Metal Nanoparticles for Improving Bactericide Functionality of Usual Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Metal Nanoparticles
2.2. Characterization of Metal Nanoparticles
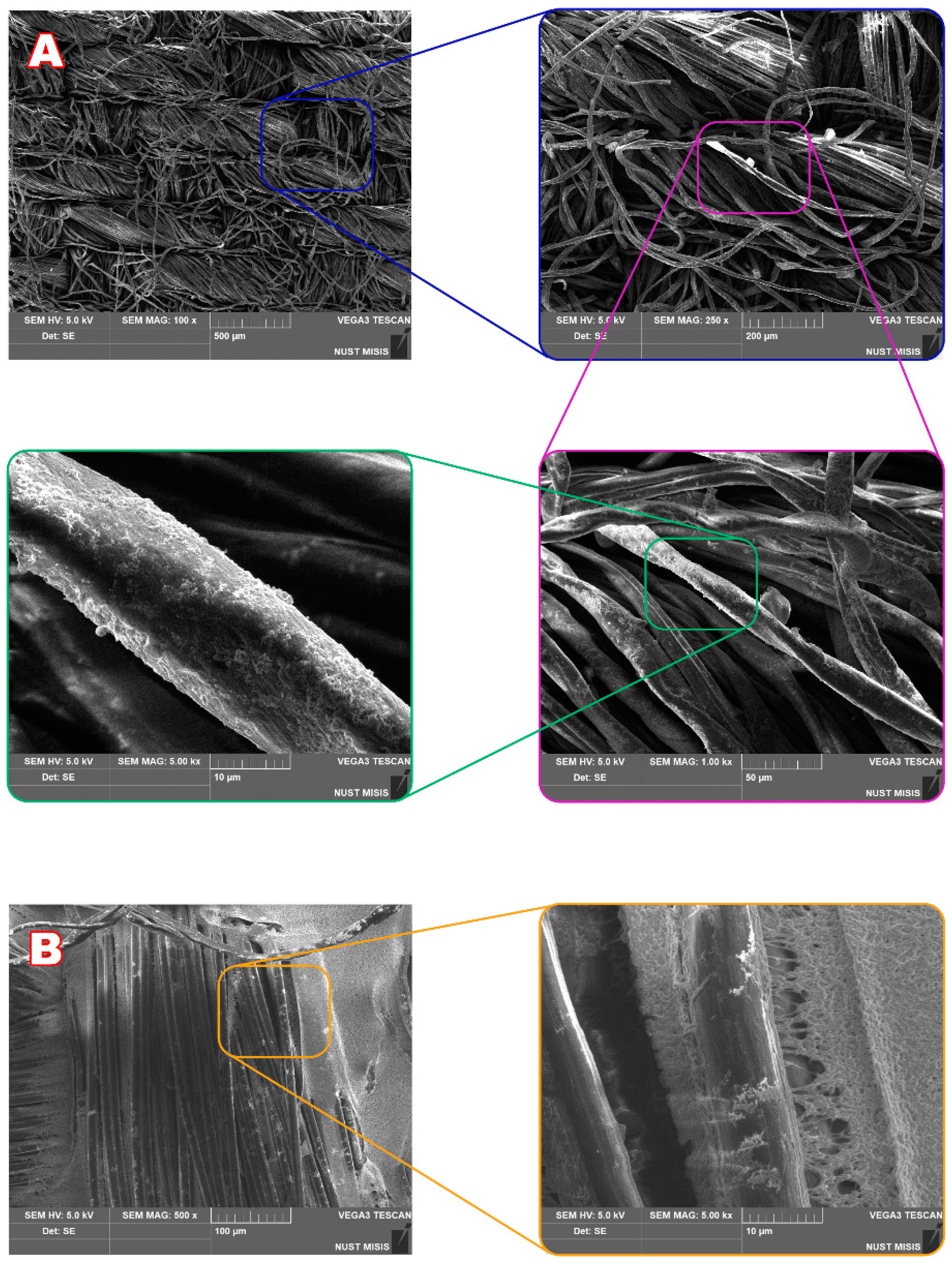
2.3. Fibrous Materials and Their Characteristics
2.4. Investigation of Bactericide Activity
3. Results
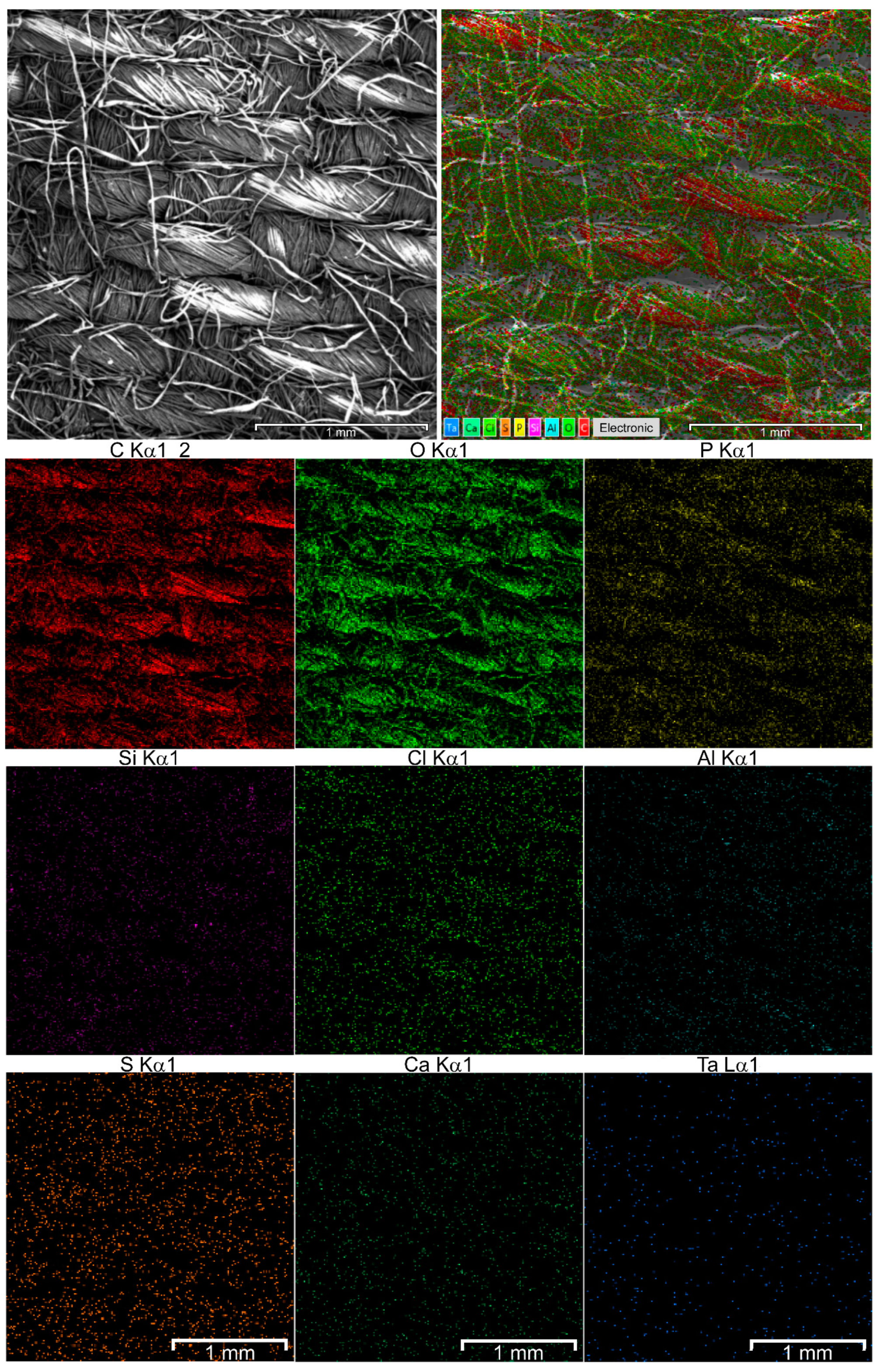
3.1. Characteristics of Metal Nanoparticles and Their Deposition onto Fibrous Material
3.2. Analysis of Bactericide Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Yun, G.; Pan, S.; Wang, T.Y.; Guo, J.; Richardson, J.J.; Caruso, F. Synthesis of Metal Nanoparticles in Metal-Phenolic Networks: Catalytic and Antimicrobial Applications of Coated Textiles. Adv. Healthc. Mater. 2018, 7, e1700934. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Konno, H.; Sasaki, S.; Nakasaka, Y.; Masuda, T. Facile Synthesis of Zeolitic Imidazolate Framework-8 (ZIF-8) Particles Immobilized on Aramid Microfibrils for Wastewater Treatment. Chem. Lett. 2018, 47, 620–623. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, G.; Dai, H.; Yang, M.; Fu, Y.; Ying, Y.; Li, Y. Biomineralization-mimetic preparation of hybrid membranes with ultra-high loading of pristine metal-organic frameworks grown on silk nanofibers for hazard collection in water. J. Mater. Chem. A 2018, 6, 3402–3413. [Google Scholar] [CrossRef]
- Smith, M.K.; Mirica, K.A. Self-Organized Frameworks on Textiles (SOFT): Conductive Fabrics for Simultaneous Sensing, Capture, and Filtration of Gases. J. Am. Chem. Soc. 2017, 139, 16759–16767. [Google Scholar] [CrossRef]
- Li, N.; Pranantyo, D.; Kang, E.-T.; Wright, D.S.; Luo, H.-K. In Situ Self-Assembled Polyoxotitanate Cages on Flexible Cellulosic Substrates: Multifunctional Coating for Hydrophobic, Antibacterial, and UV-Blocking Applications. Adv. Funct. Mater. 2018, 28, e1800345. [Google Scholar] [CrossRef]
- Robertson, J.; McGoverin, C.; Vanholsbeeck, F.; Swift, S. Optimisation of the Protocol for the LIVE/DEAD® BacLight TM Bacterial Viability Kit for Rapid Determination of Bacterial Load. Front. Microbiol. 2019, 10, e801. [Google Scholar] [CrossRef]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano 2015, 2, 630–644. [Google Scholar] [CrossRef]
- Ismayilov, I.T.; Stepanov, N.A.; Efremenko, E.N.; Abbasov, V.M. Evaluation of biocidal properties of vegetable oil-based corrosion inhibitors using bioluminescent enzymatic method. Moscow Univ. Chem. Bull. 2015, 70, 197–201. [Google Scholar] [CrossRef]
- Stepanov, N.; Senko, O.; Perminova, I.; Efremenko, E. A new approach to assess the effect of various humic compounds on the metabolic activity of cells participating in methanogenesis. Sustainability 2019, 11, 3158. [Google Scholar] [CrossRef]
- Piret, J.-P.; Bondarenko, O.M.; Boyles, M.S.P.; Himly, M.; Ribeiro, A.R.; Benetti, F.; Smal, C.; Lima, B.; Potthoff, A.; Simion, M.; et al. Pan-European inter-laboratory studies on a panel of in vitro cytotoxicity and pro-inflammation assays for nanoparticles. Arch. Toxicol. 2017, 91, 2315–2330. [Google Scholar] [CrossRef] [PubMed]
- Leont’ev, V.K.; Pogorel’skii, I.P.; Frolov, G.A.; Karasenkov, Y.N.; Gusev, A.A.; Latuta, N.V.; Borozdkin, L.L.; Stefantsova, D.S. Antibacterial Properties of Aqueous Colloid Solutions of Metal and Metal Oxide Nanoparticles against Dental Plaque Bacteria. Nanotechnol. Russ. 2018, 13, 195–198. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Lyagin, I.V.; Klyachko, N.L.; Bronich, T.; Zavyalova, N.V.; Jiang, Y.; Kabanov, A.V. A simple and highly effective catalytic nanozyme scavenger for organophosphorus neurotoxins. J. Control. Release 2017, 247, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Senko, O.; Stepanov, N.; Maslova, O.; Akhundov, R.; Ismailov, A.; Efremenko, E. Immobilized luminescent bacteria for the detection of mycotoxins under discrete and flow-through conditions. Biosensors 2019, 9, 63. [Google Scholar] [CrossRef]
- Shahidi, S.; Jamali, A.; Dalal Sharifi, S.; Ghomi, H. In-situ synthesis of CuO nanoparticles on cotton fabrics using spark discharge method to fabricate antibacterial textile. J. Nat. Fibers 2018, 15, 870–881. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Maslova, O.V.; Kholstov, A.V.; Senko, O.V.; Ismailov, A.D. Biosensitive element in the form of immobilized luminescent photobacteria for detecting ecotoxicants in aqueous flow-through systems. Luminescence 2016, 31, 1283–1289. [Google Scholar] [CrossRef]
- Aragonès, L.; Escudé, C.; Visa, P.; Salvi, L.; Mocé-Llivina, L. New insights for rapid evaluation of bactericidal activity: A semi-automated bioluminescent ATP assay. J. Appl. Microbiol. 2012, 113, 114–125. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Y.; Lin, Z.; Yao, Z.; Zhang, W. The joint effects on Photobacterium phosphoreum of metal oxide nanoparticles and their most likely coexisting chemicals in the environment. Aquat. Toxicol. 2014, 154, 200–206. [Google Scholar] [CrossRef]
- Mortimer, M.; Kasemets, K.; Heinlaan, M.; Kurvet, I.; Kahru, A. High throughput kinetic Vibrio fischeri bioluminescence inhibition assay for study of toxic effects of nanoparticles. Toxicol. In Vitro 2008, 22, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Deryabina, D.G.; Efremova, L.V.; Karimov, I.F.; Manukhov, I.V.; Gnuchikh, E.Y.; Miroshnikov, S.A. Comparative Sensitivity of the Luminescent Photobacterium phosphoreum, Escherichia coli, and Bacillus subtilis Strains to Toxic Effects of Carbon-Based Nanomaterials and Metal Nanoparticles. Microbiology 2016, 85, 198–206. [Google Scholar] [CrossRef]
- Schwegmann, H.; Feitz, A.J.; Frimmel, F.H. Influence of the zeta potential on the sorption and toxicity of iron oxide nanoparticles on S. cerevisiae and E. coli. J. Colloid Interface Sci. 2010, 347, 43–48. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015, 13, e91. [Google Scholar] [CrossRef] [PubMed]
- Kasemets, K.; Suppi, S.; Künnis-Beres, K.; Kahru, A. Toxicity of CuO nanoparticles to yeast Saccharomyces cerevisiae BY4741 wild-type and its nine isogenic single-gene deletion mutants. Chem. Res. Toxicol. 2013, 26, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Elbadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef]
- Monteserín, C.; Blanco, M.; Murillo, N.; Pérez-Márquez, A.; Maudes, J.; Gayoso, J.; Laza, J.M.; Hernáez, E.; Aranzabe, E.; Vilas, J.L. Novel Antibacterial and Toughened Carbon-Fibre/Epoxy Composites by the Incorporation of TiO2 Nanoparticles Modified Electrospun Nanofibre Veils. Polymers 2019, 11, 1524. [Google Scholar] [CrossRef] [PubMed]
- Salat, M.; Petkova, P.; Hoyo, J.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Durable antimicrobial cotton textiles coated sonochemically with ZnO nanoparticles embedded in an in-situ enzymatically generated bioadhesive. Carbohydr. Polym. 2018, 189, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.L.; Han, P.; Guo, L.C.; Cao, Y.Q.; Li, A.D.; Kong, J.Z.; Zhai, H.-F.; Wu, D. The antibacterial activity of Ta-doped ZnO nanoparticles. Nanoscale Res. Lett. 2015, 10, e336. [Google Scholar] [CrossRef] [PubMed]
 ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in ethanol. (B) Residual intensity of bioluminescence of immobilized cells P. phosphoreum treated by the Fe (
) nanoparticles obtained in ethanol. (B) Residual intensity of bioluminescence of immobilized cells P. phosphoreum treated by the Fe ( ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in water.
) nanoparticles obtained in water.
 ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in ethanol. (B) Residual intensity of bioluminescence of immobilized cells P. phosphoreum treated by the Fe (
) nanoparticles obtained in ethanol. (B) Residual intensity of bioluminescence of immobilized cells P. phosphoreum treated by the Fe ( ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in water.
) nanoparticles obtained in water.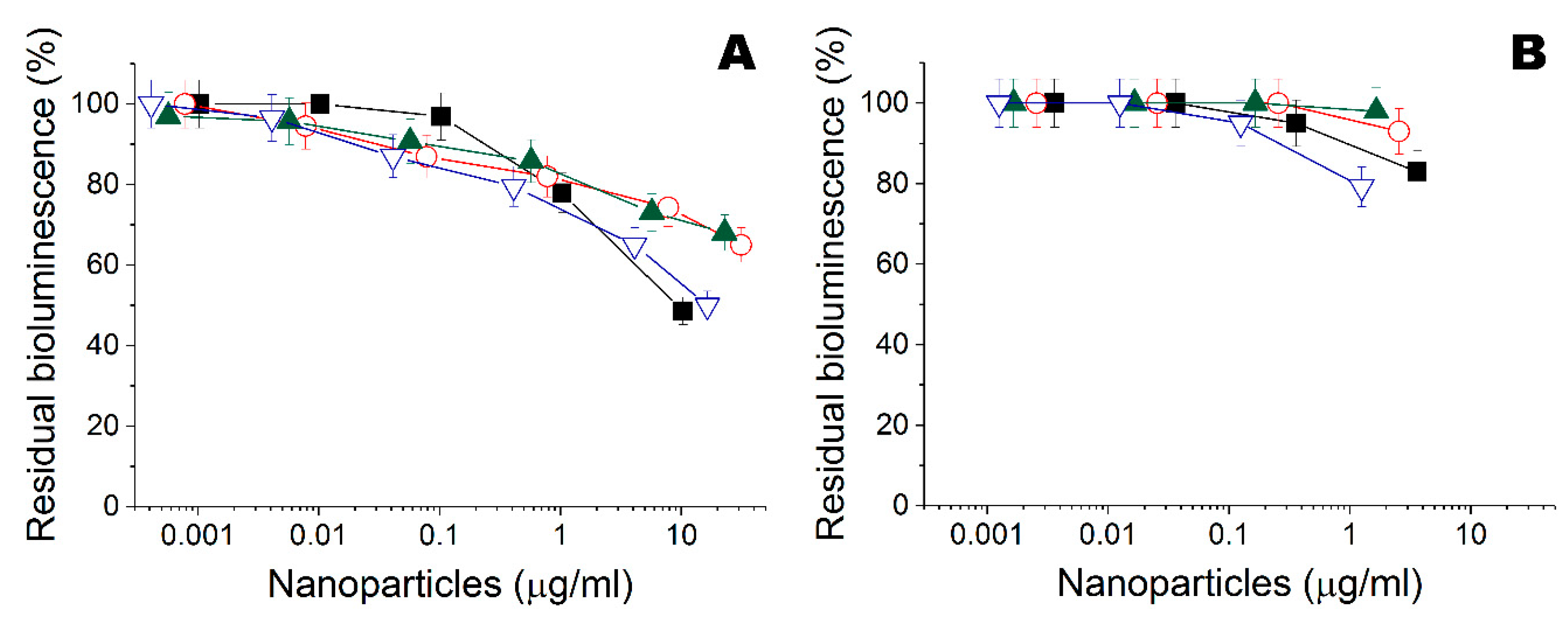
 ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in ethanol on intracellular ATP of Escherichia coli DH5α. (B) Influence of Fe (
) nanoparticles obtained in ethanol on intracellular ATP of Escherichia coli DH5α. (B) Influence of Fe ( ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in ethanol on intracellular ATP of Bacillus subtilis B-522. (C) Influence of Fe (
) nanoparticles obtained in ethanol on intracellular ATP of Bacillus subtilis B-522. (C) Influence of Fe ( ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in water on intracellular ATP of Escherichia coli DH5α. (D) Influence of Fe (
) nanoparticles obtained in water on intracellular ATP of Escherichia coli DH5α. (D) Influence of Fe ( ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in water on intracellular ATP of Bacillus subtilis B-522.
) nanoparticles obtained in water on intracellular ATP of Bacillus subtilis B-522.
 ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in ethanol on intracellular ATP of Escherichia coli DH5α. (B) Influence of Fe (
) nanoparticles obtained in ethanol on intracellular ATP of Escherichia coli DH5α. (B) Influence of Fe ( ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in ethanol on intracellular ATP of Bacillus subtilis B-522. (C) Influence of Fe (
) nanoparticles obtained in ethanol on intracellular ATP of Bacillus subtilis B-522. (C) Influence of Fe ( ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in water on intracellular ATP of Escherichia coli DH5α. (D) Influence of Fe (
) nanoparticles obtained in water on intracellular ATP of Escherichia coli DH5α. (D) Influence of Fe ( ), Ta (
), Ta ( ), Ti (
), Ti ( ), and Zn (
), and Zn ( ) nanoparticles obtained in water on intracellular ATP of Bacillus subtilis B-522.
) nanoparticles obtained in water on intracellular ATP of Bacillus subtilis B-522.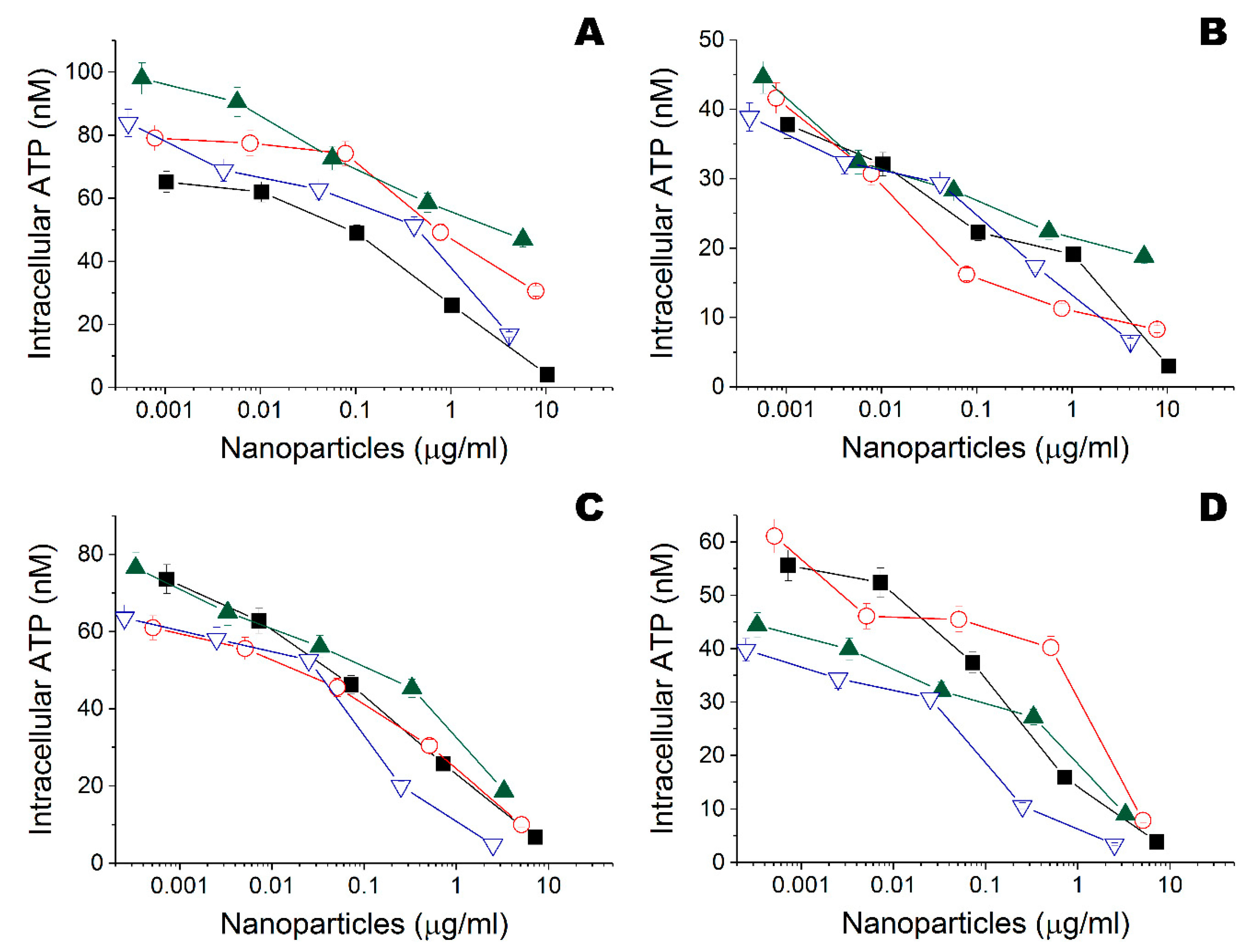
 ) and Zn (
) and Zn ( ) nanoparticles obtained in ethanol and deposited onto fibrous material, on intracellular ATP of Escherichia coli DH5α. (B) Influence of Ta (
) nanoparticles obtained in ethanol and deposited onto fibrous material, on intracellular ATP of Escherichia coli DH5α. (B) Influence of Ta ( ) and Zn (
) and Zn ( ) nanoparticles obtained in ethanol and deposited onto fibrous material, on intracellular ATP of Bacillus subtilis B-522. (C) Influence of Ta (
) nanoparticles obtained in ethanol and deposited onto fibrous material, on intracellular ATP of Bacillus subtilis B-522. (C) Influence of Ta ( ) and Zn (
) and Zn ( ) nanoparticles obtained in isopropanol and deposited onto fibrous material, on intracellular ATP of Escherichia coli DH5α. (D) Influence of Ta (
) nanoparticles obtained in isopropanol and deposited onto fibrous material, on intracellular ATP of Escherichia coli DH5α. (D) Influence of Ta ( ) and Zn (
) and Zn ( ) nanoparticles obtained in isopropanol and deposited onto fibrous material, on intracellular ATP of Bacillus subtilis B-522.
) nanoparticles obtained in isopropanol and deposited onto fibrous material, on intracellular ATP of Bacillus subtilis B-522.
 ) and Zn (
) and Zn ( ) nanoparticles obtained in ethanol and deposited onto fibrous material, on intracellular ATP of Escherichia coli DH5α. (B) Influence of Ta (
) nanoparticles obtained in ethanol and deposited onto fibrous material, on intracellular ATP of Escherichia coli DH5α. (B) Influence of Ta ( ) and Zn (
) and Zn ( ) nanoparticles obtained in ethanol and deposited onto fibrous material, on intracellular ATP of Bacillus subtilis B-522. (C) Influence of Ta (
) nanoparticles obtained in ethanol and deposited onto fibrous material, on intracellular ATP of Bacillus subtilis B-522. (C) Influence of Ta ( ) and Zn (
) and Zn ( ) nanoparticles obtained in isopropanol and deposited onto fibrous material, on intracellular ATP of Escherichia coli DH5α. (D) Influence of Ta (
) nanoparticles obtained in isopropanol and deposited onto fibrous material, on intracellular ATP of Escherichia coli DH5α. (D) Influence of Ta ( ) and Zn (
) and Zn ( ) nanoparticles obtained in isopropanol and deposited onto fibrous material, on intracellular ATP of Bacillus subtilis B-522.
) nanoparticles obtained in isopropanol and deposited onto fibrous material, on intracellular ATP of Bacillus subtilis B-522.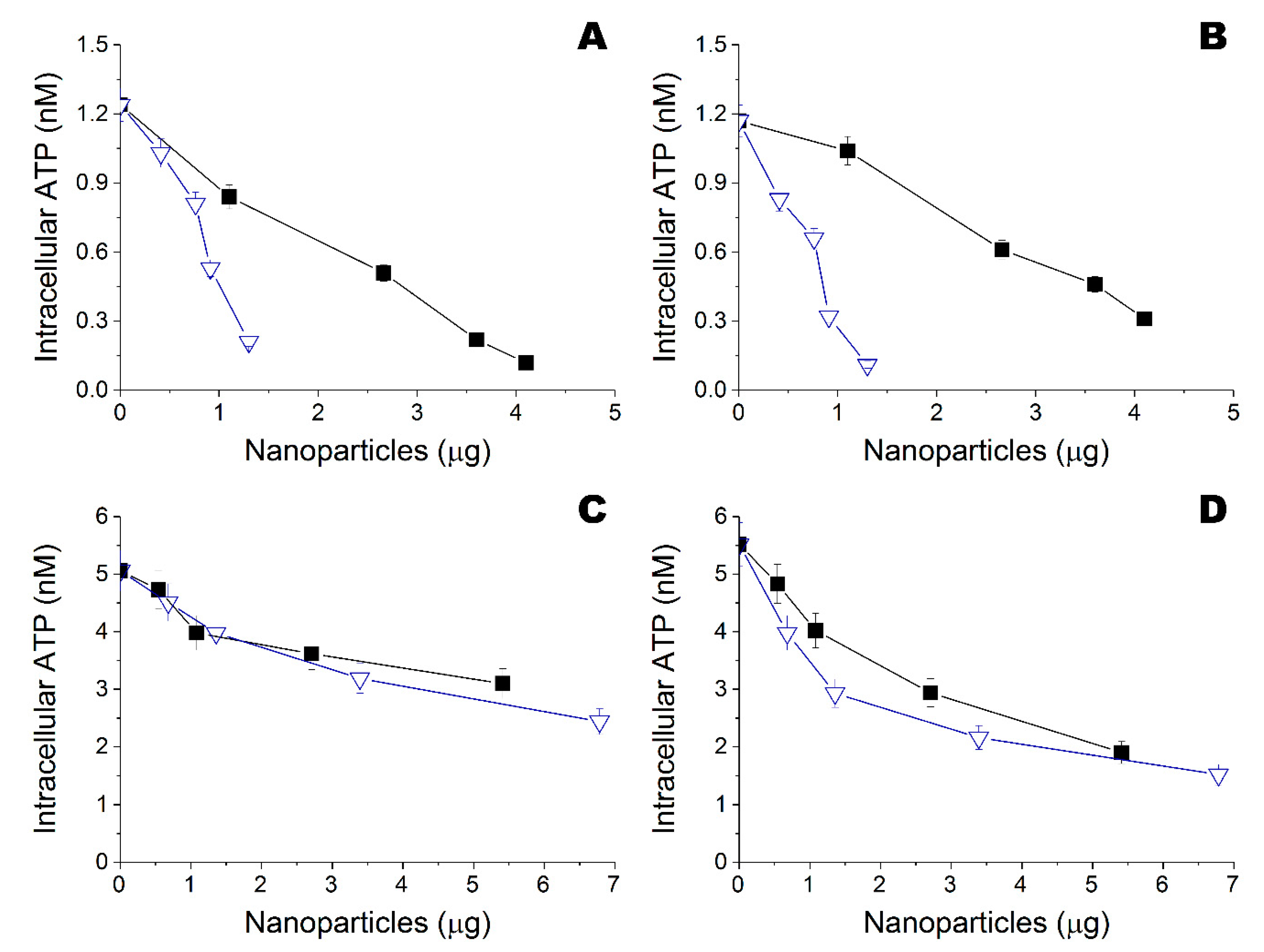
| Metal | Size by TEM (nm) | ζ-potential (mV) | Concentration (μg/mL) |
|---|---|---|---|
| FeEtOH | 2–3 | 1.4 | 7.8 |
| Fewater | 2–3 | –7.1 | 2.5 |
| TaEtOH | 1–3 | 2.7 | 18.3 |
| TaiPrOH | 1–3 | 0.05 | 15.1 |
| Tawater | 2–3 | 68.7 | 5.1 |
| TiEtOH | 1–3 | –0.8 | 5.7 |
| Tiwater | 1–2 | –0.02 | 3.3 |
| ZnEtOH | 2–5 | 6.2 | 71.7 |
| ZniPrOH | 4–10 | 1.3 | 53.1 |
| Znwater | 3–5 | 8.1 | 7.2 |
| Nanoparticles | MBC (μg/mL) | |
|---|---|---|
| E.coli | B.subtilis | |
| FeEtOH | 767 ± 139 | 36.6 ± 22.0 |
| TaEtOH | 41.9 ± 10.9 | 40.4 ± 22.0 |
| TiEtOH | 17,200 ± 15,200 | 2140 ± 1890 |
| ZnEtOH | 20.3 ± 9.7 | 27.3 ± 14.2 |
| Fewater | 34.4 ± 24.7 | 19.4 ± 5.6 |
| Tawater | 4.0 ± 1.1 | 4.8 ± 2.7 |
| Tiwater | 66.6 ± 35.3 | 26.8 ± 17.1 |
| Znwater | 23.9 ± 11.0 | 10.0 ± 3.9 |
| Antibacterial Agent | Fibrous Material | MBC (pg/cell) | |
|---|---|---|---|
| E. coli | B. subtilis | ||
| ZnEtOH nanoparticles | – | 20 ± 10 | 27 ± 14 |
| + | 105 ± 11 | 161 ± 43 | |
| TaEtOH nanoparticles | – | 42 ± 11 | 40 ± 22 |
| + | 37 ± 4 | 31 ± 1 | |
| ZniPrOH nanoparticles | + | 8970 ± 1290 | 518 ± 86 |
| TaiPrOH nanoparticles | + | 2220 ± 660 | 551 ± 229 |
| Benzalkonium chloride | + | 152 ± 16 | 133 ± 15 |
| Benzethonium chloride | + | 131 ± 13 | 106 ± 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolov, G.; Lyagin, I.; Senko, O.; Stepanov, N.; Pogorelsky, I.; Efremenko, E. Metal Nanoparticles for Improving Bactericide Functionality of Usual Fibers. Nanomaterials 2020, 10, 1724. https://doi.org/10.3390/nano10091724
Frolov G, Lyagin I, Senko O, Stepanov N, Pogorelsky I, Efremenko E. Metal Nanoparticles for Improving Bactericide Functionality of Usual Fibers. Nanomaterials. 2020; 10(9):1724. https://doi.org/10.3390/nano10091724
Chicago/Turabian StyleFrolov, George, Ilya Lyagin, Olga Senko, Nikolay Stepanov, Ivan Pogorelsky, and Elena Efremenko. 2020. "Metal Nanoparticles for Improving Bactericide Functionality of Usual Fibers" Nanomaterials 10, no. 9: 1724. https://doi.org/10.3390/nano10091724
APA StyleFrolov, G., Lyagin, I., Senko, O., Stepanov, N., Pogorelsky, I., & Efremenko, E. (2020). Metal Nanoparticles for Improving Bactericide Functionality of Usual Fibers. Nanomaterials, 10(9), 1724. https://doi.org/10.3390/nano10091724