Carbon-Supported Mo2C for Oxygen Reduction Reaction Electrocatalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation and Characterisation
2.2. Electrochemical Measurements
3. Results
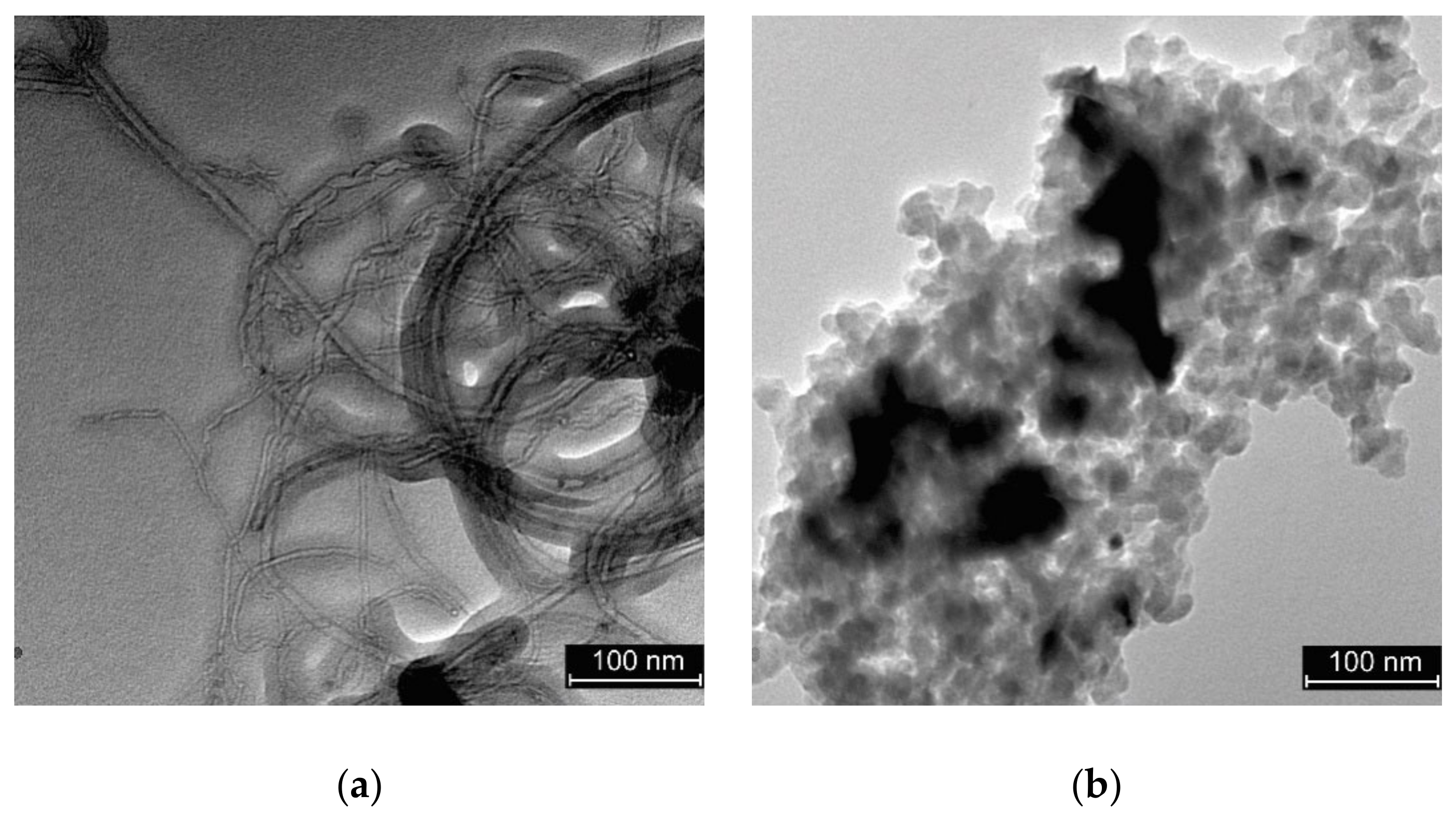
3.1. Characterisation of the Electrocatalysts
3.2. Electrical Conductivity Measurements
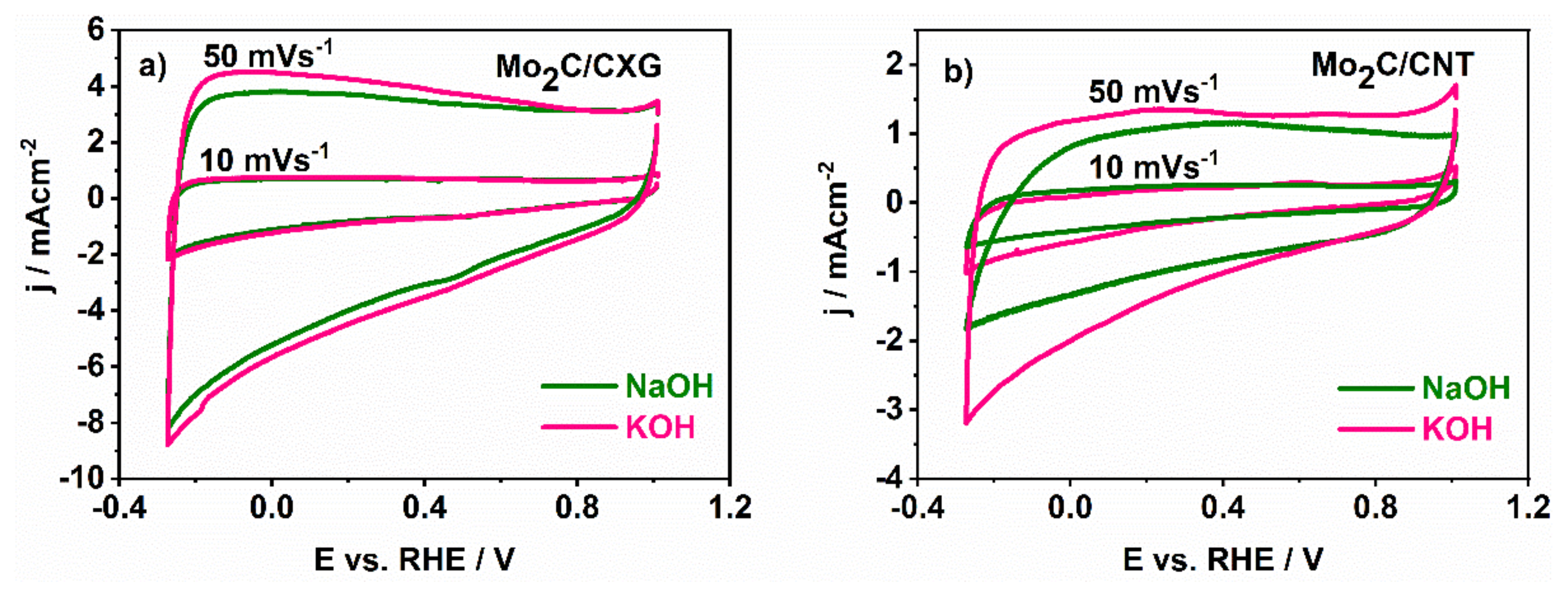
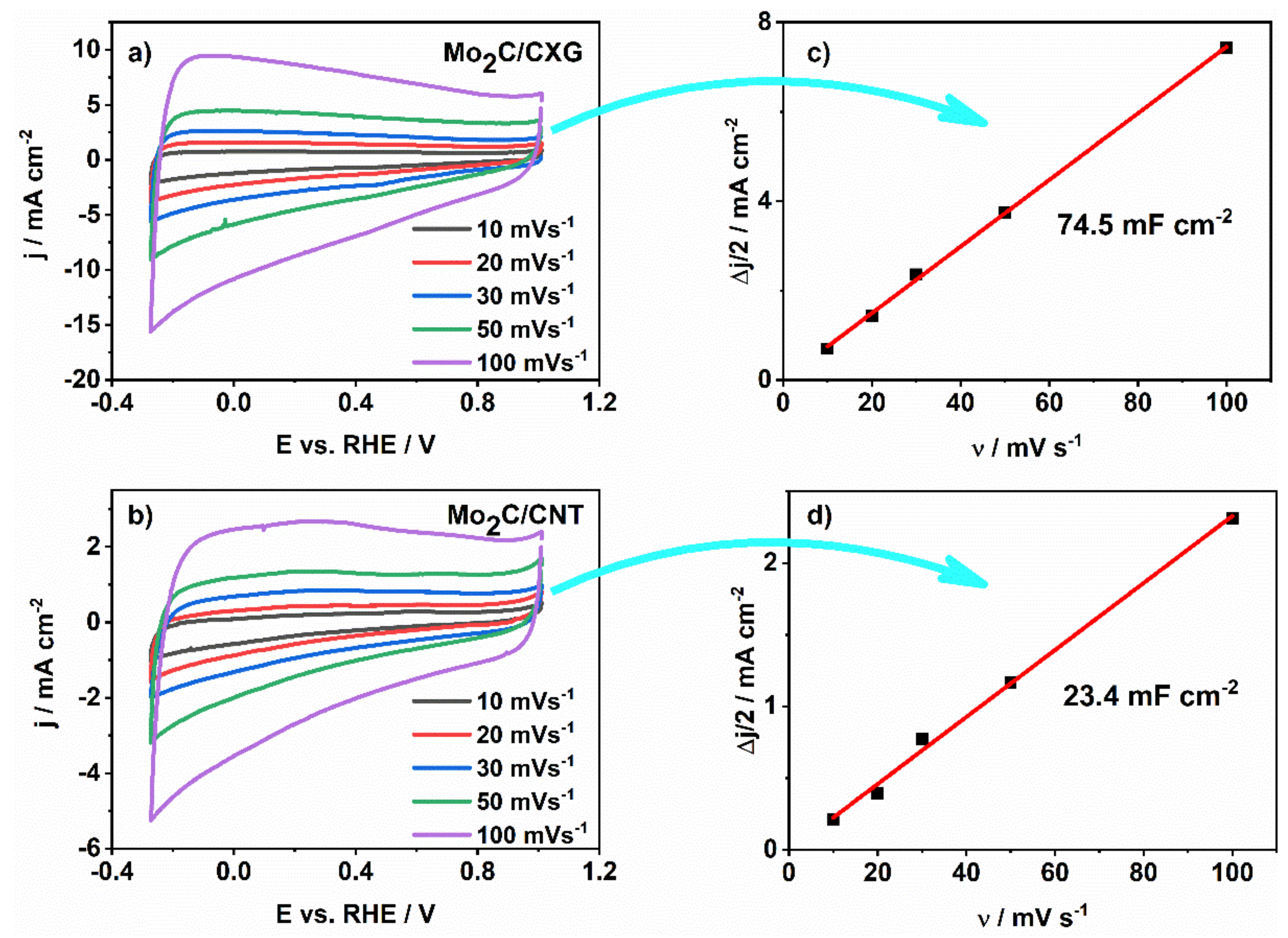
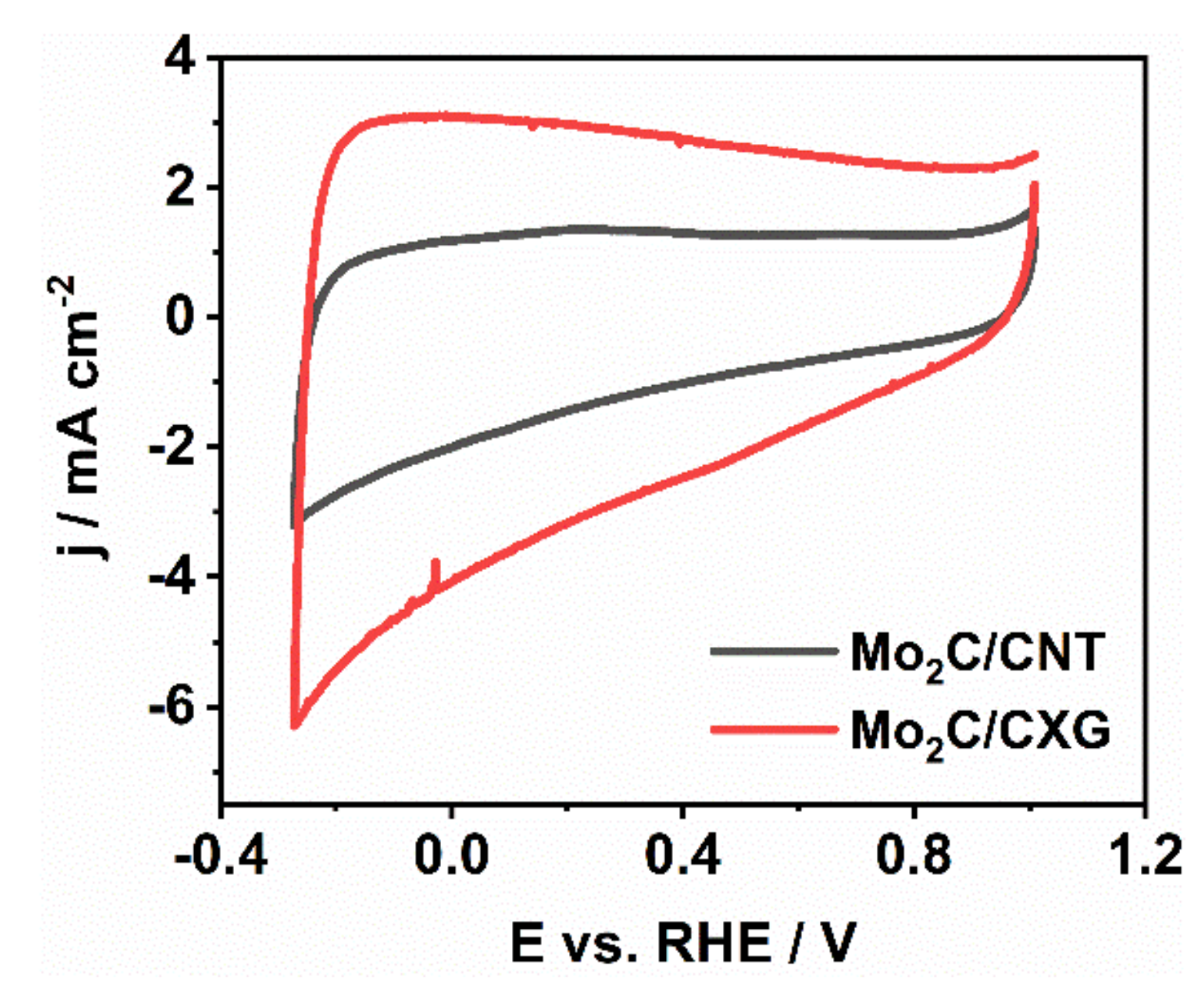
3.3. The Capacitance Behaviour of Mo2C/CXG and Mo2C/CNT
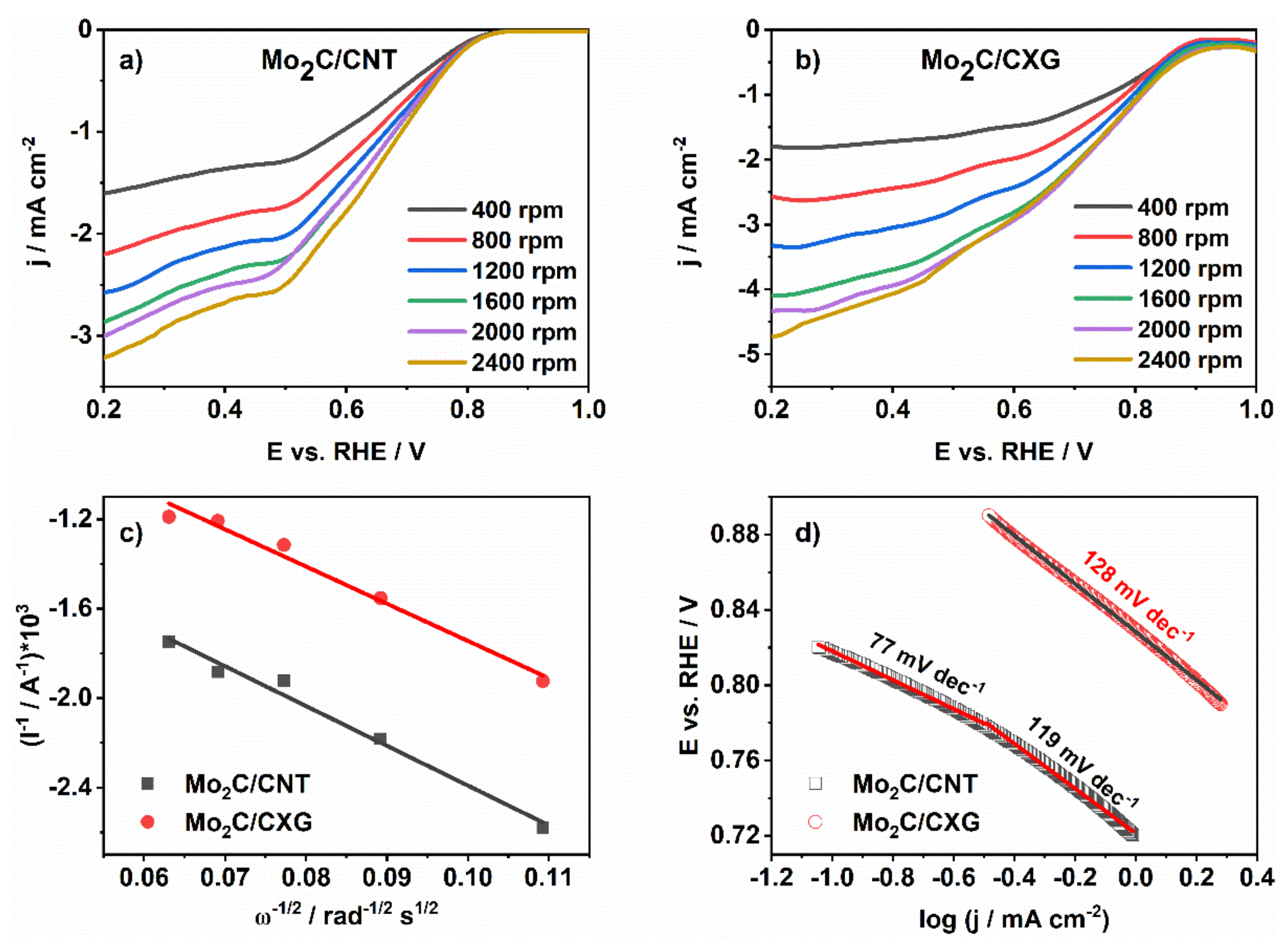
3.4. Carbon-Supported Mo2C Activity for ORR
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Adzic, R.R. Electrocatalysis. In Frontiers in Electrochemistry; Lipkowski, J., Ross, P.N., Eds.; Wiley-VCH: New York, NY, USA, 1998; Volume 3, p. 197. [Google Scholar]
- Bruce, P.G.; Hardwick, L.J.; Abraham, K.M. Lithium-air and lithium-sulfur batteries. MRS Bull. 2011, 36, 506–512. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal-air batteries: from oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172. [Google Scholar] [CrossRef] [PubMed]
- Gatto, I.; Stassi, A.; Passalacqua, E.; Aricò, A. An electro-kinetic study of oxygen reduction in polymer electrolyte fuel cells at intermediate temperatures. Int. J. Hydrogen Energy 2013, 38, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Lin, B.; Gilmore, K.; Hou, T.; Lee, S.-T.; Li, Y. Theoretical investigations on SiC2 siligraphene as promising metal-free catalyst for oxygen reduction reaction. J. Power Sources 2015, 299, 371–379. [Google Scholar] [CrossRef]
- Keith, J.A.; Jerkiewicz, G.; Jacob, T. Theoretical Investigations of the Oxygen Reduction Reaction on Pt(111). ChemPhysChem 2010, 11, 2779–2794. [Google Scholar] [CrossRef]
- Wang, B. Recent development of non-platinum catalysts for oxygen reduction reaction. J. Power Sources 2005, 152, 1–15. [Google Scholar] [CrossRef]
- Šljukić, B.; Santos, D.M.F.; Sequeira, C. Manganese dioxide electrocatalysts for borohydride fuel cell cathodes? J. Electroanal. Chem. 2013, 694, 77–83. [Google Scholar] [CrossRef]
- Ko, A.-R.; Lee, Y.-W.; Moon, J.-S.; Han, S.-B.; Cao, G.; Park, K.-W. Ordered mesoporous tungsten carbide nanoplates as non-Pt catalysts for oxygen reduction reaction. Appl. Catal., A 2014, 477, 102–108. [Google Scholar] [CrossRef]
- Tominaga, H.; Aoki, Y.; Nagai, M. Hydrogenation of CO on molybdenum and cobalt molybdenum carbides. Appl. Catal. A. 2012, 423–424, 192–204. [Google Scholar] [CrossRef]
- Esposito, D.V.; Hunt, S.T.; Kimmel, Y.C.; Chen, J.G. A new class of electrocatalysts for hydrogen production from water electrolysis: Metal monolayers supported on low-cost transition metal carbides. J. Am. Chem. Soc. 2012, 134, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Cheekatamarla, P.; Thomson, W.J. Catalytic activity of molybdenum carbide for hydrogen generation via diesel reforming. J. Power Sources 2006, 158, 477–484. [Google Scholar] [CrossRef]
- Yan, Z.; He, G.; Shen, P.K.; Luo, Z.; Xie, J.; Chen, M. MoC-graphite composite as a Pt electrocatalyst support for highly active methanol oxidation and oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4014–4022. [Google Scholar] [CrossRef]
- Elbaz, L.; Kreller, C.R.; Henson, N.J.; Brosha, E.L. Electrocatalysis of oxygen reduction with platinum supported on molybdenum carbide-carbon composite. J. Electroanal. Chem. 2014, 720–721, 34–40. [Google Scholar] [CrossRef]
- Nikolic, V.M.; Perović, I.; Gavrilov, N.M.; Pašti, I.A.; Šaponjić, A.; Vulić, P.J.; Karic, S.D.; Babić, B.M.; Kaninski, M.P. On the tungsten carbide synthesis for PEM fuel cell application—Problems, challenges and advantages. Int. J. Hydrogen Energy 2014, 39, 11175–11185. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, C.; Meng, H.; Wang, R.; Shen, P.K.; Fu, H. Oxygen reduction electrocatalysis enhanced by nanosized cubic vanadium carbide. Electrochem. Commun. 2011, 13, 763–765. [Google Scholar] [CrossRef]
- Polonský, J.; Petrushina, I.M.; Christensen, E.; Bouzek, K.; Prag, C.B.; Andersen, J.E.T.; Bjerrum, N.J. Tantalum carbide as a novel support material for anode electrocatalysts in polymer electrolyte membrane water electrolysers. Int. J. Hydrogen Energy 2012, 37, 2173–2181. [Google Scholar] [CrossRef] [Green Version]
- Horigome, M.; Kobayashi, K.; Suzuki, T.M. Impregnation of metal carbides in Raney Ni-PTFE hydrogen electrodes. Int. J. Hydrogen Energy 2007, 32, 365–370. [Google Scholar] [CrossRef]
- Huang, T.; Mao, S.; Pu, H.; Wen, Z.; Huang, X.; Ci, S.; Chen, J. Nitrogen-doped graphene-vanadium carbide hybrids as a high-performance oxygen reduction reaction electrocatalyst support in alkaline media. J. Mater. Chem. A 2013, 1, 13404–13410. [Google Scholar] [CrossRef]
- Huang, T.; Yu, J.; Han, J.; Zhang, Z.; Xing, Y.; Wen, C.; Wu, X.; Zhang, Y. Oxygen reduction catalytic characteristics of vanadium carbide and nitrogen doped vanadium carbide. J. Power Sources 2015, 300, 483–490. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, Y.; Yu, J.; Yin, J.; Gao, S. Tungsten carbide nanofibers prepared by electrospinning with high electrocatalytic activity for oxygen reduction. Int. J. Hydrogen Energy 2011, 36, 7398–7404. [Google Scholar] [CrossRef]
- Chen, W.F.; Wang, C.H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Šljukić, B.; Vujković, M.; Amaral, L.; Santos, D.M.F.; Rocha, R.P.; Sequeira, C.A.C.; Figueiredo, J.L. Carbon-supported Mo2C electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 15505–15512. [Google Scholar] [CrossRef]
- Huang, K.; Bi, K.; Liang, C.; Lin, S.; Wang, W.J.; Yang, T.Z.; Liu, J.; Zhang, R.; Fan, D.Y.; Wang, Y.G.; et al. Graphite carbon-supported Mo2C nanocomposites by a single-step solid state reaction for electrochemical oxygen reduction. PLoS One 2015, 10, e0138330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Wang, Z.; Fu, Y.; Jin, C.; Wei, Q.; Yang, R. In situ preparation of hollow Mo2C-C hybrid microspheres as bifunctional electrocatalysts for oxygen reduction and evolution reactions. J. Mater. Chem. A 2016, 4, 12583–12590. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Zhou, W.; Lin, J.; Shen, Z. Nitrogen-doped graphene-supported transition-metals carbide electrocatalysts for oxygen reduction reaction. Sci. Rep. 2015, 5, 10389. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Sun, C.; Cao, Y.; Zhu, J.; Chen, Y.; Guo, J.; Zhao, J.; Sun, Y.; Zou, G. Molybdenum carbide nanoparticles embedded in nitrogen-doped porous carbon nanofibers as a dual catalyst for hydrogen evolution and oxygen reduction reactions. Carbon 2017, 114, 628–634. [Google Scholar] [CrossRef]
- Kimmel, Y.C.; Xu, X.; Yu, W.; Yang, X.; Chen, J.G. Trends in electrochemical stability of transition metal carbides and their potential use as supports for low-cost electrocatalysts. ACS Catal. 2014, 4, 1558–1562. [Google Scholar] [CrossRef]
- Te Hsieh, C.; Lin, J.Y. Fabrication of bimetallic Pt-M (M = Fe, Co, and Ni) nanoparticle/carbon nanotube electrocatalysts for direct methanol fuel cells. J. Power Sources 2009, 188, 347–352. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Ryu, S.H. Excellent electrochemical performance of graphene-silver nanoparticle hybrids prepared using a microwave spark assistance process. Electrochim. Acta 2012, 74, 207–214. [Google Scholar] [CrossRef]
- Aricò, A.S.; Baglio, V.; Di Blasi, A.; Modica, E.; Monforte, G.; Antonucci, V. Electrochemical analysis of high temperature methanol electro-oxidation at Pt-decorated Ru catalysts. J. Electroanal. Chem. 2005, 576, 161–169. [Google Scholar] [CrossRef]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.P. Synthesis of transition metal-doped carbon xerogels by solubilization of metal salts in resorcinol-formaldehyde aqueous solution. Carbon 2004, 42, 3217–3227. [Google Scholar] [CrossRef]
- Arbizzani, C.; Righi, S.; Soavi, F.; Mastragostino, M. Graphene and carbon nanotube structures supported on mesoporous xerogel carbon as catalysts for oxygen reduction reaction in proton-exchange-membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 5038–5046. [Google Scholar] [CrossRef]
- Alegre, C.; Gálvez, M.E.; Moliner, R.; Baglio, V.; Aricò, A.S.; Lázaro, M.J. Towards an optimal synthesis route for the preparation of highly mesoporous carbon xerogel-supported Pt catalysts for the oxygen reduction reaction. Appl. Catal., B 2014, 147, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Job, N.; Pereira, M.F.R.; Lambert, S.; Cabiac, A.; Delahay, G.; Colomer, J.F.; Marien, J.; Figueiredo, J.L.; Pirard, J.P. Highly dispersed platinum catalysts prepared by impregnation of texture-tailored carbon xerogels. J. Catal. 2006, 240, 160–171. [Google Scholar] [CrossRef]
- Samant, P.V.; Fernandes, J.B.; Rangel, C.M.; Figueiredo, J.L. Carbon xerogel supported Pt and Pt-Ni catalysts for electro-oxidation of methanol in basic medium. Catal. Today 2005, 102–103, 173–176. [Google Scholar] [CrossRef]
- Job, N.; Marie, J.; Lambert, S.; Berthon-Fabry, S.; Achard, P. Carbon xerogels as catalyst supports for PEM fuel cell cathode. Energy Convers. Manage. 2008, 49, 2461–2470. [Google Scholar] [CrossRef]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.P. Porous carbon xerogels with texture tailored by pH control during sol-gel process. Carbon 2004, 42, 619–628. [Google Scholar] [CrossRef]
- Šljukic, B.; Santos, D.M.F.; Vujkovic, M.; Amaral, L.; Rocha, R.P.; Sequeira, C.A.C.; Figueiredo, J.L. Molybdenum Carbide Nanoparticles on Carbon Nanotubes and Carbon Xerogel: Low-Cost Cathodes for Hydrogen Production by Alkaline Water Electrolysis. ChemSusChem 2016, 9, 1200–1208. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Bott-Neto, J.L.; Souza, J.B.; Silva, T.L.; Beck, W.; Varanda, L.C.; Ticianelli, E.A. Electrocatalytic Activity of Different Phases of Molybdenum Carbide/Carbon and Platinum–Molybdenum Carbide/Carbon Composites toward the Oxygen Reduction Reaction. ChemElectroChem 2016, 3, 1570–1579. [Google Scholar] [CrossRef]
- Ren, J.; Huo, C.F.; Wang, J.; Li, Y.W.; Jiao, H. Surface structure and energetics of oxygen and CO adsorption on α-Mo2C(0 0 0 1). Surf. Sci. 2005, 596, 212–221. [Google Scholar] [CrossRef]
- Viñes, F.; Sousa, C.; Illas, F.; Liu, P.; Rodriguez, J.A. A systematic density functional study of molecular oxygen adsorption and dissociation on the (001) surface of group IV-VI transition metal carbides. J. Phys. Chem. C 2007, 111, 16982–16989. [Google Scholar] [CrossRef]
- Liao, L.; Bian, X.; Xiao, J.; Liu, B.; Scanlon, M.D.; Girault, H.H. Nanoporous molybdenum carbide wires as an active electrocatalyst towards the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 10088–10094. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, S.V.; Sepunaru, L.; Compton, R.G. Taking cues from nature: Hemoglobin catalysed oxygen reduction. Appl. Mater. Today 2017, 7, 82–90. [Google Scholar] [CrossRef]
- Masa, J.; Batchelor-McAuley, C.; Schuhmann, W.; Compton, R.G. Koutecky–Levich analysis applied to nanoparticle modified rotating disk electrodes: Electrocatalysis or misinterpretation. Nano Res. 2014, 7, 71–78. [Google Scholar] [CrossRef]
- Li, D.; Batchelor-McAuley, C.; Compton, R.G. Some thoughts about reporting the electrocatalytic performance of nanomaterials. Appl. Mater. Today 2020, 18, 100404. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, T.; Wang, T.; Yang, J.; Zhu, Y.; Ding, W. Molybdenum carbide promotion on Fe-N-doped carbon nanolayers facilely prepared for enhanced oxygen reduction. Nanoscale 2018, 10, 21944–21950. [Google Scholar] [CrossRef]
- Óvári, L.; Kiss, J.; Farkas, A.P.; Solymosi, F. Surface and subsurface oxidation of Mo2C/Mo(100): Low-energy ion-scattering, auger electron, angle-resolved X-ray photoelectron, and mass spectroscopy studies. J. Phys. Chem. B 2005, 109, 4638–4645. [Google Scholar] [CrossRef] [Green Version]
- Jäger, R.; Kasatkin, P.E.; Härk, E.; Lust, E. Oxygen reduction on molybdenum carbide derived micromesoporous carbon electrode in alkaline solution. Electrochem. Commun. 2013, 35, 97–99. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, M.; Liu, Y.; Wang, S.; Li, J. Molybdenum-doped mesoporous carbon/graphene composites as efficient electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 19969–19973. [Google Scholar] [CrossRef]
| Catalysts | Onset Potential / V | Current Density at 0.6 V ; / mA cm−2 | Tafel Slope / mV dec−1 | Electron Transfer Number | Reference |
|---|---|---|---|---|---|
| G-Mo2C | 0.75 | −2.3 | / | 2.1–3.2 | [25] |
| FeMo carbide/NG | 0.91 | −3.5 | / | 3.5 | [27] |
| Mo2C nanowires | 0.87 | −2.7 | 65 | 3–4 | [45] |
| Hollow Mo2C-C microspheres | 0.83 | −4.2 | 72.2 | 3.2–3.6 | [26] |
| Mo2C/NPCNFs | 0.90 | −4.6 | 60.3 | 3.8 | [28] |
| Mo2C@NC-Fe | / | / | 46 | 3.7 | [49] |
| C(Mo2C) | 0.84 | / | 57 (126) | 2.8 | [51] |
| Mo-doped MCG | 0.76 | / | 37 | 2.3 | [52] |
| Mo2C/CXG | 0.89 | −2.9 | 128 | 3.0 | This work |
| Mo2C/CNT | 0.81 | −1.6 | 77 (119) | 2.7 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenović, D.; Vujković, M.; Mentus, S.; Santos, D.M.F.; Rocha, R.P.; C. Sequeira, C.A.; Figueiredo, J.L.; Šljukić, B. Carbon-Supported Mo2C for Oxygen Reduction Reaction Electrocatalysis. Nanomaterials 2020, 10, 1805. https://doi.org/10.3390/nano10091805
Mladenović D, Vujković M, Mentus S, Santos DMF, Rocha RP, C. Sequeira CA, Figueiredo JL, Šljukić B. Carbon-Supported Mo2C for Oxygen Reduction Reaction Electrocatalysis. Nanomaterials. 2020; 10(9):1805. https://doi.org/10.3390/nano10091805
Chicago/Turabian StyleMladenović, Dušan, Milica Vujković, Slavko Mentus, Diogo M. F. Santos, Raquel P. Rocha, Cesar A. C. Sequeira, Jose Luis Figueiredo, and Biljana Šljukić. 2020. "Carbon-Supported Mo2C for Oxygen Reduction Reaction Electrocatalysis" Nanomaterials 10, no. 9: 1805. https://doi.org/10.3390/nano10091805









