Osteogenic and Anti-Inflammatory Behavior of Injectable Calcium Phosphate Loaded with Therapeutic Drugs
Abstract
1. Introduction
2. Materials and Methods
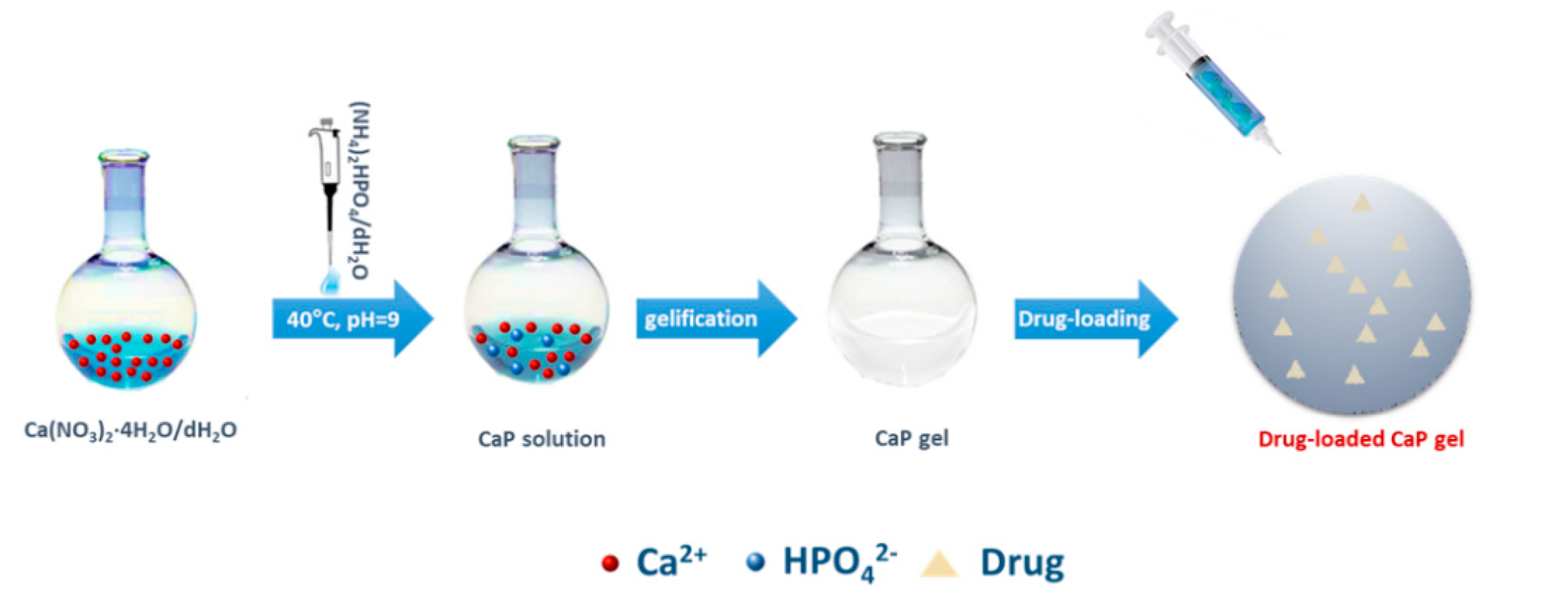
2.1. Biomaterials Synthesis
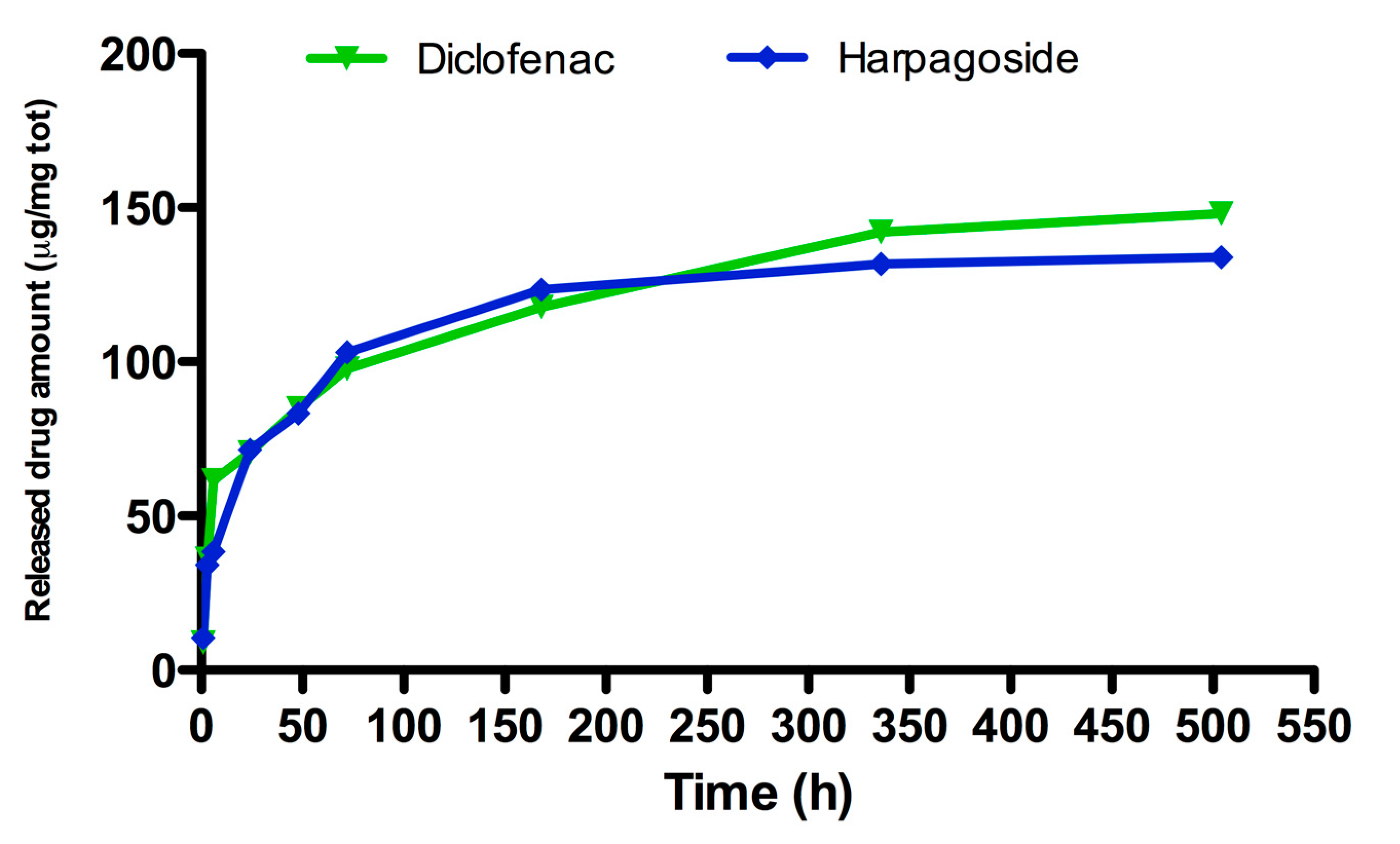
Drug-Release Study
2.2. Biological Tests
2.2.1. Osteoblasts Expansion, Culture, and Proliferation
2.2.2. Osteogenic Differentiation Study
2.2.3. Scanning Electron Microscopy Analysis for Cell Adhesion Evaluation
2.2.4. In Vitro Anti-Inflammatory Response
2.3. Statistical Analysis
3. Results
3.1. Drug Release Study
3.2. Effect of Drug-Loaded CaP Based Materials on Cell Proliferation
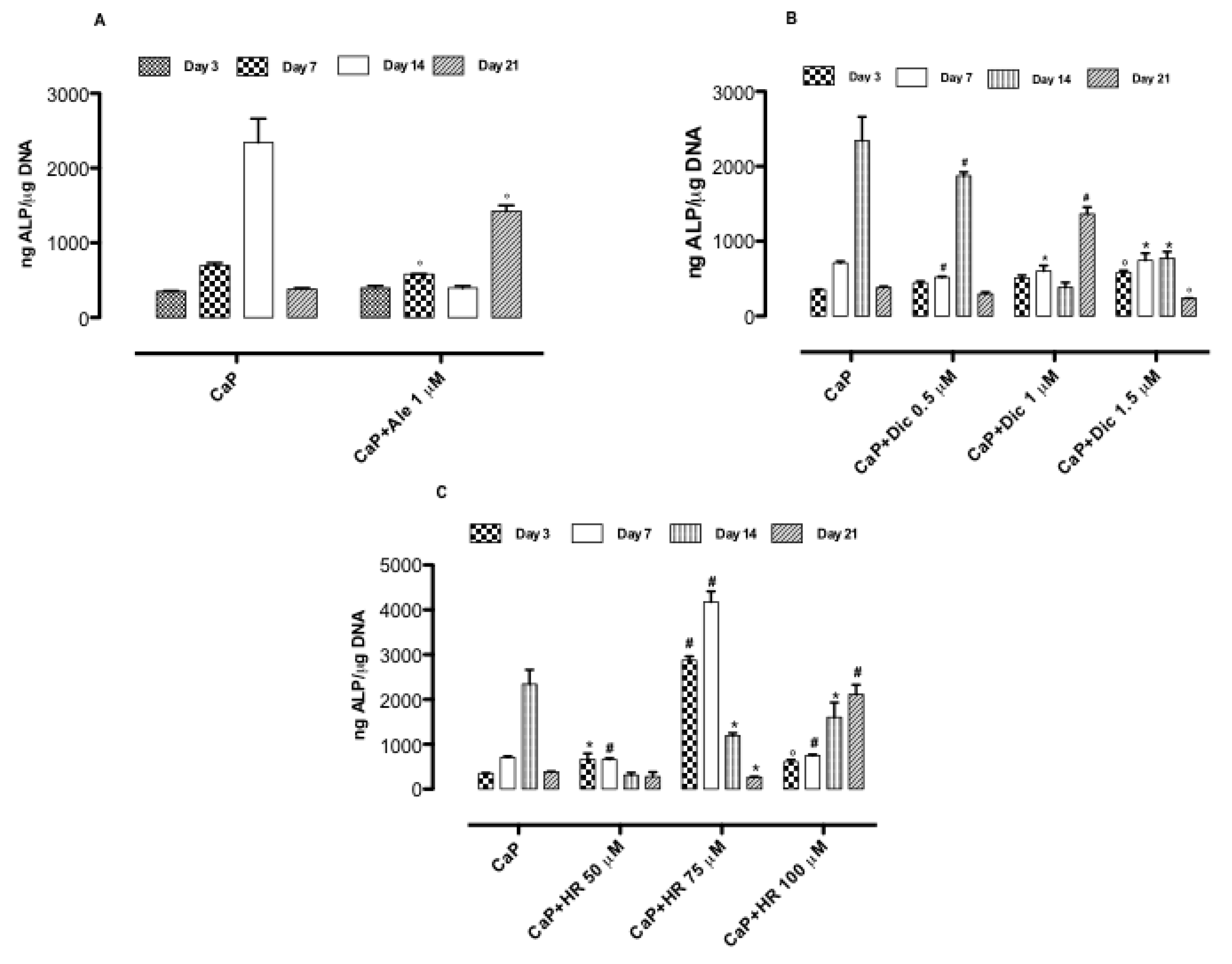
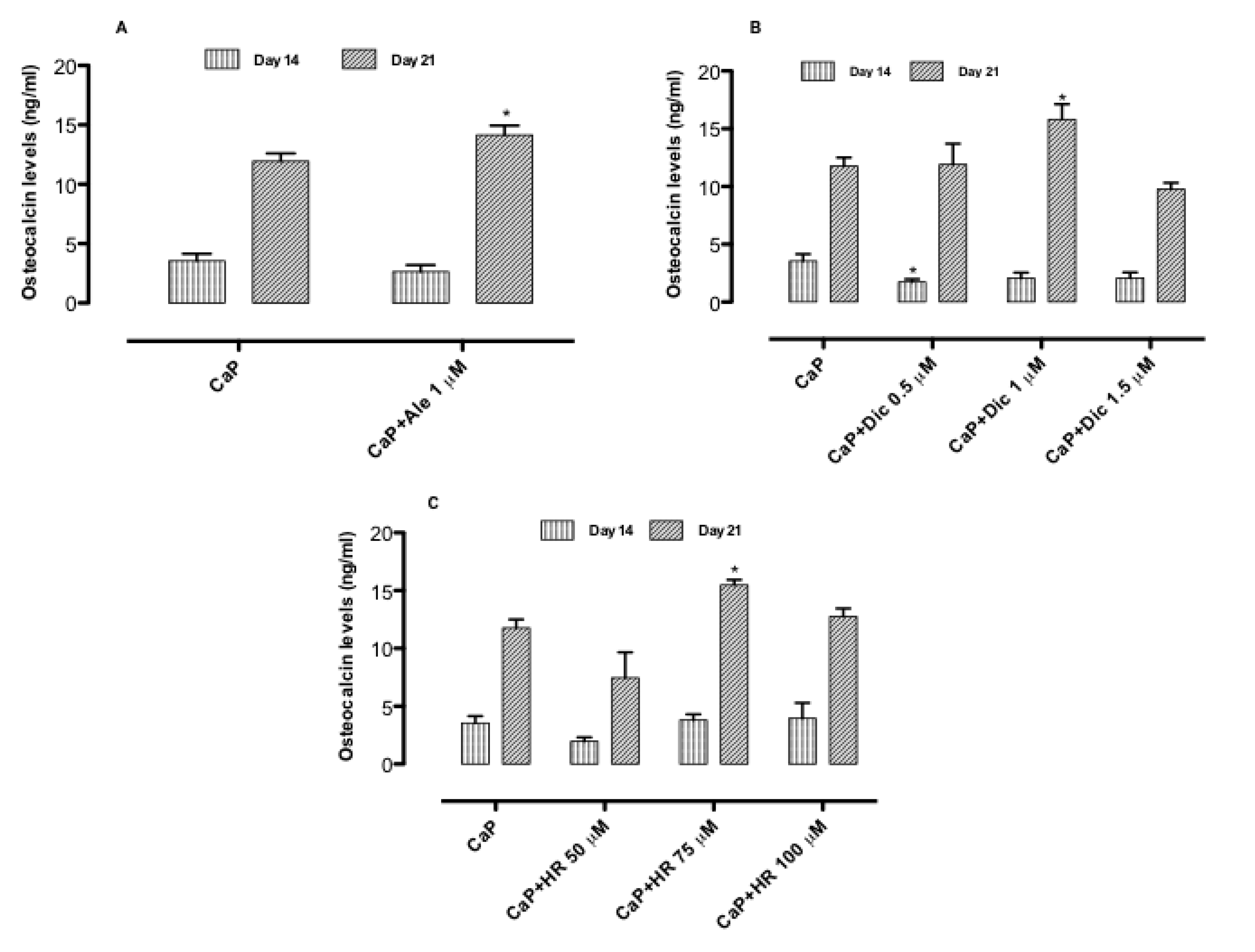
3.3. Effect of Drug-Loaded CaP-Based Materials on Osteogenesis
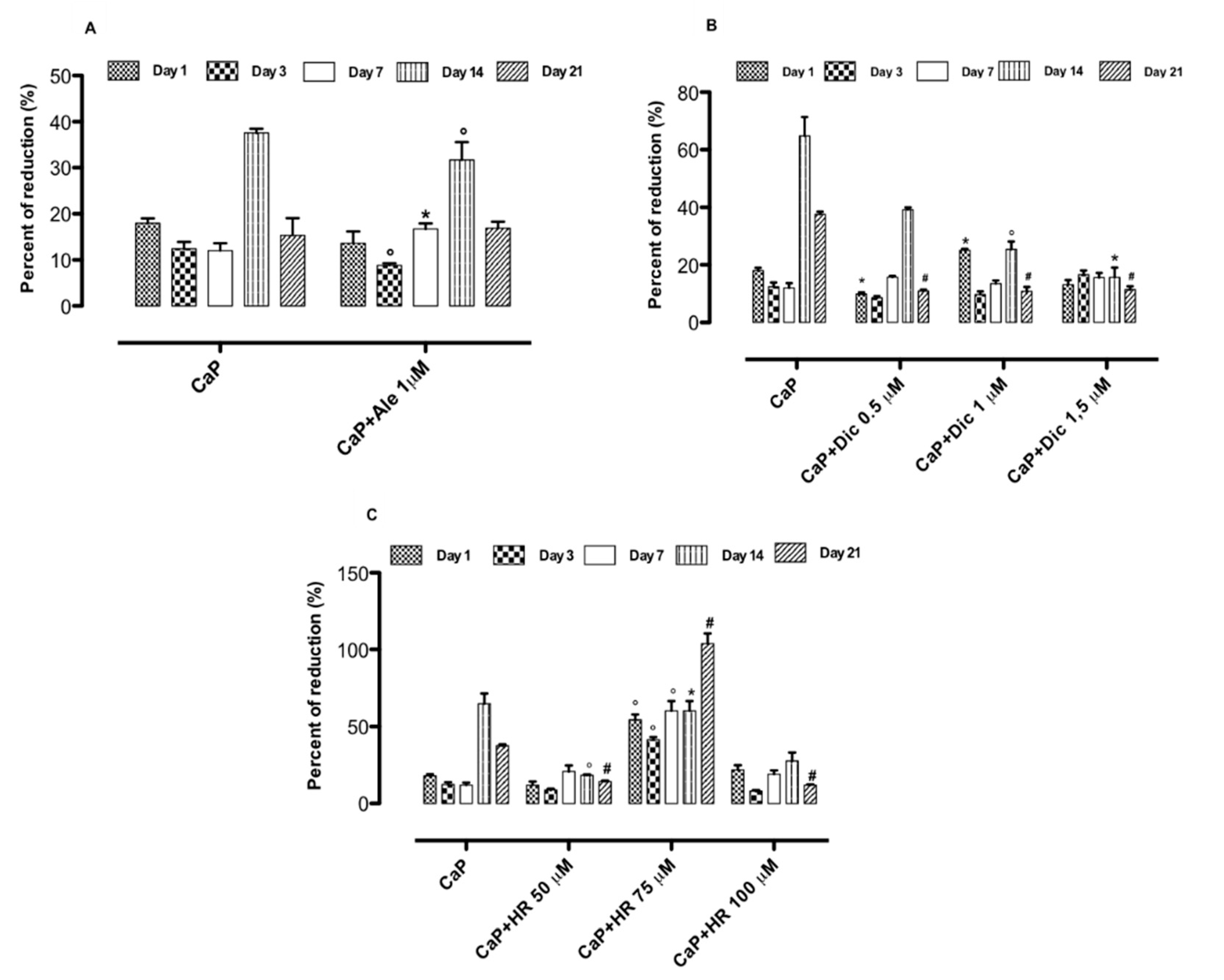
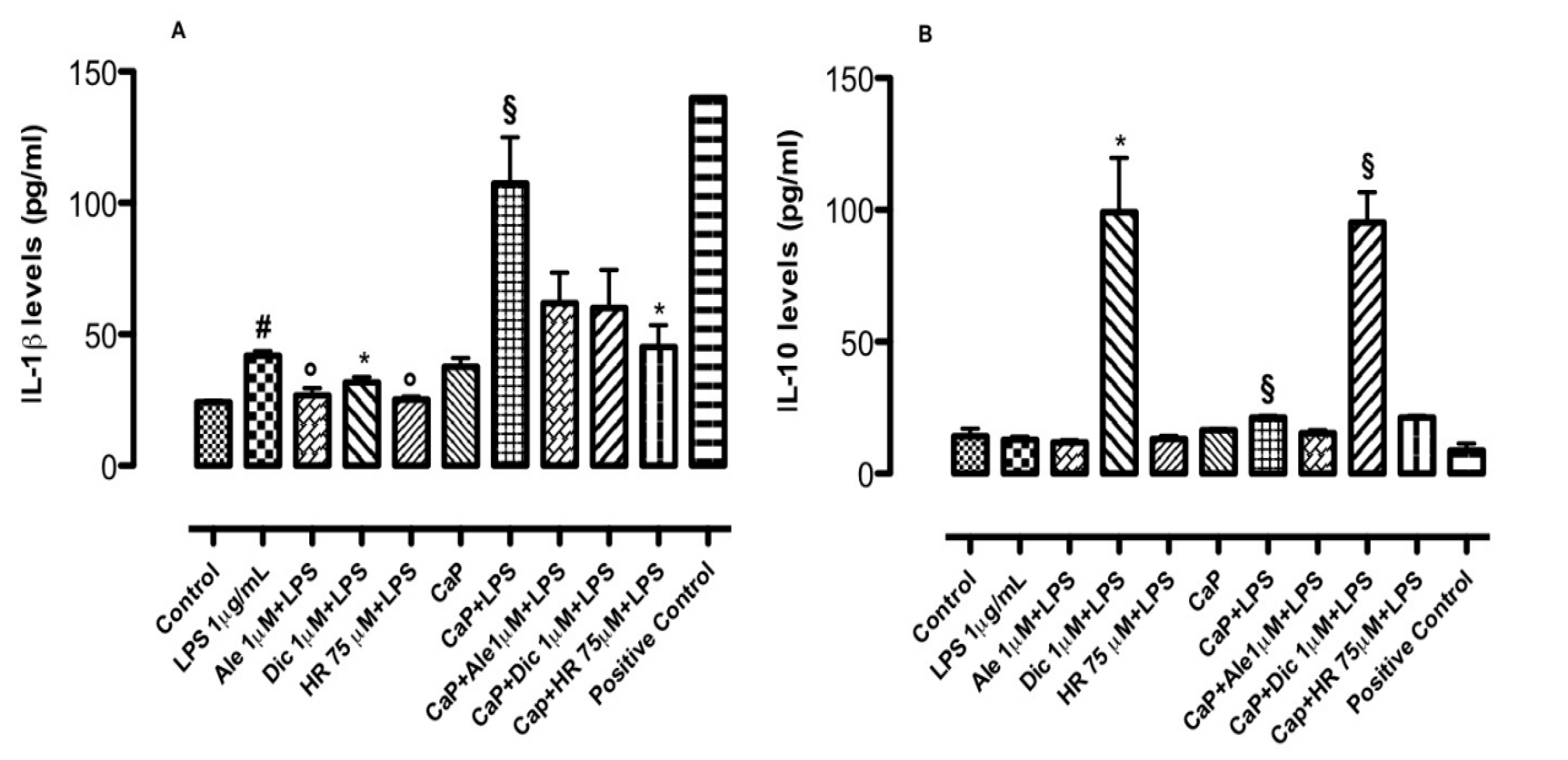
3.4. Effect of Drug-Loaded CaP-Based Materials on Basal Inflammatory Response
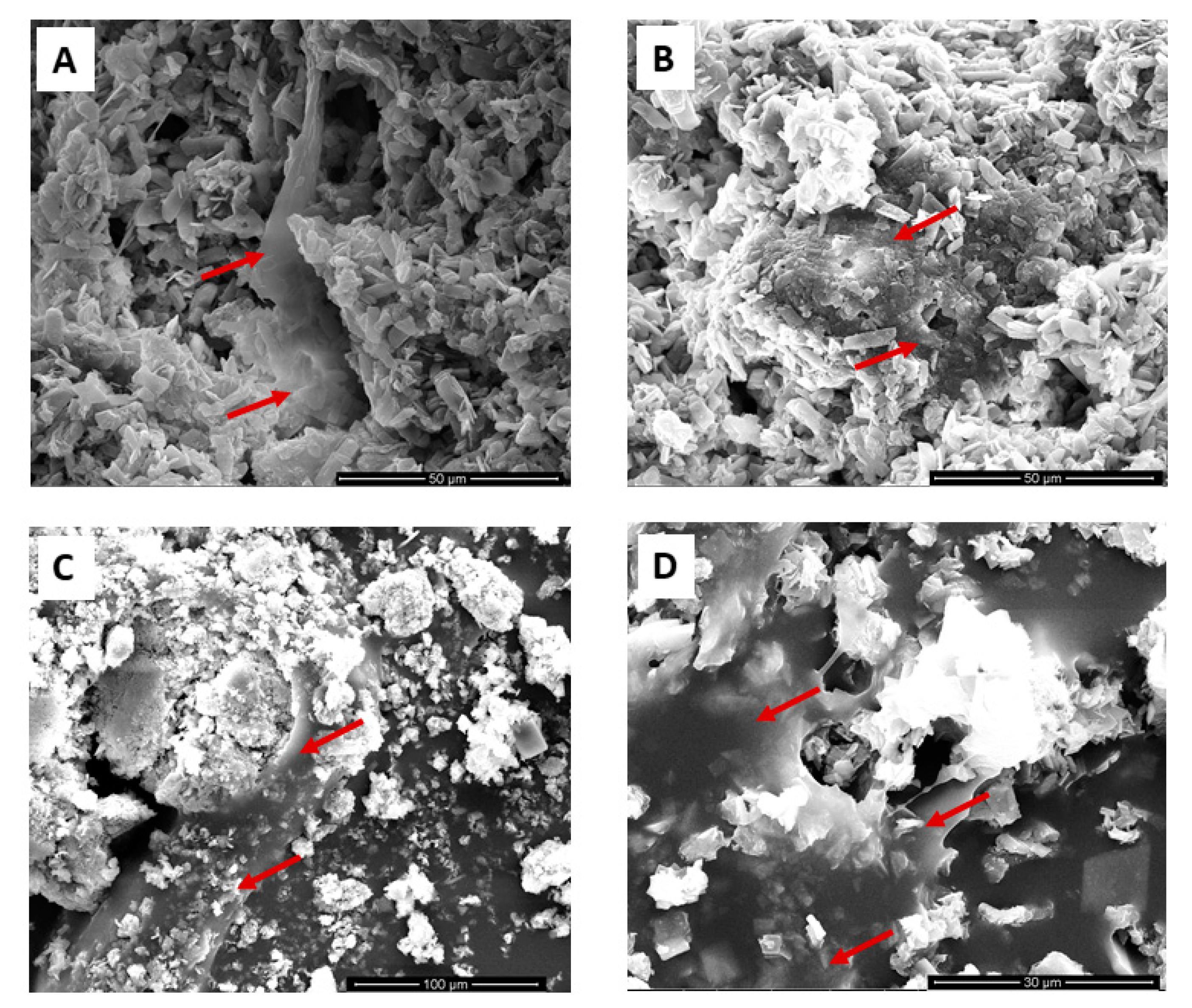
3.5. Cell Adhesion on Drugs-Loaded CaP-Based Materials: SEM Analysis
3.6. Inflammatory Co-Culture Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alt, V.; Thormann, U.; Ray, S.; Zahner, D.; Dürselen, L.; Lips, K.; El Khassawna, T.; Heiss, C.; Riedrich, A.; Schlewitz, G.; et al. A new metaphyseal bone defect model in osteoporotic rats to study biomaterials for the enhancement of bone healing in osteoporotic fractures. Acta Biomater. 2013, 9, 7035–7042. [Google Scholar] [CrossRef] [PubMed]
- Fasolino, I.; Raucci, M.G.; Soriente, A.; Demitri, C.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Osteoinductive and anti-inflammatory properties of chitosan-based scaffolds for bone regeneration. Mater. Sci. Eng. C 2019, 105, 110046. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Moon, R.J.; Dennison, E.; Harvey, N.C.; Cooper, C. Recent advances in the pathogenesis and treatment of osteoporosis. Clin. Med. 2015, 15, s92–s96. [Google Scholar] [CrossRef] [PubMed]
- Thormann, U.; Ray, S.; Sommer, U.; Sommer, U.; ElKhassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphys e al fracture defects in ovariectomized rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin. Proc. 2009, 84, 632–637. [Google Scholar] [CrossRef]
- Raucci, M.G.; Álvarez-Pérez, M.A.; Meikle, S.T.; Ambrosio, L.; Santin, M. Poly(Epsilon-Lysine) Dendrons Tethered with Phosphoserine Increase Mesenchymal Stem Cell Differentiation Potential of Calcium Phosphate Gels. Tissue Eng. Part A 2014, 20, 3–4. [Google Scholar] [CrossRef]
- Low, K.L.; Tan, S.H.; Zein, S.H.S.; Roether, J.A.; Mouriño, V.; Boccaccini, A.R. Calcium phosphate-based composites as injectable bone substitute materials: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 273–286. [Google Scholar] [CrossRef]
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.; Gericke, N. Devil’s Claw—A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012, 143, 755–771. [Google Scholar] [CrossRef]
- Denner, S.S. A Review of the Efficacy and Safety of Devilʼs Claw for Pain Associated With Degenerative Musculoskeletal Diseases, Rheumatoid, and Osteoarthritis. Holist. Nurs. Pract. 2007, 21, 203–207. [Google Scholar] [CrossRef]
- Anauate, M.C.; Torres, L.M.; De Mello, S.B.V. Effect of isolated fractions of Harpagophytum procumbens D.C. (devil’s claw) on COX-1, COX-2 activity and nitric oxide production on whole-blood assay. Phytother. Res. 2010, 24, 1365–1369. [Google Scholar] [CrossRef]
- Inaba, K.; Murata, K.; Naruto, S.; Matsuda, H. Inhibitory effects of devil’s claw (secondary root of Harpagophytum procumbens) extract and harpagoside on cytokine production in mouse macrophages. J. Nat. Med. 2010, 64, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Chen, D.; Haqqi, T.M. Delphinidin inhibits IL-1beta-induced activation of NF-kappaB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology 2013, 52, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, S.-H.; Baek, J.M.; Erkhembaatar, M.; Kim, M.S.; Yoon, K.-H.; Oh, J.; Lee, M.S. Harpagoside Inhibits RANKL-Induced Osteoclastogenesis via Syk-Btk-PLCγ2-Ca2+Signaling Pathway and Prevents Inflammation-Mediated Bone Loss. J. Nat. Prod. 2015, 78, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Fasolino, I.; Pastore, S.G.; Soriente, A.; Capeletti, L.B.; Dessuy, M.B.; Giannini, C.; Schrekker, H.S.; Ambrosio, L. Antimicrobial Imidazolium Ionic Liquids for the Development of Minimal Invasive Calcium Phosphate-Based Bionanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 42766–42776. [Google Scholar] [CrossRef]
- Prieto, E.M.; Page, J.M.; Harmata, A.J.; Guelcher, S.A. Injectable foams for regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 6, 136–154. [Google Scholar] [CrossRef]
- D’Antò, V.; Raucci, M.G.; Guarino, V.; Martina, S.; Valletta, R.; Ambrosio, L. Behaviour of human mesenchymal stem cells on chemically synthesized HA-PCL scaffolds for hard tissue regeneration. J. Tissue Eng. Regen. Med. 2013, 10, E147–E154. [Google Scholar] [CrossRef]
- Raucci, M.G.; Giugliano, D.; Longo, A.; Zeppetelli, S.; Carotenuto, G.; Ambrosio, L. Comparative facile methods for preparing graphene oxide-hydroxyapatite for bone tissue engineering. J. Tissue Eng. Regen. Med. 2016, 11, 2204–2216. [Google Scholar] [CrossRef]
- Dessì, M.; Raucci, M.G.; Zeppetelli, S.; Ambrosio, L. Design of injectable organic-inorganic hybrid for bone tissue repair. J. Biomed. Mater. Res. Part A 2012, 100, 2063–2070. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, J.H.; Abbas, A.A.; Yoon, T.R. Alendronate Enhances Osteogenic Differentiation of Bone Marrow Stromal Cells: A Preliminary Study. Clin. Orthop. Relat. Res. 2008, 467, 3121–3128. [Google Scholar] [CrossRef]
- Sidney, L.E.; Heathman, T.R.; Britchford, E.R.; Abed, A.; Rahman, C.V.; Buttery, L.D. Investigation of localized delivery of diclofenac sodium from poly(D,L-lactic acid-co-glycolic acid)/poly(ethylene glycol) scaffolds using an in vitro osteoblast inflammation model. Tissue Eng. Part A 2015, 21, 362–373. [Google Scholar] [CrossRef]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus synthetic drugs; beliefs and facts. J. Nephronpharmacol. 2015, 4, 27–30. [Google Scholar]
- Walash, M.I.; Metwally, M.E.-S.; Eid, M.I.; El-Shaheny, R. Validated spectrophotometric methods for determination of Alendronate sodium in tablets through nucleophilic aromatic substitution reactions. Chem. Cent. J. 2012, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Fasolino, I.; Caporali, M.; Serrano-Ruiz, M.; Soriente, A.; Peruzzini, M.; Ambrosio, L. Exfoliated Black Phosphorus Promotes in Vitro Bone Regeneration and Suppresses Osteosarcoma Progression through Cancer-Related Inflammation Inhibition. ACS Appl. Mater. Interfaces 2019, 11, 9333–9342. [Google Scholar] [CrossRef]
- Lowe, E.S.; Balis, F.M. Principles of Clinical Pharmacology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Borgström, F.; Lekander, I.; Ivergård, M.; Strom, O.; Svedbom, A.; Alekna, V.; Bianchi, M.L.; Clark, P.; Curiel, M.D.; Dimai, H.P.; et al. The International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS)—Quality of life during the first 4 months after fracture. Osteoporos. Int. 2013, 24, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2006, 22, 465–475. [Google Scholar] [CrossRef]
- Ensrud, K.E. Epidemiology of Fracture Risk With Advancing Age. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2013, 68, 1236–1242. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Mao, J.J. Bone Tissue Engineering and Regeneration: From Discovery to the Clinic—An Overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef]
- Ducheyne, P.; Mauck, R.L.; Smith, U.H. Biomaterials in the repair of sports injuries. Nat. Mater. 2012, 11, 652–654. [Google Scholar] [CrossRef]
- Steinert, A.F.; Rackwitz, L.; Gilbert, F.; Nöth, U.; Tuan, R.S. Concise Review: The Clinical Application of Mesenchymal Stem Cells for Musculoskeletal Regeneration: Current Status and Perspectives. Stem Cells Transl. Med. 2012, 1, 237–247. [Google Scholar] [CrossRef]
- Prinsloo, P.J.J.; Hosking, D.J. Alendronate Sodium in the Management of Osteoporosis. Ther. Clin. Risk Manag. 2006, 2, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Tóth, E.; Fork, F.T.; Lindelöw, K.; Lindström, E.; Verbaan, H.; Veress, B. Alendronate-induced severe esophagitis. A rare and severe reversible side-effect illustrated by three case reports. Lakartidningen 1998, 95, 3676–3680. [Google Scholar] [PubMed]
- Rossini, M.; Bertoldo, F.; Lovato, R.; Bortolotti, R.; Gatti, D.; Adami, S. Use of nonsteroidal anti-inflammatory drugs in patients with vertebral osteoporotic fractures. Reumatismo 2003, 54, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Cappell, M.S.; Schein, J.R. Diagnosis and treatment of nonsteroidal anti-inflammatory drug-associated uppergastrointestinal toxicity. Gastroenterol. Clin. N. Am. 2000, 29, 97–124. [Google Scholar] [CrossRef]
- Graham, D.Y.; Malaty, H.M. Alendronate and naproxenare synergistic for development of gastric ulcers. ArchIntern. Med. 2001, 161, 107–110. [Google Scholar]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-Inflammatory Iridoids of Botanical Origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef]
- Andersen, M.L.; Santos, E.H.; Seabra, M.D.L.V.; Da Silva, A.A.; Tufik, S. Evaluation of acute and chronic treatments with Harpagophytum procumbens on Freund’s adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2004, 91, 325–330. [Google Scholar] [CrossRef]
- Park, K.-W.; Yun, Y.-P.; Kim, H.J.; Song, H.-R. The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model. Int. J. Mol. Sci. 2015, 16, 26738–26753. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasolino, I.; Soriente, A.; Ambrosio, L.; Raucci, M.G. Osteogenic and Anti-Inflammatory Behavior of Injectable Calcium Phosphate Loaded with Therapeutic Drugs. Nanomaterials 2020, 10, 1743. https://doi.org/10.3390/nano10091743
Fasolino I, Soriente A, Ambrosio L, Raucci MG. Osteogenic and Anti-Inflammatory Behavior of Injectable Calcium Phosphate Loaded with Therapeutic Drugs. Nanomaterials. 2020; 10(9):1743. https://doi.org/10.3390/nano10091743
Chicago/Turabian StyleFasolino, Ines, Alessandra Soriente, Luigi Ambrosio, and Maria Grazia Raucci. 2020. "Osteogenic and Anti-Inflammatory Behavior of Injectable Calcium Phosphate Loaded with Therapeutic Drugs" Nanomaterials 10, no. 9: 1743. https://doi.org/10.3390/nano10091743
APA StyleFasolino, I., Soriente, A., Ambrosio, L., & Raucci, M. G. (2020). Osteogenic and Anti-Inflammatory Behavior of Injectable Calcium Phosphate Loaded with Therapeutic Drugs. Nanomaterials, 10(9), 1743. https://doi.org/10.3390/nano10091743




