Spherical Bi2WO6/Bi2S3/MoS2 n-p Heterojunction with Excellent Visible-Light Photocatalytic Reduction Cr(VI) Activity
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
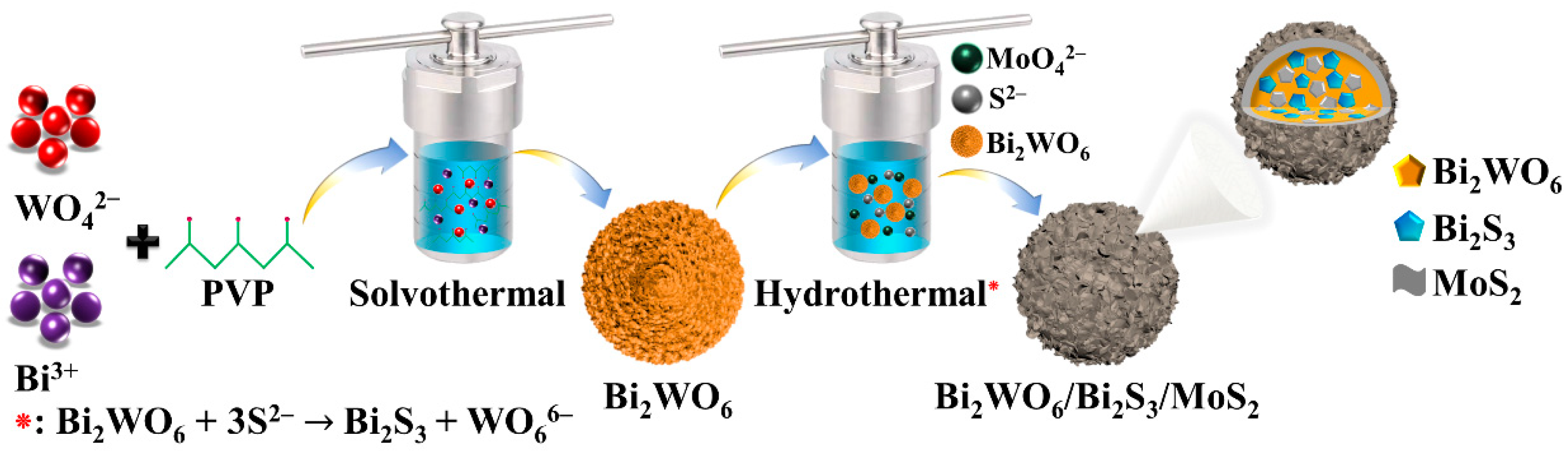
2.2. Synthesis of Spherical Bi2WO6 Nanostructures
2.3. Synthesis of Bi2WO6/Bi2S3/MoS2 n-p Heterojunction Photocatalyst
2.4. Characterization
2.5. Photocatalytic Activity Experiments
3. Results and Discussion
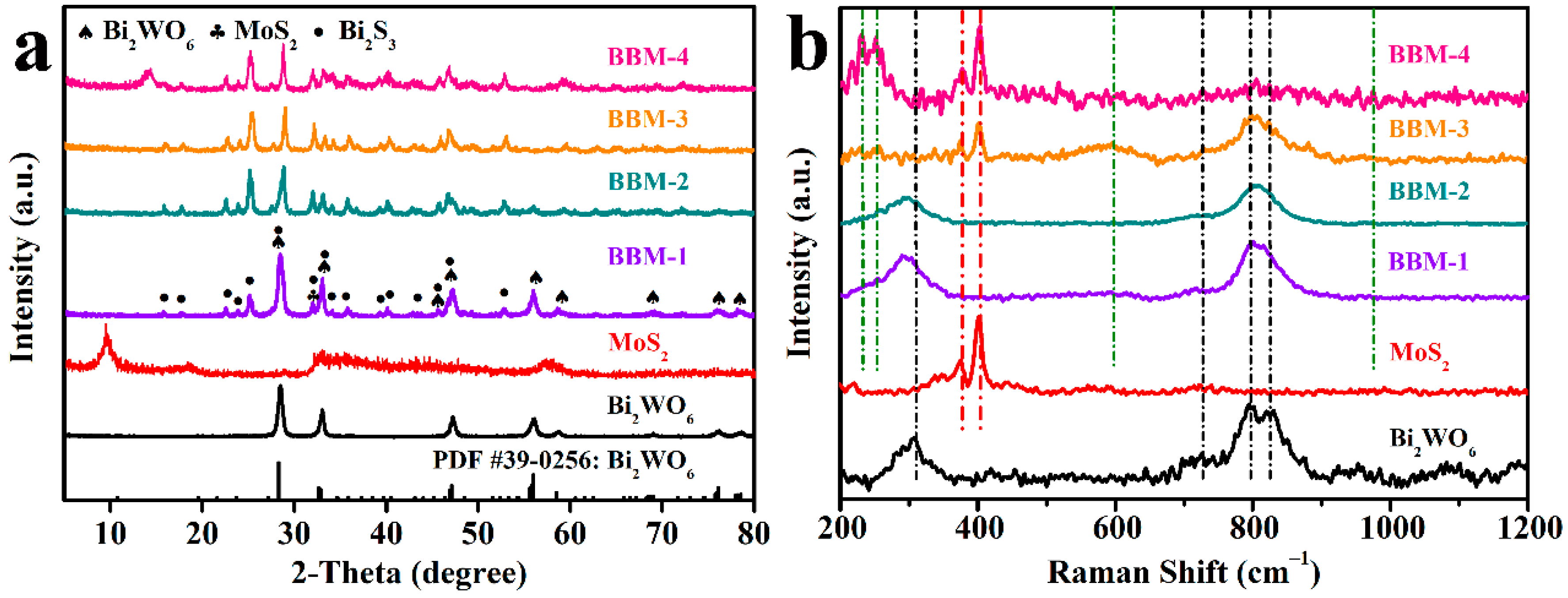
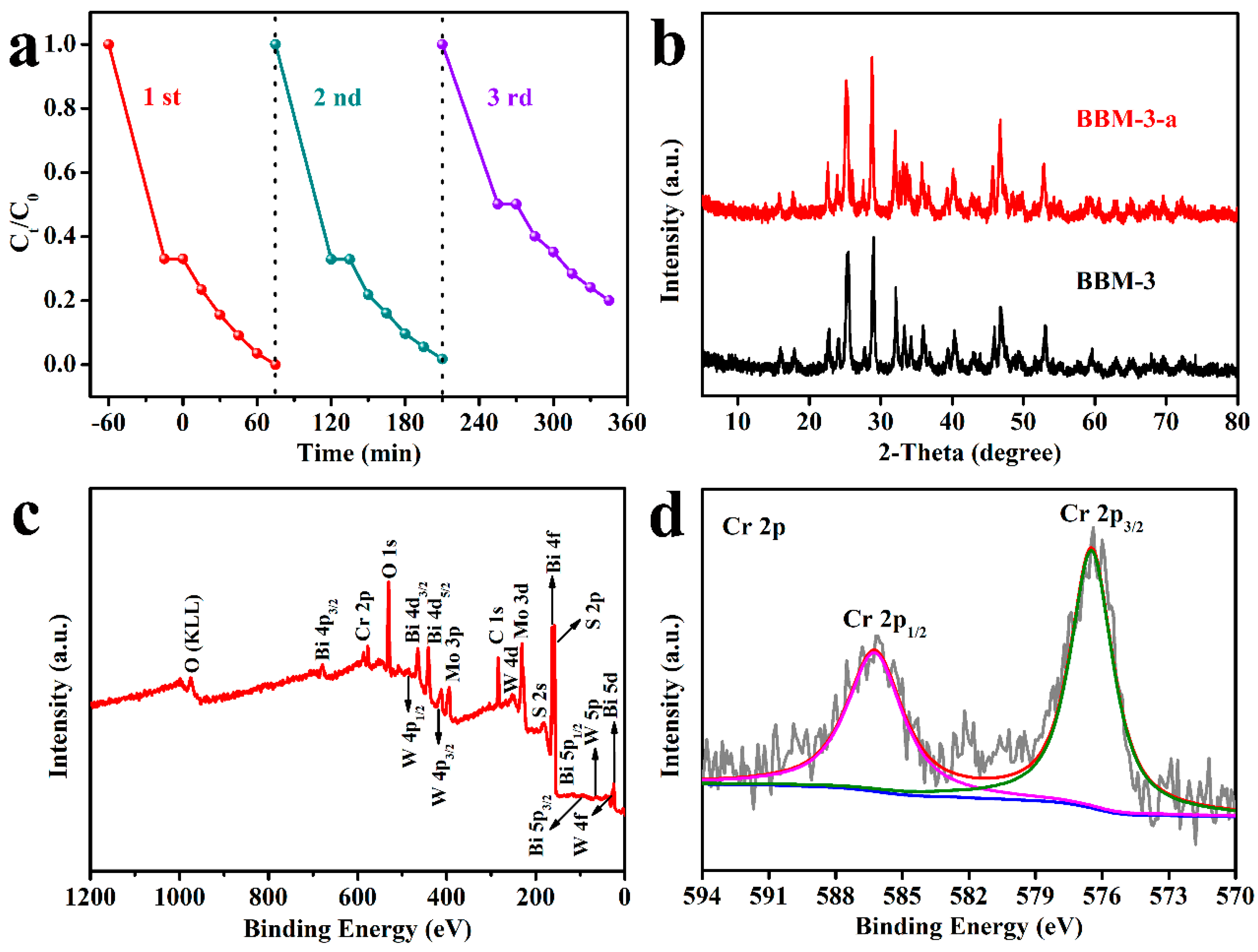
3.1. X-ray Diffraction (XRD) and Raman Analysis
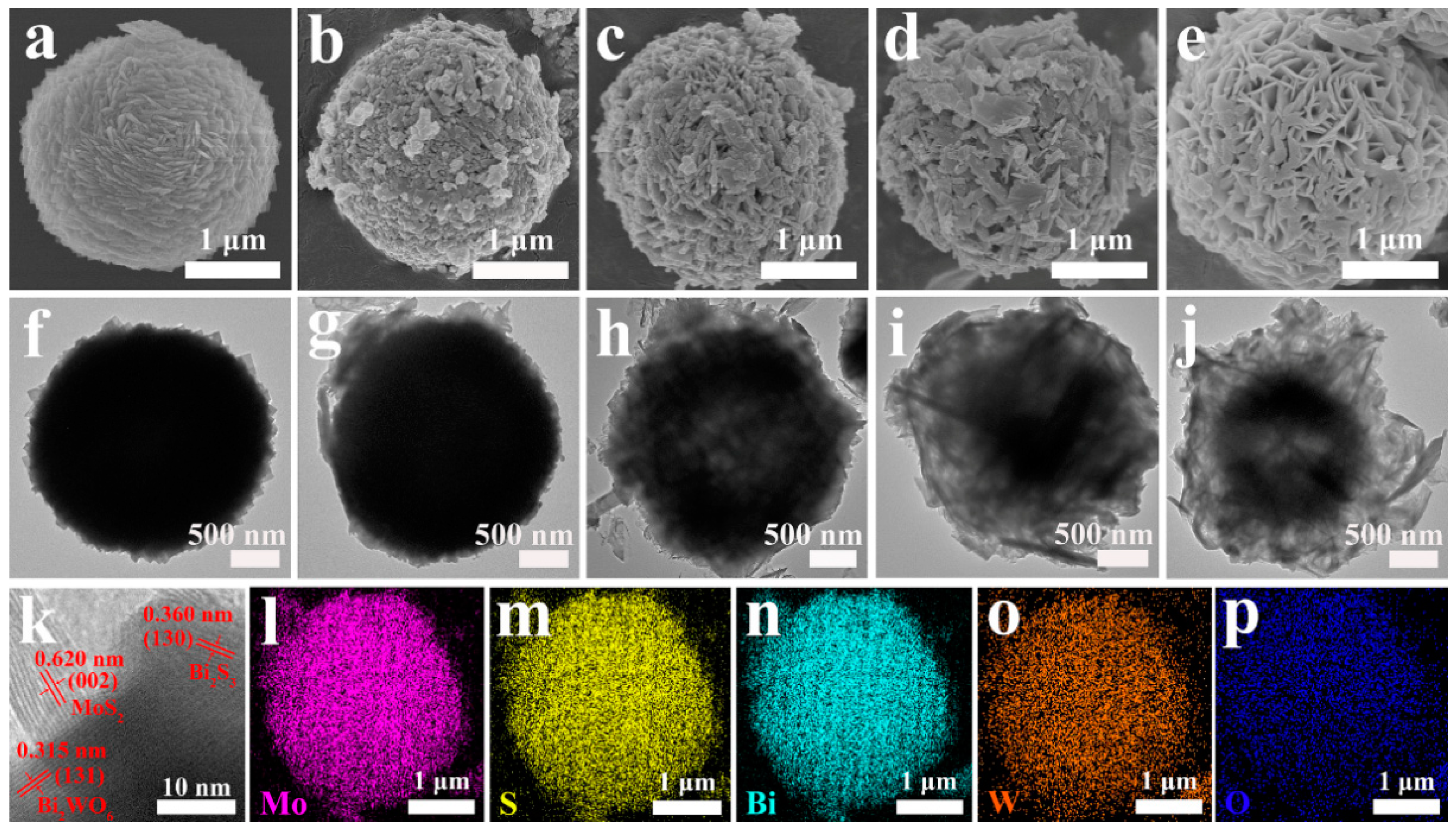
3.2. Morphology
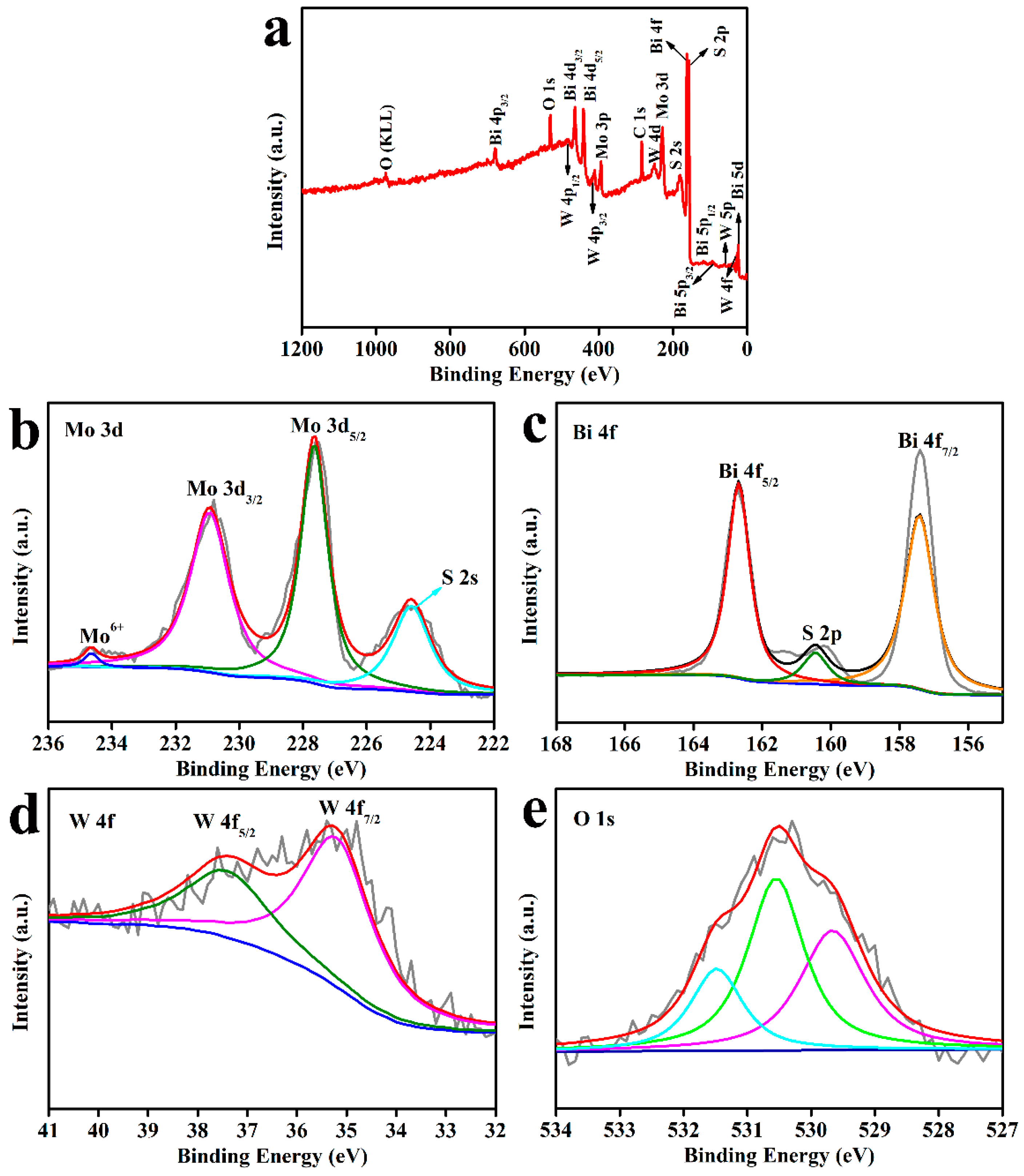
3.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.4. Brunauer–Emmett–Teller (BET) Specific Surface Area Analysis
3.5. Ultraviolet–Visible (UV–Vis) Absorption and Band Gap Positions
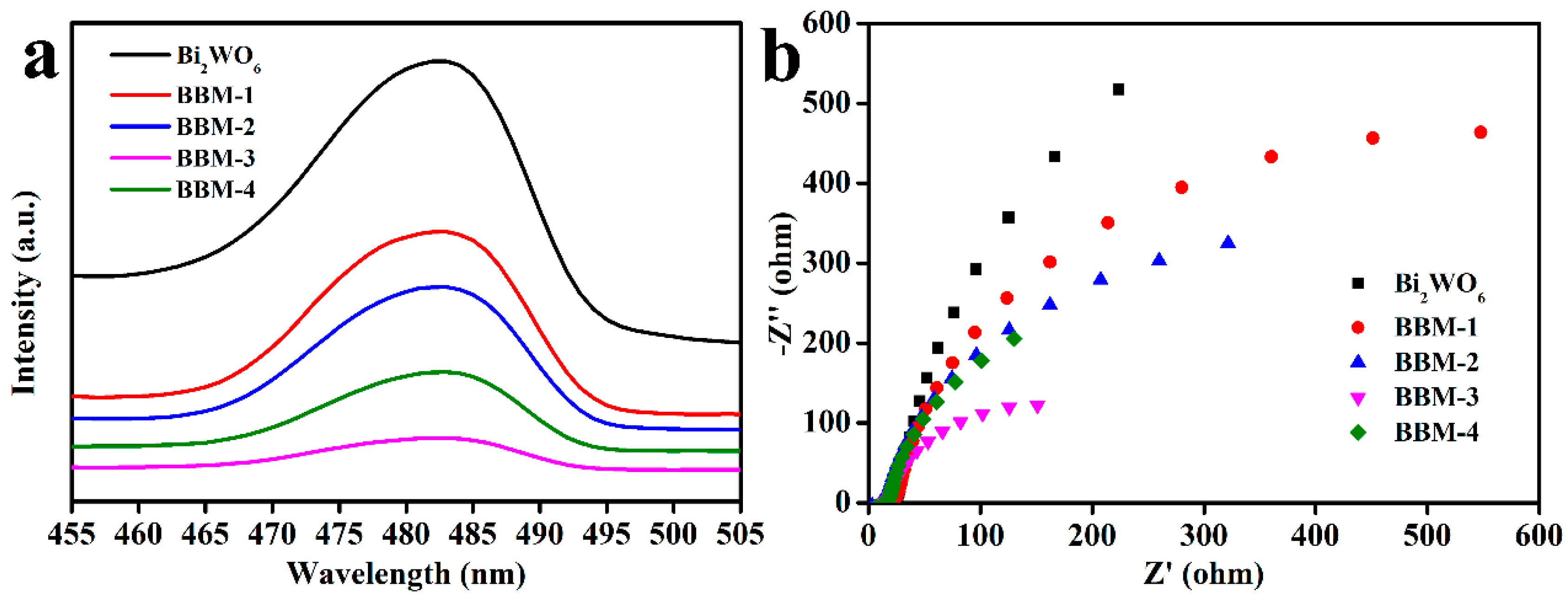
3.6. Photoelectrochemical Performance
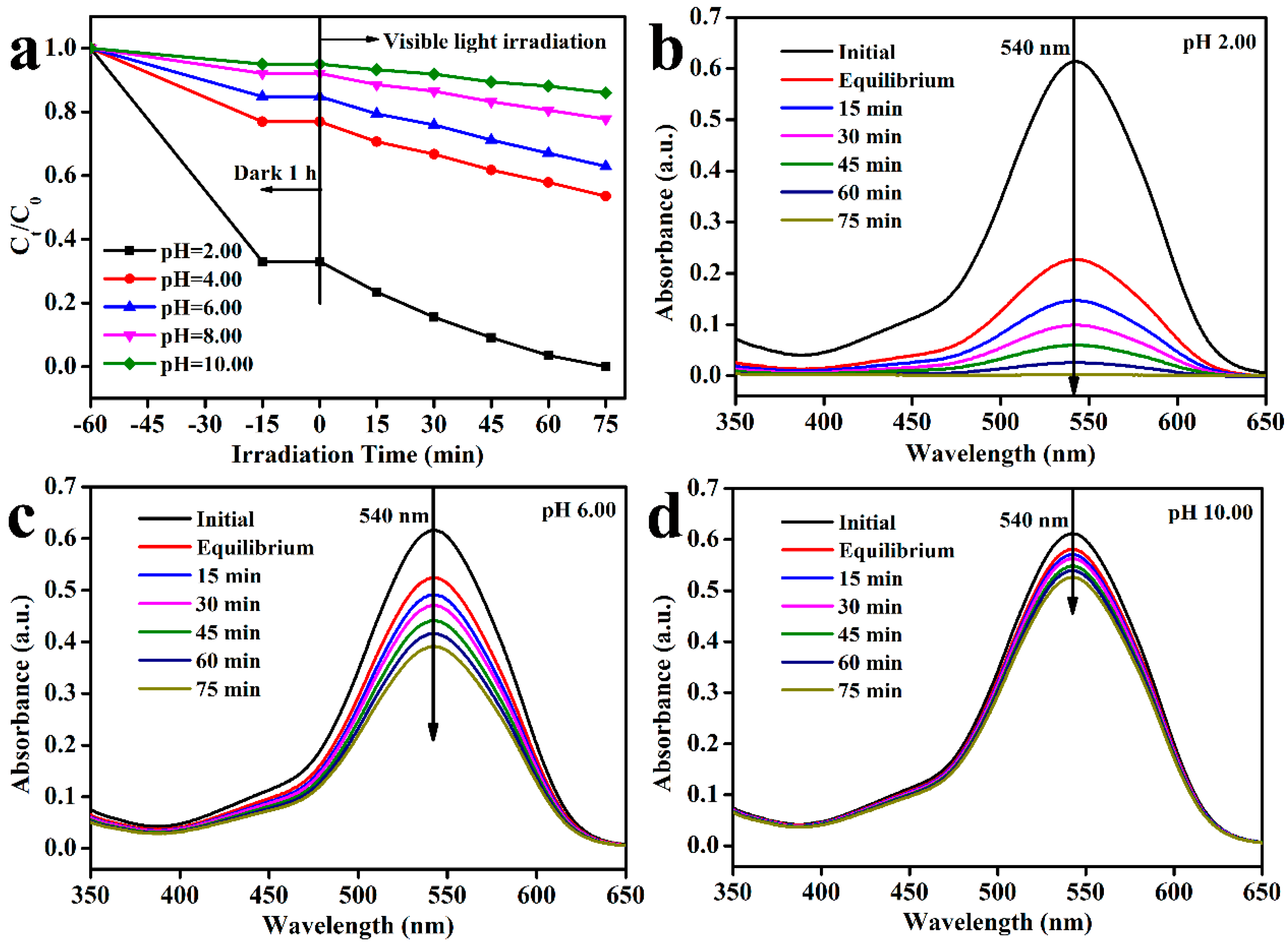
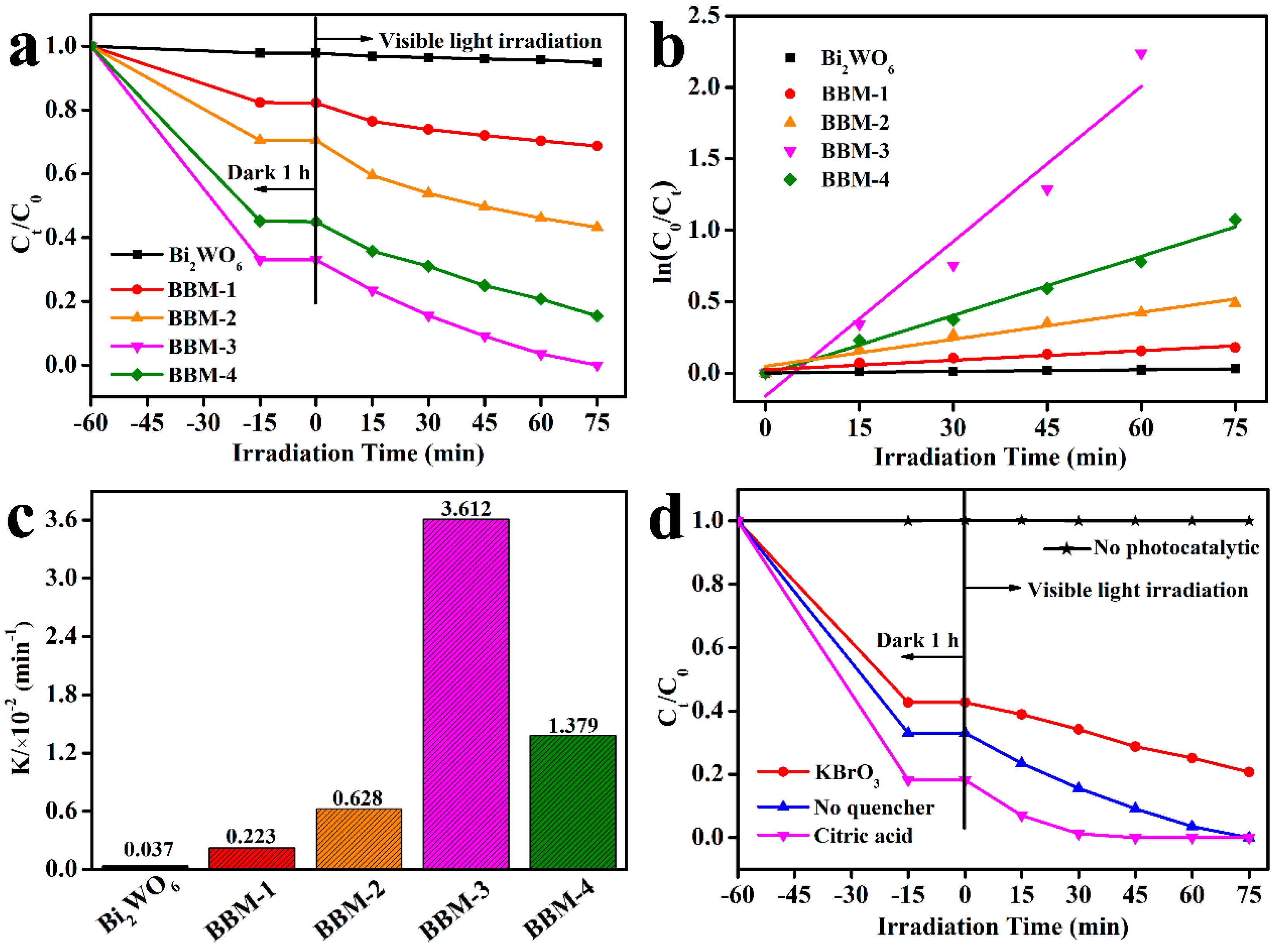
3.7. Photocatalytic Activity
3.8. Possible Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, C.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. ZnIn2S4 grown on nitrogen-doped hollow carbon spheres: An advanced catalyst for Cr(VI) reduction. J. Hazard. Mater. 2020, 391, 122205. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.S.; Zhu, X.F.; Zhu, B.C.; Jiang, C.J.; Le, Y.; Yu, J.G. Superb adsorption capacity of hierarchical calcined Ni/Mg/Al layered double hydroxides for Congo red and Cr(VI) ions. J. Hazard. Mater. 2017, 321, 801–811. [Google Scholar] [CrossRef]
- Anceschi, A.; Caldera, F.; Bertasa, M.; Cecone, C.; Trotta, F.; Bracco, P.; Zanetti, M.; Malandrino, M.; Mallon, P.E.; Scalarone, D. New poly(β-Cyclodextrin)/poly(vinyl alcohol) electrospun sub-micrometric fibers and their potential application for wastewater treatments. Nanomaterials 2020, 10, 482. [Google Scholar] [CrossRef]
- Gu, T.Y.; Dai, M.; Young, D.J.; Ren, Z.G.; Lang, J.P. Luminescent Zn(II) coordination polymers for highly selective sensing of Cr(III) and Cr(VI) in water. Inorg. Chem. 2017, 56, 4668–4678. [Google Scholar] [CrossRef]
- Li, M.Q.; Mu, Y.; Shang, H.; Mao, C.L.; Cao, S.Y.; Ai, Z.H.; Zhang, L.Z. Phosphate modification enables high efficiency and electron selectivity of nZVI toward Cr(VI) removal. Appl. Catal. B Environ. 2020, 263, 118364. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Lu, H.J.; Wang, J.K.; Hao, H.X.; Wang, T. Magnetically separable MoS2/Fe3O4/nZVI nanocomposites for the treatment of wastewater containing Cr(VI) and 4-Chlorophenol. Nanomaterials 2017, 7, 303. [Google Scholar] [CrossRef]
- Dong, R.F.; Zhong, Y.L.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Morphology-controlled fabrication of CNT@MoS2/SnS2 nanotubes for promoting photocatalytic reduction of aqueous Cr(VI) under visible light. J. Alloys Compd. 2019, 784, 282–292. [Google Scholar] [CrossRef]
- Xu, Y.L.; Chen, J.Y.; Chen, R.; Yu, P.L.; Guo, S.; Wang, X.F. Adsorption and reduction of chromium(VI) from aqueous solution using polypyrrole/calcium rectorite composite adsorbent. Water Res. 2019, 160, 148–157. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Cheng, B.; You, W.; Yu, J.G.; Ho, W.K. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr(VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef]
- Zheng, X.G.; Chen, Q.; Lv, S.H.; Fu, X.J.; Wen, J.; Liu, X.H. Enhanced visible-light photocatalytic activity of Ag QDs anchored on CeO2 nanosheets with a carbon coating. Nanomaterials 2019, 9, 1643. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.H.; Zhang, M.Z.; Tan, W.F.; Qiu, G.H.; Zheng, L.R. Improved removal capacity of magnetite for Cr(VI) by electrochemical reduction. J. Hazard. Mater. 2019, 374, 26–34. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Bourceanu, G.; Olariu, R.I.; Arsene, C. A novel polymer inclusion membrane applied in chromium(VI) separation from aqueous solutions. J. Hazard. Mater. 2011, 197, 244–253. [Google Scholar] [CrossRef]
- Shao, Z.C.; Huang, C.; Wu, Q.; Zhao, Y.J.; Xu, W.J.; Liu, Y.Y.; Dang, J.; Hou, H.W. Ion exchange collaborating coordination substitution: More efficient Cr(VI) removal performance of a water-stable CuII-MOF material. J. Hazard. Mater. 2019, 378, 120719. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Zhou, C.; Jing, Q.Y.; Tang, Q.; Mu, Y.H.; Du, A.K. Facile synthesis of g-C3N4 nanosheets/ZnO nanocomposites with enhanced photocatalytic activity in reduction of aqueous chromium(VI) under visible light. Nanomaterials 2016, 6, 173. [Google Scholar] [CrossRef]
- Li, X.K.; Li, C.X.; Xiang, D.; Zhang, C.M.; Xia, L.; Liu, X.Y.; Zheng, F.Q.; Xie, X.Y.; Zhang, Y.L.; Chen, W. Self-limiting synthesis of Au–Pd core–shell nanocrystals with a near surface alloy and monolayer Pd shell structure and their superior catalytic activity on the conversion of hexavalent chromium. Appl. Catal. B Environ. 2019, 253, 263–270. [Google Scholar] [CrossRef]
- Wang, Y.H.; Kang, C.L.; Xiao, K.K.; Wang, X.Y. Fabrication of Bi2S3/MOFs composites without noble metals for enhanced photoreduction of Cr(VI). Sep. Purif. Technol. 2020, 241, 116703. [Google Scholar] [CrossRef]
- Huang, H.W.; Cao, R.R.; Yu, S.X.; Xu, K.; Hao, W.C.; Wang, Y.G.; Dong, F.; Zhang, T.R.; Zhang, Y.H. Single-unit-cell layer established Bi2WO6 3D hierarchical architectures: Efficient adsorption, photocatalysis and dye-sensitized photoelectrochemical performance. Appl. Catal. B Environ. 2017, 219, 526–537. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Jiang, W.J.; Yao, W.Q.; Yang, H.P.; Zhu, Y.F. Self-assembled perylene diimide based supramolecular heterojunction with Bi2WO6 for efficient visible-light-driven photocatalysis. Appl. Catal. B Environ. 2018, 232, 175–181. [Google Scholar] [CrossRef]
- Ma, Y.C.; Lv, C.; Hou, J.H.; Yuan, S.T.; Wang, Y.R.; Xu, P.; Gao, G.; Shi, J.S. 3D hollow hierarchical structures based on 1D BiOCl nanorods intersected with 2D Bi2WO6 nanosheets for efficient photocatalysis under visible light. Nanomaterials 2019, 9, 322. [Google Scholar] [CrossRef]
- Cao, R.R.; Huang, H.W.; Tian, N.; Zhang, Y.H.; Guo, Y.X.; Zhang, T.R. Novel Y doped Bi2WO6 photocatalyst: Hydrothermal fabrication, characterization and enhanced visible-light-driven photocatalytic activity for Rhodamine B degradation and photocurrent generatio. Mater. Charact. 2015, 101, 166–172. [Google Scholar] [CrossRef]
- Wan, J.; Du, X.; Wang, R.M.; Liu, E.Z.; Jia, J.; Bai, X.; Hu, X.Y.; Fan, J. Mesoporous nanoplate multi-directional assembled Bi2WO6 for high efficient photocatalytic oxidation of NO. Chemosphere 2018, 193, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, Y.; Wang, R.M.; Liu, L.; Liu, E.Z.; Fan, J.; Fu, F. Effective charge kinetics steering in surface plasmons coupled two-dimensional chemical Au/Bi2WO6-MoS2 heterojunction for superior photocatalytic detoxification performance. J. Hazard. Mater. 2020, 384, 121484. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.H.; Gao, T.T.; Hu, T.T.; Zhou, G.W. Synthesis and electrochemical energy storage applications of Micro/Nanostructured spherical materials. Nanomaterials 2019, 9, 1207. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Selvaraj, S.; Kim, D.H. Construction of heterojunction photoelectrode via atomic layer deposition of Fe2O3 on Bi2WO6 for highly efficient photoelectrochemical sensing and degradation of tetracycline. Appl. Catal. B Environ. 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Liu, X.T.; Gu, S.N.; Zhao, Y.J.; Zhou, G.W.; Li, W.J. BiVO4, Bi2WO6, Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Xu, Q.L.; Zhang, L.Y.; Cheng, B.; Fan, J.J.; Yu, J.G. S-Scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Xie, T.P.; Liu, Y.; Wang, H.Q.; Wu, Z.B. Layered MoSe2/Bi2WO6 composite with P-N heterojunctions as a promising visible-light induced photocatalyst. Appl. Surf. Sci. 2018, 444, 320–329. [Google Scholar] [CrossRef]
- Jo, W.K.; Kumar, S.; Eslava, S.; Tonda, S. Construction of Bi2WO6/RGO/g-C3N4 2D/2D/2D hybrid Z-scheme heterojunctions with large interfacial contact area for efficient charge separation and high-performance photoreduction of CO2 and H2O into solar fuels. Appl. Catal. B Environ. 2018, 239, 586–598. [Google Scholar] [CrossRef]
- Meng, X.C.; Li, Z.Z.; Zeng, H.M.; Chen, J.; Zhang, Z.S. MoS2 quantum dots-interspersed Bi2WO6 heterostructures for visible light-induced detoxification and disinfection. Appl. Catal. B Environ. 2017, 210, 160–172. [Google Scholar] [CrossRef]
- Adhikari, S.; Kim, D.H. Synthesis of Bi2S3/Bi2WO6 hierarchical microstructures for enhanced visible light driven photocatalytic degradation and photoelectrochemical sensing of ofloxacin. Chem. Eng. J. 2018, 354, 692–705. [Google Scholar] [CrossRef]
- Pan, J.B.; Liu, J.J.; Ma, H.C.; Zuo, S.L.; Khan, U.A.; Yu, Y.C.; Li, B.S. Structure of flower-like hierarchical CdS QDs/Bi/Bi2WO6 heterojunction with enhanced photocatalytic activity. New J. Chem. 2018, 42, 7293–7300. [Google Scholar] [CrossRef]
- Hu, K.; Chen, C.Y.; Zhu, Y.; Zeng, G.M.; Huang, B.B.; Chen, W.Q.; Liu, S.H.; Lei, C.; Li, B.S.; Yang, Y. Ternary Z-scheme heterojunction of Bi2WO6 with reduced graphene oxide (rGO) and meso-tetra (4-carboxyphenyl) porphyrin (TCPP) for enhanced visible-light photocatalysis. J. Colloid Interface Sci. 2019, 540, 115–125. [Google Scholar] [CrossRef]
- Wan, J.; Xue, P.; Wang, R.M.; Liu, L.; Liu, E.Z.; Bai, X.; Fan, J.; Hu, X.Y. Synergistic effects in simultaneous photocatalytic removal of Cr(VI) and tetracycline hydrochloride by Z-scheme Co3O4/Ag/Bi2WO6 heterojunction. Appl. Surf. Sci. 2019, 483, 677–687. [Google Scholar] [CrossRef]
- Huang, D.L.; Li, J.; Zeng, G.M.; Xue, W.J.; Chen, S.; Li, Z.H.; Deng, R.; Yang, Y.; Cheng, M. Facile construction of hierarchical flower-like Z-scheme AgBr/Bi2WO6 photocatalysts for effective removal of tetracycline: Degradation pathways and mechanism. Chem. Eng. J. 2019, 375, 121991. [Google Scholar] [CrossRef]
- Xue, W.J.; Huang, D.L.; Li, J.; Zeng, G.M.; Deng, R.; Yang, Y.; Chen, S.; Li, Z.H.; Gong, X.M.; Li, B. Assembly of AgI nanoparticles and ultrathin g-C3N4 nanosheets codecorated Bi2WO6 direct dual Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced photocatalytic performance. Chem. Eng. J. 2019, 373, 1144–1157. [Google Scholar] [CrossRef]
- Long, L.L.; Chen, J.J.; Zhang, X.; Zhang, A.Y.; Huang, Y.X.; Rong, Q.; Yu, H.Q. Layer-controlled growth of MoS2 on self-assembled flower-like Bi2S3 for enhanced photocatalysis under visible light irradiation. NPG Asia Mater. 2016, 8, e263. [Google Scholar] [CrossRef]
- Jonjana, S.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Synthesis, characterization and photocatalysis of heterostructure AgBr/Bi2WO6 nanocomposites. Mater. Lett. 2018, 216, 92–96. [Google Scholar] [CrossRef]
- Babu, B.; Koutavarapu, R.; Shim, J.; Yoo, K. Enhanced solar light-driven photocatalytic degradation of tetracycline and organic pollutants by novel one-dimensional ZnWO4 nanorod-decorated two-dimensional Bi2WO6 nanoflakes. J. Taiwan Inst. Chem. E 2020, 110, 58–70. [Google Scholar] [CrossRef]
- Xie, J.F.; Zhang, J.J.; Li, S.; Grote, F.; Zhang, X.D.; Zhang, H.; Wang, R.X.; Lei, Y.; Pan, B.C.; Xie, Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Chen, D.D.; Fang, J.Z.; Lu, S.Y.; Zhou, G.Y.; Feng, W.H.; Yang, F.; Chen, Y.; Fang, Z.Q. Fabrication of Bi modified Bi2S3 pillared g-C3N4 photocatalyst and its efficient photocatalytic reduction and oxidation performances. Appl. Surf. Sci. 2017, 426, 427–436. [Google Scholar] [CrossRef]
- Ke, J.; Liu, J.; Sun, H.Q.; Zhang, H.Y.; Duan, X.G.; Liang, P.; Li, X.Y.; Tade, M.O.; Liu, S.M.; Wang, S.B. Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3 and p-MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl. Catal. B Environ. 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Zhou, W.J.; Yin, Z.Y.; Du, Y.P.; Huang, X.; Zeng, Z.Y.; Fan, Z.X.; Liu, H.; Wang, J.Y.; Zhang, H. Synthesis of Few-Layer MoS2 Nanosheet-Coated TiO2 Nanobelt Heterostructures for Enhanced Photocatalytic Activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Wang, Y.J.; Ling, Q.; Zhu, Y.F. Enhancement of full-spectrum photocatalytic activity over BiPO4/Bi2WO6 composites. Appl. Catal. B Environ. 2017, 200, 222–229. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, G.F.; Li, H.L.; Zhang, F.F.; Jiang, H.Y.; Tian, G.H. Controlled synthesis and exceptional photoelectrocatalytic properties of Bi2S3/MoS2/Bi2MoO6 ternary hetero-structured porous film. J. Colloid Interface Sci. 2019, 555, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Xia, X.; Lv, P.F.; Zhang, J.; Pang, Z.Y.; Li, D.W.; Cai, Y.B.; Wei, Q.F. Wintersweet Branch-Like C/C@SnO2/MoS2 Nanofibers as High-Performance Li and Na-Ion Battery Anodes. Part. Part. Syst. Charact. 2017, 34, 1700295. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Lv, P.F.; Zhang, W.; Meng, X.D.; He, H.; Zeng, X.H.; Shen, X.S. Pristine Bi2WO6 and hybrid Au-Bi2WO6 hollow microspheres with excellent photocatalytic activities. Appl. Surf. Sci. 2018, 457, 925–932. [Google Scholar] [CrossRef]
- Shao, B.B.; Liu, X.J.; Liu, Z.F.; Zeng, G.M.; Liang, Q.H.; Liang, C.; Cheng, Y.; Zhang, W.; Liu, Y.; Gong, S.X. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2019, 368, 730–745. [Google Scholar] [CrossRef]
- Song, S.S.; Wang, J.M.; Peng, T.Y.; Fu, W.L.; Zan, L. MoS2-MoO3−x hybrid cocatalyst for effectively enhanced H2 production photoactivity of AgIn5S8 nano-octahedrons. Appl. Catal. B Environ. 2018, 228, 39–46. [Google Scholar] [CrossRef]
- Pan, Q.C.; Zhang, Q.B.; Zheng, F.H.; Liu, Y.Z.; Li, Y.P.; Ou, X.; Xiong, X.H.; Yang, C.H.; Liu, M.L. Construction of MoS2/C hierarchical tubular heterostructures for high-performance sodium ion batteries. ACS Nano 2018, 12, 12578–12586. [Google Scholar] [CrossRef]
- Wang, J.Z.; Jin, J.; Wang, X.G.; Yang, S.N.; Zhao, Y.L.; Wu, Y.W.; Dong, S.Y.; Sun, J.Y.; Sun, J.H. Facile fabrication of novel BiVO4/Bi2S3/MoS2 n-p heterojunction with enhanced photocatalytic activities towards pollutant degradation under natural sunlight. J. Colloid Interface Sci. 2017, 505, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liang, X.H.; Ou, X.; Yang, X.F.; Li, Y.Z.; Yang, C.H.; Lin, Z.; Liu, M.L. Heterointerface engineering of hierarchical Bi2S3/MoS2 with self-generated rich phase boundaries for superior sodium storage performance. Adv. Funct. Mater. 2020, 30, 1910732. [Google Scholar] [CrossRef]
- Huang, H.W.; Zhou, C.; Jiao, X.C.; Yuan, H.F.; Zhao, J.W.; He, C.Q.; Hofkens, J.; Roeffaers, M.B.J.; Long, J.L.; Steele, J.A. Subsurface defect engineering in single-unit-cell Bi2WO6 monolayers boosts solar-driven photocatalytic performance. ACS Catal. 2020, 10, 1439–1443. [Google Scholar] [CrossRef]
- Qian, X.F.; Yue, D.T.; Tian, Z.Y.; Reng, M.; Zhu, Y.; Kan, M.; Zhang, T.Y.; Zhao, Y.X. Carbon quantum dots decorated Bi2WO6 nanocomposite with enhanced photocatalytic oxidation activity for VOCs. Appl. Catal. B Environ. 2016, 193, 16–21. [Google Scholar] [CrossRef]
- Song, N.N.; Zhang, M.H.; Zhou, H.; Li, C.Y.; Liu, G.; Zhong, S.; Zhang, S.Y. Synthesis and properties of Bi2WO6 coupled with SnO2 nano-microspheres for improved photocatalytic reduction of Cr6+ under visible light irradiation. Appl. Surf. Sci. 2019, 495, 143551. [Google Scholar] [CrossRef]
- Bai, X.; Du, Y.Y.; Hu, X.Y.; He, Y.D.; He, C.L.; Liu, E.Z.; Fan, J. Synergy removal of Cr(VI) and organic pollutants over RP-MoS2/rGO photocatalyst. Appl. Catal. B Environ. 2018, 239, 204–213. [Google Scholar] [CrossRef]
- Zheng, J.H.; Zhang, L. Incorporation of CoO nanoparticles in 3D marigold flower-like hierarchical architecture MnCo2O4 for highly boosting solar light photo-oxidation and reduction ability. Appl. Catal. B Environ. 2018, 237, 1–8. [Google Scholar] [CrossRef]
- Yang, L.J.; Hu, Y.D.; Zhang, L. Architecting Z-scheme Bi2S3@CoO with 3D chrysanthemums-like architecture for both photoeletro-oxidization and -reduction performance under visible light. Chem. Eng. J. 2019, 378, 122092. [Google Scholar] [CrossRef]
- Babu, B.; Koutavarapu, R.; Shim, J.; Yoo, K. Enhanced visible-light-driven photoelectrochemical and photocatalytic performance of Au-SnO2 quantum dot-anchored g-C3N4 nanosheets. Sep. Purif. Technol. 2020, 240, 116652. [Google Scholar] [CrossRef]
- Babu, B.; Koutavarapu, R.; Shim, J.; Yoo, K. Facile one-pot synthesis of gold/tin oxide quantum dots for visible light catalytic degradation of methylene blue: Optimization of plasmonic effect. J. Alloys Compd. 2020, 812, 152081. [Google Scholar]
- Zhang, G.P.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.C.; Zhou, C.Q.; Yang, Z.J.; Xue, H.G.; Dionysiou, D.D. A new high efficiency visible-light photocatalyst made of SnS2 and conjugated derivative of polyvinyl alcohol and its application to Cr(VI) reduction. Chem. Eng. J. 2017, 324, 140–153. [Google Scholar] [CrossRef]
- Wang, Y.J.; Bao, S.Y.; Liu, Y.Q.; Yang, W.W.; Yu, Y.S.; Feng, M.; Li, K.F. Efficient photocatalytic reduction of Cr(VI) in aqueous solution over CoS2/g-C3N4-rGO nanocomposites under visible light. Appl. Surf. Sci. 2020, 510, 145495. [Google Scholar] [CrossRef]
- Alam, U.; Khan, A.; Bahnemann, D.; Muneer, M. Synthesis of Co doped ZnWO4 for simultaneous oxidation of RhB and reduction of Cr(VI) under UV-light irradiation. J. Environ. Chem. Eng. 2018, 6, 4885–4898. [Google Scholar] [CrossRef]
- Patnaik, S.; Swain, G.; Parida, K.M. Highly efficient charge transfer through a double Z-scheme mechanism by a Cu-promoted MoO3/g-C3N4 hybrid nanocomposite with superior electrochemical and photocatalytic performance. Nanoscale 2018, 10, 5950–5964. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.X.; Yi, X.H.; Zhao, C.; Wang, P.; Deng, J.G.; Wang, C.C. Photocatalytic Cr(VI) elimination over BUC-21/N-K2Ti4O9 composites: Big differences in performance resulting from small differences in composition. Chin. J. Catal. 2021, 42, 259–270. [Google Scholar] [CrossRef]
- Ren, Z.X.; Liu, X.J.; Zhuge, Z.H.; Gong, Y.Y.; Sun, C.Q. MoSe2/ZnO/ZnSe hybrids for efficient Cr(VI) reduction under visible light irradiation. Chin. J. Catal. 2020, 41, 180–187. [Google Scholar] [CrossRef]
- Xia, Y.; Gang, R.Q.; Xu, L.; Huang, S.J.; Zhou, L.X.; Wang, J. Nanorod-pillared mesoporous rGO/ZnO/Au hybrids for photocatalytic Cr(VI) reduction: Enhanced Cr(VI) adsorption and solar energy harvest. Ceram. Int. 2020, 46, 1487–1493. [Google Scholar] [CrossRef]
- Yang, L.; Xu, C.; Wan, F.C.; He, H.H.; Gu, H.S.; Xiong, J. Synthesis of RGO/BiOI/ZnO composites with efficient photocatalytic reduction of aqueous Cr(VI) under visible-light irradiation. Mater. Res. Bull. 2019, 112, 154–158. [Google Scholar] [CrossRef]
- Xu, F.; Chen, H.M.; Xu, C.Y.; Wu, D.P.; Gao, Z.Y.; Zhang, Q.; Jiang, K. Ultra-thin Bi2WO6 porous nanosheets with high lattice coherence for enhanced performance for photocatalytic reduction of Cr(VI). J. Colloid Interface Sci. 2018, 525, 97–106. [Google Scholar] [CrossRef]
- Luo, S.; Ke, J.; Yuan, M.Q.; Zhang, Q.; Xie, P.; Deng, L.D.; Wang, S.B. CuInS quantum dots embedded in Bi2WO6 nanoflowers for enhanced visible light photocatalytic removal of contaminants. Appl. Catal. B Environ. 2018, 221, 215–222. [Google Scholar] [CrossRef]
- Zhang, C.M.; Chen, G.; Li, C.M.; Sun, J.X.; Lv, C.D.; Fan, S.; Xing, W.N. In situ fabrication of Bi2WO6/MoS2/RGO heterojunction with nanosized interfacial contact via confined space effect toward enhanced photocatalytic properties. ACS Sustain. Chem. Eng. 2016, 4, 5936–5942. [Google Scholar] [CrossRef]
- Jiang, Z.K.; Chen, K.X.; Zhang, Y.C.; Wang, Y.Y.; Wang, F.; Zhang, G.S.; Dionysioue, D.D. Magnetically recoverable MgFe2O4/conjugated polyvinyl chloride derivative nanocomposite with higher visible-light photocatalytic activity for treating Cr(VI)-polluted water. Sep. Purif. Technol. 2020, 236, 116272. [Google Scholar] [CrossRef]
- Hua, E.B.; Jin, S.; Wang, X.R.; Ni, S.; Liu, G.; Xu, X.X. Ultrathin 2D type-II p-n heterojunctions La2Ti2O7/In2S3 with efficient charge separations and photocatalytic hydrogen evolution under visible light illumination. Appl. Catal. B Environ. 2019, 245, 733–742. [Google Scholar] [CrossRef]
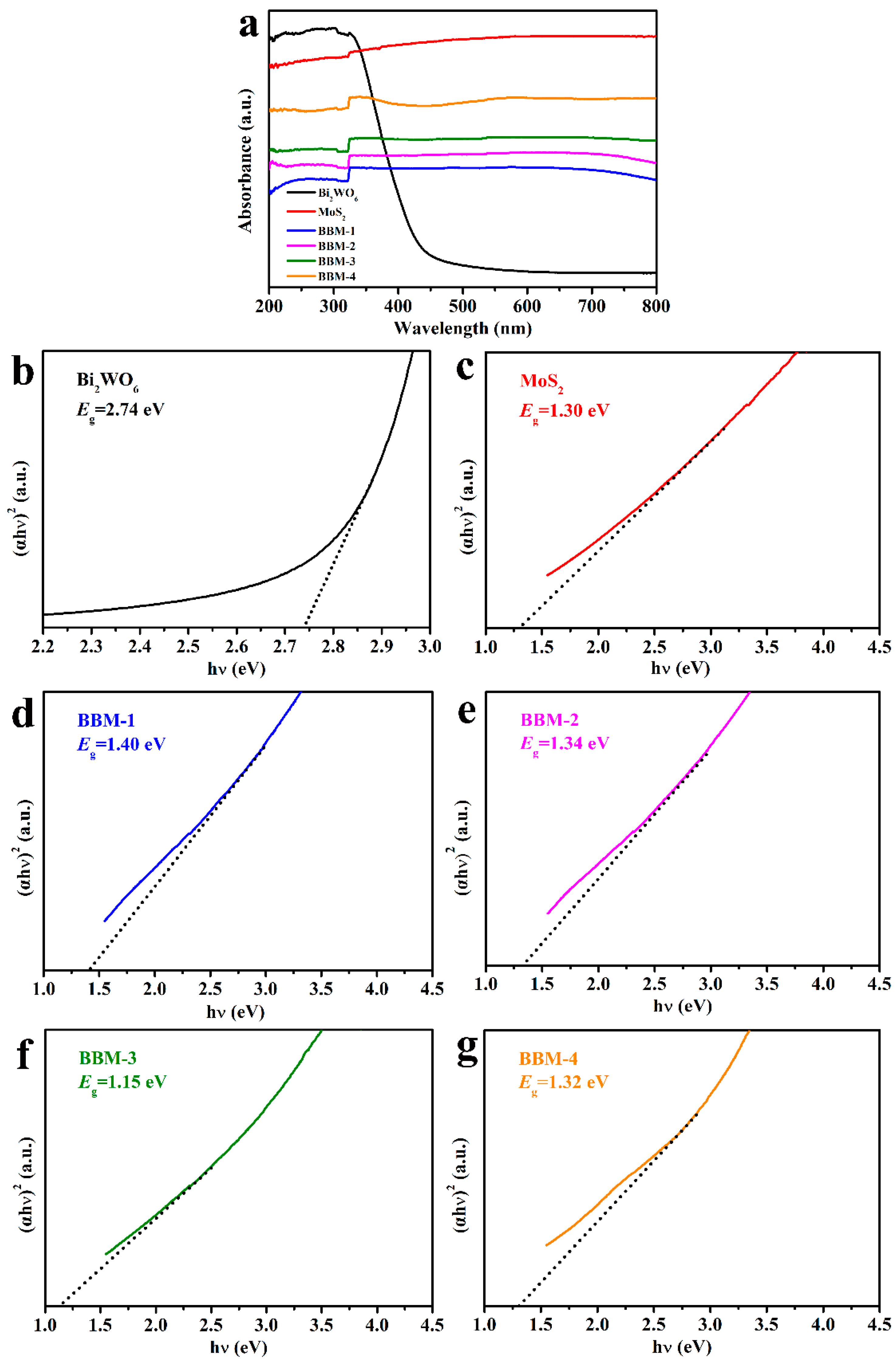
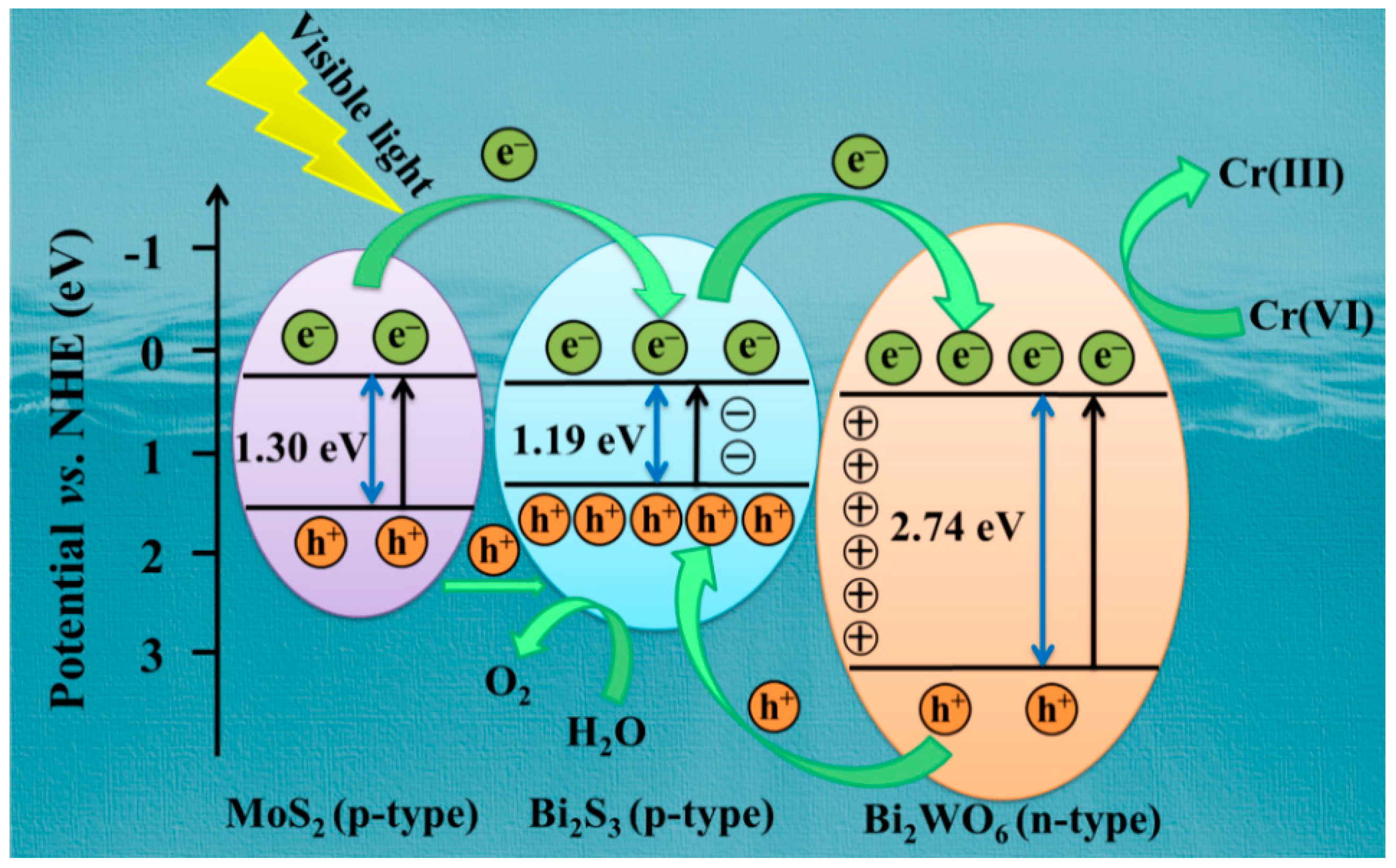
| Materials | Eg (eV) | X (eV) | Ee (eV) | ECB vs. NHE 1 (eV) | EVB vs. NHE 1 (eV) |
|---|---|---|---|---|---|
| Bi2WO6 | 2.74 | 6.20 | 4.50 | 0.33 | 3.07 |
| Bi2S3 | 1.19 [51] | 5.27 | 4.50 | 0.18 | 1.37 |
| MoS2 | 1.30 | 5.32 | 4.50 | 0.17 | 1.47 |
| Materials/Amount (mg) | Cr(VI) Solution Volume (mL)/Concentration (mg L−1) | Time (min) | Photocatalytic Removal Rate | Publication Date | Ref. |
|---|---|---|---|---|---|
| CoS2/g-C3N4-rGO/10 | 20/20 | 120 | 99.8 | 2020 | [63] |
| MoSe2/ZnO/ZnSe/80 | 80/20 | 180 | 100 | 2020 | [67] |
| rGO/ZnO/Au/50 | 50/10 | 40 | 97 | 2020 | [68] |
| Bi2MoO6/ZnO/100 | 50/50 | 150 | 100 | 2019 | [61] |
| RGO/BiOI/ZnO/100 | 150/10 | 180 | 92 | 2019 | [69] |
| Bi2WO6+Oxalic/60 | 50/10 | 120 | 100 | 2018 | [70] |
| CuInS2 QDs/Bi2WO6/20 | 40/10 | 300 | 90 | 2018 | [71] |
| Bi2WO6/MoS2/RGO+Lactic acid/30 | 100/10 | 80 | 100 | 2016 | [72] |
| Bi2WO6/Bi2S3/MoS2/20 | 50/40 | 75 | 100 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Hu, T.; Gong, Q.; Wang, Q.; Sun, B.; Gao, T.; Cao, P.; Zhou, G. Spherical Bi2WO6/Bi2S3/MoS2 n-p Heterojunction with Excellent Visible-Light Photocatalytic Reduction Cr(VI) Activity. Nanomaterials 2020, 10, 1813. https://doi.org/10.3390/nano10091813
Ren J, Hu T, Gong Q, Wang Q, Sun B, Gao T, Cao P, Zhou G. Spherical Bi2WO6/Bi2S3/MoS2 n-p Heterojunction with Excellent Visible-Light Photocatalytic Reduction Cr(VI) Activity. Nanomaterials. 2020; 10(9):1813. https://doi.org/10.3390/nano10091813
Chicago/Turabian StyleRen, Jing, Tingting Hu, Qinghua Gong, Qian Wang, Bin Sun, Tingting Gao, Pei Cao, and Guowei Zhou. 2020. "Spherical Bi2WO6/Bi2S3/MoS2 n-p Heterojunction with Excellent Visible-Light Photocatalytic Reduction Cr(VI) Activity" Nanomaterials 10, no. 9: 1813. https://doi.org/10.3390/nano10091813
APA StyleRen, J., Hu, T., Gong, Q., Wang, Q., Sun, B., Gao, T., Cao, P., & Zhou, G. (2020). Spherical Bi2WO6/Bi2S3/MoS2 n-p Heterojunction with Excellent Visible-Light Photocatalytic Reduction Cr(VI) Activity. Nanomaterials, 10(9), 1813. https://doi.org/10.3390/nano10091813







