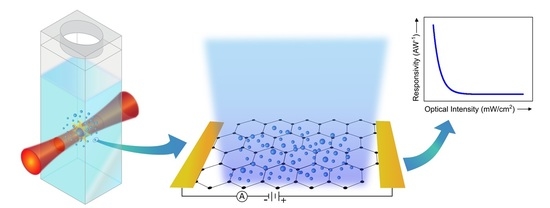
ZnO Nanoparticle/Graphene Hybrid Photodetectors via Laser Fragmentation in Liquid
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
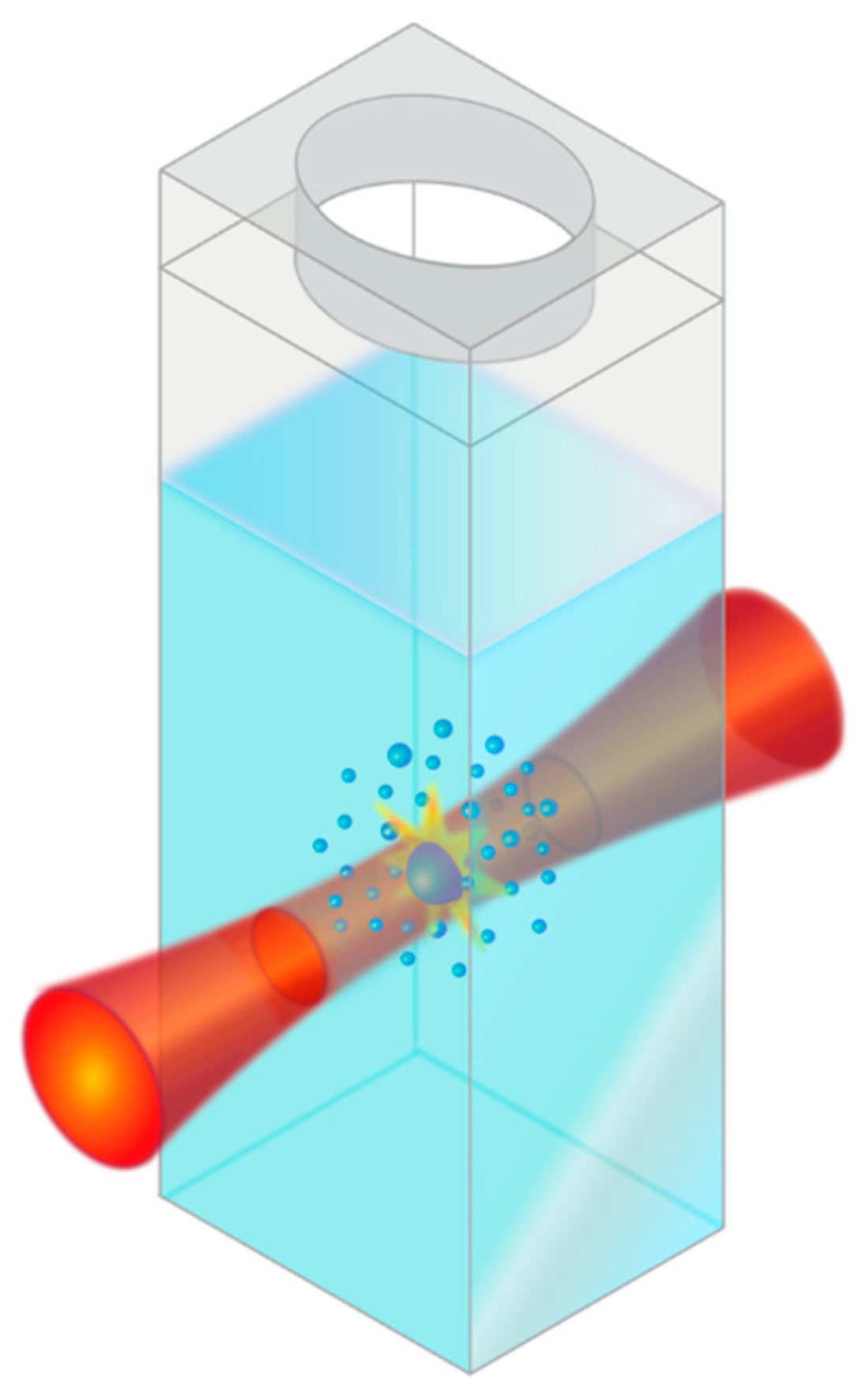
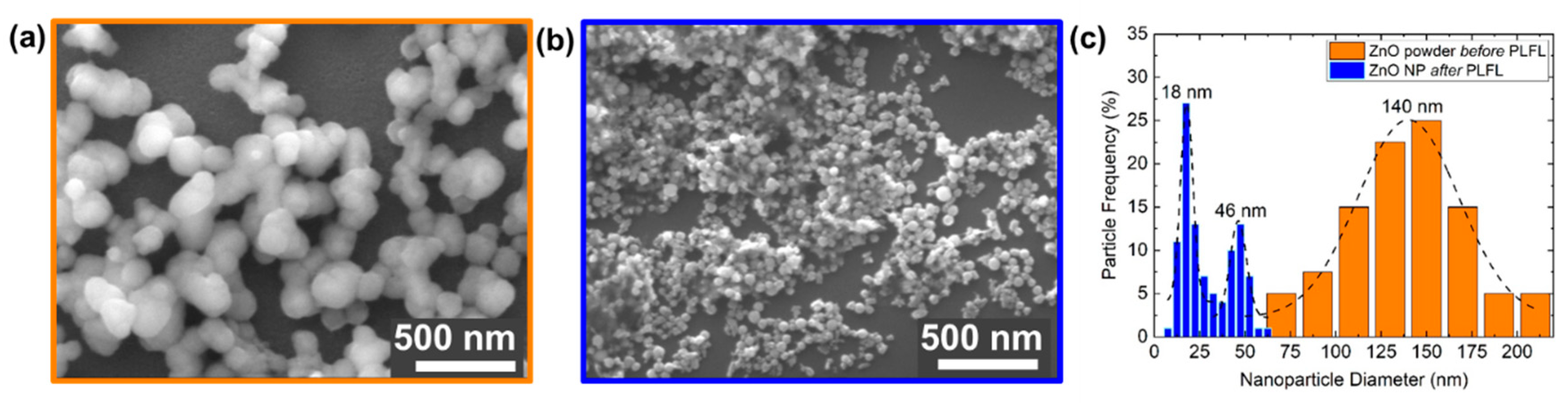
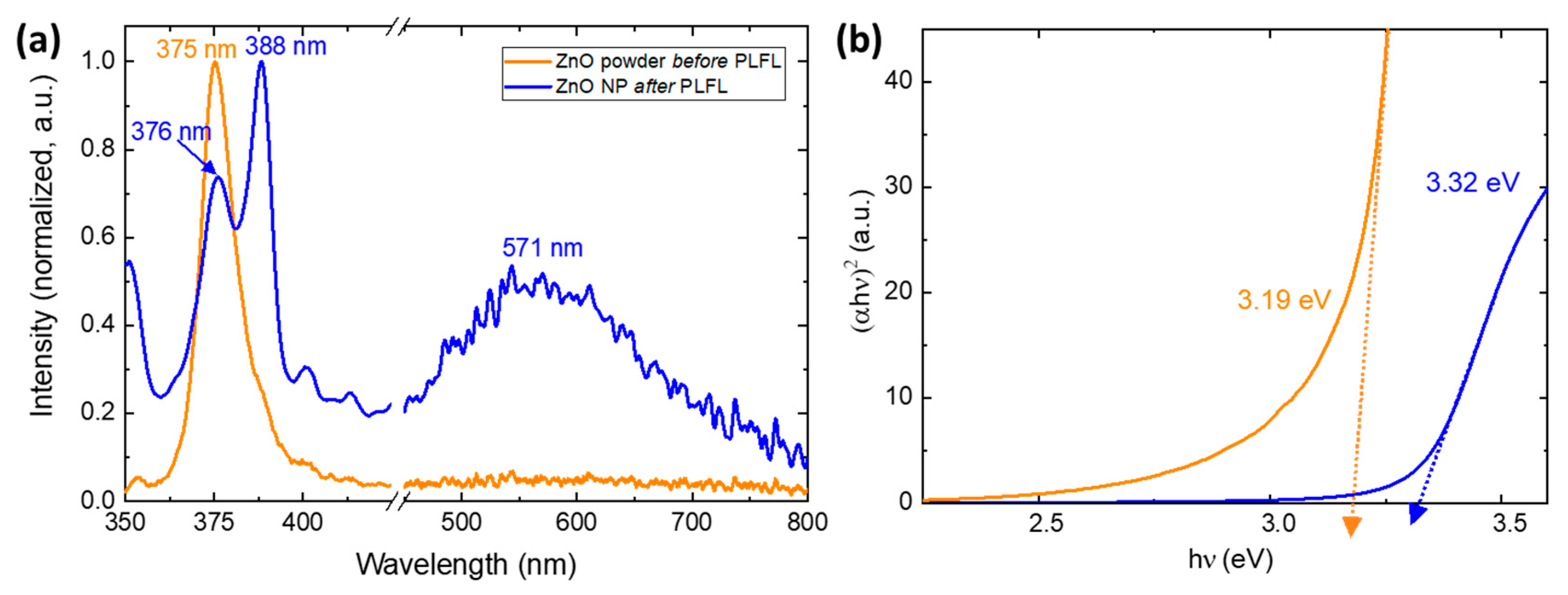
3.1. Pulsed Laser Fragmentation in Liquid (PLFL)
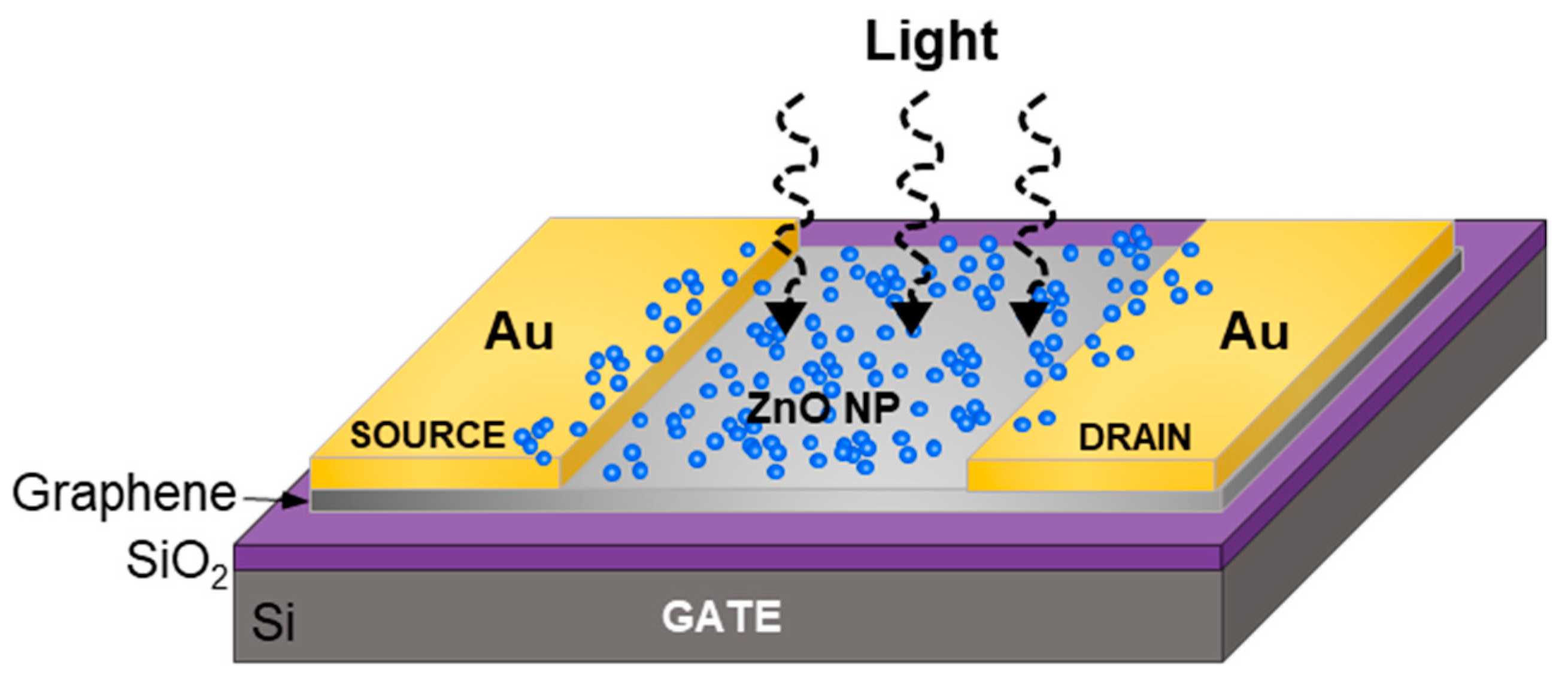
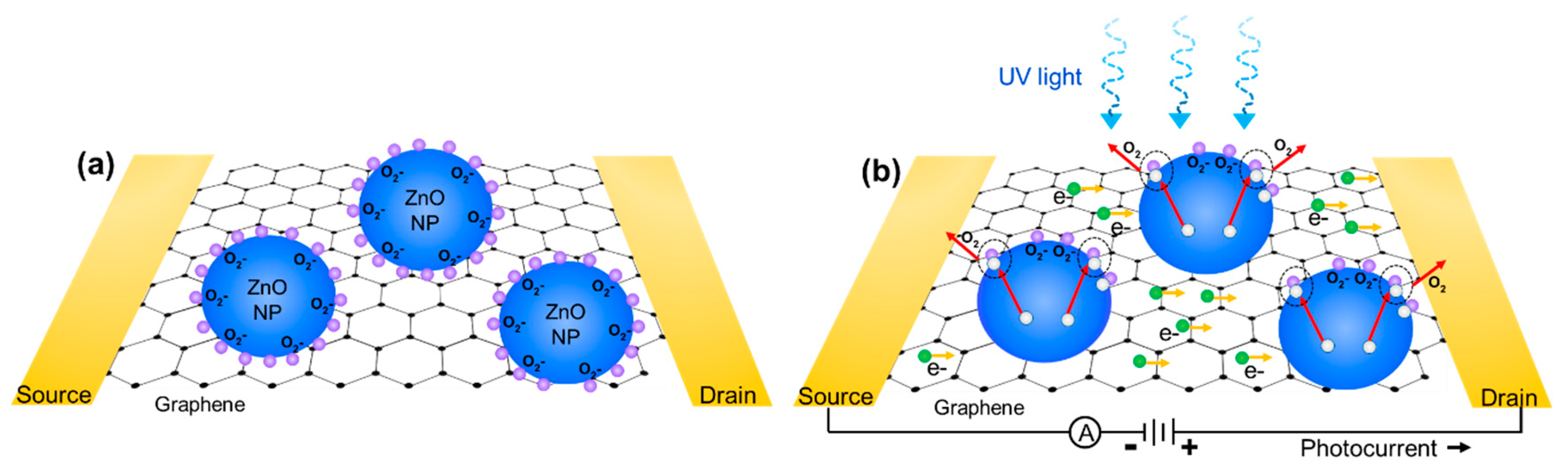
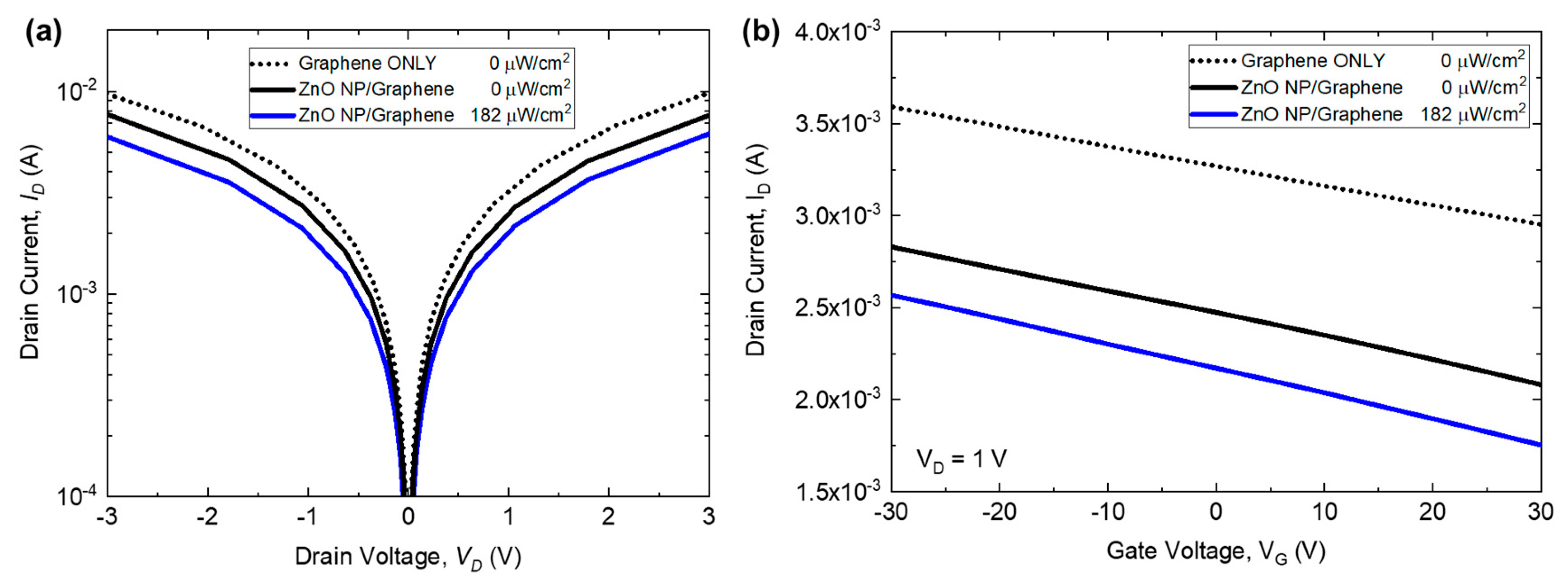
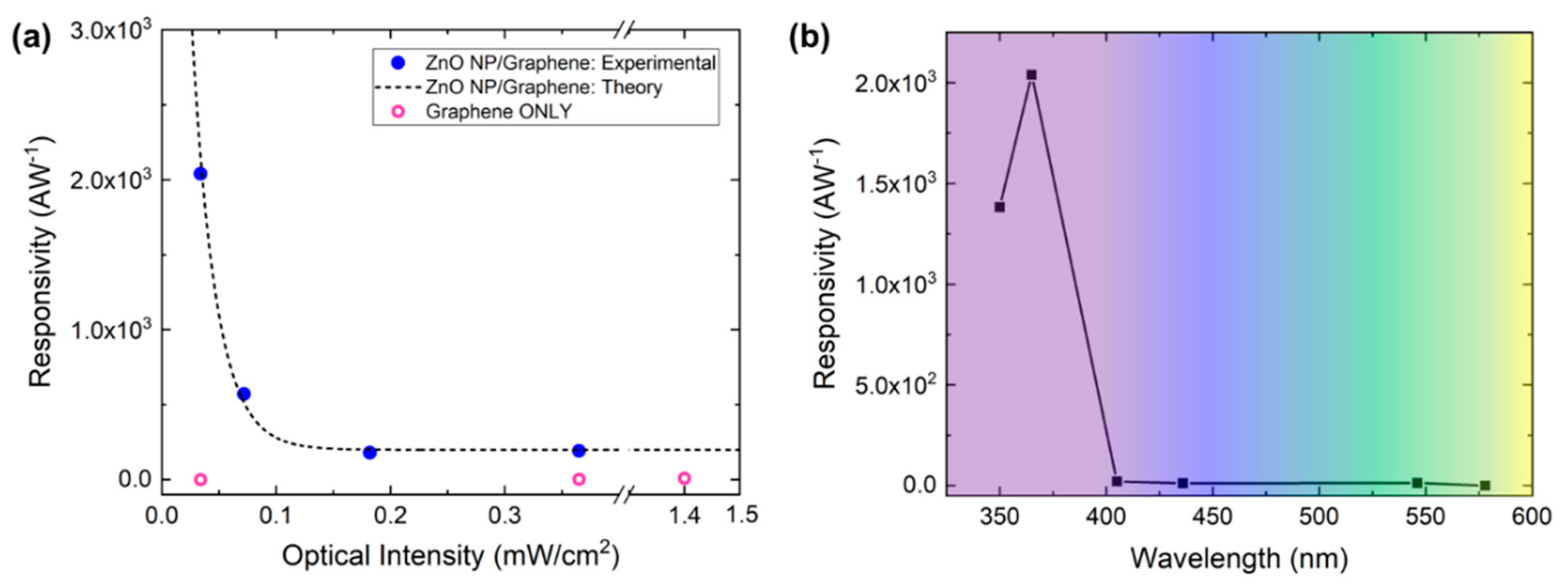
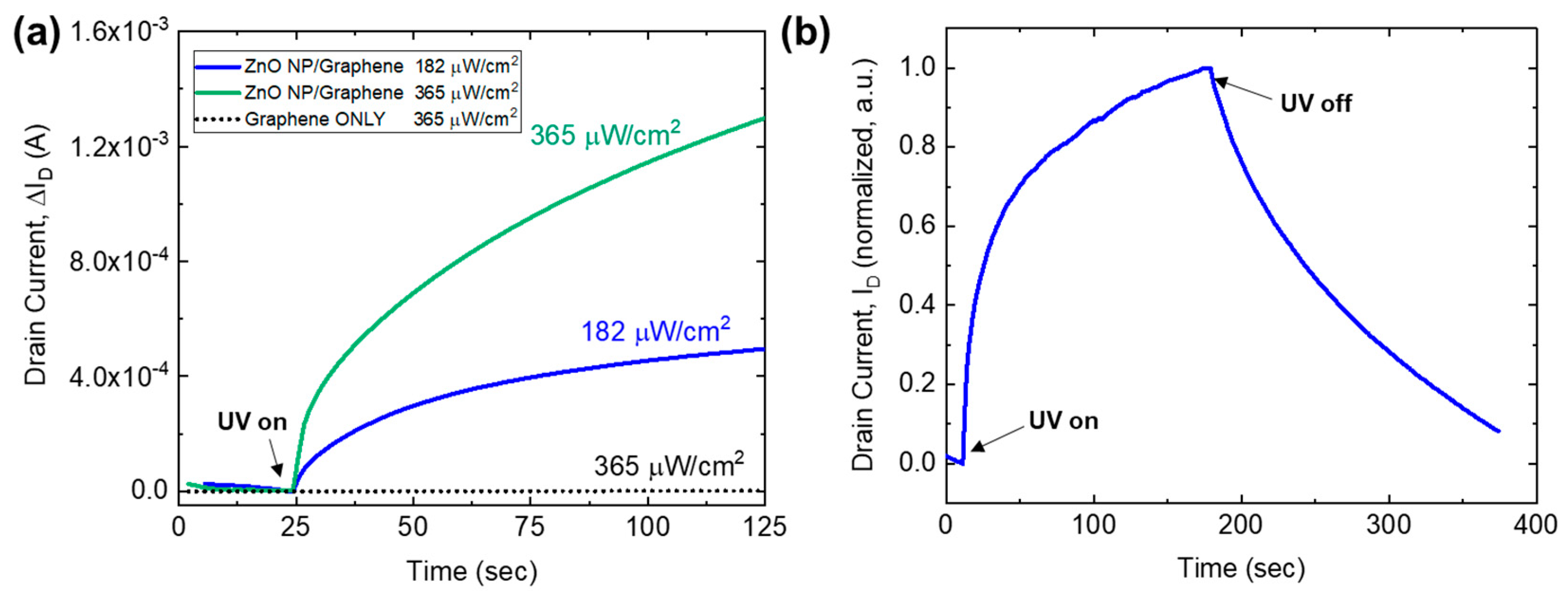
3.2. ZnO Nanoparticle/Graphene Phototransistors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Phys. 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dawlaty, J.M.; Shivaraman, S.; Chandrashekhar, M.; Rana, F.; Spencer, M.G. Measurement of ultrafast carrier dynamics in epitaxial graphene. Appl. Phys. Lett. 2008, 92, 042116. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chang, Y.-C.; Norris, T.B.; Zhong, Z. Graphene photodetectors with ultra-broadband and high responsivity at room temperature. Nat. Nanotechnol. 2014, 9, 273–278. [Google Scholar] [CrossRef]
- Konstantatos, G.; Badioli, M.; Gaudreau, L.; Osmond, K.; Bernechea, M.; de Arquer, F.P.G.; Gatti, F.; Koppens, F.H.L. Hybrid graphene-quantum dot phototransistors with ultrahigh gain. Nat. Nanotechnol. 2012, 7, 363–368. [Google Scholar] [CrossRef]
- Liang, F.-X.; Gao, Y.; Xie, C.; Tong, X.-W.; Li, Z.-J.; Luo, L.-B. Recent advances in the fabrication of graphene-ZnO heterojunctions for optoelectronic device applications. J. Mater. Chem. C 2018, 6, 3815–3833. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Hu, X.; Zhang, D.; Tao, Y.; Liu, Z.; He, Y.; Haque, A.; Liu, Z.; Wu, T.; et al. Narrow bandgap oxide nanoparticles coupled with graphene for high performance mid-infrared photodetection. Nat. Commun. 2018, 9, 4299. [Google Scholar] [CrossRef]
- Look, D.C.; Reynolds, D.C.; Sizelove, J.R.; Jones, R.L.; Litton, C.W.; Cantwell, G.; Harsh, W.C. Electrical properties of bulk ZnO. Solid State Commun. 1998, 105, 399–401. [Google Scholar] [CrossRef]
- Jun, J.H.; Seong, H.; Cho, K.; Moon, B.-M.; Kim, S. Ultraviolet photodetectors based on ZnO nanoparticles. Ceram. Int. 2009, 35, 2797–2801. [Google Scholar] [CrossRef]
- Gong, M.; Liu, Q.; Cook, B.; Kattel, B.; Wang, T.; Chan, W.-L.; Ewing, D.; Casper, M.; Stramel, A.; Wu, J.Z. All-Printable ZnO Quantum Dots/Graphene van der Waals Heterostructures for Ultrasensitive Detection of Ultraviolet Light. ACS Nano 2017, 11, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Q.; Gong, M.; Cook, B.; Ewing, D.; Casper, M.; Stramel, A.; Wu, J. Transfer-free and printable graphene/ZnO-nanoparticle nanohybrid photodetectors with high performance. J. Mater. Chem. C 2017, 5, 6427–6432. [Google Scholar] [CrossRef]
- Guo, W.; Xu, S.; Wu, Z.; Wang, N.; Loy, M.M.T.; Du, S. Oxygen-Assisted Charge Transfer Between ZnO Quantum Dots and Graphene. Small 2013, 9, 3031–3036. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Gao, J.; Chow, P.; Sun, H.; Xin, G.; Sharma, P.; Lian, J.; Koratkar, N.A.; Sawyer, S. Organic-Inorganic Heterointerfaces for Ultrasensitive Detection of Ultraviolet Light. Nano Lett. 2015, 15, 3787–3792. [Google Scholar] [CrossRef]
- Xu, Q.; Cheng, Q.; Zhong, J.; Cai, W.; Zhang, Z.; Wu, Z.; Zhang, F. A metal-semiconductor-metal detector based on ZnO nanowires grown on a graphene layer. Nanotechnology 2014, 25, 055501. [Google Scholar] [CrossRef]
- Dang, V.Q.; Trung, T.Q.; Duy, L.T.; Kim, B.-Y.; Siddiqui, S.; Lee, W.; Lee, N.-E. High-performance flexible ultraviolet (UV) phototransistor using hybrid channel of vertical ZnO nanorods and graphene. ACS Appl. Mater. Interface 2015, 7, 11032–11040. [Google Scholar] [CrossRef]
- Dang, V.Q.; Trung, T.Q.; Kim, D.-I.; Duy, L.T.; Hwang, B.-U.; Lee, D.-W.; Kim, B.-Y.; Toan, L.D.; Lee, N.-E. Ultrahigh responsivity in graphene-ZnO nanorod hybrid UV photodetector. Small 2015, 11, 3054–3065. [Google Scholar] [CrossRef]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.R.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO Nanowire UV Photodetectors with High Internal Gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, D.Y.; Lee, S. Low-power graphene/ZnO Schottky UV photodiodes with enhanced lateral schottky barrier homogeneity. Nanomaterials 2019, 9, 799. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Kim, D.Y.; Song, E.B.; Kim, S.M. Back-gate tuning of Schottky barrier height in graphene/zinc-oxide photodiodes. Appl. Phys. Lett. 2013, 102, 242114. [Google Scholar] [CrossRef]
- Lee, H.; An, N.; Jeong, S.; Kang, S.; Kwon, S.; Lee, J.; Lee, Y.; Kim, D.Y.; Lee, S. Strong dependence of photocurrent on illumination-light colors for ZnO/graphene Schottky diode. Curr. Appl. Phys. 2017, 17, 552–556. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Zhan, J.-Y.; Liao, Y.-M.; Lin, T.-Y.; Hsieh, Y.-P.; Chen, Y.-F. Self-powered and broadband photodetectors based on graphene/ZnO/silicon triple junctions. Appl. Phys. Lett. 2016, 109, 053501. [Google Scholar] [CrossRef]
- Hongsith, N.; Wongrat, E.; Kerdcharoen, T.; Choopun, S. Sensor response formula for sensor based on ZnO nanostructures. Sens. Actuators B Chem. 2010, 144, 67–72. [Google Scholar] [CrossRef]
- Zhang, D.; Gökcev, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Kamat, P.V.; Flumiani, M.; Hartland, G.V. Picosecond Dynamics of Silver Nanoclusters. Photoejection of Electrons and Fragmentation. J. Phys. Chem. B 1998, 102, 3123–3128. [Google Scholar] [CrossRef]
- Werner, D.; Furube, A.; Okamoto, T.; Hashimoto, S. Femtosecond Laser-Induced Size Reduction of Aqueous Gold Nanoparticles: In Situ and Pump-Probe Spectroscopy Investigations Revealing Coulomb Explosion. J. Phys. Chem. C 2011, 115, 8503–8512. [Google Scholar] [CrossRef]
- Takami, A.; Kurita, J.; Koda, S. Laser-Induced Size Reduction of Noble Metal Particles. J. Phys. Chem. B 1999, 103, 1226–1232. [Google Scholar] [CrossRef]
- Kuzmin, P.G.; Shafeev, G.A.; Serkov, A.A.; Kirichenko, N.A.; Shcherbina, M.E. Laser-assisted fragmentation of Al particles suspended in liquid. Appl. Surf. Sci. 2014, 294, 15–19. [Google Scholar] [CrossRef]
- Chubilleau, C.; Lenoir, B.; Migot, S.; Dauscher, A. Laser fragmentation in liquid medium: A new way for the synthesis of PbTe nanoparticles. J. Colloid Interface Sci. 2011, 357, 13–17. [Google Scholar] [CrossRef]
- Singh, S.C.; Mishra, S.K.; Srivastava, R.K.; Gopal, R. Optical Properties of Selenium Quantum Dots Produced with Laser Irradation of Water Suspended Se Nanoparticles. J. Phys. Chem. C 2010, 114, 17374–17384. [Google Scholar] [CrossRef]
- Usui, H.; Sasaki, T.; Koshizaki, N. Optical Transmittance of Indium Tin Oxide Nanoparticles Prepared by Laser-Induced Fragmentation in Water. J. Phys. Chem. B 2006, 110, 12890–12895. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.; Barcikowski, S. Quantification of mass-specific laser energy input converted into particle properties during picosecond pulsed laser fragmentation of zinc oxide and boron carbide in liquids. Appl. Surf. Sci. 2015, 348, 22–29. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, S.; Cai, W. Reshaping Formation and Luminescence Evolution of ZnO Quantum Dots by Laser-Induced Fragmentation in Liquid. J. Phys. Chem. C 2011, 115, 5038–5043. [Google Scholar] [CrossRef]
- Suk, J.W.; Kitt, A.; Magnuson, C.W.; Hao, Y.; Ahmed, S.; An, J.; Swan, A.K.; Goldberg, B.B.; Ruoff, R.S. Transfer of CVD-grown monolayer graphene onto arbitrary substrates. ACS Nano 2011, 5, 6916–6924. [Google Scholar] [CrossRef]
- Liang, X.; Sperling, B.A.; Calizo, I.; Cheng, G.; Hacker, C.A.; Zhang, Q.; Obeng, Y.; Yan, K.; Peng, H.; Li, Q.; et al. Toward clean and crackless transfer of graphene. ACS Nano 2011, 5, 9144–9153. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper films. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Spanhel, L.; Anderson, M.A. Semiconductor cluster in the sol-gel process: Quantized aggregation, gelation, and crystal growth in concentrated ZnO colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhang, L.; Yuan, J.; Yan, S.; Wang, C. Low-temperature synthesis of ZnO nanoparticles by solid-state pyrolytic reaction. Nanotechnology 2003, 14, 11–15. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Yan, Z.; Chrisey, D.B. Pulsed laser ablation in liquid for micro-/nanostructure generation. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 204–223. [Google Scholar] [CrossRef]
- Tan, D.; Zhou, S.; Qiu, J.; Khusro, N. Preparation of functional nanomaterials with femtosecond laser ablation in solution. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 50–68. [Google Scholar] [CrossRef]
- Singh, S.C.; Gopal, R. Synthesis of colloidal zinc oxide nanoparticles by pulsed laser ablation in aqueous media. Phys. E Low Dimens. Syst. Nanostruct. 2008, 40, 724–730. [Google Scholar] [CrossRef]
- Al-Nassar, S.I.; Hussein, F.I.; Mahmoud, A.K. The effect of laser pulse energy on ZnO nanoparticles formation by liquid phase pulse laser ablation. J. Mater. Res. Technol. 2019, 8, 4026–4031. [Google Scholar] [CrossRef]
- Huang, H.; Lai, J.; Lu, J.; Li, Z. Pulsed laser ablation of bulk target and particle products in liquid for nanomaterial fabrication. AIP Adv. 2019, 9, 015307. [Google Scholar] [CrossRef]
- Naher, U.; Bjornholm, S.; Frauendorf, S.; Garcias, F.; Guet, C. Fission of Metal Clusters. Phys. Rep. 1997, 285, 245–320. [Google Scholar] [CrossRef]
- Siebeneicher, S.; Waag, F.; Castillo, M.E.; Shvartsman, V.V.; Lupascu, D.C.; Gökce, B. Laser Fragmentation Synthesis of Colloidal Bismuth Ferrite Particles. Nanomaterials 2020, 10, 359. [Google Scholar] [CrossRef]
- Ling, D.; Hackett, M.J.; Hyeon, T. Surface ligands in synthesis, modification, assembly and biomedical applications of nanoparticles. Nanotoday 2014, 9, 457–477. [Google Scholar] [CrossRef]
- Richter, T.V.; Stelzl, F.; Schulz-Gericke, J.; Kerscher, B.; Würfel, U.; Niggermann, M.; Ludwigs, S. Room temperature vacuum-induced ligand removal and patterning of ZnO nanoparticles: From semiconducting films towards printed electronics. J. Mater. Chem. 2010, 20, 874–879. [Google Scholar] [CrossRef]
- Luo, J.; Dai, X.; Bai, S.; Jin, Y.; Ye, Z.; Guo, X. Ligand Exchange of Colloidal ZnO Nanocrystals from the High Temperatuer and Nonaqueous Approach. Nano Micro Lett. 2013, 5, 274–280. [Google Scholar] [CrossRef]
- O’Toole, P.I.; Hudson, T.S. New High-Density Packings of Similarly Sized Binary Spheres. J. Phys. Chem. C 2011, 115, 19037–19040. [Google Scholar] [CrossRef]
- Goh, E.G.; Xu, X.; McCormick, P.G. Effect of particle size on the UV absorbance of zinc oxide nanoparticles. Scr. Mater. 2014, 78–79, 49–52. [Google Scholar] [CrossRef]
- Thareja, R.K.; Shukla, S. Synthesis and characterization of zinc oxide nanoparticles by laser ablation of zinc in liquid. Appl. Surf. Sci. 2007, 253, 8889–8895. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, L.; Wang, C.; Iun, N.; Qi, Y.; Xiang, D. Origin of visible photoluminescence of ZnO quantum dots: Defect-dependent and size-dependent. J. Phys. Chem. C 2010, 114, 9651–9658. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Leung, Y.H. Optical Properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef]
- Ischenko, V.; Polarz, S.; Grote, D.; Stavarache, V.; Fink, K.; Driess, M. Zinc Oxide Nanoparticles with Defects. Adv. Funct. Mater. 2005, 15, 1945–1954. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Lu, C.-J.; Lv, Z.-H. Photoluminescence of ZnO nanoparticles and nanorods. Optik 2016, 127, 1421–1423. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Choy, W.C.H.; Roy, V.A.L.; Leung, Y.H.; Kwong, C.Y.; Cheah, K.W.; Rao, T.K.G.; Chan, W.K.; Lui, H.F.; Surya, C. Photoluminescence and Electron Paramagnetic Resonance of ZnO Tetrapod Structures. Adv. Funct. Mater. 2004, 14, 856–864. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis and photoluminescence characterization of ZnO nanoparticles. J. Lumin. 2013, 134, 213–219. [Google Scholar] [CrossRef]
- Xu, C.H.; Si, J.; Xu, Y.; Hou, X. Femtosecond Laser-Assisted Synthesis of ZnO Nanoparticles in Solvent with Visible Emission for Temperature Sensing. Nano Brief Rep. Rev. 2019, 14, 1950054. [Google Scholar] [CrossRef]
- Liu, Q.; Gong, M.; Cook, B.; Ewing, D.; Casper, M.; Stramel, A.; Wu, J. Fused nanojunctions of electron-depleted ZnO Nanoparticles for extraordinary performance in ultraviolet detection. Adv. Mater. Interface 2017, 4, 1601064. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charipar, K.; Kim, H.; Piqué, A.; Charipar, N. ZnO Nanoparticle/Graphene Hybrid Photodetectors via Laser Fragmentation in Liquid. Nanomaterials 2020, 10, 1648. https://doi.org/10.3390/nano10091648
Charipar K, Kim H, Piqué A, Charipar N. ZnO Nanoparticle/Graphene Hybrid Photodetectors via Laser Fragmentation in Liquid. Nanomaterials. 2020; 10(9):1648. https://doi.org/10.3390/nano10091648
Chicago/Turabian StyleCharipar, Kristin, Heungsoo Kim, Alberto Piqué, and Nicholas Charipar. 2020. "ZnO Nanoparticle/Graphene Hybrid Photodetectors via Laser Fragmentation in Liquid" Nanomaterials 10, no. 9: 1648. https://doi.org/10.3390/nano10091648
APA StyleCharipar, K., Kim, H., Piqué, A., & Charipar, N. (2020). ZnO Nanoparticle/Graphene Hybrid Photodetectors via Laser Fragmentation in Liquid. Nanomaterials, 10(9), 1648. https://doi.org/10.3390/nano10091648