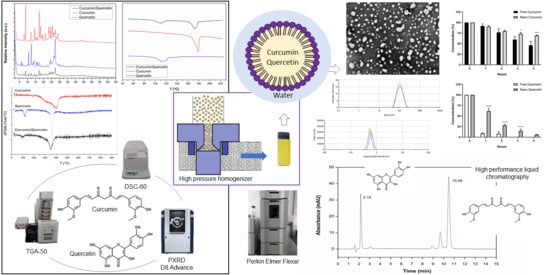
Curcumin and Quercetin-Loaded Nanoemulsions: Physicochemical Compatibility Study and Validation of a Simultaneous Quantification Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Analytical Method Development
2.2. Materials for Nanoemulsion Production and Characterization
2.3. Compatibility Study of Curcumin and Quercetin
2.3.1. Preparation of Curcumin/Quercetin Binary Mixtures
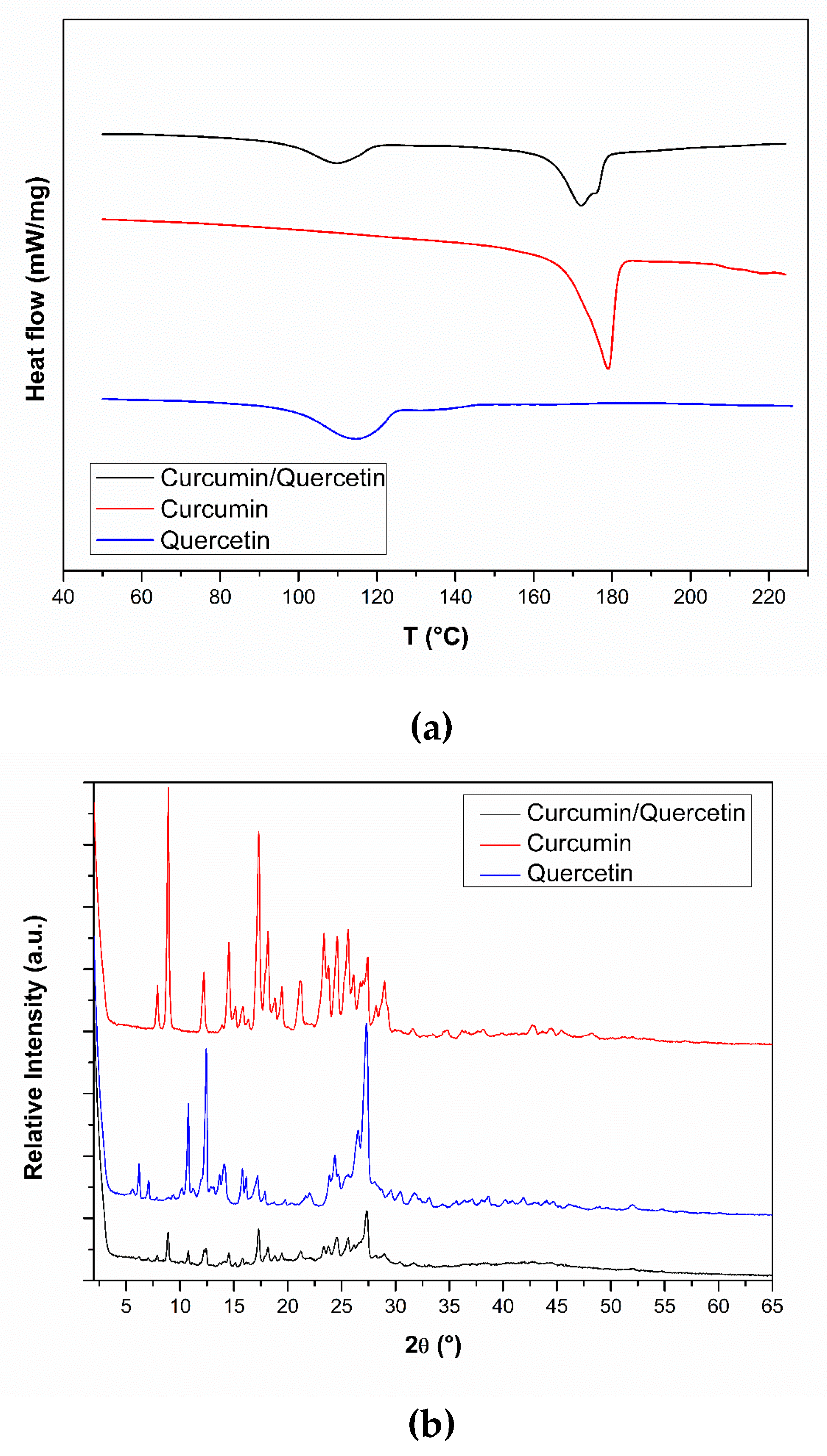
2.3.2. Thermal Analyses
2.3.3. X-ray Powder Diffraction (PXRD)
2.3.4. Equipment and Chromatographic Conditions
2.3.5. Preparation of Stock and Working Solutions
2.3.6. HPLC Method Validation
Linearity Range
Selectivity and System Suitability
Sensitivity
Precision and Accuracy
Robustness
Stability
2.3.7. Application of the Method
Nanoemulsion Preparation
Size and Zeta Potential Measurements
Determination of Curcumin and Quercetin Concentrations in the Nanoemulsion
Morphologic Evaluation
2.4. Data Presentation and Statistical Analysis
3. Results
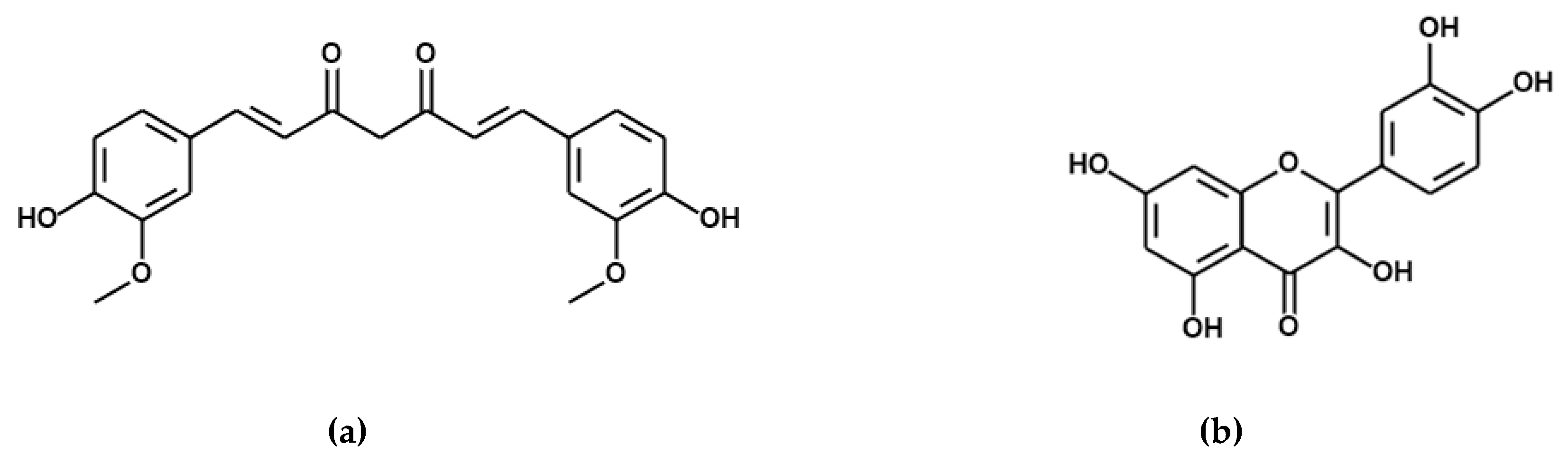
3.1. Pre-Formulation Studies with Curcumin and Quercetin
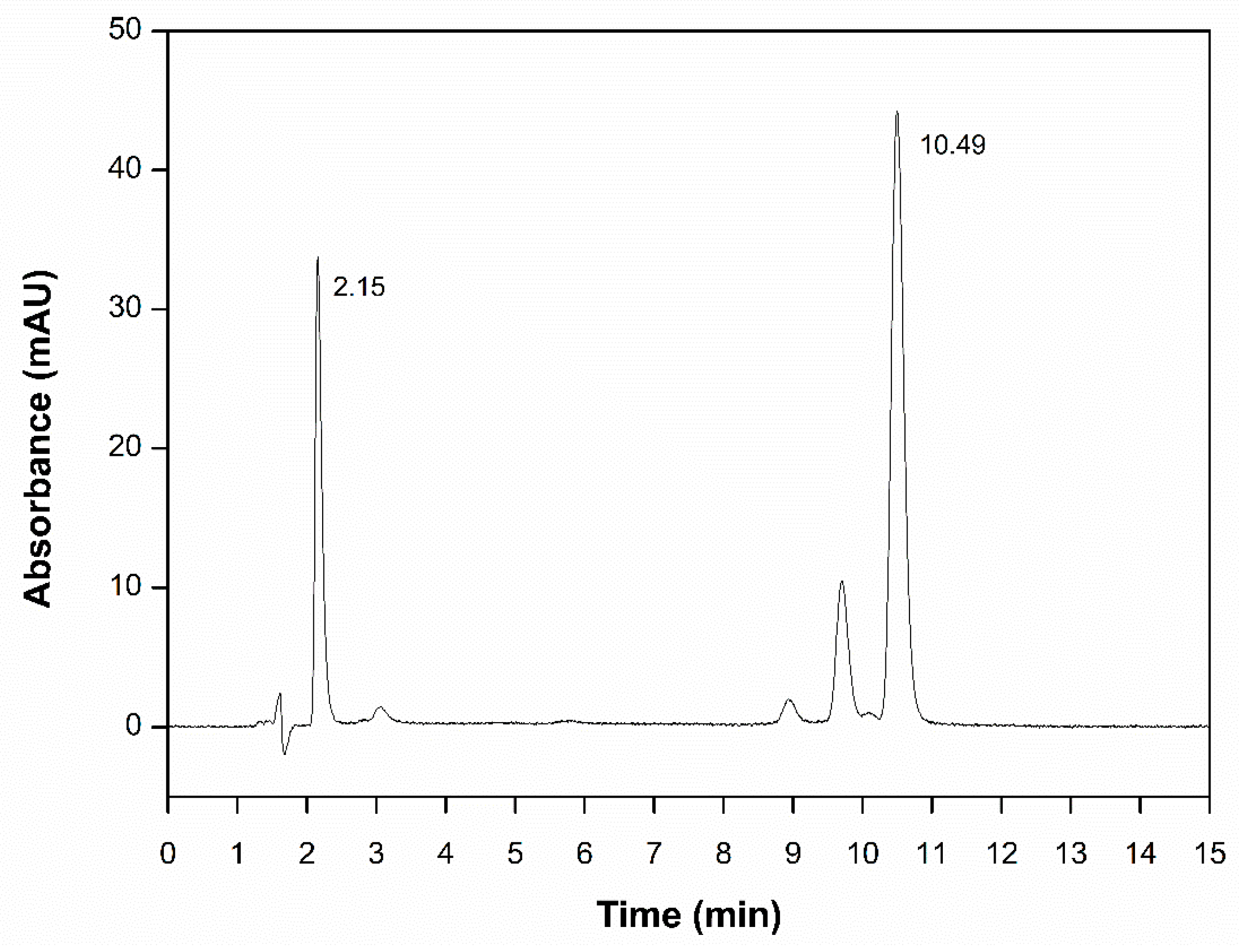
3.2. Method Development and Validation
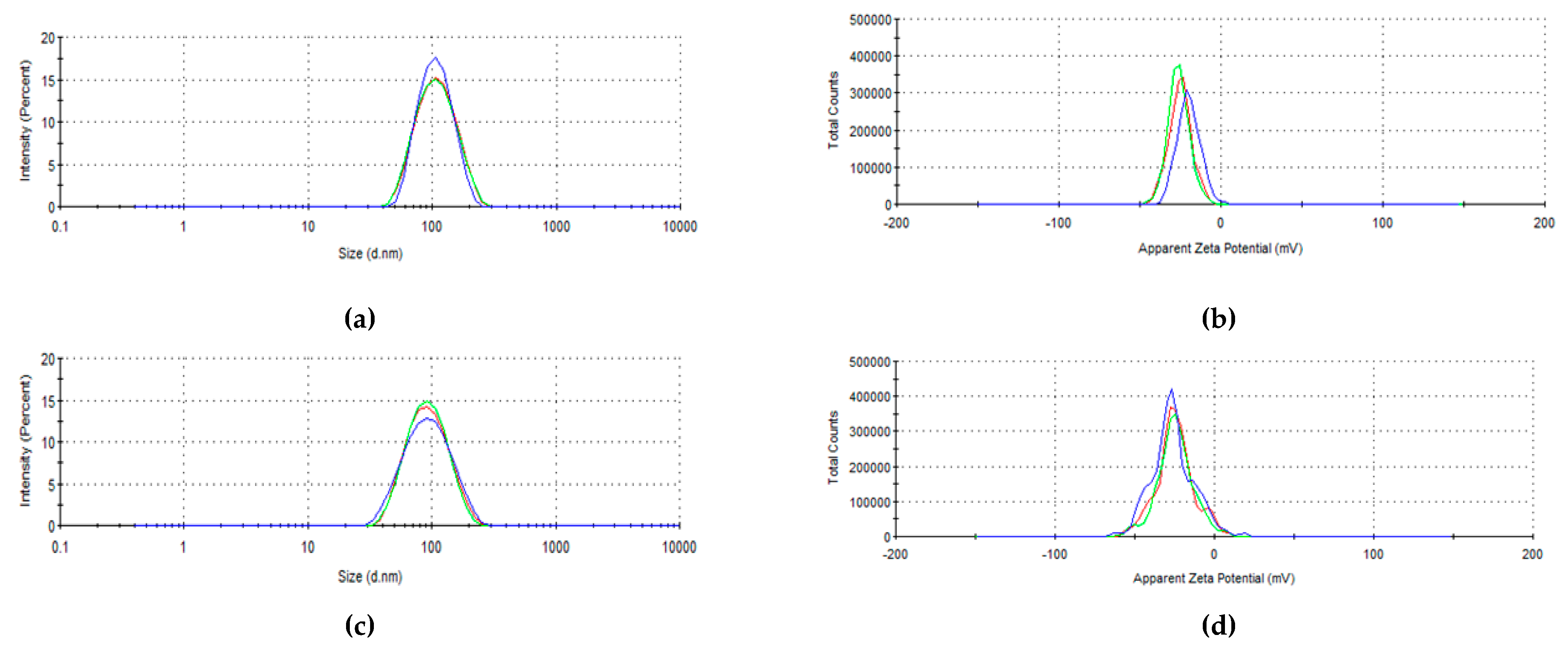
3.3. Size, Polydispersity Index, and Zeta Potential
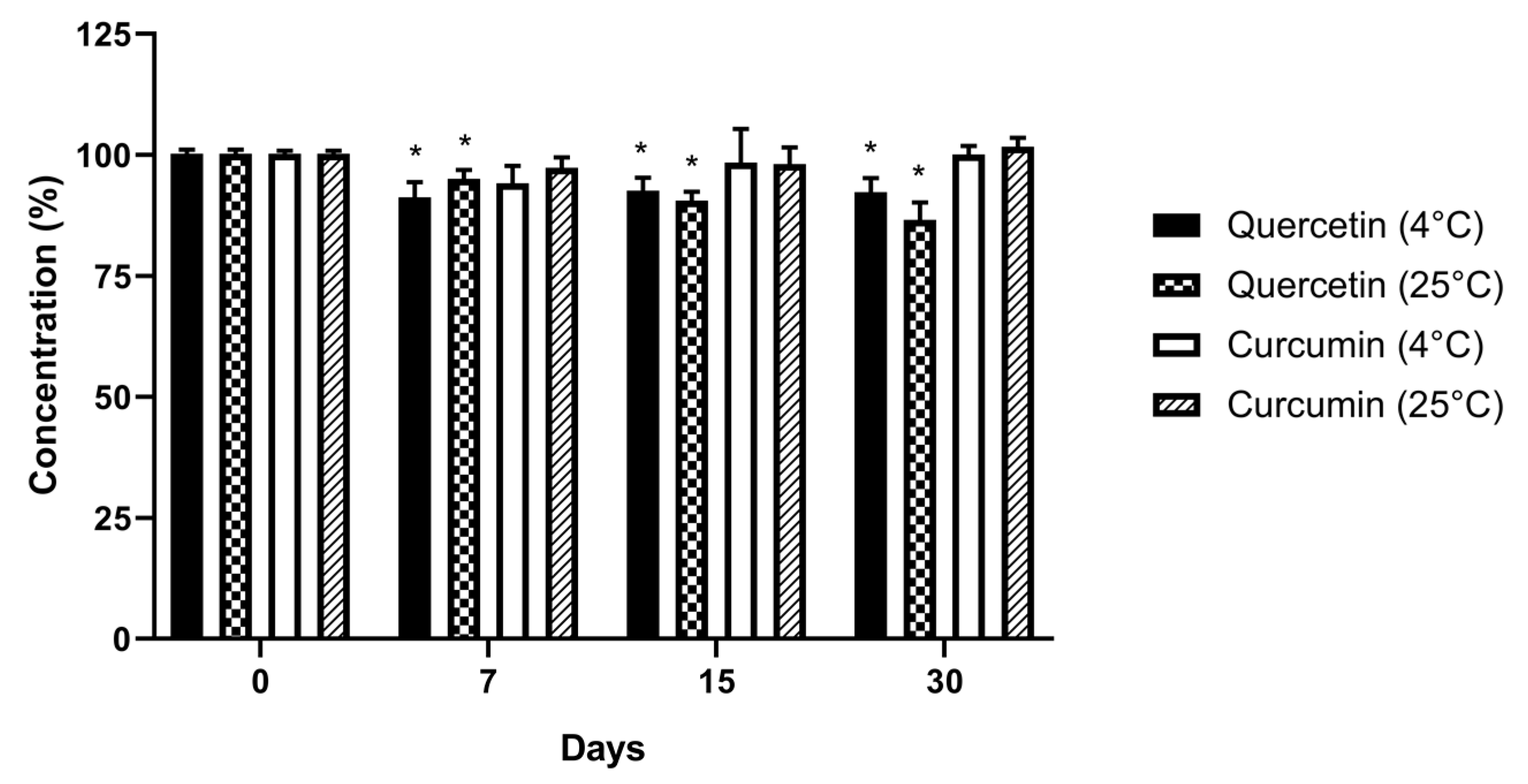
3.4. Determination of the Concentration of Curcumin and Quercetin in Nanoemulsion Using HPLC Analyses
3.5. Transmission Electron Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Cheong, E. Synthetic and naturally occurring COX-2 inhibitors suppress proliferation in a human oesophageal adenocarcinoma cell line (OE33) by inducing apoptosis and cell cycle arrest. Carcinogenesis 2004, 25, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 2017, 171, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Gamba, P.; Badilli, U.; Gargiulo, S.; Maina, M.; Guina, T.; Calfapietra, S.; Biasi, F.; Cavalli, R.; Poli, G.; et al. Loading into Nanoparticles Improves Quercetin’s Efficacy in Preventing Neuroinflammation Induced by Oxysterols. PLoS ONE 2014, 9, e96795. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R. Studies on Dissolution Behaviour of Nanoparticulate Curcumin Formulation. Adv. Nanopart. 2013, 2, 51–59. [Google Scholar] [CrossRef]
- Sarker, M.R.; Franks, S.F. Efficacy of curcumin for age-associated cognitive decline: A narrative review of preclinical and clinical studies. GeroScience 2018, 40, 73–95. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Motevalian, M.; Fatima, S.; Faraji, F.; Mozaffari, S. The Neuroprotective Effect of Curcumin Against Nicotine-Induced Neurotoxicity is Mediated by CREB–BDNF Signaling Pathway. Neurochem. Res. 2017, 42, 2921–2932. [Google Scholar] [CrossRef]
- Tsai, Y.-M.; Chien, C.-F.; Lin, L.-C.; Tsai, T.-H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood–brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell Longev. 2019, 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Garaschuk, O. Imaging microcircuit function in healthy and diseased brain. Exp. Neurol. 2013, 242, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Verdile, G.; Fuller, S.J.; Martins, R.N. The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis. 2015, 84, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Cimini, A.; Peluso, G.; Giordano, A.; Melone, M. Nano-delivery systems for encapsulation of dietary polyphenols: An experimental approach for neurodegenerative diseases and brain tumors. Biochem. Pharmacol. 2018, 154, 303–317. [Google Scholar] [CrossRef]
- Pereira, G.; Rawling, T.; Pozzoli, M.; Pazderka, C.; Chen, Y.; Dunstan, C.; Murray, M.; Sonvico, F. Nanoemulsion-Enabled Oral Delivery of Novel Anticancer ω-3 Fatty Acid Derivatives. Nanomaterials 2018, 8, 825. [Google Scholar] [CrossRef]
- Perrone, L.; Sampaolo, S.; Melone, M. Bioactive Phenolic Compounds in the Modulation of Central and Peripheral Nervous System Cancers: Facts and Misdeeds. Cancers 2020, 12, 454. [Google Scholar] [CrossRef]
- Di Martino, P.; Censi, R.; Gigliobianco, M.R.; Zerrillo, L.; Magnoni, F.; Agas, D.; Quaglia, W.; Lupidi, G. Nano-medicine Improving the Bioavailability of Small Molecules for the Prevention of Neurodegenerative Diseases. CPD 2017, 23, 1897–1908. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.-M.; Zhang, P.-Y. Protective effects of curcumin and quercetin during benzo(a)pyrene induced lung carcinogenesis in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 1736–1743. [Google Scholar]
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ghadi, Z.; Dinarvand, R.; Asemi, N.; Amiri, F.; Ebrahimnejad, P. Preparation, characterization and in vivo evaluation of novel hyaluronan containing niosomes tailored by Box-Behnken design to co-encapsulate curcumin and quercetin. Eur. J. Pharm. Sci. 2019, 130, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.; Pal, M.; Ray, R. Synergism of co-delivered nanosized antioxidants displayed enhanced anticancer efficacy in human colon cancer cell lines. Bioact. Mater. 2017, 2, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Mansourizadeh, F.; Alberti, D.; Bitonto, V.; Tripepi, M.; Sepehri, H.; Khoee, S.; Crich, S. Efficient synergistic combination effect of Quercetin with Curcumin on breast cancer cell apoptosis through their loading into Apo ferritin cavity. Coll. Surf. B Biointerfaces 2020, 191, 110982. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Coll. Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Thakur, N.; Garg, G.; Sharma, P.K.; Kumar, N. Nanoemulsions: A Review on Various Pharmaceutical Application. Glob. J. Pharmacol. 2012, 6, 222–225. [Google Scholar] [CrossRef]
- Bonferoni, M.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “Nose-to-Brain” Drug Delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef]
- Comfort, C.; Garrastazu, G.; Pozzoli, M.; Sonvico, F. Opportunities and Challenges for the Nasal Administration of Nanoemulsions. Curr. Top. Med. Chem. 2015, 15, 356–368. [Google Scholar] [CrossRef]
- Prasad, C.; Bhatia, E.; Banerjee, R. Curcumin Encapsulated Lecithin Nanoemulsions: An Oral Platform for Ultrasound Mediated Spatiotemporal Delivery of Curcumin to the Tumor. Sci. Rep. 2020, 10, 8587. [Google Scholar] [CrossRef]
- Ellis, L.Z.; Cohen, J.L.; High, W.; Stewart, L. Melanoma In Situ Treated Successfully Using Imiquimod After Nonclearance with Surgery: Review of the Literature. Dermatol. Surg. 2012, 38, 937–946. [Google Scholar] [CrossRef]
- Algahtani, M.; Ahmad, M.; Nourein, I.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kumar, R.; Mehta, S. Formulation of saponin stabilized nanoemulsion by ultrasonic method and its role to protect the degradation of quercitin from UV light. Ultrason. Sonochem. 2016, 31, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Strickley, R.G. Solubilizing Excipients in Oral and Injectable Formulations. Pharm. Res. 2004, 2, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.M.D. Enhanced neuroprotective effect of fish oil in combination with quercetin against 3-nitropropionic acid induced oxidative stress in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 83–92. [Google Scholar] [CrossRef] [PubMed]
- ICH Q2 (R1). Validation of Analytical Procedures: Text and Methodology; ICH: Geneva, Switzerland, 1995. [Google Scholar]
- Center for Drug Evaluation and Research, US Food and Drug Administration. Reviwer Guidance, Validation of Chromatographic Methods; Center for Drug Evaluation and Research, US Food and Drug Administration: Rockville, MD, USA, 1994.
- Borghetti, G.S.; Costa, I.M.; Petrovick, P.R.; Pereira, V.P.; Bassani, V.L. Characterization of different samples of quercetin in solid-state: Indication of polymorphism occurrence. Pharmazie 2006, 61, 802–804. [Google Scholar] [PubMed]
- Borghetti, G.S.; Carini, J.P.; Honorato, S.B.; Ayala, A.P.; Moreira, J.C.F.; Bassani, V.L. Physicochemical properties and thermal stability of quercetin hydrates in the solid state. Thermochim. Acta 2012, 539, 109–114. [Google Scholar] [CrossRef]
- Olejniczak, S.; Potrzebowski, M.J. Solid state NMR studies and density functional theory (DFT) calculations of conformers of quercetin. Org. Biomol. Chem. 2004, 2, 2315. [Google Scholar] [CrossRef]
- Kiran, G.; Kulkarni, A.P. Design, Synthesis and Evaluation of Quercetin-Meclofenamic acid Conjugate: A Mutual Pro-drug for Safer NSAIDs. Asian J. Med. Pharm. Res. 2013, 3, 18–23. [Google Scholar]
- Sayyar, Z.; Jafarizadeh-Malmiri, H. Temperature Effects on Thermodynamic Parameters and Solubility of Curcumin O/W Nanodispersions Using Different Thermodynamic Models. Int. J. Food Eng. 2019, 15. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, W.; Wang, F.; Chen, Z. Simultaneous determination of three curcuminoids in Curcuma longa L. by high performance liquid chromatography coupled with electrochemical detection. J. Pharm. Anal. 2014, 4, 325–330. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Sonvico, F. Development and validation of a RP-HPLC method for the simultaneous detection and quantification of simvastatin’s isoforms and coenzyme Q10 in lecithin/chitosan nanoparticles. J. Pharm. Biomed. Anal. 2018, 155, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Baharate, S.S.; Bharate, S.B.; Bajaj, A.N. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: A comprehensive review. J. Excip. Food Chem. 2010, 1, 3–26. [Google Scholar] [CrossRef]
- Ceschel, G.C.; Badiello, R.; Ronchi, C.; Maffei, P. Degradation of components in drug formulations: A comparison between HPLC and DSC methods. J. Pharm. Biomed. Anal. 2003, 32, 1067–1072. [Google Scholar] [CrossRef]
- Fornaguera, C.; Solans, C. Analytical Methods to Characterize and Purify Polymeric Nanoparticles. Int. J. Pol. Sci. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.; Benoit, J. A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharm. Res. 2002, 19, 875–880. [Google Scholar] [CrossRef]
- Dora, C.L.; Silva, L.F.C.; Putaux, J.-L.; Nishiyama, Y.; Pignot-Paintrand, I.; Borsali, R.; Lemos-Senna, E. Poly(ethylene glycol) Hydroxystearate-Based Nanosized Emulsions: Effect of Surfactant Concentration on Their Formation and Ability to Solubilize Quercetin. J. Biomed. Nanotechnol. 2012, 8, 202–210. [Google Scholar] [CrossRef]
- Riccardi, C.; Fàbrega, C.; Grijalvo, S.; Vitiello, G.; D’Errico, G.; Eritja, R.; Montesarchio, D. AS1411-decorated niosomes as effective nanocarriers for Ru(iii)-based drugs in anticancer strategies. J. Mater. Chem. B 2018, 6, 5368–5384. [Google Scholar] [CrossRef]
- Lv, S.; Gu, J.; Zhang, R.; Zhang, Y.; Tan, H.; McClements, D. Vitamin E Encapsulation in Plant-Based Nanoemulsions Fabricated Using Dual-Channel Microfluidization: Formation, Stability, and Bioaccessibility. J. Agric. Food Chem. 2018, 66, 10532–10542. [Google Scholar] [CrossRef]
- Real, D.; Hoffmann, S.; Leonardi, D.; Salomon, C.; Goycoolea, F. Chitosan-based nanodelivery systems applied to the development of novel triclabendazole formulations. PLoS ONE 2018, 13, e0207625. [Google Scholar] [CrossRef]



| Time (min) | Eluent A (%) | Eluent B (%) | Flow Rate (mL/min) |
|---|---|---|---|
| 0.01 | 60 | 40 | 1.0 |
| 5.00 | 60 | 40 | 1.0 |
| 6.00 | 50 | 50 | 1.0 |
| 15.00 | 50 | 50 | 1.0 |
| Parameter | Condition |
|---|---|
| Column temperature | 35 °C |
| 40 °C | |
| 45 °C | |
| Mobile phase composition (aqueous phosphoric acid 1%:acetonitrile) | 35:65/45:55 |
| 40:60/50:50 | |
| 45:55/55:45 | |
| pH | 2.3 |
| 2.6 | |
| 2.9 |
| Formulation | PEG 660-Stearate (%; w/v) * | Castor Oil (mg) | Purified Fish Oil (mg) | Egg Lecithin (mg) | CUR (mg/mL) | QU (mg/mL) |
|---|---|---|---|---|---|---|
| CQ NE | 1.5 | 2400 | 2400 | 1200 | 0.75 | 0.75 |
| NE | 1.5 | 2400 | 2400 | 1200 | - | - |
| Parameters | Validation Results | |
|---|---|---|
| Curcumin | Linearity | Calibration range (µg/mL): 0.5–12.5 |
| y = 40,565x − 272.07 (R2 = 0.9998) | ||
| LOD | 0.005 µg/mL | |
| LOQ | 0.017 µg/mL | |
| Slope | 40,565 ± 201 | |
| Intercept | −272.07 ± 1246.54 | |
| Quercetin | Linearity | Calibration range (µg/mL): 0.5–12.5 |
| y = 18,043x + 129.07 (R2 = 0.9997) | ||
| LOD | 0.14 µg/mL | |
| LOQ | 0.48 µg/mL | |
| Slope | 18,043 ± 118 | |
| Intercept | 129.07 ± 733.51 |
| Concentration (µg/mL) | Curcumin | Quercetin | ||||
|---|---|---|---|---|---|---|
| Concentration Found (µg/mL) | Accuracy (%) | Precision (%) | Concentration Found (µg/mL) | Accuracy (%) | Precision (%) | |
| 0.5 | 0.497 ± 0.005 | 99.40 ± 1.10 | 1.20 | 0.518 ± 0.012 | 103.60 ± 2.54 | 2.69 |
| 1.0 | 1.024 ± 0.008 | 102.40 ± 0.85 | 0.87 | 1.008 ± 0.012 | 100.80 ± 1.85 | 2.08 |
| 2.5 | 2.503 ± 0.014 | 100.12 ± 0.56 | 0.59 | 2.501 ± 0.020 | 100.04 ± 0.88 | 0.87 |
| 5.0 | 5.005 ± 0.049 | 100.10 ± 0.98 | 0.99 | 4.993 ± 0.020 | 99.86 ± 0.40 | 0.40 |
| 7.5 | 7.517 ± 0.023 | 100.22 ± 0.30 | 0.30 | 7.489 ± 0.074 | 99.85 ± 0.99 | 1.10 |
| 10.0 | 10.019 ± 0.019 | 100.19 ± 0.19 | 0.20 | 9.982 ± 0.030 | 99.82 ± 0.30 | 0.34 |
| 12.5 | 12.579 ± 0.090 | 100.63 ± 0.76 | 0.86 | 12.496 ± 0.062 | 99.96 ± 0.49 | 0.53 |
| Analyte | N | K´ | T | α | Rs |
|---|---|---|---|---|---|
| QU | 3506 | 0.47 | 0.968 | - | - |
| CUR | 20774 | 6.18 | 0.909 | 13.14QU/CUR | 10.83QU/CUR |
| Samples | Intra-Day Precision (Repeatability) | Inter-Day Precision (Repeatability) | ||||
|---|---|---|---|---|---|---|
| Curcumin (µg/mL) | Concentration Found (µg/mL) | Accuracy (%) | Precision (%) | Concentration Found (µg/mL) | Accuracy (%) | Precision (%) |
| 0.5 | 0.50 | 101.63 ± 4.2 | 5.9 | 0.49 | 99.46 ± 1.10 | 1.2 |
| 2.5 | 2.49 | 99.98 ± 1.0 | 1.5 | 2.50 | 100.12 ± 0.56 | 0.5 |
| 12.5 | 12.65 | 101.26 ± 1.0 | 1.4 | 12.57 | 100.63 ± 0.76 | 0.8 |
| Quercetin (µg/mL) | Concentration Found (µg/mL) | Accuracy (%) | Precision (%) | Concentration Found (µg/mL) | Accuracy (%) | Precision (%) |
| 0.5 | 0.46 | 93.60 ± 4.64 | 7.6 | 0.51 | 103.73 ± 2.54 | 2.6 |
| 2.5 | 2.46 | 98.68 ± 1.84 | 3.2 | 2.50 | 100.06 ± 0.88 | 0.8 |
| 12.5 | 12.43 | 99.44 ± 0.67 | 1.1 | 12.49 | 99.97 ± 0.49 | 0.4 |
| Variable | Value | Curcumin Content a (μg/mL) | RSD a (%) | Quercetin Content a (μg/mL) | RSD a (%) |
|---|---|---|---|---|---|
| Column temperature (°C) | 35 | 2.53 ± 0.04 | 1.67 | 2.54 ± 0.02 | 0.82 |
| 40 | 2.50 ± 0.03 | 1.59 | 2.50 ± 0.03 | 1.87 | |
| 45 | 2.51 ± 0.03 | 1.41 | 2.49 ± 0.01 | 0.40 | |
| Mobile phase composition (1% phosphoric acid:acetonitrile, v/v; pH 2.6) | 35:65/45:55 | 2.51 ± 0.06 | 2.39 | 2.51 ± 0.06 | 2.58 |
| 40:60/50:50 | 2.50 ± 0.03 | 1.59 | 2.50 ± 0.03 | 1.87 | |
| 45:55/55:45 | 2.51 ± 0.03 | 1.31 | 2.54 ± 0.05 | 2.09 | |
| pH of the mobile phase | 2.3 | 2.50 ± 0.02 | 0.89 | 2.49 ± 0.04 | 1.94 |
| 2.6 | 2.50 ± 0.03 | 1.59 | 2.50 ± 0.03 | 1.87 | |
| 2.9 | 2.50 ± 0.03 | 1.25 | 2.49 ± 0.09 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaz, G.R.; Clementino, A.; Bidone, J.; Villetti, M.A.; Falkembach, M.; Batista, M.; Barros, P.; Sonvico, F.; Dora, C. Curcumin and Quercetin-Loaded Nanoemulsions: Physicochemical Compatibility Study and Validation of a Simultaneous Quantification Method. Nanomaterials 2020, 10, 1650. https://doi.org/10.3390/nano10091650
Vaz GR, Clementino A, Bidone J, Villetti MA, Falkembach M, Batista M, Barros P, Sonvico F, Dora C. Curcumin and Quercetin-Loaded Nanoemulsions: Physicochemical Compatibility Study and Validation of a Simultaneous Quantification Method. Nanomaterials. 2020; 10(9):1650. https://doi.org/10.3390/nano10091650
Chicago/Turabian StyleVaz, Gustavo Richter, Adryana Clementino, Juliana Bidone, Marcos Antonio Villetti, Mariana Falkembach, Matheus Batista, Paula Barros, Fabio Sonvico, and Cristiana Dora. 2020. "Curcumin and Quercetin-Loaded Nanoemulsions: Physicochemical Compatibility Study and Validation of a Simultaneous Quantification Method" Nanomaterials 10, no. 9: 1650. https://doi.org/10.3390/nano10091650
APA StyleVaz, G. R., Clementino, A., Bidone, J., Villetti, M. A., Falkembach, M., Batista, M., Barros, P., Sonvico, F., & Dora, C. (2020). Curcumin and Quercetin-Loaded Nanoemulsions: Physicochemical Compatibility Study and Validation of a Simultaneous Quantification Method. Nanomaterials, 10(9), 1650. https://doi.org/10.3390/nano10091650