Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
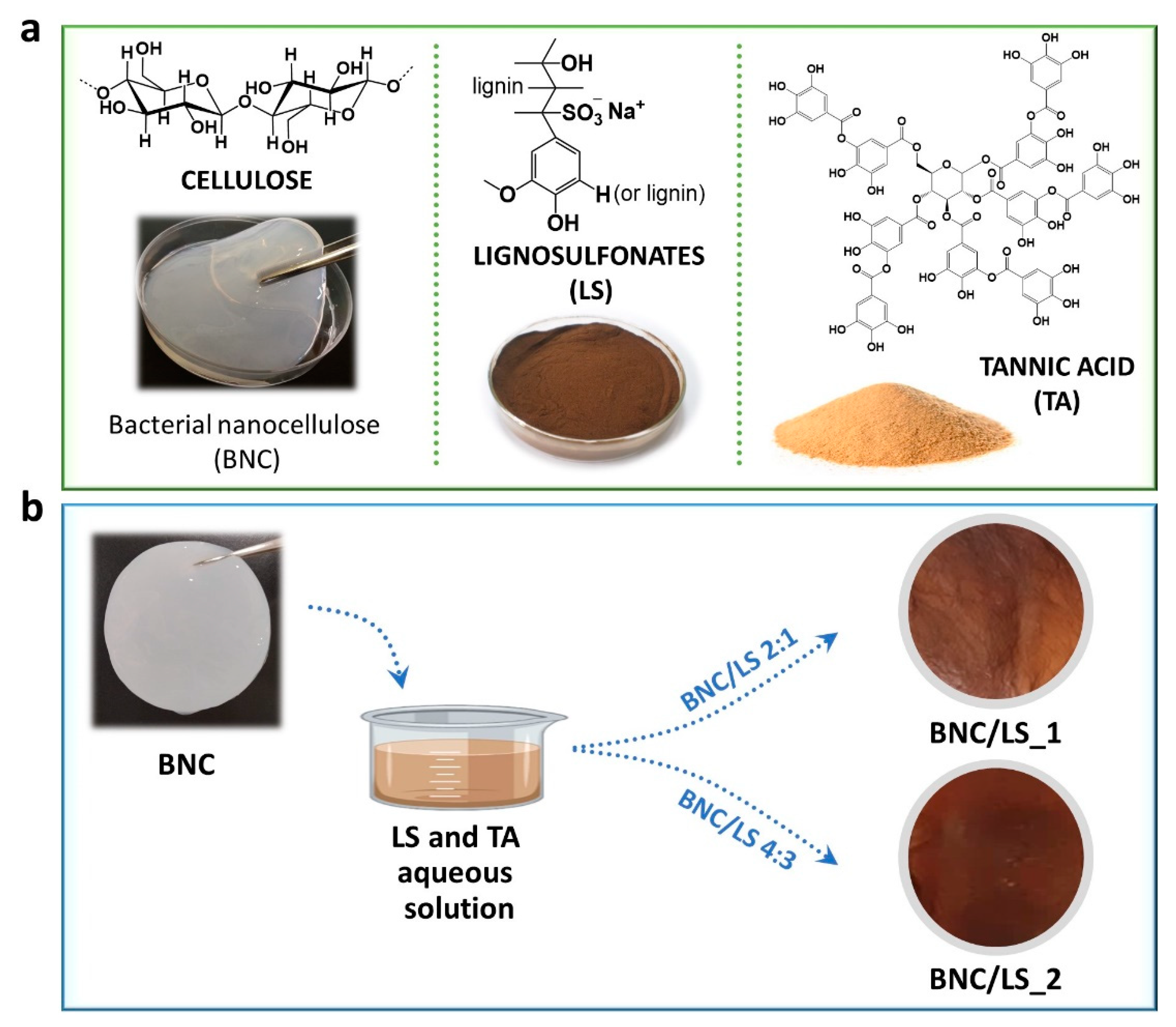
2.2. Preparation of the BNC/LS-Based Membranes
2.3. Characterization Methods
2.3.1. Thickness
2.3.2. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.3.3. Scanning Electron Microscopy (SEM) Combined with Energy Dispersive X-ray Spectroscopy (EDS)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Tensile Testing
2.3.6. Moisture-Uptake Capacity
2.3.7. Ionic Conductivity
3. Results and Discussion
3.1. Membrane Production and Characterization (Structure and Morphology)
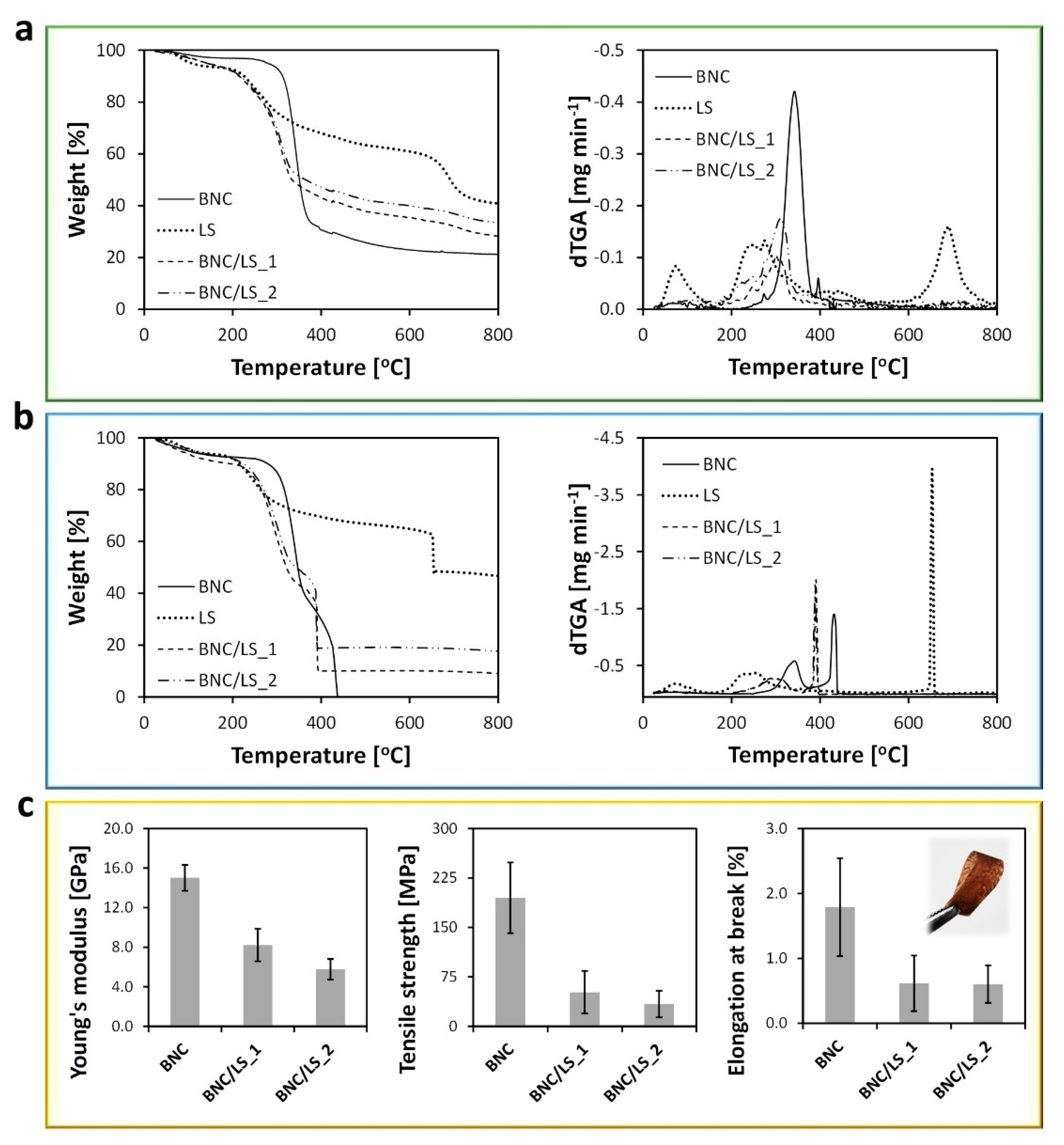
3.2. Thermal and Mechanical Properties
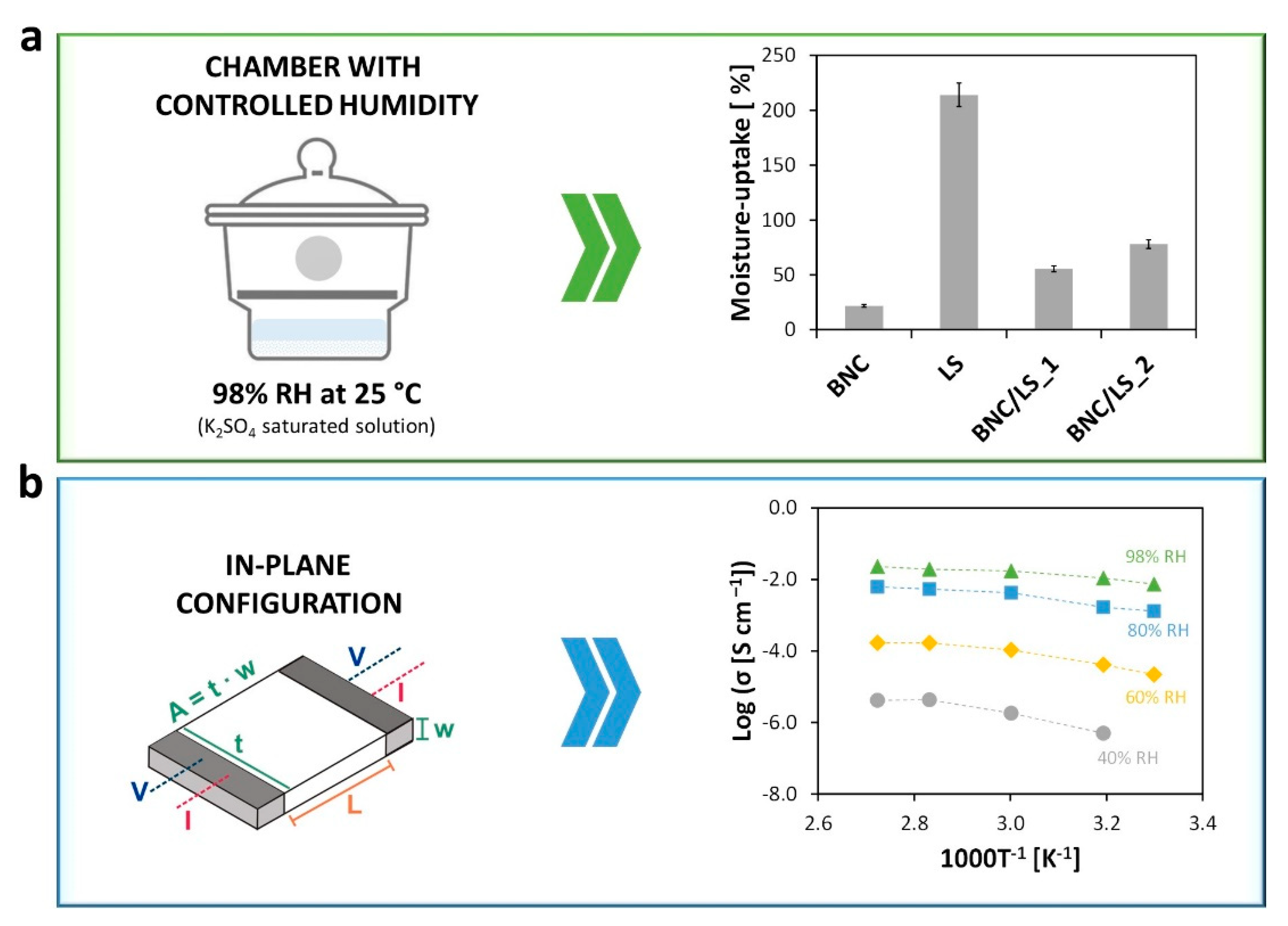
3.3. Moisture-Uptake Capacity and Ionic Conductivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scofield, M.E.; Liu, H.; Wong, S.S. A concise guide to sustainable PEMFCs: Recent advances in improving both oxygen reduction catalysts and proton exchange membranes. Chem. Soc. Rev. 2015, 44, 5836–5860. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Jacek, P.; Dourado, F.; Gama, M.; Bielecki, S. Molecular aspects of bacterial nanocellulose biosynthesis. Microb. Biotechnol. 2019, 12, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Gadim, T.D.; Loureiro, F.J.; Vilela, C.; Rosero-Navarro, N.C.; Silvestre, A.; Freire, C.; Figueiredo, F.M. Protonic conductivity and fuel cell tests of nanocomposite membranes based on bacterial cellulose. Electrochim. Acta 2017, 233, 52–61. [Google Scholar] [CrossRef]
- Vilela, C.; Silva, A.C.; Domingues, E.M.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2019, 230, 115604. [Google Scholar] [CrossRef]
- Yue, L.; Xie, Y.; Zheng, Y.; He, W.; Guo, S.; Sun, Y.; Zhang, T.; Liu, S. Sulfonated bacterial cellulose/polyaniline composite membrane for use as gel polymer electrolyte. Compos. Sci. Technol. 2017, 145, 122–131. [Google Scholar] [CrossRef]
- Vilela, C.; Martins, A.P.C.; Sousa, N.; Silvestre, A.; Figueiredo, F.M.L.; Freire, C. Poly(bis[2-(methacryloyloxy)ethyl] phosphate)/Bacterial Cellulose Nanocomposites: Preparation, Characterization and Application as Polymer Electrolyte Membranes. Appl. Sci. 2018, 8, 1145. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Figueiredo, A.G.P.R.; Rosero-Navarro, N.C.; Vilela, C.; Gamelas, J.A.F.; Barros-Timmons, A.; Neto, C.P.; Silvestre, A.; Freire, C.; Figueiredo, F.M.L. Nanostructured Bacterial Cellulose–Poly(4-styrene sulfonic acid) Composite Membranes with High Storage Modulus and Protonic Conductivity. ACS Appl. Mater. Interfaces 2014, 6, 7864–7875. [Google Scholar] [CrossRef]
- Vilela, C.; Cordeiro, D.M.; Boas, J.V.; Barbosa, P.; Nolasco, M.; Vaz, P.D.; Rudić, S.; Ribeiro-Claro, P.; Silvestre, A.J.; Oliveira, V.B.; et al. Poly(4-styrene sulfonic acid)/bacterial cellulose membranes: Electrochemical performance in a single-chamber microbial fuel cell. Bioresour. Technol. Rep. 2020, 9, 100376. [Google Scholar] [CrossRef]
- Jiang, G.-P.; Zhang, J.; Qiao, J.; Jiang, Y.-M.; Zarrin, H.; Chen, Z.; Hong, F.N. Bacterial nanocellulose/Nafion composite membranes for low temperature polymer electrolyte fuel cells. J. Power Sources 2015, 273, 697–706. [Google Scholar] [CrossRef]
- Gadim, T.D.; Vilela, C.; Loureiro, F.J.; Silvestre, A.; Freire, C.; Figueiredo, F.M. Nafion® and nanocellulose: A partnership for greener polymer electrolyte membranes. Ind. Crop. Prod. 2016, 93, 212–218. [Google Scholar] [CrossRef]
- Santos, F.M.; Barbosa, P.C.; Pereira, R.F.; Silva, M.M.; Gonçalves, H.M.; Nunes, S.C.; Figueiredo, F.L.; Valente, A.J.M.; Bermudez, V.D.Z. Proton conducting electrolytes composed of chondroitin sulfate polysaccharide and citric acid. Eur. Polym. J. 2020, 124, 109453. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. High Temperature Proton Conduction in Nanocellulose Membranes: Paper Fuel Cells. Chem. Mater. 2016, 28, 4805–4814. [Google Scholar] [CrossRef]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value-Added Chemicals from Biomass Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; U.S. Department of Energy: Washington, DC, USA, 2007.
- Moreno, A.; Sipponen, M.H. Lignin-based smart materials: A roadmap to processing and synthesis for current and future applications. Mater. Horiz. 2020. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Barati, S.; Abdollahi, M.; Ghazi, M.M.; Khoshandam, B. High temperature proton exchange porous membranes based on polybenzimidazole/lignosulfonate blends: Preparation, morphology and physical and proton conductivity properties. Int. J. Hydrogen Energy 2019, 44, 30440–30453. [Google Scholar] [CrossRef]
- Zhang, X.; Benavente, J.; Garcia-Valls, R. Lignin-based membranes for electrolyte transference. J. Power Sources 2005, 145, 292–297. [Google Scholar] [CrossRef]
- Gonggo, S.T.; Bundjali, B.; Hariyawati, K.; Arcana, I.M. The influence of nano-silica on properties of sulfonated polystyrene-lignosulfonate membranes as proton exchange membranes for direct methanol fuel cell application. Adv. Polym. Technol. 2017, 37, 1859–1867. [Google Scholar] [CrossRef]
- Keshk, S.M.A.S. Physical properties of bacterial cellulose sheets produced in presence of lignosulfonate. Enzym. Microb. Technol. 2006, 40, 9–12. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. Phys. Chem. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Ghazali, N.A.; Naganawa, S.; Masuda, Y. Feasibility Study of Tannin-Lignosulfonate Drilling Fluid System for Drilling Geothermal Prospect. In Proceedings of the 43rd Workshop on Geothermal Reservoir Engineering, Standford University, Stanford, CA, USA, 12–14 February 2018. SGP-TR-213. [Google Scholar]
- Chen, W.; Li, N.; Ma, Y.; Minus, M.L.; Benson, K.; Lu, X.; Wang, X.; Ling, X.; Zhu, H. Superstrong and Tough Hydrogel through Physical Cross-Linking and Molecular Alignment. Biomacromolecules 2019, 20, 4476–4484. [Google Scholar] [CrossRef] [PubMed]
- Erel-Unal, I.; Sukhishvili, S.A. Hydrogen-Bonded Multilayers of a Neutral Polymer and a Polyphenol. Macromolecules 2008, 41, 3962–3970. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef]
- Vilela, C.; Oliveira, H.; Almeida, A.; Silvestre, A.; Freire, C. Nanocellulose-based antifungal nanocomposites against the polymorphic fungus Candida albicans. Carbohydr. Polym. 2019, 217, 207–216. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, T.; Zhu, M.-F. A comparison of the surface properties of lignin and sulfonated lignins by FTIR spectroscopy and wicking technique. Colloids Surf. Physicochem. Eng. Asp. 2008, 320, 57–60. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall Ltd.: London, UK, 1975; ISBN 0412138506. [Google Scholar]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanoparticle Res. 2017, 19, 273. [Google Scholar] [CrossRef]
- Pa’e, N.; Salehudin, M.H.; Hassan, N.D.; Marsin, A.M.; Muhamad, I.I. Thermal behavior of bacterial cellulose-based hydrogels with other composites and related instrumental analysis. In Cellulose-Based Superabsorbent Hydrogels; Polymers and Polymeric Composites: A Reference Series; Mondal, M.I.H., Ibrahim, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 763–787. [Google Scholar]
- Yao, J.; Odelius, K.; Hakkarainen, M. Carbonized lignosulfonate-based porous nanocomposites for adsorption of environmental contaminants. Funct. Compos. Mater. 2020, 1, 1–12. [Google Scholar]
- Vilela, C.; Gadim, T.D.O.; Silvestre, A.; Freire, C.; Figueiredo, F.M.L. Nanocellulose/poly(methacryloyloxyethyl phosphate) composites as proton separator materials. Cellulose 2016, 23, 3677–3689. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, N.; Pinto, R.J.B.; Silvestre, A.; Figueiredo, F.M.; Freire, C. Exploiting poly(ionic liquids) and nanocellulose for the development of bio-based anion-exchange membranes. Biomass Bioenergy 2017, 100, 116–125. [Google Scholar] [CrossRef]
- DuPontTM Nafion® N115, N117, N1110—Ion Exchange Materials, Product Bulletin P-12. Available online: http://fuelcellearth.com/pdf/nafion-N115-N117-N1110.pdf (accessed on 7 July 2020).
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.-T.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Saito, M.; Arimura, N.; Hayamizu, K.; Okada, T. Mechanisms of Ion and Water Transport in Perfluorosulfonated Ionomer Membranes for Fuel Cells. J. Phys. Chem. B 2004, 108, 16064–16070. [Google Scholar] [CrossRef]
- Petrowsky, M.; Frech, R. Temperature Dependence of Ion Transport: The Compensated Arrhenius Equation. J. Phys. Chem. B 2009, 113, 5996–6000. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Domingues, E.M.; Sousa, N.; Ferreira, P.; Figueiredo, F.M.L. Protonic conductivity and viscoelastic behaviour of Nafion® membranes with periodic mesoporous organosilica fillers. Int. J. Hydrog. Energy 2014, 39, 5338–5349. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Domingues, E.M.; Sousa, N.; Ferreira, P.; Figueiredo, F.M. Meso-structured organosilicas as fillers for Nafion® membranes. Solid State Ion. 2014, 262, 324–327. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.A.; Kuznetsov, A.M.; Ulstrup, J.; Walbran, S. Mechanisms of Proton Conductance in Polymer Electrolyte Membranes. J. Phys. Chem. B 2001, 105, 3646–3662. [Google Scholar] [CrossRef]
- Miyake, T.; Rolandi, M. Grotthuss mechanisms: From proton transport in proton wires to bioprotonic devices. J. Phys. Condens. Matter 2015, 28, 023001. [Google Scholar] [CrossRef]
- Cele, N.; Ray, S.S. Recent Progress on Nafion-Based Nanocomposite Membranes for Fuel Cell Applications. Macromol. Mater. Eng. 2009, 294, 719–738. [Google Scholar] [CrossRef]
- Gaur, S.S.; Dhar, P.; Sonowal, A.; Sharma, A.; Kumar, A.; Katiyar, V. Thermo-mechanically stable sustainable polymer based solid electrolyte membranes for direct methanol fuel cell applications. J. Membr. Sci. 2017, 526, 348–354. [Google Scholar] [CrossRef]
- Esmaielzadeh, S.; Ahmadizadegan, H. Construction of proton exchange membranes under ultrasonic irradiation based on novel fluorine functionalizing sulfonated polybenzimidazole/cellulose/silica bionanocomposite. Ultrason. Sonochem. 2018, 41, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.C.; Pereira, R.F.P.; Sousa, N.; Silva, M.M.; Almeida, P.C.; Figueiredo, F.M.L.; Bermudez, V.D.Z. Eco-Friendly Red Seaweed-Derived Electrolytes for Electrochemical Devices. Adv. Sustain. Syst. 2017, 1, 1700070. [Google Scholar] [CrossRef]

| Membrane | WBNC:WLSa | WLS/Vtotal [mg cm−3] a | Thickness [μm] |
|---|---|---|---|
| BNC | – | – | 69 ± 11 |
| BNC/LS_1 | 2:1 | 395 ± 16 | 75 ± 10 |
| BNC/LS_2 | 4:3 | 547 ± 19 | 85 ± 9 |
| Temperature [°C] | Ionic Conductivity [S cm−1] | |||
|---|---|---|---|---|
| 40% RH | 60% RH | 80% RH | 98% RH | |
| 30 | – | 2.2 × 10−5 | 1.3 × 10−3 | 7.3 × 10−3 |
| 40 | 5.0 × 10−7 | 4.1 × 10−5 | 1.7 × 10−3 | 1.1 × 10−2 |
| 60 | 1.8 × 10−6 | 1.1 × 10−4 | 4.2 × 10−3 | 1.7 × 10−2 |
| 80 | 4.3 × 10−6 | 1.7 × 10−4 | 5.4 × 10−3 | 1.9 × 10−2 |
| 94 | 4.1 × 10−6 | 1.7 × 10−4 | 6.1 × 10−3 | 2.3 × 10−2 |
| Components a | Conductivity a | Ref. | |
|---|---|---|---|
| Partially biobased | |||
| CNCs/CH/PVA | 0.642 mS cm−1 (IP, 25 °C, fully hydrated) | [46] | |
| CNFs/PBI | 66.6 mS cm−1 (IP, 140 °C) | [47] | |
| BNC/Nafion® | 140 mS cm−1 (IP, 94 °C, 98% RH) | [12] | |
| BNC/PSSA | 185 mS cm−1 (IP, 94 °C, 98% RH) | [5] | |
| BNC/PMOEP | 100 mS cm−1 (TP, 80 °C, 98% RH) | [35] | |
| BNC/P(bisMEP) | 30 mS cm−1 (IP, 80 °C, 98% RH) | [8] | |
| k-carrageenan/IL | 186 mS cm−1 (IP, 60 °C, 98% RH) | [48] | |
| LS/PBI | 187 mS cm−1 (IP, 160 °C, anhydrous) | [18] | |
| LS/PSSA/nano-silica | 406 mS cm−1 (TP, 25 °C, fully hydrated) | [20] | |
| Fully biobased | |||
| BNC/LS | 23 mS cm−1 (IP, 94 °C, 98% RH) | Present study | |
| BNC/Fucoidan | 1.6 mS cm−1 (TP, 94 °C, 98% RH) | [6] | |
| CNCs | 2.5 mS cm−1 (TP, 90 °C, 100% RH) 4.6 mS cm−1 (TP, 120 °C, 100% RH) | [14] | |
| Chondroitin sulfate/citric acid | 37 mS cm−1 (TP, 25 °C, 98% RH) | [13] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, C.; Morais, J.D.; Silva, A.C.Q.; Muñoz-Gil, D.; Figueiredo, F.M.L.; Silvestre, A.J.D.; Freire, C.S.R. Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells. Nanomaterials 2020, 10, 1713. https://doi.org/10.3390/nano10091713
Vilela C, Morais JD, Silva ACQ, Muñoz-Gil D, Figueiredo FML, Silvestre AJD, Freire CSR. Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells. Nanomaterials. 2020; 10(9):1713. https://doi.org/10.3390/nano10091713
Chicago/Turabian StyleVilela, Carla, João D. Morais, Ana Cristina Q. Silva, Daniel Muñoz-Gil, Filipe M. L. Figueiredo, Armando J. D. Silvestre, and Carmen S. R. Freire. 2020. "Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells" Nanomaterials 10, no. 9: 1713. https://doi.org/10.3390/nano10091713
APA StyleVilela, C., Morais, J. D., Silva, A. C. Q., Muñoz-Gil, D., Figueiredo, F. M. L., Silvestre, A. J. D., & Freire, C. S. R. (2020). Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells. Nanomaterials, 10(9), 1713. https://doi.org/10.3390/nano10091713










