Microwave-Assisted Rapid Synthesis of Reduced Graphene Oxide-Based Gum Tragacanth Hydrogel Nanocomposite for Heavy Metal Ions Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Reduced Graphene Oxide (RGO)
2.3. Synthesis of Gum Tragacanth-cl-N,N-dimethylacrylamide (GT-cl-poly(DMA)) Hydrogel
2.4. Synthesis of Reduced Graphene Oxide Incorporated Gum Tragacanth-cl-N,N-dimethylacrylamide (GT-cl-poly(DMA)/RGO) Hydrogel Composite
2.5. Characterization
2.6. Swelling Study
2.7. Adsorption of Hg2+ and Cr6+
3. Results
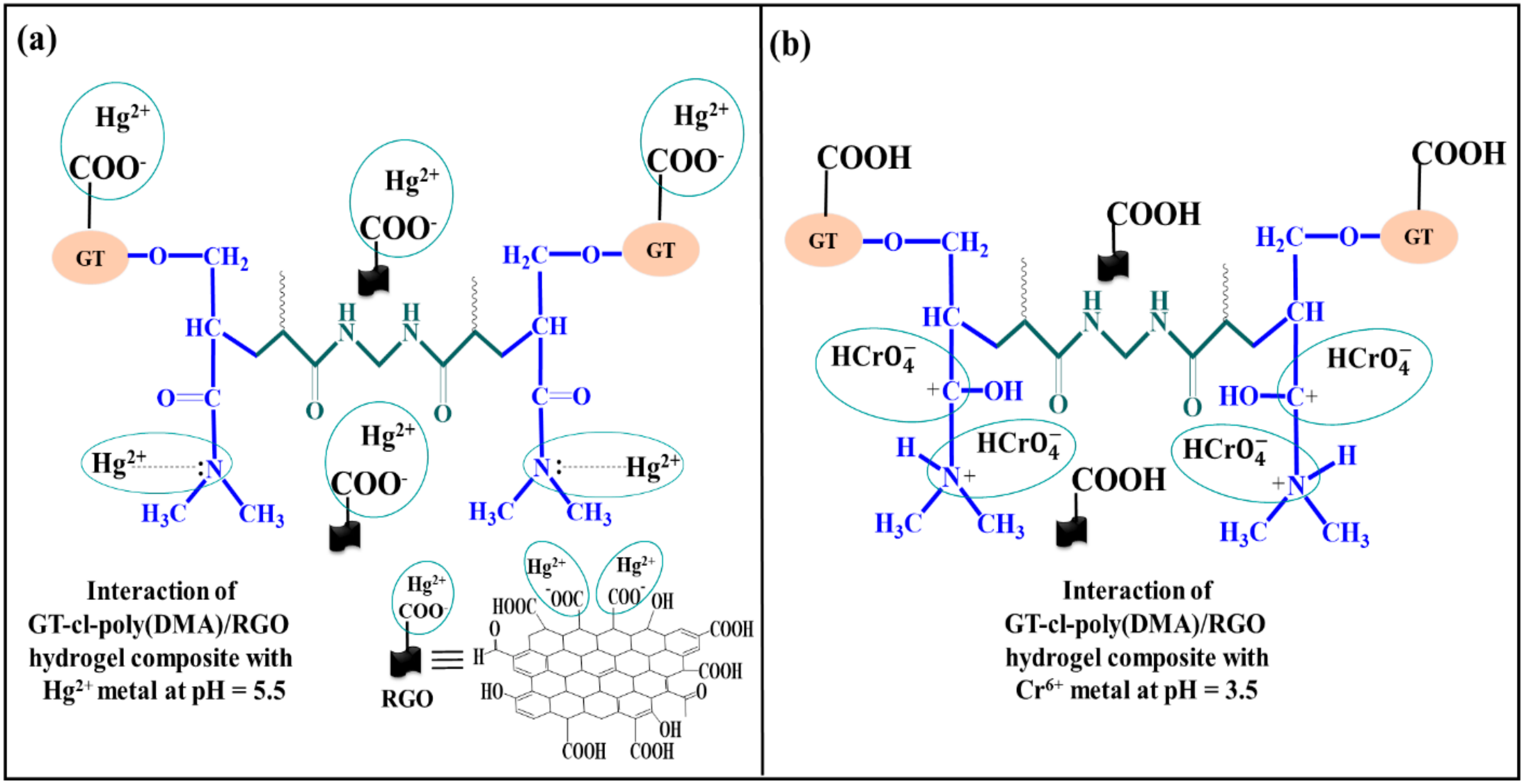
3.1. Mechanism for Synthesis of Gum Tragacanth-cl-N,N-dimethylacrylamide Hydrogel
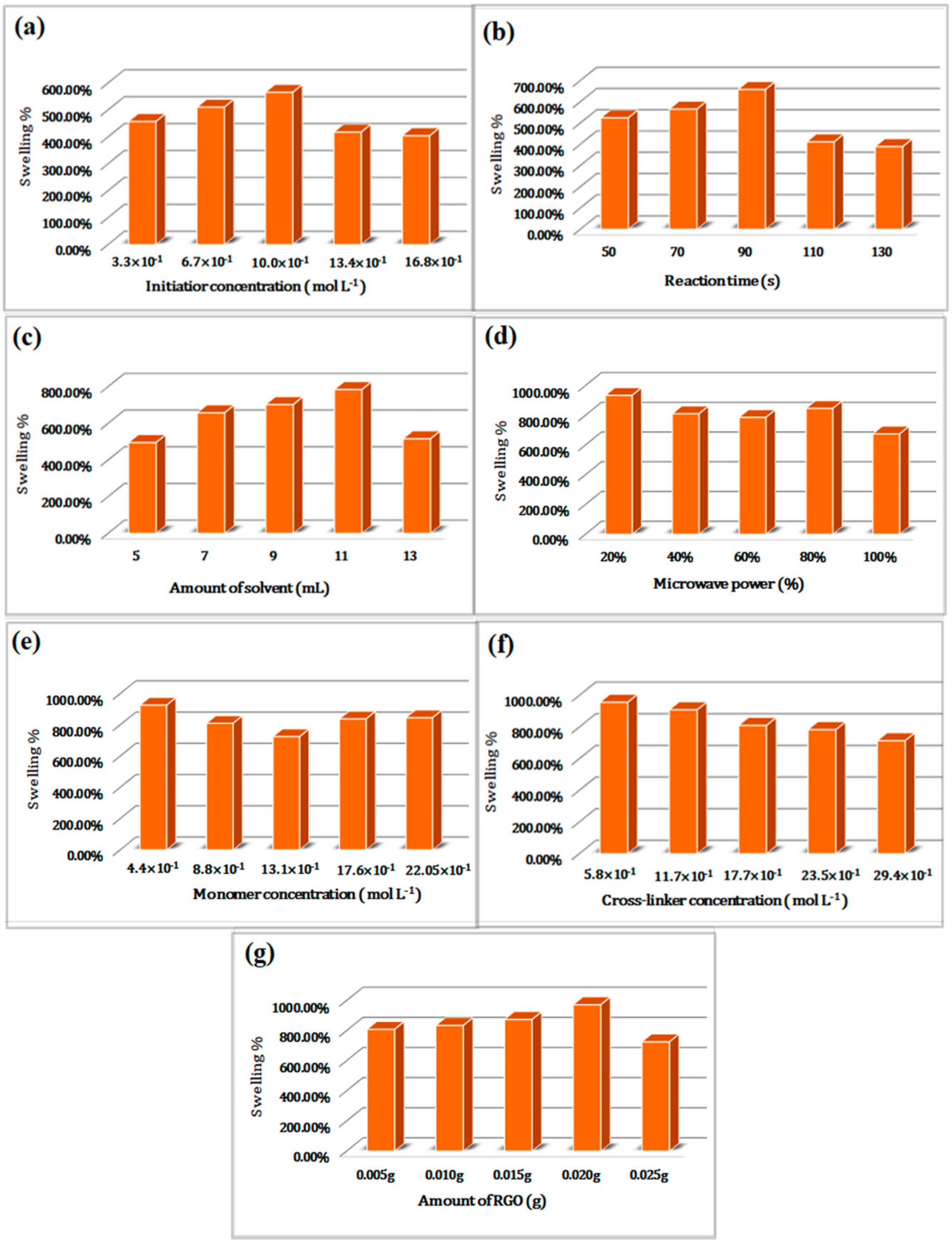
3.2. Optimization of Swelling for Reduced Graphene Oxide Incorporated Gum Tragacanth-cl-N,N-dimethylacrylamide Hydrogel Composite
3.2.1. Initiator (KPS) Concentration
3.2.2. Reaction Time
3.2.3. Solvent
3.2.4. Microwave Power
3.2.5. Monomer (DMA) Concentration
3.2.6. Cross-Linker (NMBA)
3.2.7. RGO Loading
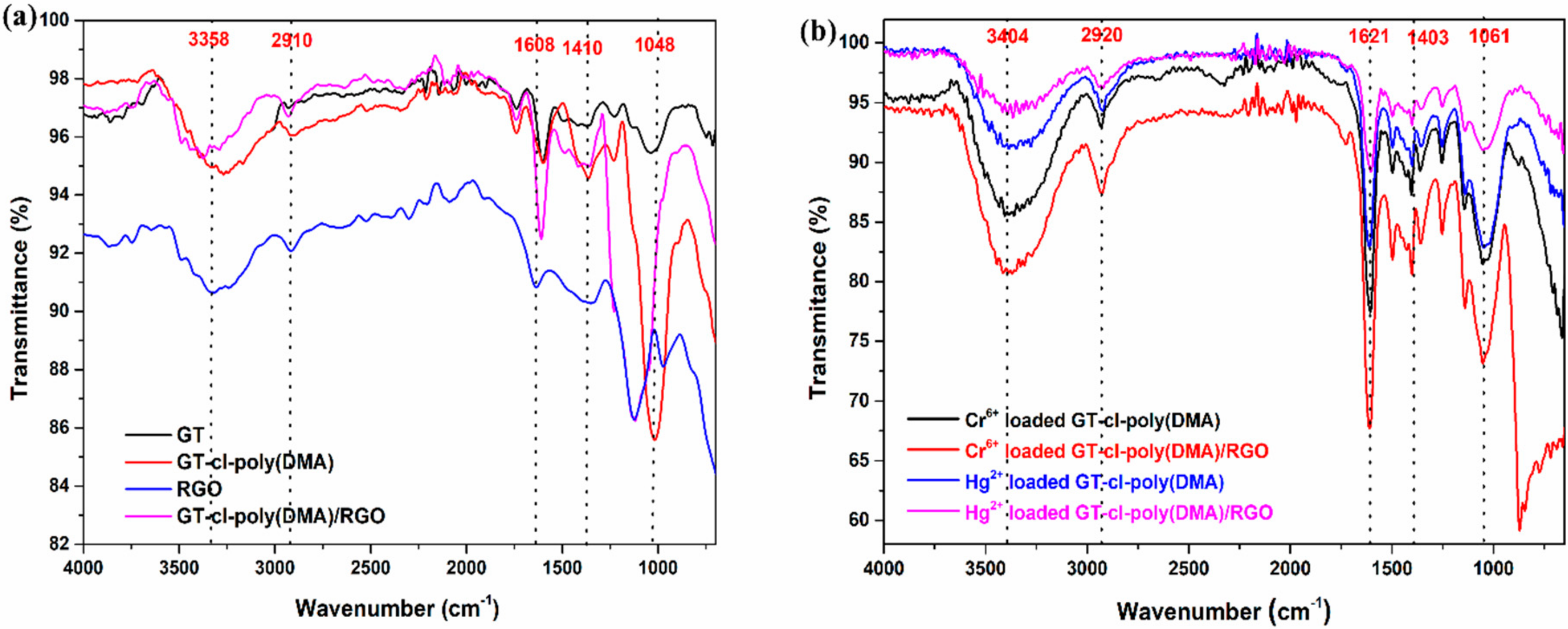
3.3. FTIR
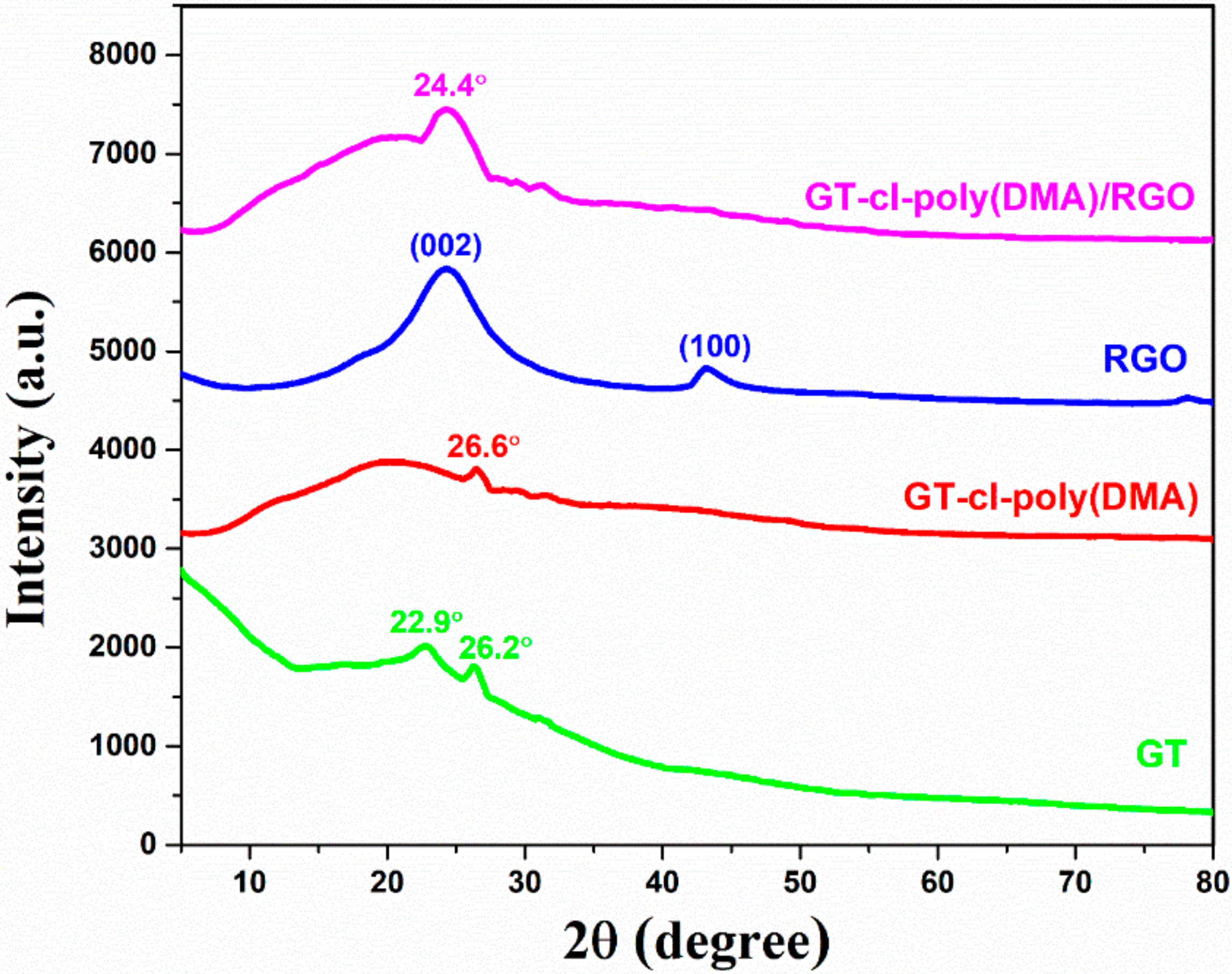
3.4. XRD
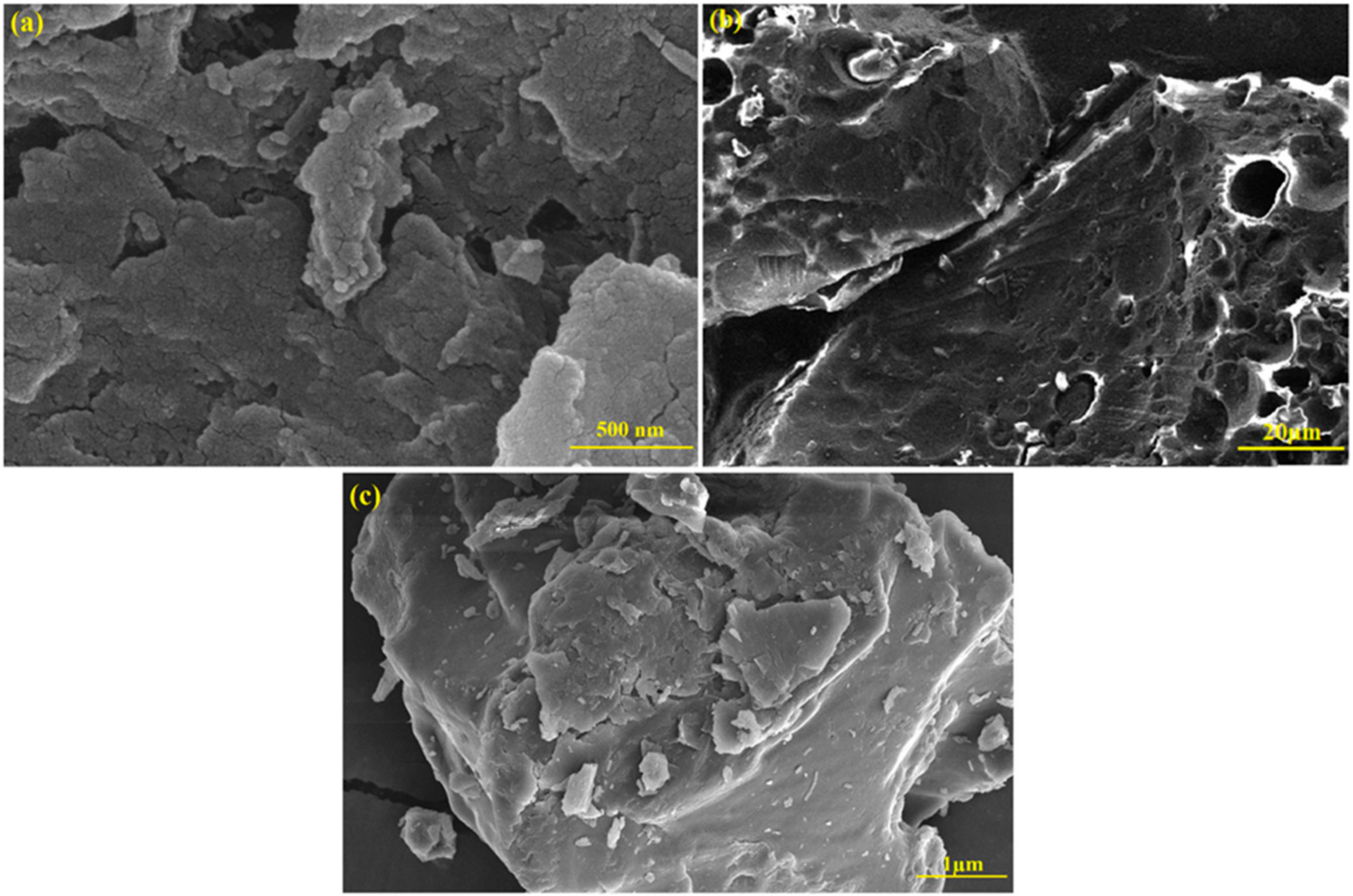
3.5. SEM
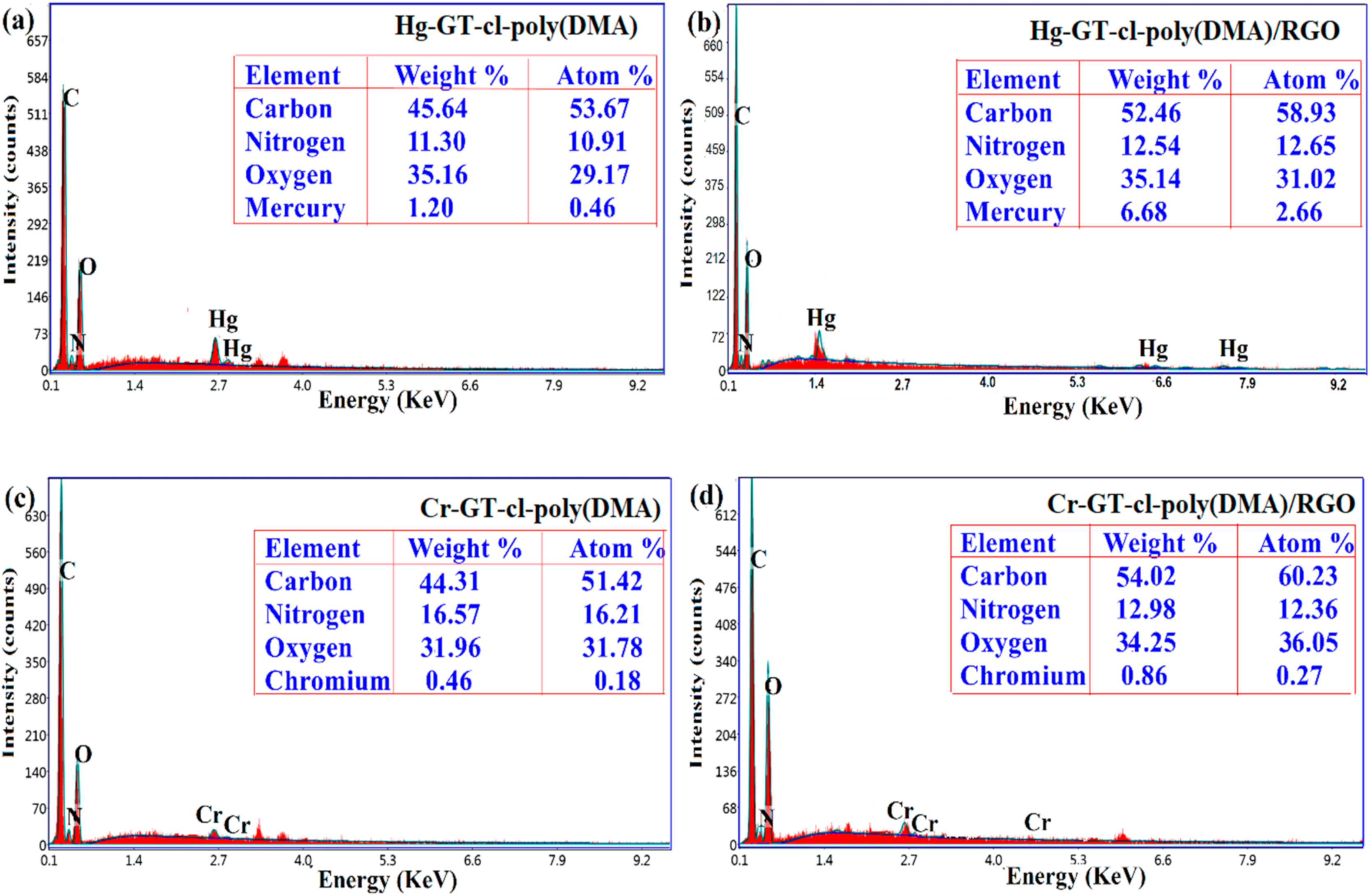
3.6. EDS
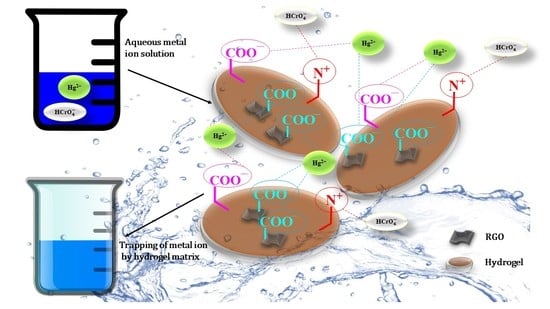
3.7. Application of Gum Tragacanth-cl-N,N-dimethylacrylamide (GT-cl-poly(DMA)) Hydrogel and Reduced Graphene Oxide Incorporated Gum Tragacanth-cl-N,N-dimethylacrylamide (GT-cl-poly(DMA)/RGO) Hydrogel Composite for Removal of Hg2+ and Cr6+
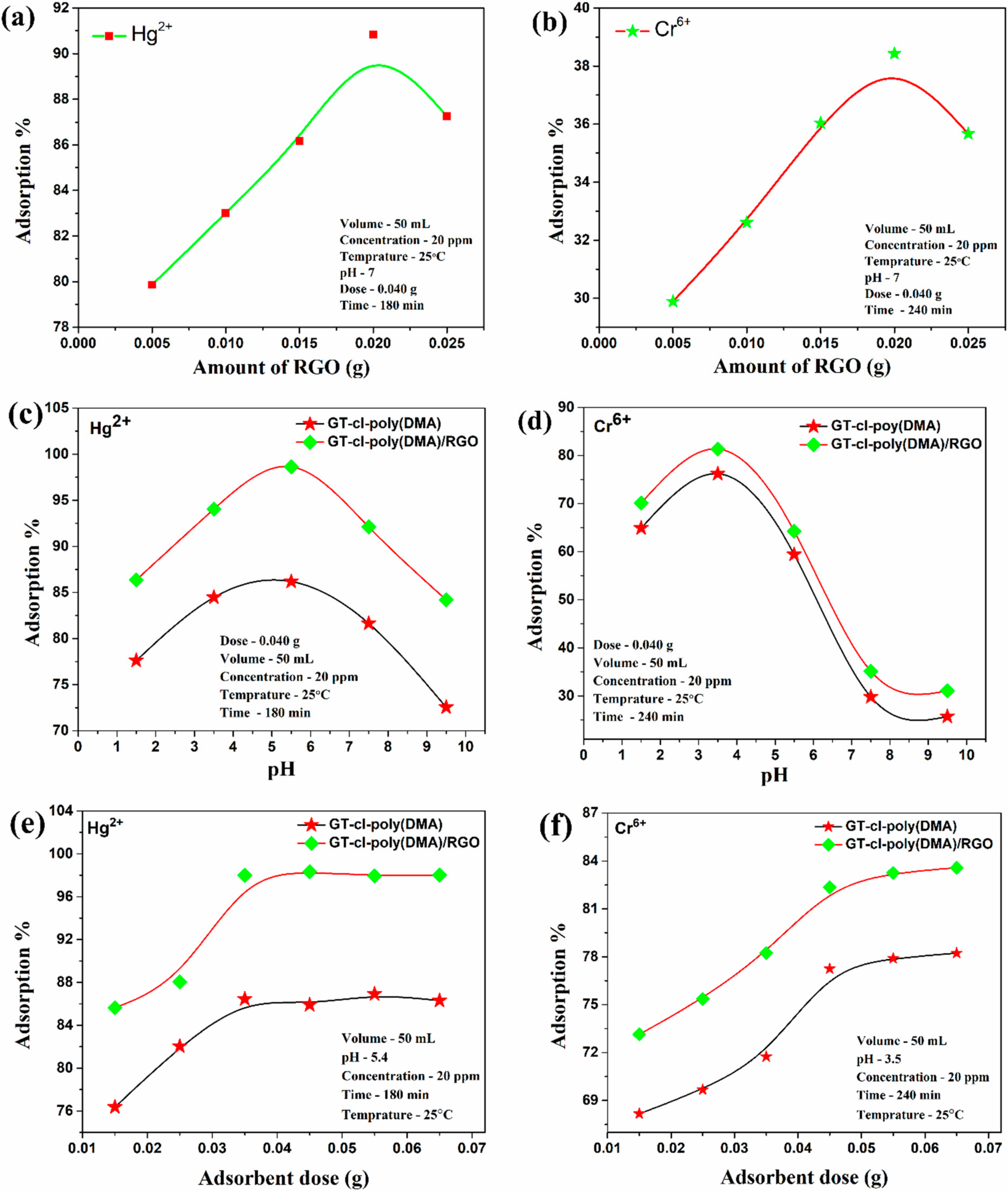
3.7.1. Influence of RGO Loading on the Removal of Hg2+ and Cr6+
3.7.2. Influence of pH on Removal of Hg2+ and Cr6+ by GT-cl-poly(DMA) Hydrogel and GT-cl-poly(DMA)/RGO Hydrogel Composite
3.7.3. Influence of GT-cl-poly(DMA) Hydrogel and GT-cl-poly(DMA)/RGO Hydrogel Composite Dose for Removal of Hg2+ and Cr6+
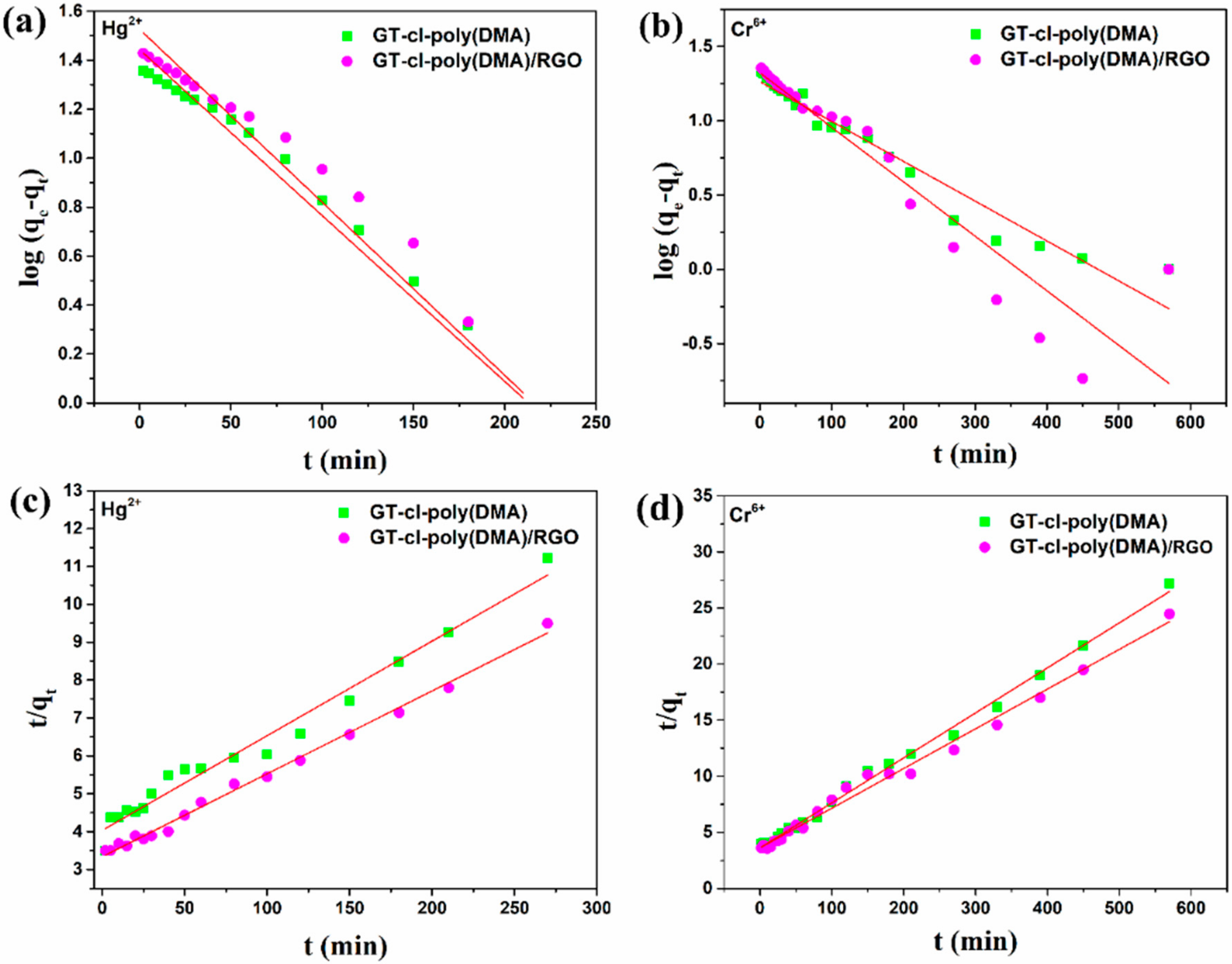
3.8. Adsorption Kinetics
3.9. Adsorption Isotherms
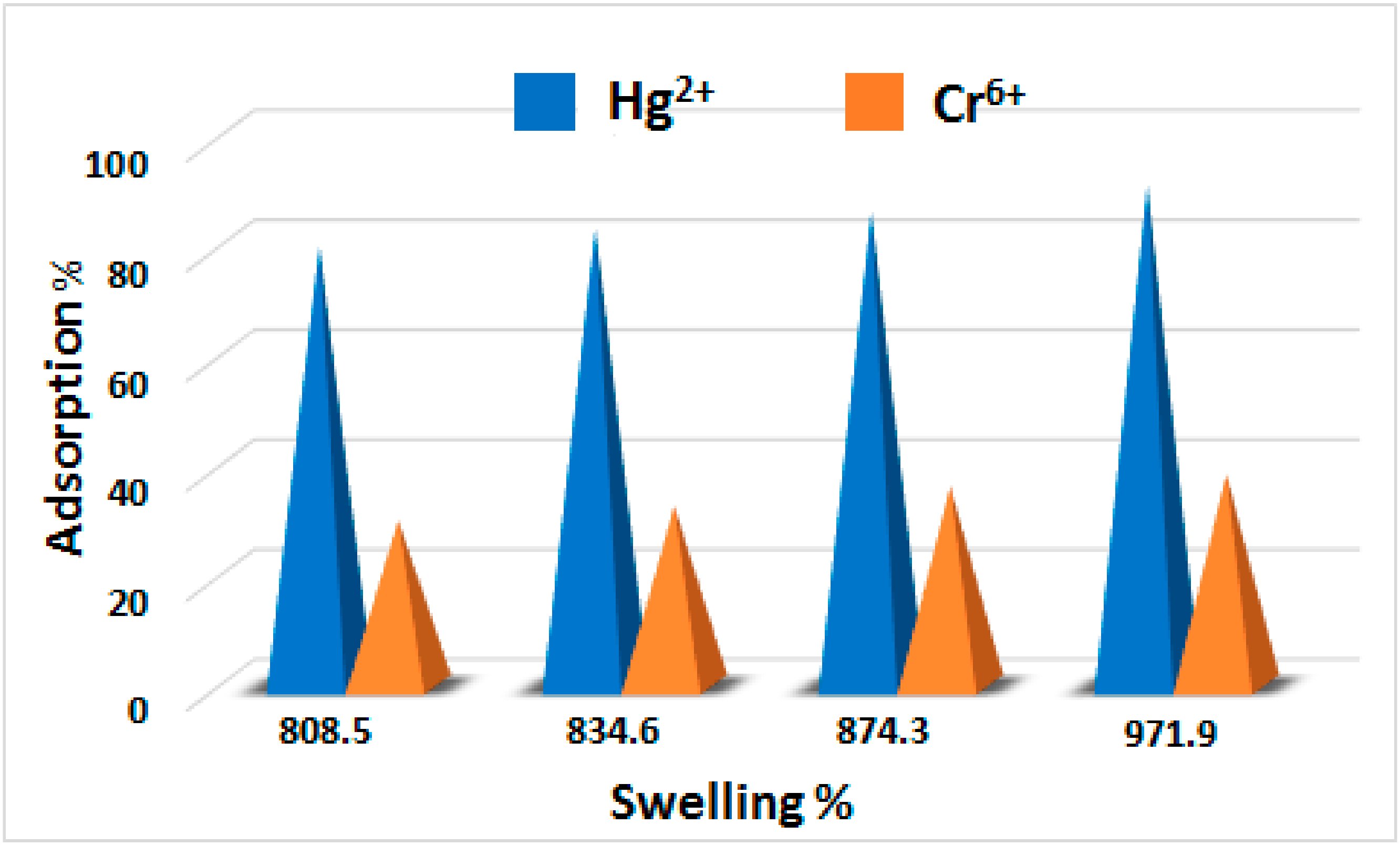
3.10. Relationship between the Adsorption and Swelling
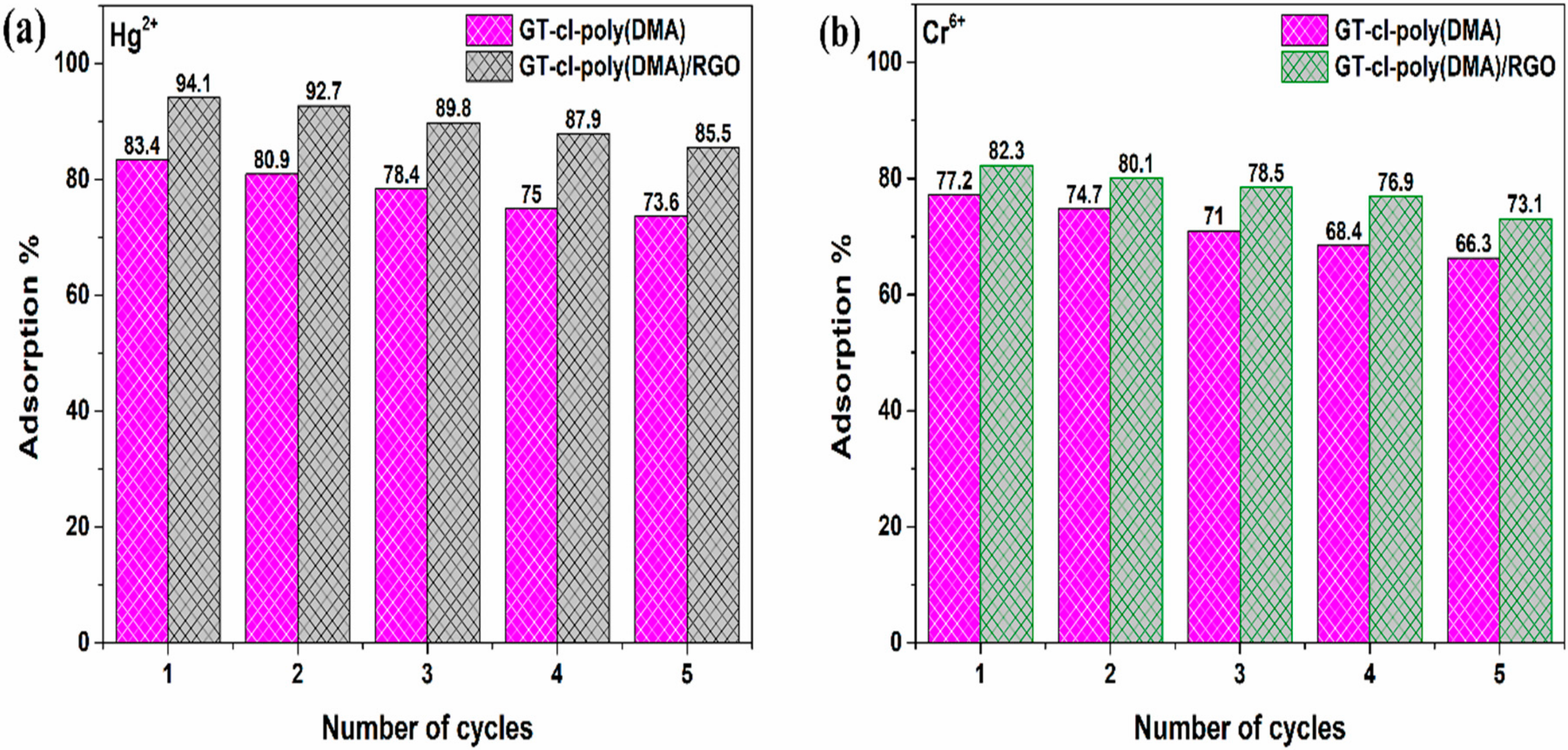
3.11. Adsorption-Desorption Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siddiqui, E.; Pandey, J. Assessment of heavy metal pollution in water and surface sediment and evaluation of ecological risks associated with sediment contamination in the Ganga River: A basin-scale study. Environ. Sci. Pollut. Res. 2019, 26, 10926–10940. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent approaches in guar gum hydrogel synthesis for water purification. Int. J. Polym. Anal. Chem. 2018, 23, 621–632. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef]
- Hussain, I.; Sayed, S.M.; Fu, G. Facile and cost-effective synthesis of glycogen-based conductive hydrogels with extremely flexible, excellent self-healing and tunable mechanical properties. Int. J. Biol. Macromol. 2018, 118, 1463–1469. [Google Scholar] [CrossRef]
- Chaudhary, J.; Thakur, S.; Sharma, M.; Gupta, V.K.; Thakur, V.K. Development of Biodegradable Agar-Agar/Gelatin-Based Superabsorbent Hydrogel as an Efficient Moisture-Retaining Agent. Biomolecules 2020, 10, 939. [Google Scholar] [CrossRef]
- Nazarzadeh, E.Z.; Makvandi, P.; Tay, F.R. Recent progress in the industrial and biomedical applications of tragacanth gum: A review. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mat. Today 2019, 16, 213–246. [Google Scholar] [CrossRef]
- Nejatian, M.; Abbasi, S.; Azarikia, F. Gum Tragacanth: Structure, characteristics and applications in foods. Int. J. Biol. Macromol. 2020, 160, 846–860. [Google Scholar] [CrossRef]
- Mallakpour, S.; Abdolmaleki, A.; Tabesh, F. Ultrasonic-assisted manufacturing of new hydrogel nanocomposite biosorbent containing calcium carbonate nanoparticles and tragacanth gum for removal of heavy metal. Ultrason. Sonochem. 2018, 41, 572–581. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Tavakol, M. Synthesis of composite hydrogel of glutamic acid, gum tragacanth, and anionic polyacrylamide by electron beam irradiation for uranium (VI) removal from aqueous samples: Equilibrium, kinetics, and thermodynamic studies. Carbohydr. Polym. 2019, 206, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.-Q.; Bao, R.-Y.; Liu, Z.; Yang, W.; Xie, B.-H.; Yang, M.-B. Self-assembled sponge-like chitosan/reduced graphene oxide/montmorillonite composite hydrogels without cross-linking of chitosan for effective Cr (VI) sorption. ACS Sustain. Chem. Eng. 2017, 5, 1557–1566. [Google Scholar] [CrossRef]
- Zhuang, Y.-T.; Zhang, X.; Wang, D.-H.; Yu, Y.-L.; Wang, J.-H. Three-dimensional molybdenum disulfide/graphene hydrogel with tunable heterointerfaces for high selective Hg (II) scavenging. J. Colloid Interf. Sci. 2018, 514, 715–722. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seo, H.; Lee, T.G. Removal of Hg (II) ions from aqueous solution by poly (allylamine-co-methacrylamide-co-dimethylthiourea). J. Ind. Eng. Chem. 2020, 84, 82–86. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, J.; Xiao, Y.; Zhang, C.; Wang, Q.; Zheng, W. Preparation sulfhydryl functionalized paramagnetic Ni0. 25Zn0. 75Fe2O4 microspheres for separating Pb (II) and Hg (II) ions from aqueous solution. Colloids Surf. A 2020, 586, 124205. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Zhang, J.; Pan, Y.; Huang, Z.; Xiao, H. Adsorption of Hg (II) ions from aqueous solution by diethylenetriaminepentaacetic acid-modified cellulose. Int. J. Biol. Macromol. 2019, 122, 149–156. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg (II), Cd (II) and Zn (II) ions from aqueous solutions. J. Hazard. Mater. 2012, 209, 240–249. [Google Scholar] [CrossRef]
- Li, S.-S.; Wang, X.-L.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Cui, L.; Li, Z.-C. Upon designing carboxyl methylcellulose and chitosan-derived nanostructured sorbents for efficient removal of Cd (II) and Cr (VI) from water. Int. J. Biol. Macromol. 2020, 143, 640–650. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; Yang, B.; Teng, H. Application of Carboxymethyl Cellulose–Stabilized Sulfidated Nano Zerovalent Iron for Removal of Cr (VI) in Simulated Groundwater. Water Air Soil Pollut. 2019, 230, 113. [Google Scholar] [CrossRef]
- Espinoza-Sánchez, M.A.; Arévalo-Niño, K.; Quintero-Zapata, I.; Castro-González, I.; Almaguer-Cantú, V. Cr (VI) adsorption from aqueous solution by fungal bioremediation based using Rhizopus sp. J. Environ. Manage. 2019, 251, 109595. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Jin, Y.; Song, T.; Liang, J.; Bai, X.; Yu, S.; Teng, C.; Wang, X.; Qu, J.; Huang, X. Removal of Cr (VI) by surfactant modified Auricularia auricula spent substrate: Biosorption condition and mechanism. Environ. Sci. Pollut. Res. 2017, 24, 17626–17641. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Gupta, V.K.; Agarwal, S.; Dev, K.; Pathania, D. Controlled release of antibiotic amoxicillin drug using carboxymethyl cellulose-cl-poly (lactic acid-co-itaconic acid) hydrogel. Intern. J. Biol. Macromol. 2017, 101, 612–620. [Google Scholar] [CrossRef]
- Pathania, D.; Verma, C.; Negi, P.; Tyagi, I.; Asif, M.; Kumar, N.S.; Al-Ghurabi, E.H.; Agarwal, S.; Gupta, V.K. Novel nanohydrogel based on itaconic acid grafted tragacanth gum for controlled release of ampicillin. Carbohydr. Polym. 2018, 196, 262–271. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; KhaN, N.R.; Hua, Z.; Huo, J.; Li, Y. Microwave assisted chitosan-polyethylene glycol hydrogel membrane synthesis of curcumin for open incision wound healing. Pharmazie 2020, 75, 118–123. [Google Scholar]
- Kaur, S.; Jindal, R.; Kaur Bhatia, J. Synthesis and RSM-CCD optimization of microwave-induced green interpenetrating network hydrogel adsorbent based on gum copal for selective removal of malachite green from waste water. Polym. Eng. Sci. 2018, 58, 2293–2303. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Inter. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Ramontja, J. Microwave assisted synthesis of xanthan gum-cl-poly(acrylic acid) based-reduced graphene oxide hydrogel composite for adsorption of methylene blue and methyl violet from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 255–269. [Google Scholar] [CrossRef]
- Rahmani, Z.; Sahraei, R.; Ghaemy, M. Preparation of spherical porous hydrogel beads based on ion-crosslinked gum tragacanth and graphene oxide: Study of drug delivery behavior. Carbohydr. Polym. 2018, 194, 34–42. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Číková, E.; Omastová, M. Development and characterization of composite fibers based on tragacanth gum and polyvinylpyrrolidone. Compos. B Eng. 2019, 169, 79–87. [Google Scholar] [CrossRef]
- Pandey, V.S.; Verma, S.K.; Yadav, M.; Behari, K. Guar gum-gN,N′-dimethylacrylamide: Synthesis, characterization and applications. Carbohydr. Polym. 2014, 99, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tesfay Reda, A.; Zhang, D. Reduced Graphene Oxide/ZIF-67 Aerogel Composite Material for Uranium Adsorption in Aqueous Solutions. ACS Omega 2020, 5, 8012–8022. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, Y.; Ding, H.; Wu, Z.; Yang, X.; Li, Z.; Huang, W.; Xie, X.; Tao, K.; Wang, X. Green Synthesis of 3D Chemically Functionalized Graphene Hydrogel for High-Performance NH3 and NO2 Detection at Room Temperature. ACS Appl. Mater. Inter. 2020, 12, 20623–20632. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F. Tragacanth gum based hydrogel nanocomposites for the adsorption of methylene blue: Comparison of linear and non-linear forms of different adsorption isotherm and kinetics models. Int. J. Biol. Macromol. 2019, 133, 754–766. [Google Scholar] [CrossRef]
- Khraisheh, M.A.M.; Al-Ghouti, M.A.; Allen, S.J.; Ahmad, M.N.M. The effect of pH, temperature, and molecular size on the removal of dyes from textile effluent using manganese oxides-modified diatomite. Water Environ. Res. 2004, 76, 2655–2663. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.; Abu-Dieyeh, M.; Khraisheh, M. Adsorptive removal of mercury from water by adsorbents derived from date pits. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Mei, J.; Zhang, H.; Li, Z.; Ou, H. A novel tetraethylenepentamine crosslinked chitosan oligosaccharide hydrogel for total adsorption of Cr (VI). Carbohydr. Polym. 2019, 224, 115154. [Google Scholar] [CrossRef]
- Li, Y.-S.; Li, T.-T.; Song, X.-F.; Yang, J.-Y.; Liu, G.; Qin, J.-T.; Dong, Z.-B.; Chen, H.-G.; Liu, Y. Enhanced adsorption-photocatalytic reduction removal for Cr (VI) based on functionalized TiO2 with hydrophilic monomers by pre-radiation induced grafting-ring opening method. Appl. Surf. Sci. 2020, 514, 145789. [Google Scholar] [CrossRef]
- Vilela, P.B.; Dalalibera, A.; Duminelli, E.C.; Becegato, V.A.; Paulino, A.T. Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ. Sci. Pollut. Res. 2019, 26, 28481–28489. [Google Scholar] [CrossRef]



| Serial Number | Adsorbent | Hg2+ Adsorption Capacity (mg g−1) | Cr6+ Adsorption Capacity (mg g−1) | References |
|---|---|---|---|---|
| 1. | Poly(allylamine-co-methacrylamide-co-dimethylthiourea) | 198.23 | - | [16] |
| 2. | Sulfhydryl-functional paramagnetic solid-phase adsorbent | 51.32 | - | [17] |
| 3. | Diethylenetriaminepentaacetic acid-modified cellulose adsorbent | 476.2 | - | [18] |
| 4. | Cross-linked magnetic chitosan-phenylthiourea resin | 135.5 | - | [19] |
| 5. | Carboxyl methylcellulose and chitosan-derived nanostructured | - | 347.0 | [20] |
| 6. | Carboxymethyl cellulose–stabilized sulfidated nano zerovalent iron | - | 355.9 | [21] |
| 7. | Fungal strain (Rhizopus sp.) | - | 9.95 | [22] |
| 8. | Surfactant-modified Auricularia auricula spent substrate | - | 21.74 | [23] |
| 9. | GT-cl-poly(DMA) hydrogel and GT-cl-poly(DMA)/RGO hydrogel composite | 636.94 and 666.66 | - | Present work |
| 10. | GT-cl-poly(DMA) hydrogel and GT-cl-poly(DMA)/RGO hydrogel composite | - | 416.66 and 476.19 | Present work |
| Serial Number | Sample | Synthetic Route | Time for Synthesis (s) | Swelling Percentage (%) | References |
|---|---|---|---|---|---|
| 1. | Carboxymethyl cellulose-cl-poly(lactic acid- co-itaconic acid) hydrogel | Microwave assisted method | 90 s | 332% | [24] |
| 2. | Tragacanth gum-g-poly(itaconic acid) hydrogel | Microwave-assisted method | 220 s | 800% | [25] |
| 3. | Chitosan-polyethylene glycol hydrogel membrane | Microwave assisted method | 120 s | 96.4% | [26] |
| 4. | IPN [(GcA-coll)-cl-poly (AAm-ip-AA)] | Microwave assisted method | 150 s | 382.1% | [27] |
| 5. | GT-cl-poly(DMA) hydrogel | Microwave assisted method | 90 s | 957.2% | Present work |
| 6. | GT-cl-poly(DMA)/RGO hydrogel composite | Microwave assisted method | 90 s | 971.9% | Present work |
| Serial Number | Sample Name | GT (g) | KPS (g) | DMA (mL) | NMBA (g) | Solvent (mL) | RGO (g) | Swelling % |
|---|---|---|---|---|---|---|---|---|
| 1. | GT-cl-poly(DMA) hydrogel | 0.500 | 0.030 | 0.5 | 0.030 | 11 | - | 957.2% |
| 2. | GT-cl-poly(DMA)/RGO hydrogel composite | 0.500 | 0.030 | 0.5 | 0.030 | 11 | 0.020 | 971.9% |
| Kinetic Model | Parameters | GT-cl-poly(DMA) | GT-cl-poly(DMA)/RGO | ||
|---|---|---|---|---|---|
| Hg2+ | Cr6+ | Hg2+ | Cr6+ | ||
| Pseudo-first-order kinetics | R2 | 0.946 | 0.931 | 0.909 | 0.863 |
| qe (cal) | 25.7 | 19.0 | 33.5 | 21.0 | |
| qe (exp) | 28.2 | 22.6 | 40.8 | 25.9 | |
| k1 | 0.011 | 0.008 | 0.016 | 0.006 | |
| Pseudo-second-order kinetics | R2 | 0.989 | 0.995 | 0.994 | 0.989 |
| qe (cal) | 29.4 | 25 | 45.8 | 28.3 | |
| qe (exp) | 28.2 | 22.6 | 40.8 | 25.9 | |
| k2 | 5.90 | 4.42 | 1.42 | 3.44 | |
| Isotherm Models | Temperature | Parameters | GT-cl-poly(DMA) | GT-cl-poly(DMA)/RGO | ||
|---|---|---|---|---|---|---|
| Hg2+ | Cr6+ | Hg2+ | Cr6+ | |||
| Langmuir | 25 °C | qm (mg g−1) | 591.7 | 289.8 | 628.9 | 423.7 |
| b (L mg−1) | 0.014 | 0.010 | 0.036 | 0.020 | ||
| RL | 0.405–0.121 | 0.839–0.370 | 0.217–0.052 | 0.856–0.339 | ||
| R2 | 0.987 | 0.964 | 0.996 | 0.989 | ||
| 35 °C | qm (mg g−1) | 621.1 | 362.3 | 662.2 | 467.2 | |
| b (L mg−1) | 0.015 | 0.008 | 0.035 | 0.019 | ||
| RL | 0.400–0.119 | 0.874–0.452 | 0.220–0.054 | 0.869–0.371 | ||
| R2 | 0.960 | 0.962 | 0.994 | 0.989 | ||
| 45 °C | qm (mg g−1) | 625 | 401.6 | 666.6 | 473.9 | |
| b (L mg−1) | 0.016 | 0.007 | 0.046 | 0.022 | ||
| RL | 0.385–0.112 | 0.887–0.438 | 0.167–0.039 | 0.864–0.353 | ||
| R2 | 0.963 | 0.956 | 0.995 | 0.9623 | ||
| Freundlich | 25 °C | KF (mg g−1) | 1.144 | 0.765 | 1.393 | 1.05 |
| n | 1.72 | 1.70 | 2.12 | 1.58 | ||
| R2 | 0.889 | 0.898 | 0.905 | 0.955 | ||
| 35 °C | KF (mg g−1) | 1.156 | 0.679 | 1.389 | 1.038 | |
| N | 1.69 | 1.47 | 2.04 | 1.52 | ||
| R2 | 0.891 | 0.895 | 0.914 | 0.955 | ||
| 45 °C | KF (mg g−1) | 1.168 | 0.726 | 1.438 | 1.080 | |
| n | 1.66 | 1.49 | 2.17 | 1.53 | ||
| R2 | 0.868 | 0.876 | 0.898 | 0.924 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, B.; Thakur, S.; Trache, D.; Yazdani Nezhad, H.; Thakur, V.K. Microwave-Assisted Rapid Synthesis of Reduced Graphene Oxide-Based Gum Tragacanth Hydrogel Nanocomposite for Heavy Metal Ions Adsorption. Nanomaterials 2020, 10, 1616. https://doi.org/10.3390/nano10081616
Sharma B, Thakur S, Trache D, Yazdani Nezhad H, Thakur VK. Microwave-Assisted Rapid Synthesis of Reduced Graphene Oxide-Based Gum Tragacanth Hydrogel Nanocomposite for Heavy Metal Ions Adsorption. Nanomaterials. 2020; 10(8):1616. https://doi.org/10.3390/nano10081616
Chicago/Turabian StyleSharma, Bhawna, Sourbh Thakur, Djalal Trache, Hamed Yazdani Nezhad, and Vijay Kumar Thakur. 2020. "Microwave-Assisted Rapid Synthesis of Reduced Graphene Oxide-Based Gum Tragacanth Hydrogel Nanocomposite for Heavy Metal Ions Adsorption" Nanomaterials 10, no. 8: 1616. https://doi.org/10.3390/nano10081616
APA StyleSharma, B., Thakur, S., Trache, D., Yazdani Nezhad, H., & Thakur, V. K. (2020). Microwave-Assisted Rapid Synthesis of Reduced Graphene Oxide-Based Gum Tragacanth Hydrogel Nanocomposite for Heavy Metal Ions Adsorption. Nanomaterials, 10(8), 1616. https://doi.org/10.3390/nano10081616