem. Int. Ed. 2008, 47, 5163–5166. [Google Scholar] [CrossRef]
- Maeda, Y.; Pittella, F.; Nomoto, T.; Takemoto, H.; Nishiyama, N.; Miyata, K.; Kataoka, K. Fine-Tuning of Charge-Conversion Polymer Structure for Efficient Endosomal Escape of SiRNA-Loaded Calcium Phosphate Hybrid Micelles. Macromol. Rapid Commun. 2014, 35, 1211–1215. [Google Scholar] [CrossRef]
- Convertine, A.J.; Benoit, D.S.W.; Duvall, C.L.; Hoffman, A.S.; Stayton, P.S. Development of a Novel Endosomolytic Diblock Copolymer for SiRNA Delivery. J. Control. Release 2009, 133, 221–229. [Google Scholar] [CrossRef]
- Ding, H.; Portilla-Arias, J.; Patil, R.; Black, K.L.; Ljubimova, J.Y.; Holler, E. The Optimization of Polymalic Acid Peptide Copolymers for Endosomolytic Drug Delivery. Biomaterials 2011, 32, 5269–5278. [Google Scholar] [CrossRef]
- Wang, S.; Chen, R. PH-Responsive Lysine-Based, Hyperbranched Polymers Mimicking Endosomolytic Cell-Penetrating Peptides for Efficient Intracellular Delivery. Chem. Mater. 2017, 29, 5806–5815. [Google Scholar] [CrossRef]
- Jones, R.A.; Cheung, C.Y.; Black, F.E.; Zia, J.K.; Stayton, P.S.; Hoffman, A.S.; Wilson, M.R. Poly(2-Alkylacrylic Acid) Polymers Deliver Molecules to the Cytosol by PH-Sensitive Disruption of Endosomal Vesicles. Biochem. J. 2003, 372, 65–75. [Google Scholar] [CrossRef]
- Reyes-Ortega, F. PH-Responsive Polymers: Properties Synthesis and Applications. In Smart Polymers and their Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 45–92. [Google Scholar] [CrossRef]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Watarai, S.; Kono, K. A Liposome-Based Antigen Delivery System Using PH-Sensitive Fusogenic Polymers for Cancer Immunotherapy. Biomaterials 2013, 34, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Fujita, S.; Matsumura, K. Enhanced Protein Internalization and Efficient Endosomal Escape Using Polyampholyte-Modified Liposomes and Freeze Concentration. Nanoscale 2016, 8, 15888–15901. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Hatakeyama, H.; Sato, Y.; Akita, H.; Takayama, K.; Kobayashi, S.; Futaki, S.; Harashima, H. Endosomal Escape and the Knockdown Efficiency of Liposomal-SiRNA by the Fusogenic Peptide ShGALA. Biomaterials 2011, 32, 5733–5742. [Google Scholar] [CrossRef]
- Yao, L.; Daniels, J.; Wijesinghe, D.; Andreev, O.A.; Reshetnyak, Y.K. PHLIP-Mediated Delivery of PEGylated Liposomes to Cancer Cells. J. Control. Release 2013, 167, 228–237. [Google Scholar] [CrossRef]
- Burks, S.R.; Legenzov, E.A.; Martin, E.W.; Li, C.; Lu, W.; Kao, J.P.Y. Co-Encapsulating the Fusogenic Peptide INF7 and Molecular Imaging Probes in Liposomes Increases Intracellular Signal and Probe Retention. PLoS ONE 2015, 10, e0120982. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ranjan, S.; Zhang, W.; Zou, J.; Pyykkö, I.; Kinnunen, P.K.J. Novel Endosomolytic Peptides for Enhancing Gene Delivery in Nanoparticles. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 544–553. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Marchitto, J.; Nguyen, T.D.T.; Marasini, R.; Celia, C.; Aryal, S. PH-Responsive Cationic Liposome for Endosomal Escape Mediated Drug Delivery. Colloids Surf. B Biointerfaces 2020, 188, 110804. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A Strategy for Overcoming the PEG Dilemma in Efficient Drug Delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef]
- Kanamala, M.; Palmer, B.D.; Ghandehari, H.; Wilson, W.R.; Wu, Z. PEG-Benzaldehyde-Hydrazone-Lipid Based PEG-Sheddable PH-Sensitive Liposomes: Abilities for Endosomal Escape and Long Circulation. Pharm. Res. 2018, 35. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Guo, M.; Weng, X.; Zhang, W.; Owens, G.; Chen, Z. Modified Green Synthesis of Fe3O4@SiO2 Nanoparticles for PH Responsive Drug Release. Mater. Sci. Eng. C 2020, 112, 110900. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yan, Y.; Liu, X.; Yan, H.; Liu, K.; Zhang, H.; Cao, Y. Multilayer Nanoparticles with a Magnetite Core and a Polycation Inner Shell as PH-Responsive Carriers for Drug Delivery. Nanoscale 2010, 2, 434–441. [Google Scholar] [CrossRef]
- Gawali, S.L.; Barick, K.C.; Shetake, N.G.; Rajan, V.; Pandey, B.N.; Kumar, N.N.; Priyadarsini, K.I.; Hassan, P.A. PH-Labile Magnetic Nanocarriers for Intracellular Drug Delivery to Tumor Cells. ACS Omega 2019, 4, 11728–11736. [Google Scholar] [CrossRef] [PubMed]
- Adimoolam, M.G.; Amreddy, N.; Nalam, M.R.; Sunkara, M.V. A Simple Approach to Design Chitosan Functionalized Fe3O4 Nanoparticles for PH Responsive Delivery of Doxorubicin for Cancer Therapy. J. Magn. Magn. Mater. 2018, 448, 199–207. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Y.; Li, J.; Luo, Z.; Hu, Y.; Zhang, B.; Liu, J.; Zhou, J.; Cai, K. Hydrazone-Bearing PMMA-Functionalized Magnetic Nanocubes as PH-Responsive Drug Carriers for Remotely Targeted Cancer Therapy in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2014, 6, 7395–7407. [Google Scholar] [CrossRef]
- Chen, R. Polymers in Drug Delivery: Concepts Developments and Potential. In Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalised Treatment; Springer: Dordrecht, The Netherlands, 2013; pp. 1–34. [Google Scholar] [CrossRef]
- Soe, T.H.; Nanjo, T.; Watanabe, K.; Ohtsuki, T. Relation of Photochemical Internalization to Heat PH and Ca2+ Ions. Photochem. Photobiol. 2019, 95, 1395–1402. [Google Scholar] [CrossRef]
- Berg, K.; Folini, M.; Prasmickaite, L.; Selbo, P.; Bonsted, A.; Engesaeter, B.; Zaffaroni, N.; Weyergang, A.; Dietzea, A.; Maelandsmo, G.; et al. Photochemical Internalization: A New Tool for Drug Delivery. Curr. Pharm. Biotechnol. 2007, 8, 362–372. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.; Kim, J.; Kim, T.-L.; Kim, W.J. Photothermally Triggered Cytosolic Drug DeliveryviaEndosome Disruption Using a Functionalized Reduced Graphene Oxide. ACS Nano 2013, 7, 6735–6746. [Google Scholar] [CrossRef]
- Seabra, A.B. Iron Oxide Magnetic Nanoparticles in Photodynamic Therapy: A Promising Approach Against Tumor Cells. In Metal Nanoparticles in Pharma; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–20. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal Therapy and Photoacoustic Imaging via Nanotheranostics in Fighting Cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Prasmickaite, L.; Høgset, A.; Berg, K. Evaluation of Different Photosensitizers for Use in Photochemical Gene Transfection. Photochem. Photobiol. 2007, 73, 388–395. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, J.M.; Haque, S. Strategies in the Design of Endosomolytic Agents for Facilitating Endosomal Escape in Nanoparticles. Biochimie 2019, 160, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Selbo, P.K.; Weyergang, A.; Høgset, A.; Norum, O.-J.; Berstad, M.B.; Vikdal, M.; Berg, K. Photochemical Internalization Provides Time- and Space-Controlled Endolysosomal Escape of Therapeutic Molecules. J. Control. Release 2010, 148, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Pucelik, B.; Sułek, A.; Dbrowski, J.M. Bacteriochlorins and Their Metal Complexes as NIR-Absorbing Photosensitizers: Properties Mechanisms, and Applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Kloeckner, J.; Prasmickaite, L.; HØgset, A.; Berg, K.; Wagner, E. Photochemically Enhanced Gene Delivery of EGF Receptor-Targeted DNA Polyplexes. J. Drug Target. 2004, 12, 205–213. [Google Scholar] [CrossRef]
- Wong, J.J.W.; Berstad, M.B.; Fremstedal, A.S.V.; Berg, K.; Patzke, S.; Sørensen, V.; Peng, Q.; Selbo, P.K.; Weyergang, A. Photochemically-Induced Release of Lysosomal Sequestered Sunitinib: Obstacles for Therapeutic Efficacy. Cancers 2020, 12, 417. [Google Scholar] [CrossRef]
- Liang, W.; Lam, J.K.W. Endosomal Escape Pathways for Non-Viral Nucleic Acid Delivery Systems. In Molecular Regulation of Endocytosis; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Jayakumar, M.K.G.; Bansal, A.; Huang, K.; Yao, R.; Li, B.N.; Zhang, Y. Near-Infrared-Light-Based Nano-Platform Boosts Endosomal Escape and Controls Gene Knockdown in Vivo. ACS Nano 2014, 8, 4848–4858. [Google Scholar] [CrossRef]
- Zhang, Z.; Jayakumar, M.K.G.; Zheng, X.; Shikha, S.; Zhang, Y.; Bansal, A.; Poon, D.J.J.; Chu, P.L.; Yeo, E.L.L.; Chua, M.L.K.; et al. Upconversion Superballs for Programmable Photoactivation of Therapeutics. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion Nanoparticles in Biological Labeling Imaging, and Therapy. Analyst 2010, 135, 1839. [Google Scholar] [CrossRef]
- Fernandez-Villamarin, M.; Brooks, L.; Mendes, P.M. The Role of Photochemical Reactions in the Development of Advanced Soft Materials for Biomedical Applications. Adv. Opt. Mater. 2019, 7, 1900215. [Google Scholar] [CrossRef]
- Dai, X.; Du, T.; Han, K. Engineering Nanoparticles for Optimized Photodynamic Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6342–6354. [Google Scholar] [CrossRef]
- Bhana, S.; Lin, G.; Wang, L.; Starring, H.; Mishra, S.R.; Liu, G.; Huang, X. Near-Infrared-Absorbing Gold Nanopopcorns with Iron Oxide Cluster Core for Magnetically Amplified Photothermal and Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 11637–11647. [Google Scholar] [CrossRef]
- Hou, X.; Zhou, H.; Wang, L.; Tang, J.; Chen, C.; Jiang, G.; Liu, Y. Multifunctional near-Infrared Dye-Magnetic Nanoparticles for Bioimaging and Cancer Therapy. Cancer Lett. 2017, 390, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Ding, Y.; Wang, K.; Xing, Z.; Sun, X.; Guo, W.; Hong, X.; Zhu, X.; Liu, Y. Enhanced Photothermal-Photodynamic Therapy for Glioma Based on near-Infrared Dye Functionalized Fe3O4 Superparticles. Chem. Eng. J. 2020, 381, 122693. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Sun, Y.; Huang, P.; Yang, X.-X.; Zhou, X.-P. Studies on Preparation of Photosensitizer Loaded Magnetic Silica Nanoparticles and Their Anti-Tumor Effects for Targeting Photodynamic Therapy. Nanoscale Res. Lett. 2009, 4, 400–408. [Google Scholar] [CrossRef]
- Chen, H.; Liu, F.; Lei, Z.; Ma, L.; Wang, Z. Fe2O3@Au Core@Shell NanoparticleGraphene Nanocomposites as Theranostic Agents for Bioimaging and Chemo-Photothermal Synergistic Therapy. RSC Adv. 2015, 5, 84980–84987. [Google Scholar] [CrossRef]
- Cheng, J.; Tan, G.; Li, W.; Li, J.; Wang, Z.; Jin, Y. Preparation Characterization and in Vitro Photodynamic Therapy of a Pyropheophorbide-a-Conjugated Fe3O4 Multifunctional Magnetofluorescence Photosensitizer. RSC Adv. 2016, 6, 37610–37620. [Google Scholar] [CrossRef]
- Jalani, G.; Tam, V.; Vetrone, F.; Cerruti, M. Seeing Targeting and Delivering with Upconverting Nanoparticles. J. Am. Chem. Soc. 2018, 140, 10923–10931. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Rahman, P.; Green, M. The Synthesis of Rare Earth Fluoride Based Nanoparticles. Nanoscale 2009, 1, 214. [Google Scholar] [CrossRef]
- Gnanasammandhan, M.K.; Bansal, A.; Zhang, Y. Light-Activated Endosomal Escape Using Upconversion Nanoparticles for Enhanced Delivery of Drugs. In Nanoscale Imaging Sensing, and Actuation for Biomedical Applications X; Cartwright, A.N., Nicolau, D.V., Eds.; SPIE: San Francisco, CA, USA, 2013. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, G.; Hu, J.; Liu, S. Near-Infrared Light-Activated Photochemical Internalization of Reduction-Responsive Polyprodrug Vesicles for Synergistic Photodynamic Therapy and Chemotherapy. Biomacromolecules 2017, 18, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-F.; Chen, B.-C.; Chen, B.; Li, X.-J.; Liao, H.-L.; Huang, H.-M.; Guo, Z.-J.; Zhang, W.-Y.; Wu, L. Detection of AB Oligomers Based on Magnetic-Field-Assisted Separation of Aptamer-Functionalized Fe3O4 Magnetic Nanoparticles and BaYF 5:Yb, Er Nanoparticles as Upconversion Fluorescence Labels. Talanta 2017, 170, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, C.; Shen, B.; Zhu, X.; Li, Y.; Shi, L.; Zhang, Y.; Liu, J.; Wang, Y.; Sun, L. Upconversion-Magnetic Carbon Sphere for Near Infrared Light-Triggered Bioimaging and Photothermal Therapy. Theranostics 2019, 9, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, W.; Liao, Z.; Yang, S.; Yang, S.; Li, X.; Zuo, F.; Luo, J. Role of Mn2+ Doping in the Preparation of Core-Shell Structured Fe3O4@Upconversion Nanoparticles and Their Applications in T1/T2-Weighted Magnetic Resonance Imaging Upconversion Luminescent Imaging and Near-Infrared Activated Photodynamic Therapy. Nanomaterials 2018, 8, 466. [Google Scholar] [CrossRef] [PubMed]
- Fraire, J.C.; Houthaeve, G.; Liu, J.; Raes, L.; Vermeulen, L.; Stremersch, S.; Brans, T.; Barriga, G.G.-D.; Keulenaer, S.D.; Nieuwerburgh, F.V.; et al. Vapor Nanobubble Is the More Reliable Photothermal Mechanism for Inducing Endosomal Escape of SiRNA without Disturbing Cell Homeostasis. J. Control. Release 2020, 319, 262–275. [Google Scholar] [CrossRef]
- Hu, J.-J.; Cheng, Y.-J.; Zhang, X.-Z. Recent Advances in Nanomaterials for Enhanced Photothermal Therapy of Tumors. Nanoscale 2018, 10, 22657–22672. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Pu, K.; Chattopadhyay, N.; Rao, J. Recent Advances of Semiconducting Polymer Nanoparticles in in Vivo Molecular Imaging. J. Control. Release 2016, 240, 312–322. [Google Scholar] [CrossRef]
- Farani, M.R.; Khadiv-Parsi, P.; Riazi, G.H.; Ardestani, M.S.; Rad, H.S. PEGylation of Graphene/Iron Oxide Nanocomposite: Assessment of Release of Doxorubicin Magnetically Targeted Drug Delivery and Photothermal Therapy. Appl. Nanosci. 2020, 10, 1205–1217. [Google Scholar] [CrossRef]
- Lu, Q.; Dai, X.; Zhang, P.; Tan, X.; Zhong, Y.; Yao, C.; Song, M.; Song, G.; Zhang, Z.; Peng, G.; et al. Fe3O4@Au Composite Magnetic Nanoparticles Modified with Cetuximab for Targeted Magneto-Photothermal Therapy of Glioma Cells. Int. J. Nanomed. 2018, 13, 2491–2505. [Google Scholar] [CrossRef]
- van der Aa, M.A.E.M.; Mastrobattista, E.; Oosting, R.S.; Hennink, W.E.; Koning, G.A.; Crommelin, D.J.A. The Nuclear Pore Complex: The Gateway to Successful Nonviral Gene Delivery. Pharm. Res. 2006, 23, 447–459. [Google Scholar] [CrossRef]
- Chen, K.; Guo, L.; Zhang, J.; Chen, Q.; Wang, K.; Li, C.; Li, W.; Qiao, M.; Zhao, X.; Hu, H.; et al. A Gene Delivery System Containing Nuclear Localization Signal: Increased Nucleus Import and Transfection Efficiency with the Assistance of RanGAP1. Acta Biomater. 2017, 48, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, P.; Chen, Z.; Lal, S.; Zhang, L.; Gassensmith, J.J.; Qin, Z. Rock the Nucleus: Significantly Enhanced Nuclear Membrane Permeability and Gene Transfection by Plasmonic Nanobubble Induced Nanomechanical Transduction. Chem. Commun. 2018, 54, 2479–2482. [Google Scholar] [CrossRef]
- Lechardeur, D.; Verkman, A.; Lukacs, G. Intracellular Routing of Plasmid DNA during Non-Viral Gene Transfer. Adv. Drug Deliv. Rev. 2005, 57, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Remaut, K.; Sanders, N.N.; Fayazpour, F.; Demeester, J.; Smedt, S.C.D. Influence of Plasmid DNA Topology on the Transfection Properties of DOTAP/DOPE Lipoplexes. J. Control. Release 2006, 115, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Kudo, A.; Minoura, A.; Yamaguti, M.; Khalil, I.A.; Moriguchi, R.; Masuda, T.; Danev, R.; Nagayama, K.; Kogure, K. Multi-Layered Nanoparticles for Penetrating the Endosome and Nuclear Membrane via a Step-Wise Membrane Fusion Process. Biomaterials 2009, 30, 2940–2949. [Google Scholar] [CrossRef] [PubMed]
- Colin, M.; Moritz, S.; Fontanges, P.; Kornprobst, M.; Delouis, C.; Keller, M.; Miller, A.D.; Capeau, J.; Coutelle, C.; Brahimi-Horn, M.C. The Nuclear Pore Complex Is Involved in Nuclear Transfer of Plasmid DNA Condensed with an OligolysineRGD Peptide Containing Nuclear Localisation Properties. Gene Ther. 2001, 8, 1643–1653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xing, R.; Liu, G.; Zhu, J.; Hou, Y.; Chen, X. Functional Magnetic Nanoparticles for Non-Viral Gene Delivery and MR Imaging. Pharm. Res. 2013, 31, 1377–1389. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhou, C.; Jiao, Y. Hydrophobic Modifications of Cationic Polymers for Gene Delivery. Prog. Polym. Sci. 2010, 35, 1144–1162. [Google Scholar] [CrossRef]
- Kami, D.; Kitani, T.; Kishida, T.; Mazda, O.; Toyoda, M.; Tomitaka, A.; Ota, S.; Ishii, R.; Takemura, Y.; Watanabe, M.; et al. Pleiotropic Functions of Magnetic Nanoparticles for Ex Vivo Gene Transfer. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1165–1174. [Google Scholar] [CrossRef]
- Arsianti, M.; Lim, M.; Marquis, C.P.; Amal, R. Assembly of Polyethylenimine-Based Magnetic Iron Oxide Vectors: Insights into Gene Delivery. Langmuir 2010, 26, 7314–7326. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.; Eudes, F.; Shim, Y.-S. Cell-Penetrating Peptides: Nanocarrier for Macromolecule Delivery in Living Cells. IUBMB Life 2010, 62, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Vernon, M.; Dean, D.; Dobson, J. DNA Targeting Sequence Improves Magnetic Nanoparticle-Based Plasmid DNA Transfection Efficiency in Model Neurons. Int. J. Mol. Sci. 2015, 16, 19369–19386. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Chen, B.; Tang, Z.; Zhang, J.; Li, L.; Tang, J. Enhanced Intracellular Hyperthermia Efficiency by Magnetic Nanoparticles Modified with Nucleus and Mitochondria Targeting Peptides. J. Nanosci. Nanotechnol. 2016, 16, 6560–6566. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, R.; Chaudhuri, A. Cationic Transfection Lipids in Gene Therapy: Successes Set-Backs, Challenges and Promises. Curr. Med. Chem. 2003, 10, 1297–1306. [Google Scholar] [CrossRef]
- Martínez-Negro, M.; Barrán-Berdón, A.L.; Aicart-Ramos, C.; Moyá, M.L.; De Ilarduya, C.T.; Aicart, E.; Junquera, E. Transfection of Plasmid DNA by Nanocarriers Containing a Gemini Cationic Lipid with an Aromatic Spacer or Its Monomeric Counterpart. Colloids Surf. B Biointerfaces 2018, 161, 519–527. [Google Scholar] [CrossRef]
- Zhi, D.F.; Zhang, S.B.; Wang, B.; Zhao, Y.N.; Yang, B.L.; Yu, S.J. Transfection Efficiency of Cationic Lipids with Different Hydrophobic Domains in Gene Delivery. Bioconj. Chem. 2010, 21, 563–577. [Google Scholar] [CrossRef]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R.; Martinez-Navarrete, G.; Soto-Sánchez, C.; Diaz-Tahoces, A.; Aviles-Trigueros, M.; et al. The Influence of the Polar Head-Group of Synthetic Cationic Lipids on the Transfection Efficiency Mediated by Niosomes in Rat Retina and Brain. Biomaterials 2016, 77, 267–279. [Google Scholar] [CrossRef]
- Zhi, D.; Bai, Y.; Yang, J.; Cui, S.; Zhao, Y.; Chen, H.; Zhang, S. A Review on Cationic Lipids with Different Linkers for Gene Delivery. Adv. Colloid Interface Sci. 2018, 253, 117–140. [Google Scholar] [CrossRef]
- Lee, H.Y.; Mohammed, K.A.; Nasreen, N. Nanoparticle-Based Targeted Gene Therapy for Lung Cancer. Am. J. Cancer Res. 2016, 6, 1118–1134. [Google Scholar]
- Du, C.-X.; Zhang, T.-B.; Dong, S.-L.; Han, L.; Liang, X.-J.; Li, L.-H.; Wei, Y. A Magnetic Gene Delivery Nanosystem Based on Cationic Liposomes. J. Mater. Sci. 2016, 51, 8461–8470. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, J.; Deng, L.; Xiong, Y.; Chen, J. Preparation and Characterization of Magnetic Cationic Liposome in Gene Delivery. Int. J. Pharm. 2009, 366, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hirao, K.; Sugita, T.; Kubo, T.; Igarashi, K.; Tanimoto, K.; Murakami, T.; Yasunaga, Y.; Ochi, M. Targeted Gene Delivery to Human Osteosarcoma Cells with Magnetic Cationic Liposomes under a Magnetic Field. Int. J. Oncol. 2003. [Google Scholar] [CrossRef]
- Hu, J.; Hu, K.; Cheng, Y. Tailoring the Dendrimer Core for Efficient Gene Delivery. Acta Biomater. 2016, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, E.J.; Na, D.H. Recent Progress in Dendrimer-Based Nanomedicine Development. Arch. Pharmacal Res. 2018, 41, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM Dendrimer—Cell Membrane Interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Mignani, S.; Shen, M.; Shi, X. Dendrimer-Based Magnetic Iron Oxide Nanoparticles: Their Synthesis and Biomedical Applications. Drug Discov. Today 2016, 21, 1873–1885. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Li, L.; Cheng, Y. A Fluorinated Dendrimer Achieves Excellent Gene Transfection Efficacy at Extremely Low Nitrogen to Phosphorus Ratios. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Khalilov, R.; Mostafavi, E.; Annabi, N.; Kafshdooz, T.; Abasi, E.; Herizchi, R.; Kavetskyy, T.; Saghfi, S.; Nasibova, A.; et al. Role of Dendirmers in Advanced Drug Delivery and Biomedical Applications: A Review. Exp. Oncol. 2018, 40, 178–183. [Google Scholar] [CrossRef]
- Nasr, M.; Elmowafy, E.; Soliman, M.E. The Evolution of Dendrimers to Composite Dendrimers: A Review of the State of the Art. In Nanoparticles in Pharmacotherapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–249. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gothwal, A.; Iyer, A.K.; Jain, K.; Chourasia, M.K.; Gupta, U. Dendrimer Nanohybrid Carrier Systems: An Expanding Horizon for Targeted Drug and Gene Delivery. Drug Discov. Today 2018, 23, 300–314. [Google Scholar] [CrossRef]
- Ray, S.; Li, Z.; Hsu, C.-H.; Hwang, L.-P.; Lin, Y.-C.; Chou, P.-T.; Lin, Y.-Y. Dendrimer- and Copolymer-Based Nanoparticles for Magnetic Resonance Cancer Theranostics. Theranostics 2018, 8, 6322–6349. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Acosta, J.R.; Iriarte-Mesa, C.; Ortega, G.A.; Díaz-García, A.M. DNAIron Oxide Nanoparticles Conjugates: Functional Magnetic Nanoplatforms in Biomedical Applications. Top. Curr. Chem. 2020, 378. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Shirzadfar, H.; Tavassoli-Kafrani, E. Dendrimers and Dendrimers-Grafted Superparamagnetic Iron Oxide Nanoparticles: Synthesis Characterization, Functionalization, and Biological Applications in Drug Delivery Systems. In Nano- and Microscale Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 75–94. [Google Scholar] [CrossRef]
- Taratula, O.; Garbuzenko, O.; Savla, R.; Wang, Y.A.; He, H.; Minko, T. Multifunctional Nanomedicine Platform for Cancer Specific Delivery of SiRNA by Superparamagnetic Iron Oxide Nanoparticles-Dendrimer Complexes. Curr. Drug Deliv. 2011, 8, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Castro, R.; Rodrigues, J.; Shi, X.; Tomás, H. PAMAM Dendrimer/PDNA Functionalized-Magnetic Iron Oxide Nanoparticles for Gene Delivery. J. Biomed. Nanotechnol. 2015, 11, 1370–1384. [Google Scholar] [CrossRef]
- Thomas, R.G.; Muthiah, M.; Moon, M.J.; Park, I.-K.; Jeong, Y.Y. SPION Loaded Poly(L-Lysine)/Hyaluronic Acid Micelles as MR Contrast Agent and Gene Delivery Vehicle for Cancer Theranostics. Macromol. Res. 2017, 25, 446–451. [Google Scholar] [CrossRef]
- Plank, C.; Scherer, F.; Schillinger, U.; Bergemann, C.; Anton, M. Magnetofection: Enhancing and Targeting Gene Delivery with Superparamagnetic Nanoparticles and Magnetic Fields. J. Liposome Res. 2003, 13, 29–32. [Google Scholar] [CrossRef]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically Enhanced Nucleic Acid Delivery. Ten Years of MagnetofectionProgress and Prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef]
- Vaca, M.L.A.; Pasquevich, G.A.; Mykhaylyk, O.; Mele, N.G.; Goya, R.G.; Sánchez, F.H. Physics of in Vitro Magnetofection. Effect of Magnetic Transport and Redistribution of Nanoparticles. J. Magn. Magn. Mater. 2020, 503, 166606. [Google Scholar] [CrossRef]
- Lu, C.-W.; Hsiao, J.-K.; Liu, H.-M.; Wu, C.-H. Characterization of an Iron Oxide Nanoparticle Labelling and MRI-Based Protocol for Inducing Human Mesenchymal Stem Cells into Neural-like Cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kamau, S.W. Enhancement of the Efficiency of Non-Viral Gene Delivery by Application of Pulsed Magnetic Field. Nucleic Acids Res. 2006, 34, e40. [Google Scholar] [CrossRef]
- Subramanian, M.; Miaskowski, A.; Jenkins, S.I.; Lim, J.; Dobson, J. Remote Manipulation of Magnetic Nanoparticles Using Magnetic Field Gradient to Promote Cancer Cell Death. Appl. Phys. A 2019, 125. [Google Scholar] [CrossRef]
- Yapici, M.K.; Nabulsi, A.A.; Rizk, N.; Boularaoui, S.M.; Christoforou, N.; Lee, S. Alternating Magnetic Field Plate for Enhanced Magnetofection of Iron Oxide Nanoparticle Conjugated Nucleic Acids. J. Magn. Magn. Mater. 2019, 469, 598–605. [Google Scholar] [CrossRef]
- Prosen, L.; Hudoklin, S.; Cemazar, M.; Stimac, M.; Tratar, U.L.; Ota, M.; Scancar, J.; Romih, R.; Sersa, G. Magnetic Field Contributes to the Cellular Uptake for Effective Therapy with Magnetofection Using Plasmid DNA Encoding against Mcam in B16F10 Melanoma In Vivo. Nanomedicine 2016, 11, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.-A.M.; De la Fuente, J.; Pelaz, B.; Furlani, E.P.; Mullin, M.; Berry, C.C. The Effect of Static Magnetic Fields and Tat Peptides on Cellular and Nuclear Uptake of Magnetic Nanoparticles. Biomaterials 2010, 31, 4392–4400. [Google Scholar] [CrossRef] [PubMed]
- Badieyan, Z.S.; Pasewald, T.; Mykhaylyk, O.; Rudolph, C.; Plank, C. Efficient Ex Vivo Delivery of Chemically Modified Messenger RNA Using Lipofection and Magnetofection. Biochem. Biophys. Res. Commun. 2017, 482, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, X.; Zeljic, K.; Shan, S.; Qiu, Z.; Wang, Z. Effect of PEGylated Magnetic PLGA-PEI Nanoparticles on Primary Hippocampal Neurons: Reduced Nanoneurotoxicity and Enhanced Transfection Efficiency with Magnetofection. ACS Appl. Mater. Interfaces 2019, 11, 38190–38204. [Google Scholar] [CrossRef]
- Hryhorowicz, M.; Grześkowiak, B.; Mazurkiewicz, N.; Śledziński, P.; Lipiński, D.; Słomski, R. Improved Delivery of CRISPR/Cas9 System Using Magnetic Nanoparticles into Porcine Fibroblast. Mol. Biotechnol. 2018, 61, 173–180. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Lin, Y.-H.; Lin, S.-Y.; Li, Y.-N.; Chiang, C.-S.; Chang, C.-W. Magnetic Ternary Nanohybrids for Nonviral Gene Delivery of Stem Cells and Applications on Cancer Therapy. Theranostics 2019, 9, 2411–2423. [Google Scholar] [CrossRef]
- Cen, C.; Wu, J.; Zhang, Y.; Luo, C.; Xie, L.; Zhang, X.; Yang, X.; Li, M.; Bi, Y.; Li, T.; et al. Improving Magnetofection of Magnetic Polyethylenimine Nanoparticles into MG-63 Osteoblasts Using a Novel Uniform Magnetic Field. Nanoscale Res. Lett. 2019, 14. [Google Scholar] [CrossRef]
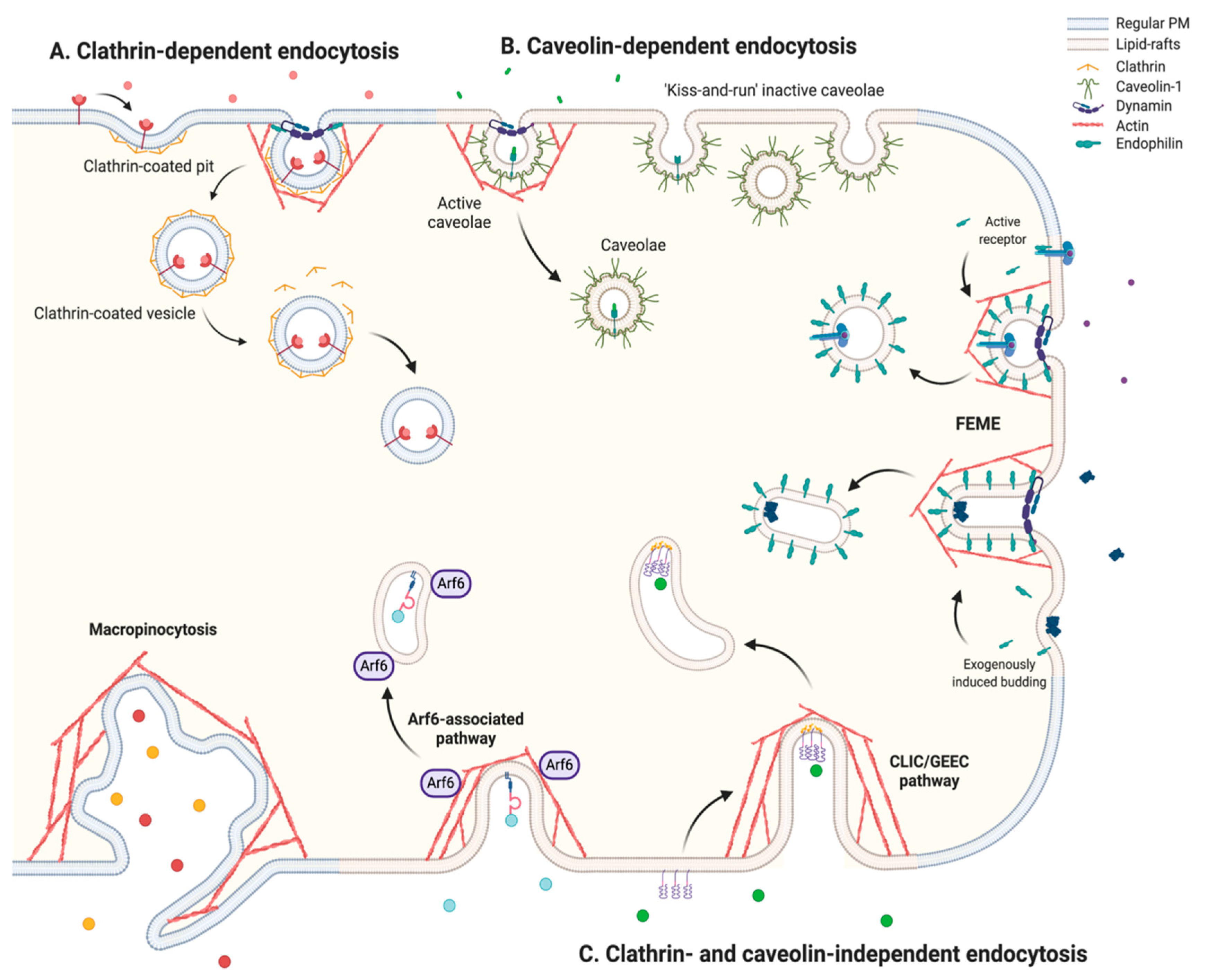
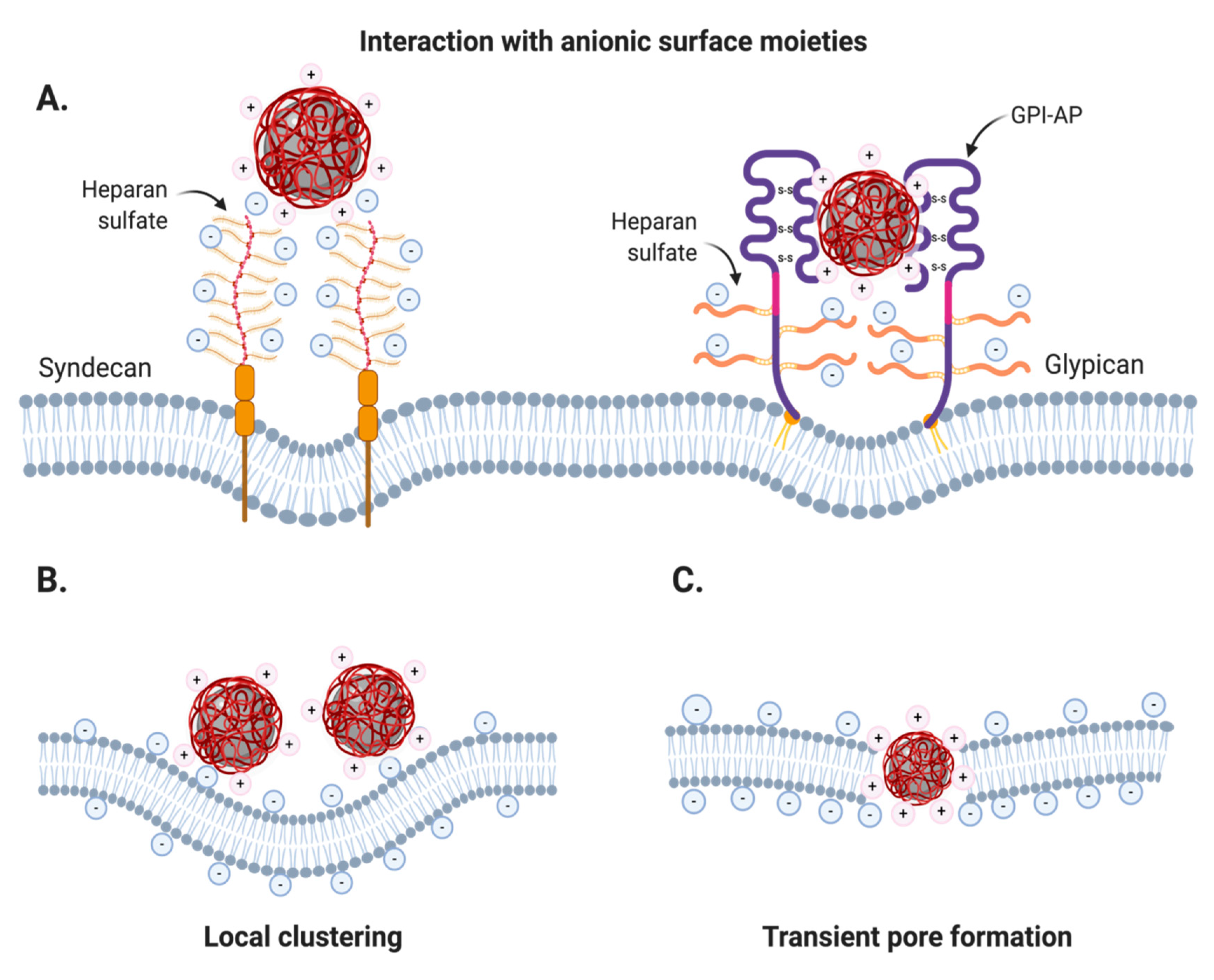
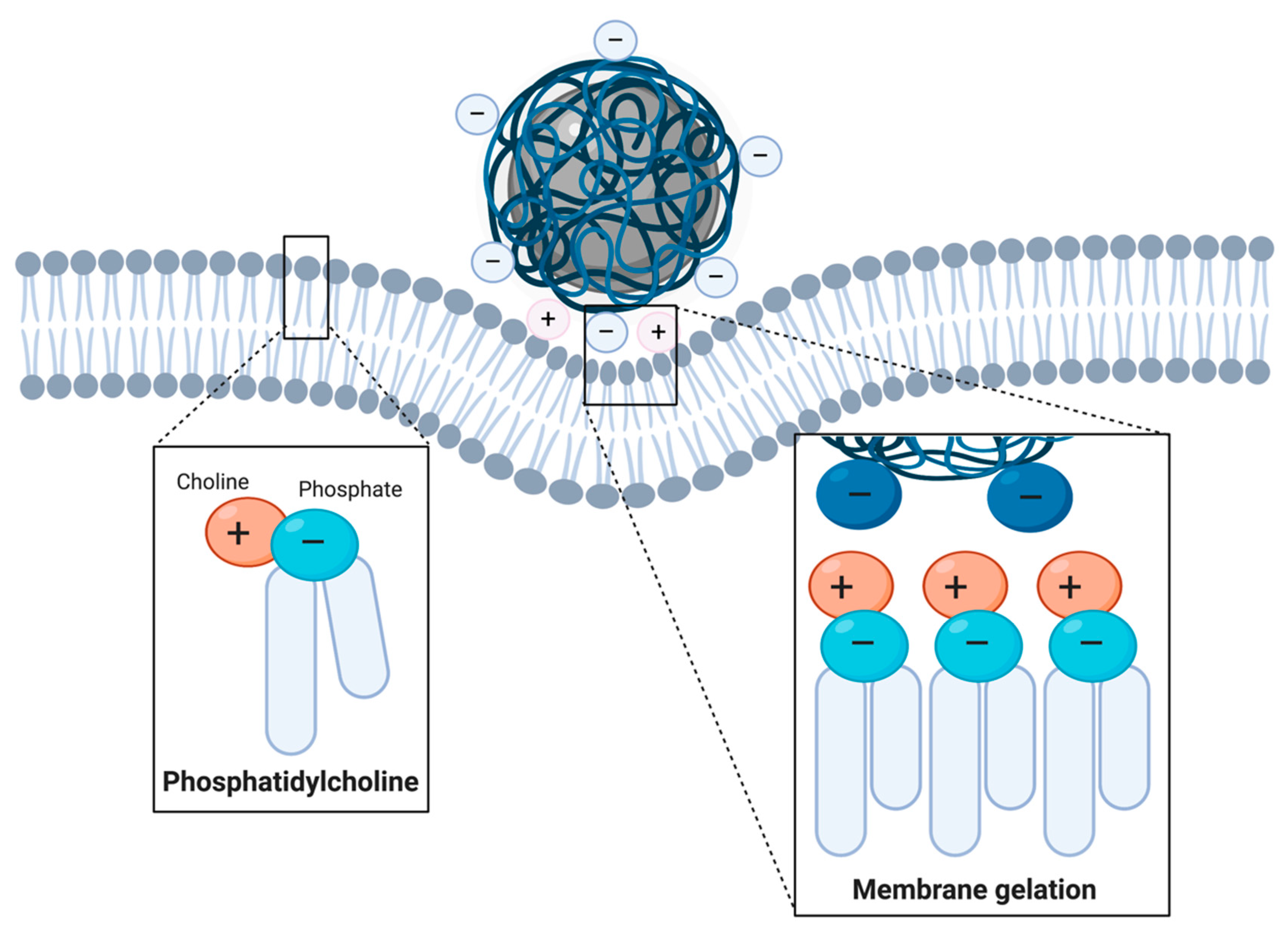
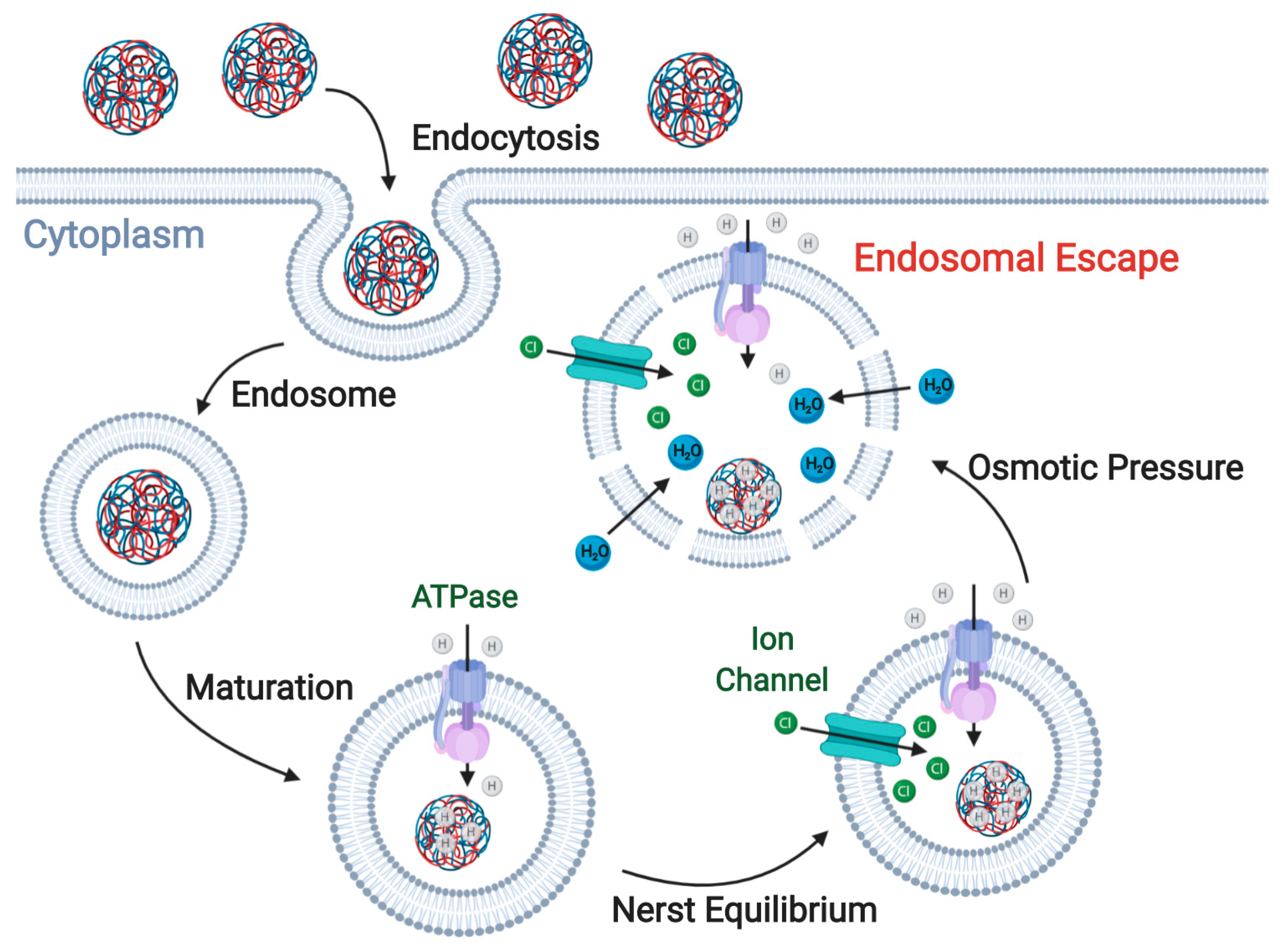
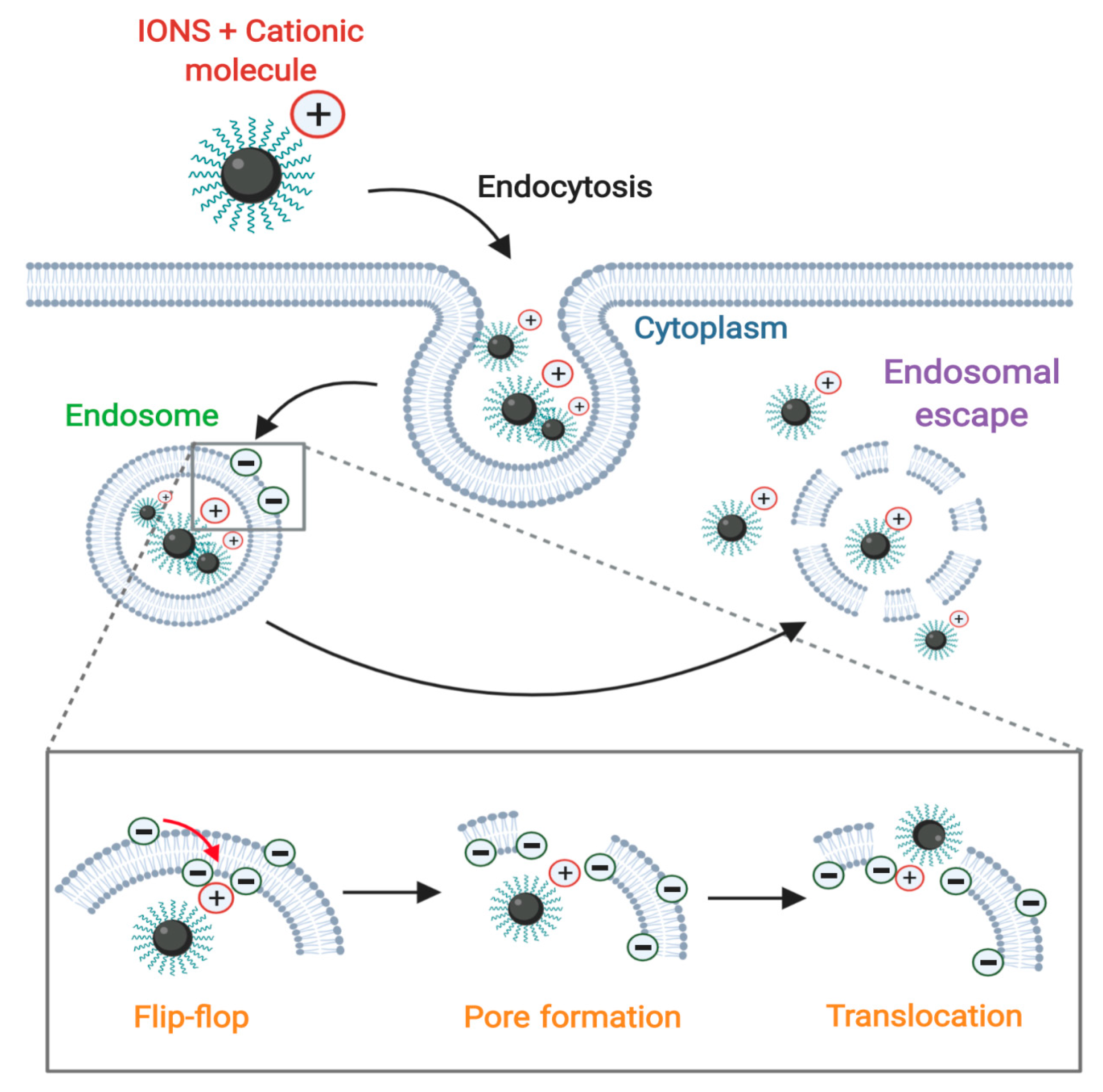
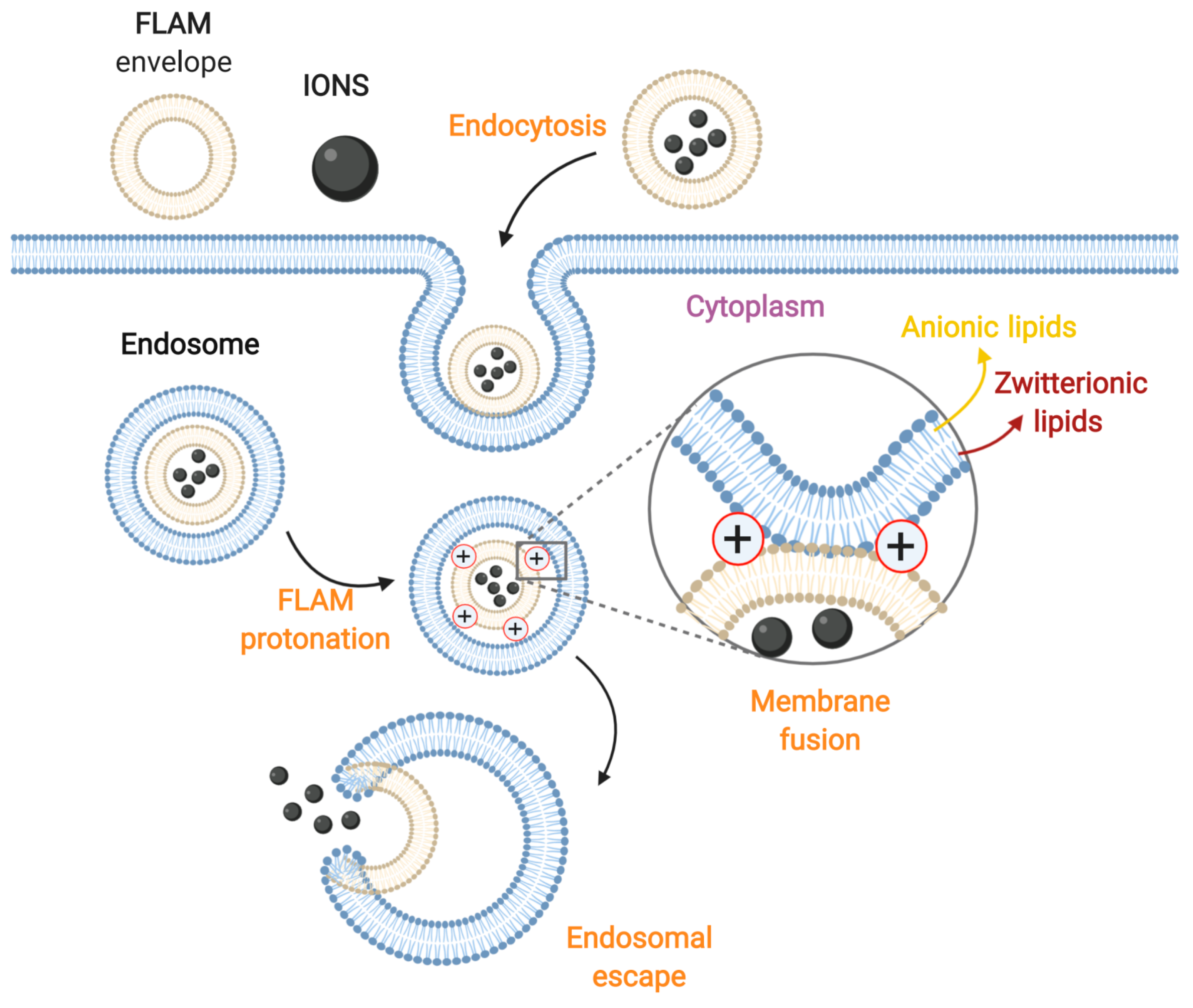
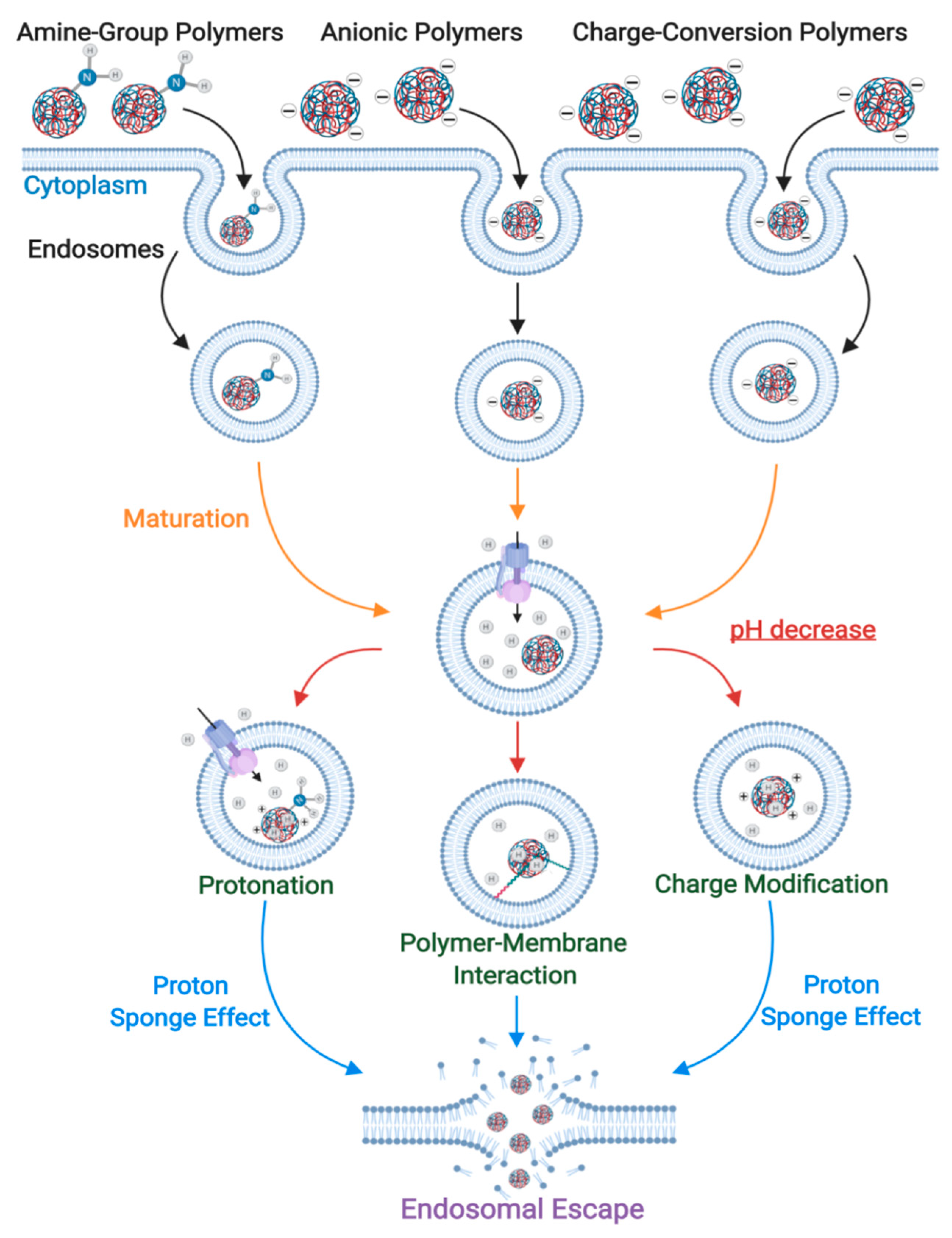
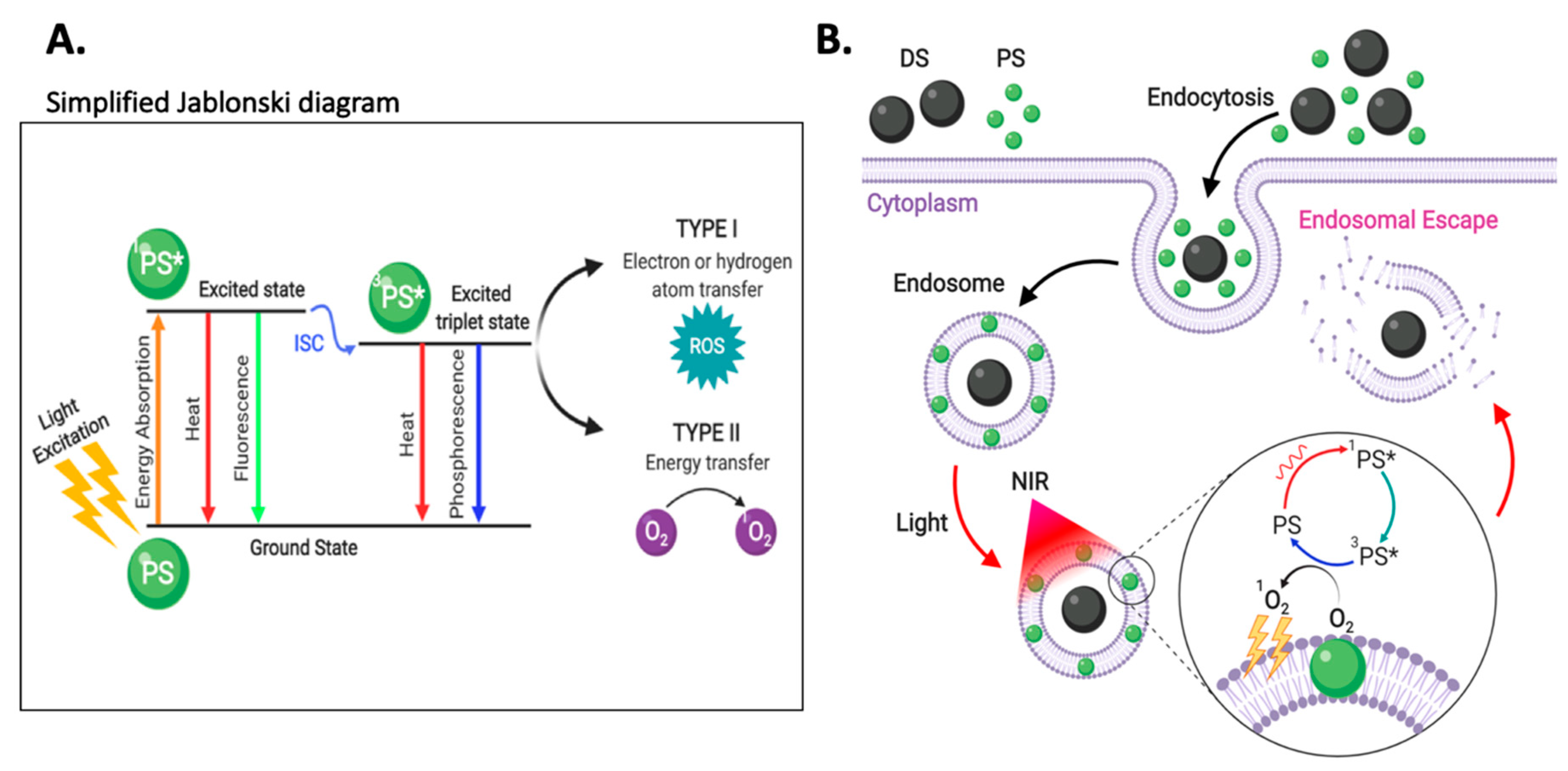
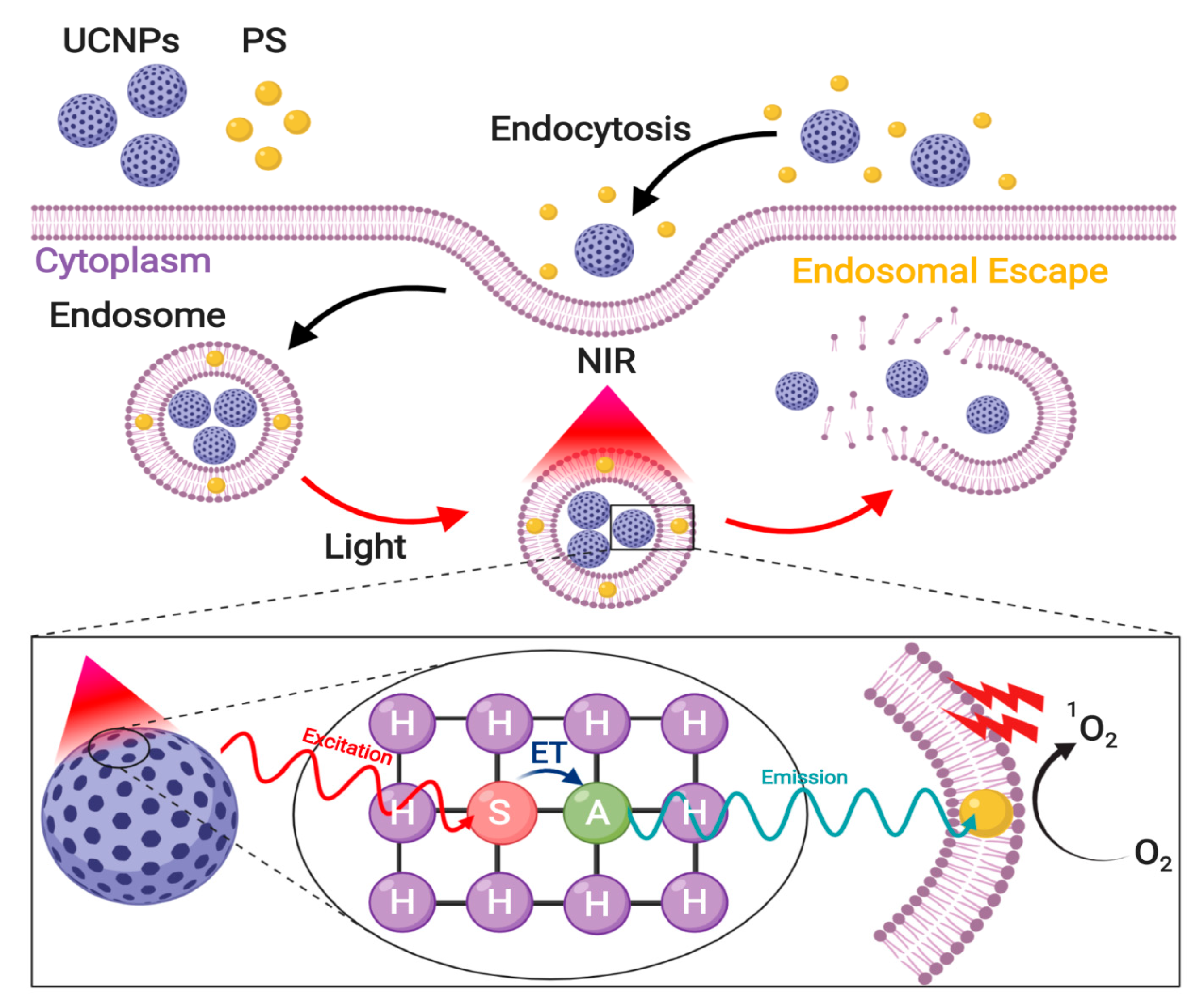
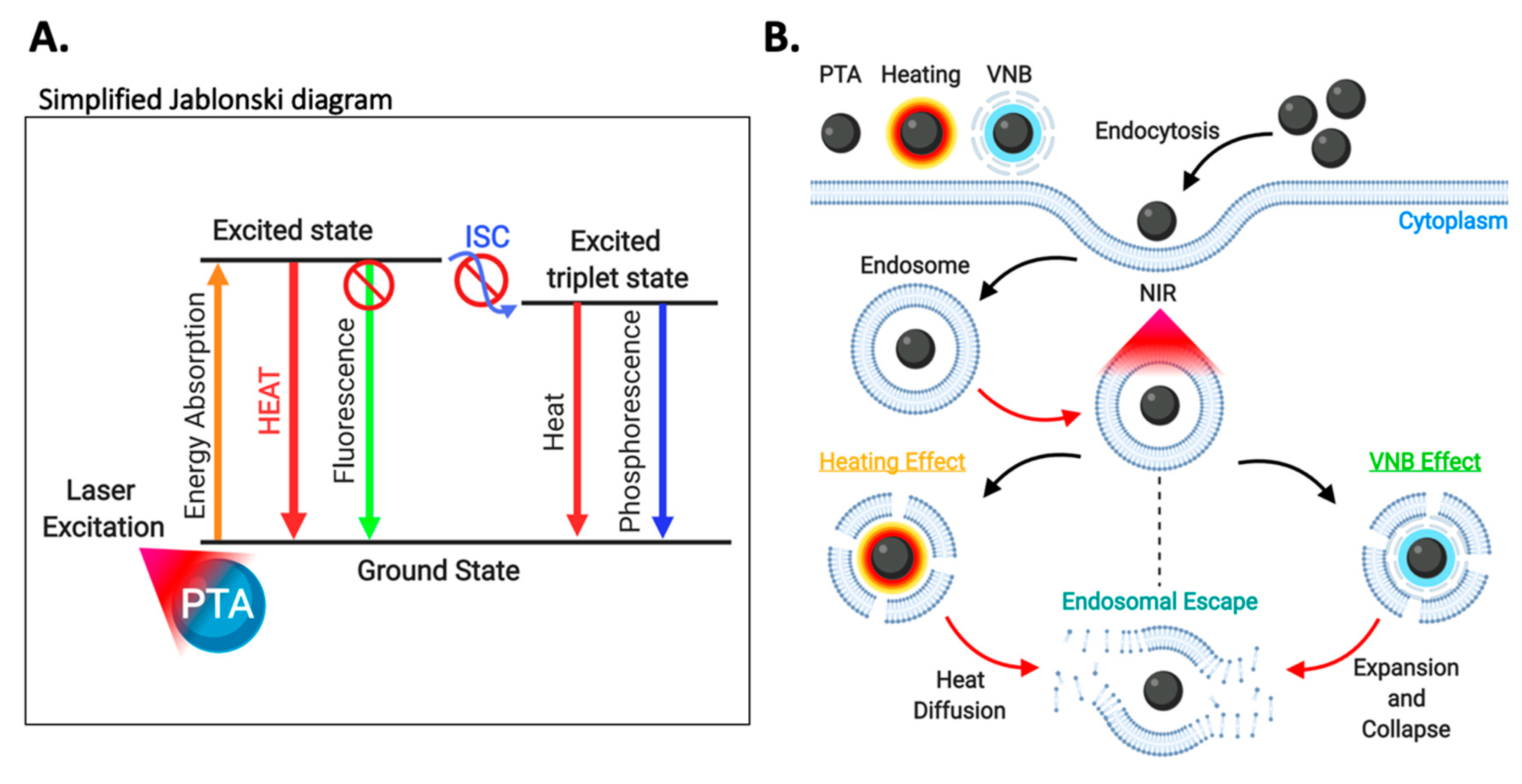
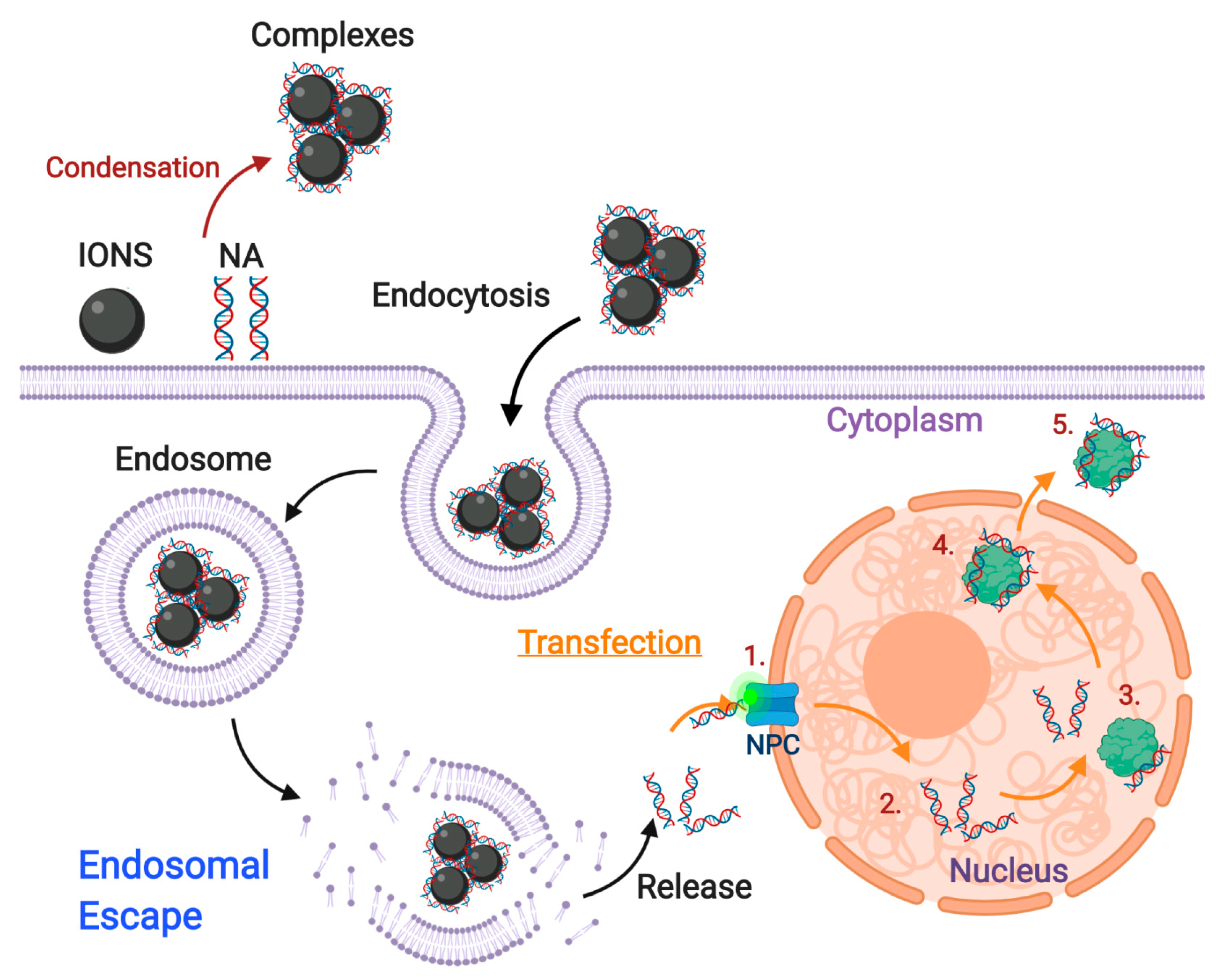
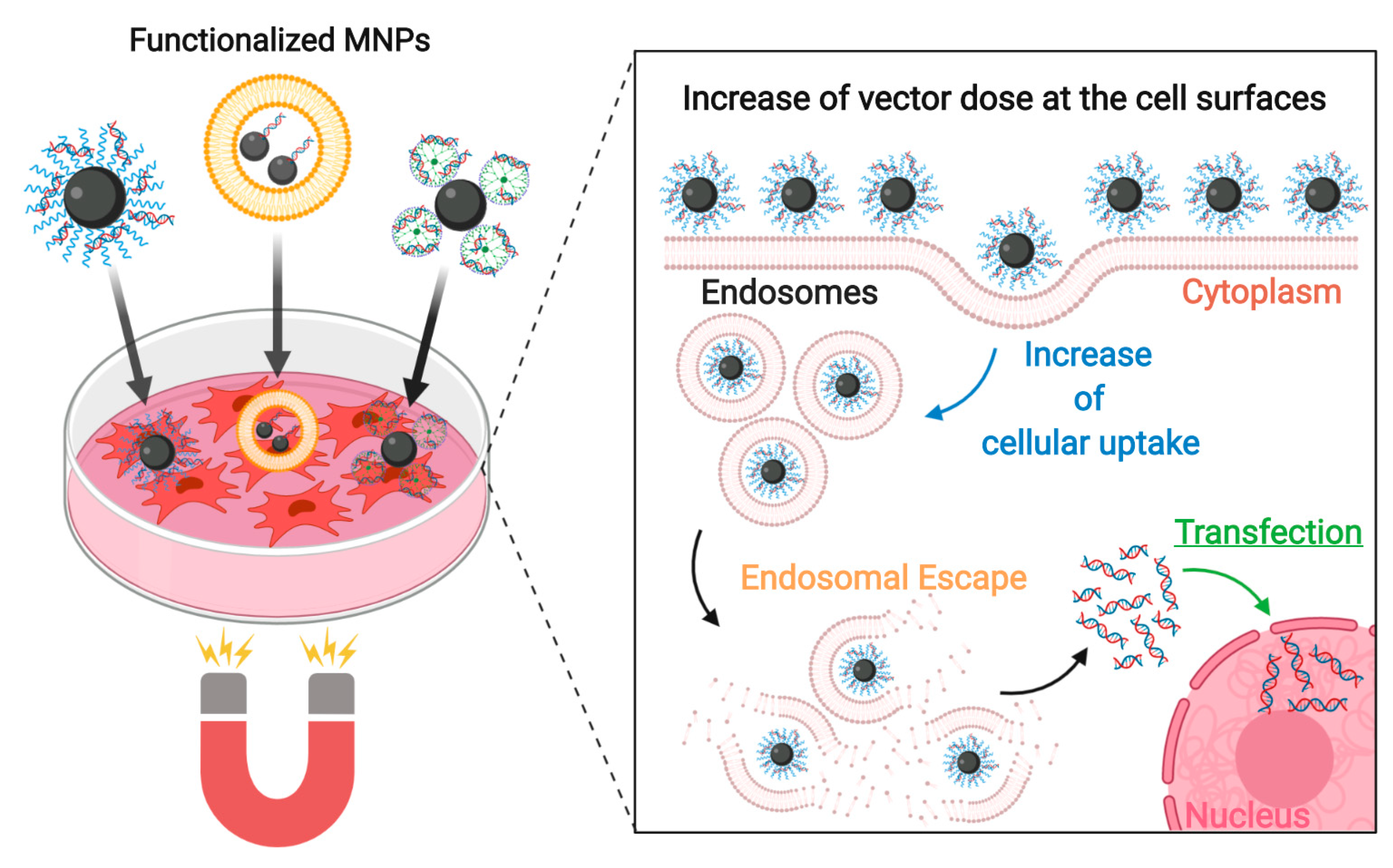
| Coating | Structure | Zeta Potential (mV) | Hydrodynamic Diameter Water (nm) 1 | Hydrodynamic Diameter Serum (nm) 1 | Cell Viability (24 h) | Main Endocytic Mechanism(s) | Internalized Tissue | Ref |
|---|---|---|---|---|---|---|---|---|
| Cationic | ||||||||
| Chitosan (CS) | Core-shell | ~4.2 | ~122.4 | Not reported | ≥90% at 30 µg/mL NPs a | Clathrin-dependent | Rat NSCs | [97] |
| CS-thioglycolic acid | Core-shell | 21 ± 5.27 | 94 ± 20 | 91 ± 8 nm | ≥80% at 300 µg/mL NPs a | Not specified | Human umbilical cord EPCs | [127] |
| Poly(vinylalcohol/vinylamine) | Core-shell | Positive | ~24 | Not reported | ~100% (up to 123 µg/mL Fe) a | Clathrin-dependent | Me300 | [128] |
| [129] | ||||||||
| diethylaminoethyl-dextran (DEAE-DEX) | Core-shell | ~26 | ~150 | Not reported | ≥90% (up to 500 µg/mL Fe) a | Clathrin- and caveolin-independent Macropinocytosis | A-549 | [130] |
| PEI-Zonyl FSA/DNA | Core-shell | ~52.2 (w/o DNA) | 144 ± 0.2 | Not reported | ≥80% (up to 0.1 µM Fe)b | Caveolin-dependent Clathrin-dependent | HEK293 | [131] |
| PEI-Pluronic F-127/DNA | Core-shell | ~61.7 (w/o DNA) | 160 ± 1.4 | Not reported | ≥80% (up to 0.05 µM Fe) b | Caveolin-dependent Clathrin-dependent | HEK293 | [132] |
| Lactosylated N-alkyl-PEI2k | Micellar | ~28.7 | 75 ± 6 | Not reported | ~100% (up to 15 µg/mL Fe) b | Not specified | RAW 264.7 | [122] |
| PEI-stearic acid/PEG-poly(L-glutamic acid) | Polymeric nanosphere | ~8 | 150 ± 25 | Not reported | ~100% (up to 6.3 µg/mL Fe) b | Not specified | MSCs | [126] |
| PEI/siRNA | Core-shell | ~25.7 (w/o siRNA) | ~43.56 | Not reported | ≥90% at 2 µg/mL NPs (w/o siRNA) a | Not specified | U-87 & U-251 | [98] |
| ≤50% at 2 µg/mL (anti-tumor siRNA) a | ||||||||
| PEI-decorated poly(glycidyl methacrylate) | Polymeric nanosphere | Positive | ~160 | Not reported | ~100% (up to 250 µg/mL NPs) b | Clathrin- and caveolin-independent | Rat PC12 | [132] |
| PEG-g-PEI/siRNA | Core-shell | 34.38 ± 1.66 | 93.8 ± 0.6 | Not reported | Non-significant cytotoxicity | Not specified | SGC-7901 | [124] |
| 15.1 ± 0.64 (siRNA) | ||||||||
| PEI-dextran/miRNA | Core-shell | 32.5 ± 0.62 (w/o miRNA) | 148.67 ± 1.52 | Not reported | ≥80% (up to 150 µg/mL NPs) a | Not specified | U2 | [133] |
| PEG-g-Chitosan/PEI/siRNA | Core-shell | 19.6 ± 5.7 (siRNA) | 111.9 ± 52.4 | ~115 nm | Non-significant cytotoxicity (concentration not specified) | Not specified | Rat C6 | [134] |
| Lipofectamine-Endoderm | Core-shell | −2.45 ± 0.53 * | ~181 (PBS) | Not reported | ≥80% (up to 50 µg/mL Fe) a,b | Clathrin-dependent Macropinocytosis | HeLa | [135] |
| Poly-L-lysine (PLL) | Core-shell | ~16.9 | ~24 | Not reported | ≥90% (up to 25 µg/mL NPs) a | Not specified | NSCs | [136] |
| PLL-dextran | Core-shell | 50 ± 2 | 115 ± 30 | Not reported | ≥80% (at 24 µg/mL NPs) a | Not specified | HepG2 | [137] |
| Maltodextrin | 25 ± 1.5 | 60 ± 13.1 | Not reported | Not reported | Clathrin-dependent | 16HBE14o | [138] | |
| D6DOM/pDNA | Core-shell | 9 ± 1.2 | 71 ± 12 | 146 ± 29 nm | ≥90% a and ≥85% b (up to 47 µg/mL NPs) | Not specified | MKN-74 & NUGC-4 | [139] |
| gH625-cysteine-PEG-Cy5.5 | Core-shell | ~4.08 | 97.8 ±1.2 | Not reported | Non-significant cytotoxicity | Not specified | MDA-MB-231 | [140] |
| PF14-SCO/Chitosan | Core-shell | ~37 | ~370 | Not reported | Non-significant cytotoxicity | Not specified | HeLa | [141] |
| PF1221-SCO/Chitosan | Core-shell | ~23 | ~420 | Not reported | Non-significant cytotoxicity | Not specified | HeLa | [141] |
| Poly(maleic anhydride-alt-1-decene)-dimethylamino propylamine- Protamine/siRNA | Core-shell | 30.5 ± 2 | ~30 | Not reported | ≥90% (up to 65 nM NPs) a | Not specified | MCF-7, U251 | [142] |
| 26.4 ± 3 (siRNA) | ||||||||
| Anionic | ||||||||
| PEG-b-poly(e-caprolactone)-g-poly(acrylic acid) | Core-shell | −29 ± 1.9 | 208.5 ± 4.6 | Not reported | ~100% (up to 500 µg/mL NPs) a | Clathrin-dependent | CRL-5802 | [143] |
| DNA-PEG | Core-shell | −25.2 ± 0.8 | 55.8 ± 7.7 | 74.7 ± 4.4 | ≥80% (up to 100 µg/mL NPs) a | Clathrin- and caveolin-independent Phagocytosis Clathrin-dependent Macropinocytosis SR-A involved | RAW 264.7 | [144] |
| Carboxy-dextran | Core-shell | ~−8.02 | ~60.32 | Not reported | ≥90% (up to 100 µg/mL Fe) a | Clathrin-dependent Macropinocytosis SR-A involved | Human macrophages | [145] |
| Carboxymethyl- dextran | Core-shell | ~−48 | 45 ± 7 | Not reported | Not reported | Macropinocytosis Caveolin-dependent Clathrin-dependent | Caco2 | [146] |
| Dextran sulfate | Core-shell | ~−45 | ~60 | Not reported | ≥90% (up to 5 mM NPs) | Not specified SR-A involved | BV2 | [147] |
| Silica | Core-shell | ~−59 | ~17 | ~136 | Non-significant cytotoxicity (50 µg/mL Fe) | Caveolin-dependent | HeLa | [148] |
| PEG-silane | Core-shell | ~−14 | ~30 | ~157 | Non-significant cytotoxicity (50 µg/mL Fe) | Caveolin-dependent Clathrin- and caveolin-independent Macropinocytosis | HeLa | [148] |
| Carboxilic acid-silane | Core-shell | ~47 | ~30 | ~133 | Non-significant cytotoxicity (50 µg/mL Fe) | Caveolin-dependent | HeLa | [148] |
| Dimercapto-succinate (DMSA) | Core-shell | −49 ± 2 −9 ± 1 (serum) | 65 ± 4 | 128 ± 54 | Not reported | Clathrin-dependent Macropinocytosis | Rat microglial cells | [149] |
| Dimercapto-succinate (DMSA) | Core-shell | −44 ± 14 −14 ± 5 (serum) | 50 ± 2 | 116 ± 13 | ≥90% up to 2 mM NPs (6 hrs) b | Clathrin-dependent | Cerebellar granule neurons | [150] |
| Dimercapto-succinate (DMSA) | Core-shell | Not reported | ~10 | Not reported | ~100% (up to 50 µg/mL NPs) a | Clathrin-dependent Caveolin-dependent Macropinocytosis SR-A involved | RAW 264.7 | [151] |
| Glucose | Core-shell | ~−40 | 40–45 (PBS) | Not reported | ≥90% (up to 100 µg/mL NPs) b | Caveolae-dependent | Vero cells | [152] |
| N-(trimethoxysilyl propyl) ethylenediamine triacetate | Core-shell | −39 ± 3 | ~8 | Not reported | Not reported | Caveolae-dependent | Mouse BMECs | [153] |
| None | - | ~−35 | 20–200 | Not reported | ≥90% (up to 50 µg/mL NPs) a | Clathrin-dependent | Caco2 | [154] |
| MamC-DOXO | Core-shell | 9.6 ± 1 | 36 ± 12 | 11–300 nm | ≥90% up to 30 µg/mL NPs (w/o DOXO) a | Not specified | HUVECs, KBV1, HeLa | [155] |
| −7 ± 0.3 (serum) | ≤50% for more than 10 µg/mL NPs (DOXO) a | |||||||
| Rhodium citrate | Core-shell | −35 ± 6 | 120 ± 1 | Not reported | Not reported | Clathrin-dependent | MDA-MD231, MCF7 | [156] |
| Citrate | Core-shell | Negative (not specified) | 8.7–11 | Not reported | Not reported | Clathrin-dependent Caveolin-dependent | HUVECs | [157] |
| Target | Main Endocytic Mechanism(s) | Targeting Agent | Coating | Target Cells | Application | Ref |
|---|---|---|---|---|---|---|
| LOX-1 receptor | Clathrin- and caveolin-independent [212] | LOX-1 antibody | Poly(ethylenglycol) (PEG) | Activated foam macrophages | Imaging probe for detecting early diabetic nephropathy (DN) | [213] |
| OxLDL | anti-OxLDL-PEG | Activated foam macrophages | Imaging of atheroschlerotic plaque lesions | [214] | ||
| Transferrin receptor (TFR) | Clathrin-dependent | Transferrin | Dimercaptosuccinic acid (DMSA) | C6 | Imaging probe for glioma | [184] |
| Ammoniated glucose-oligosaccharides-FITC | 4T1 | Not specified | [185,186] | |||
| Chitosan/Doxorubicin (DOX) | U251 | Drug delivery | [187] | |||
| Dextran-spermine | BBB (in vivo) | Drug delivery in vivo | [215] | |||
| Poly-L-lisine | HeLa | Not specified | [216] | |||
| RI7217 monoclonal antibody | DSPE-PEG-Muscone/Cholesterol/EPC liposomes | BBB and U87-MG in vivo (Mice) | Drug delivery in vivo | [217] | ||
| OX26 monoclonal antibody | Soy PC/DDAB/mPEG2000-PE liposomes | Rat BCECs in vitro and rat BBB in vivo | Targeted delivery to the brain | [218] | ||
| EGF receptor | Clathrin-dependent, Caveolin-dependent, Clathrin-and caveolin-independent | EGF | Amino-dextran | C6 | Cancer imaging probe | [188] |
| Carboxymethyldextran (CMD) | Caco-2 | Not specified | [219] | |||
| Nibotuzumab | Silica | A431 | Not specified | [191] | ||
| Cetuximab | PEG-dextran | A431 | Imaging probe | [190] | ||
| Short-chain EGFR antibody fragments (ScFv) | Poly(ethylene oxide)-poly(γ-methacryloxypropyl trimethoxysilane) | SK-BR-3 & MDA-MB-231 | Imaging probe | [192] | ||
| VEGF receptor | Clathrin-dependent, Caveolin-dependent | Bevacizumab | PEO-b-PγMPS-NIR830 | 4T1 | Imaging probe | [193] |
| Anti-VEGF | Poly(aspartate)-g-poly(ethylene glycol)-dodecylamine-hydrazone-(adriamycin-levulinic acid) micelles | HepG2 | Imaging probe | [194] | ||
| Human epidermal receptor 2 (HER-2) | Clathrin-dependent | Trastuzumab | PEG-SH | SK-BR-3 | Drug delivery | [195] |
| Anti-HER2 affibody | Polybutylacrylate-polyethylacrylate-polymethacrylic acid-NIR830 | SKOV3 | Imaging probe | [196] | ||
| Folate receptor | Clathrin- and caveolin-independent | Folate | PEG | U87-MG | Chemotherapy and hyperthermia | [200] |
| No additional coating | 22Rv.1, LnCaP | Imaging probe and hyperthermia treatments | [220] | |||
| Polyethilenimine (PEI) | KB | Imaging probe | [201] | |||
| PEG-poly(e-caprolactone) | BEL-7402 | Tumor imaging | [221] | |||
| LRP1 | Clathrin-dependent | Lactoferrin | Poly(maleic anhydride-alt-1-octadecene) (PMAO) | C6 | Imaging of brain glioma | [222] |
| Angiopep-2 | Pluronic-poly(acyrlic acid) (PF12-PAA) | BMECs | Delivery to the brain | [223] | ||
| CD44 | Clathrin- and caveolin-independent Clathrin-dependent [199] | Hyaluronic acid | Hyaluronic acid-C16 | MDA-MB-231, NIH/3T3 | Cancer imaging and therapy | [202] |
| Anti-CD44 | DMSA | Panc-1, MBA-MB-231 | Cancer therapy | [205] | ||
| CMD | HNSCC | Cancer hyperthermia | [203] | |||
| IGF1 receptor | Clathrin-dependent, Caveolin-dependent | IGF1 | Amphiphilic polymer | MIAPaCa-2 | Drug delivery in vivo | [224] |
| Anti-insulin-like-growth-factor binding protein 7 (anti-IGFBP7) | Dextran-Cy5.5 | BBB and U87 MG in vivo | Imaging probe | [225] | ||
| uMUC-1 | Clathrin-dependent | EPPT1 | Streptavidin-conjugated dextran | 6606PDA (Mouse) | Cancer theranostic platform | [207] |
| Membrane-bound matrix metalloproteinase (MMP-2) | Clathrin-dependent, Caveolin-dependent [226] | Chlorotoxin | PEG-g-chitosan/PEI | C6 | Imaging probe and siRNA delivery to cancer cells | [136] |
| Carbonic anhydrase IX (CA-IX) | Caveolin-dependent [227] | M75 monoclonal antibody | Poly-L-lysine (PLL) | CA-IX cDNA-transfected C33a cells | Targeting of hypoxic cells (Cancer) | [208] |
| CD22 | Clathrin-dependent [228] | Anti-CD22 | Amphiphilic polymer/PEI | preB-ALL | Cancer therapy for preB-ALL cells | [209] |
| Cholecytoskinin-2 receptor (CCK2R) | Clathrin-dependent | CCK | DY647-PEG | HEK293 stably expressing CCK2R | Cancer therapy | [229] |
| αvβ3 integrin | Clathrin-dependent, Caveolin-dependent, Clathrin- and caveolin-independent [230] | RGD peptide | PEG | U87 MG | Imaging probe and drug delivery in vivo | [210] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueda-Gensini, L.; Cifuentes, J.; Castellanos, M.C.; Puentes, P.R.; Serna, J.A.; Muñoz-Camargo, C.; Cruz, J.C. Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape. Nanomaterials 2020, 10, 1816. https://doi.org/10.3390/nano10091816
Rueda-Gensini L, Cifuentes J, Castellanos MC, Puentes PR, Serna JA, Muñoz-Camargo C, Cruz JC. Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape. Nanomaterials. 2020; 10(9):1816. https://doi.org/10.3390/nano10091816
Chicago/Turabian StyleRueda-Gensini, Laura, Javier Cifuentes, Maria Claudia Castellanos, Paola Ruiz Puentes, Julian A. Serna, Carolina Muñoz-Camargo, and Juan C. Cruz. 2020. "Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape" Nanomaterials 10, no. 9: 1816. https://doi.org/10.3390/nano10091816
APA StyleRueda-Gensini, L., Cifuentes, J., Castellanos, M. C., Puentes, P. R., Serna, J. A., Muñoz-Camargo, C., & Cruz, J. C. (2020). Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape. Nanomaterials, 10(9), 1816. https://doi.org/10.3390/nano10091816








