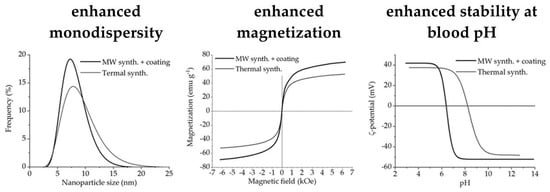
Microwave-Assisted Synthesis of Water-Dispersible Humate-Coated Magnetite Nanoparticles: Relation of Coating Process Parameters to the Properties of Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Samples
2.2.1. General Procedure
2.2.2. Synthesis of Uncoated Magnetite Nanoparticles
2.2.3. Synthesis of Humate-Coated Magnetite Nanoparticles
2.3. Samples Characterization
3. Results and Discussions
3.1. Structural Analysis
3.2. Fourier Transform Infrared Spectroscopy
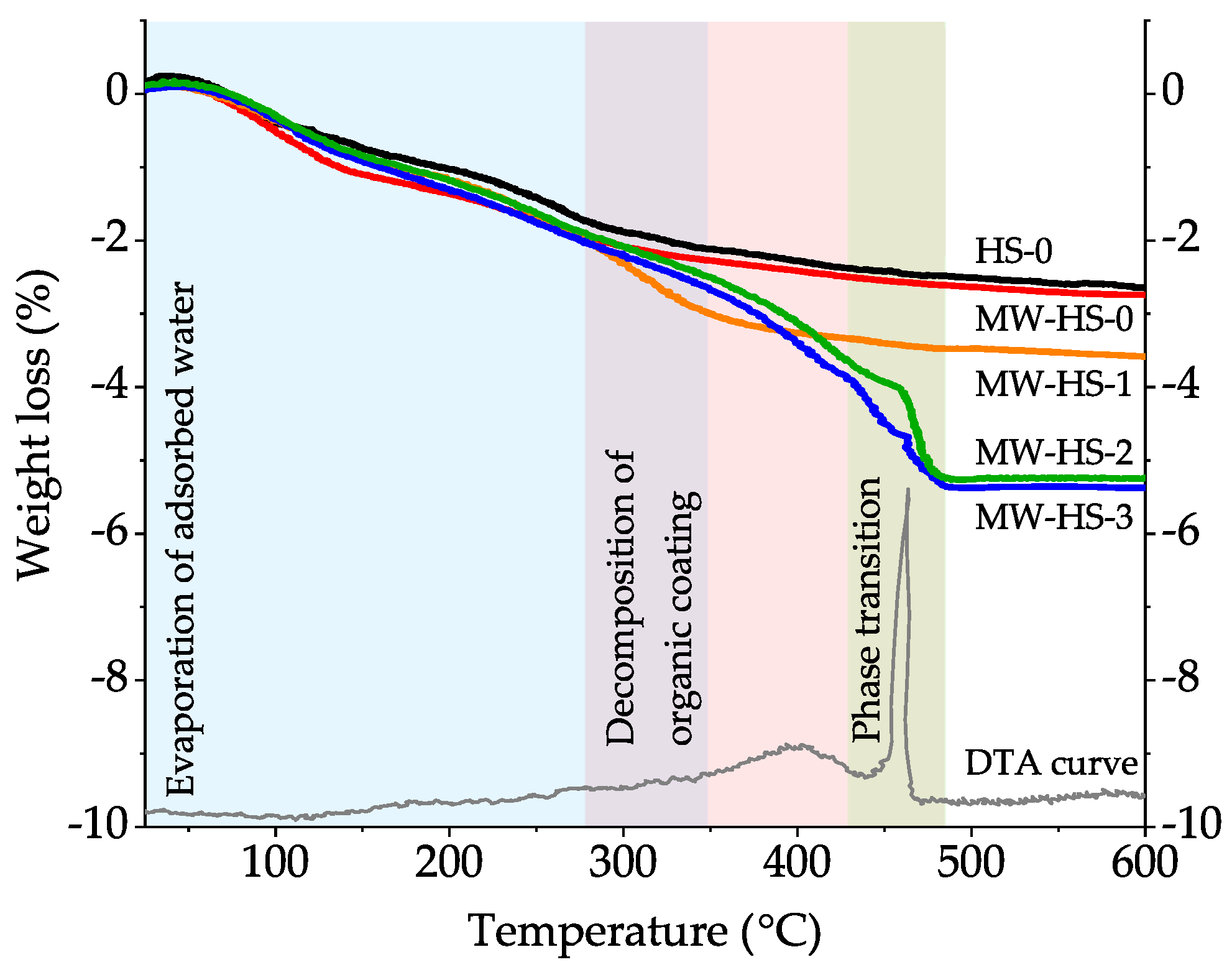
3.3. Thermal Analysis
3.4. Magnetic Measurements
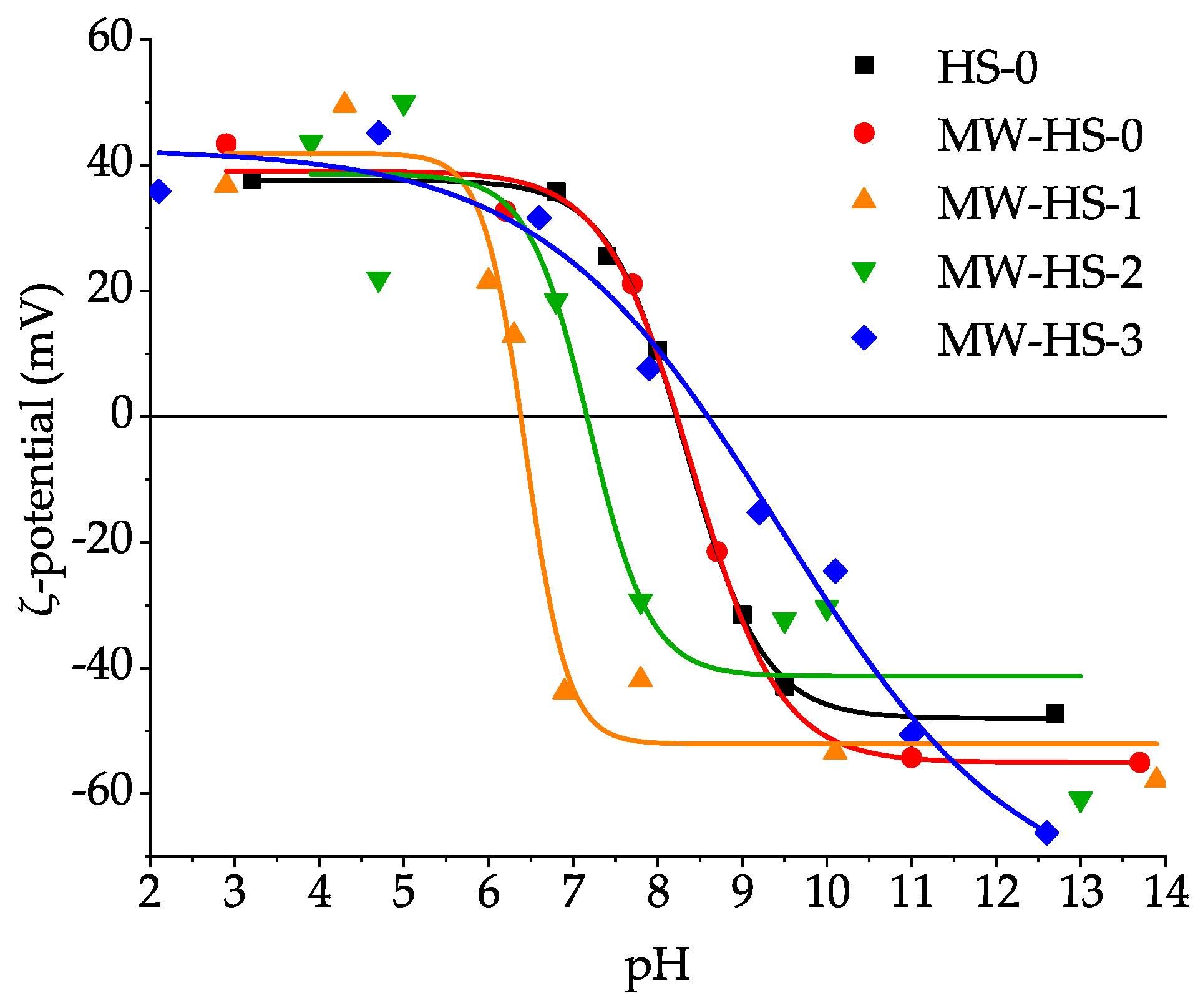
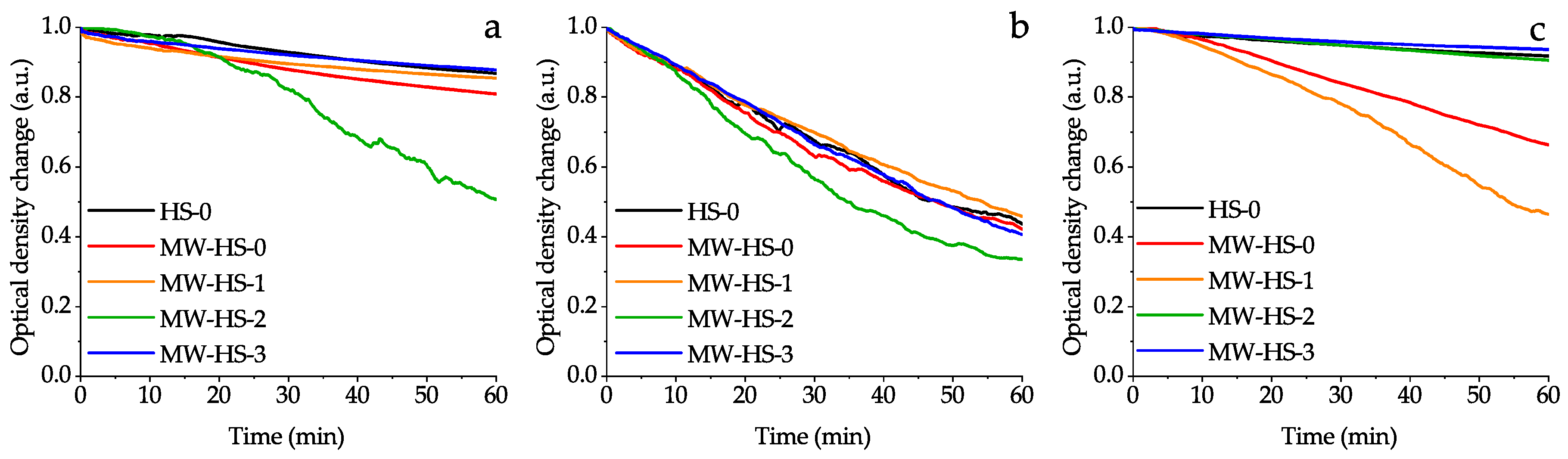
3.5. Colloid Properties
3.6. Summary
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Su, C. Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef] [PubMed]
- Tursunov, O.; Kustov, L.; Kustov, A.A. Brief Review of Carbon Dioxide Hydrogenation to Methanol Over Copper and Iron Based Catalysts. Oil Gas Sci. Technol.–Revue d’IFP Energies nouvelles 2017, 72, 30. [Google Scholar] [CrossRef]
- Dobrowolski, J.W.; Bedla, D.; Czech, T.; Gambuś, F.; Górecka, K.; Kiszczak, W.; Kuźniar, T.; Mazur, R.; Nowak, A.; Śliwka, M.; et al. Integrated Innovative Biotechnology for Optimization of Environmental Bioprocesses and a Green Economy. In Optimization and Applicability of Bioprocesses; Springer: Singapore, 2017; pp. 27–71. [Google Scholar]
- Sharma, R.K.; Dutta, S.; Sharma, S.; Zboril, R.; Varma, R.S.; Gawande, M.B. Fe3O4 (iron oxide)-supported nanocatalysts: Synthesis, characterization and applications in coupling reactions. Green Chem. 2016, 18, 3184–3209. [Google Scholar] [CrossRef]
- Tarasov, A.L.; Kostyukhin, E.M.; Kustov, L.M. Gasification of metal-containing coals and carbons via their reaction with carbon dioxide. Mendeleev Commun. 2018, 28, 530–532. [Google Scholar] [CrossRef]
- Skomski, R. Nanomagnetics. J. Phys. Condens. Matter 2003, 15, R841–R896. [Google Scholar] [CrossRef]
- De, B.; Yadav, A.; Khan, S.; Kar, K.K. A Facile Methodology for the Development of a Printable and Flexible All-Solid-State Rechargeable Battery. ACS Appl. Mater. Interfaces 2017, 9, 19870–19880. [Google Scholar] [CrossRef]
- Bruck, A.M.; Cama, C.A.; Gannett, C.N.; Marschilok, A.C.; Takeuchi, E.S.; Takeuchi, K.J. Nanocrystalline iron oxide based electroactive materials in lithium ion batteries: The critical role of crystallite size, morphology, and electrode heterostructure on battery relevant electrochemistry. Inorg. Chem. Front. 2016, 3, 26–40. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Javed, Y.; Akhtar, K.; Anwar, H.; Jamil, Y. MRI based on iron oxide nanoparticles contrast agents: Effect of oxidation state and architecture. J. Nanopart. Res. 2017, 19, 366. [Google Scholar] [CrossRef]
- Hu, Y.; Mignani, S.; Majoral, J.-P.; Shen, M.; Shi, X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem. Soc. Rev. 2018, 47, 1874–1900. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Katz, E. Magnetic Nanoparticles. Magnetochemistry 2020, 6, 6. [Google Scholar] [CrossRef]
- Majidi, S.; Zeinali Sehrig, F.; Farkhani, S.M.; Soleymani Goloujeh, M.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef]
- Duan, M.; Shapter, J.G.; Qi, W.; Yang, S.; Gao, G. Recent progress in magnetic nanoparticles: Synthesis, properties, and applications. Nanotechnology 2018, 29, 452001. [Google Scholar] [CrossRef]
- Katz, E. Synthesis, Properties and Applications of Magnetic Nanoparticles and Nanowires—A Brief Introduction. Magnetochemistry 2019, 5, 61. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Kostyukhin, E.M.; Kustov, L.M. Microwave-assisted synthesis of magnetite nanoparticles possessing superior magnetic properties. Mendeleev Commun. 2018, 28, 559–561. [Google Scholar] [CrossRef]
- Vikanova, K.; Redina, E.; Kapustin, G.; Nissenbaum, V.; Mishin, I.; Kostyukhin, E.; Kustov, L. Template-free one-step synthesis of micro-mesoporous CeO2–ZrO2 mixed oxides with a high surface area for selective hydrogenation. Ceram. Int. 2020, 46, 13980–13988. [Google Scholar] [CrossRef]
- Kostyukhin, E.M.; Kustov, A.L.; Kustov, L.M. One-step hydrothermal microwave-assisted synthesis of LaFeO3 nanoparticles. Ceram. Int. 2019, 45, 14384–14388. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Madani, M.A. Review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhao, X.; Zhao, T.; Wang, H.; Wang, P.; Wu, F.; Giesy, J.P. Magnetic Nanoparticles Interaction with Humic Acid: In the Presence of Surfactants. J. Environ. Sci. Technol. 2016, 50, 8640–8648. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, A.Y.; Lebedev, V.A.; Shirshin, E.A.; Rumyantsev, A.M.; Volikov, A.B.; Zherebker, A.; Garshev, A.V.; Goodilin, E.A.; Perminova, I.V. Non-classical growth of water-redispersible spheroidal gold nanoparticles assisted by leonardite humate. CrystEngComm 2017, 19, 876–886. [Google Scholar] [CrossRef]
- Jung, B.; O’Carroll, D.; Sleep, B. The influence of humic acid and clay content on the transport of polymer-coated iron nanoparticles through sand. Sci. Total Environ. 2014, 496, 155–164. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Goldt, A.E.; Sorkina, T.A.; Perminova, I.V.; Pankratov, D.A.; Goodilin, E.A.; Tretyakov, Y.D. Constrained growth of anisotropic magnetic δ-FeOOH nanoparticles in the presence of humic substances. CrystEngComm 2012, 14, 8097. [Google Scholar] [CrossRef]
- Chekanova, A.E.; Sorkina, T.A.; Dubov, A.L.; Nikiforov, V.N.; Davydova, G.A.; Selezneva, I.I.; Goodilin, E.A.; Trusov, L.A.; Korolev, V.V.; Aref’ev, I.M.; et al. New environmental nontoxic agents for the preparation of core-shell magnetic nanoparticles. Mendeleev Commun. 2009, 19, 72–74. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Sorkina, T.A.; Goldt, A.E.; Pankratov, D.A.; Perminova, I.V.; Goodilin, E.A. Mössbauer spectroscopy of frozen solutions as a stepwise control tool in preparation of biocompatible humic-stabilized feroxyhyte nanoparticles. Hyperfine Interact. 2013, 219, 113–120. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Guo, X.; Dang, Z.; Li, X.; Zhang, Q. Effects of humic acids on the aggregation and sorption of nano-TiO2. Chemosphere 2015, 119, 171–176. [Google Scholar] [CrossRef]
- Mert, E.H.; Yıldırım, H.; Üzümcü, A.T.; Kavas, H. Synthesis and characterization of magnetic polyHIPEs with humic acid surface modified magnetic iron oxide nanoparticles. React. Funct. Polym. 2013, 73, 175–181. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Jiang, G. Coating Fe3O4 Magnetic Nanoparticles with Humic Acid for High Efficient Removal of Heavy Metals in Water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Cai, Q.; Xu, W.; Yang, M.; Cai, Y.; Dionysiou, D.D.; O’Shea, K.E. Cr(VI) Adsorption and Reduction by Humic Acid Coated on Magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Zhang, D.; Zhang, S.; Zhang, X.; Meng, Z.; Cai, Y. Humic acid coated Fe3O4 magnetic nanoparticles as highly efficient Fenton-like catalyst for complete mineralization of sulfathiazole. J. Hazard. Mater. 2011, 190, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, P.; Wu, Z.; Zhang, L.; Zeng, G.; Zhou, C. Adsorption of methylene blue onto humic acid-coated Fe3O4 nanoparticles. Colloids Surf. A 2013, 435, 85–90. [Google Scholar] [CrossRef]
- Schepetkin, I.; Khlebnikov, A.; Kwon, B.S. Medical drugs from humus matter: Focus on mumie. Drug Dev. Res. 2002, 57, 140–159. [Google Scholar] [CrossRef]
- Van Rensburg, C.E.J. The Antiinflammatory Properties of Humic Substances: A Mini Review. Phytother. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef]
- Jansen van Rensburg, C.E.; Naude, P.J. Potassium Humate Inhibits Complement Activation and the Production of Inflammatory Cytokines In Vitro. Inflammation 2009, 32, 270–276. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef]
- Tombácz, E.; Tóth, I.Y.; Nesztor, D.; Illés, E.; Hajdú, A.; Szekeres, M.; Vékás, L. Adsorption of organic acids on magnetite nanoparticles, pH-dependent colloidal stability and salt tolerance. Colloids Surf. A 2013, 435, 91–96. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Tijing, L.D.; Zhou, J.L.; Kim, J.H.; Shon, H.K. Stability of Fe-oxide nanoparticles coated with natural organic matter under relevant environmental conditions. Water Sci. Technol. 2014, 70, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Hajdú, A.; Illés, E.; Tombácz, E.; Borbáth, I. Surface charging, polyanionic coating and colloid stability of magnetite nanoparticles. Colloids Surf. A 2009, 347, 104–108. [Google Scholar] [CrossRef]
- Govan, J.; Gun’ko, Y. Recent Advances in the Application of Magnetic Nanoparticles as a Support for Homogeneous Catalysts. Nanomaterials 2014, 4, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Illés, E.; Tombácz, E. The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite. Colloids Surf. A 2003, 230, 99–109. [Google Scholar] [CrossRef]
- Jolivet, J.-P.; Chanéac, C.; Tronc, E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem. Commun. 2004, 477–483. [Google Scholar] [CrossRef]
- Saxena, N.; Singh, M. Efficient synthesis of superparamagnetic magnetite nanoparticles under air for biomedical applications. J. Magn. Magn. Mater. 2017, 429, 166–176. [Google Scholar] [CrossRef]
- Scardi, P.; Ortolani, M.; Leoni, M. WPPM: Microstructural Analysis beyond the Rietveld Method. Mater. Sci. Forum 2010, 651, 155–171. [Google Scholar] [CrossRef]
- Stingaciu, M.; Andersen, H.L.; Granados-Miralles, C.; Mamakhel, A.; Christensen, M. Magnetism in CoFe2 O4 nanoparticles produced at sub- and near-supercritical conditions of water. CrystEngComm 2017, 19, 3986–3996. [Google Scholar] [CrossRef]
- Leoni, M.; Confente, T.; Scardi, P. PM2K: A flexible program implementing Whole Powder Pattern Modelling. Z. Kristallogr. Suppl 2006, 23, 249–254. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-oriented analysis of gaseous, liquid and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
- Kirichenko, O.A.; Kapustin, G.I.; Tkachenko, O.P.; Nissenbaum, V.D.; Mishin, I.V.; Davshan, N.A.; Redina, E.A.; Kustov, L.M. Synthesis and characterization of novel Au/θ-Al2-xFexO3 nanomaterials with high thermal stability in catalytic oxidation of carbon monoxide. Mater. Res. Bull. 2016, 80, 139–149. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Pankina, G.V.; Lunin, V.V. Magnetometric methods of investigation of supported catalysts. Russ. Chem. Rev. 2011, 80, 579–604. [Google Scholar] [CrossRef]
- Abramenko, N.B.; Demidova, T.B.; Abkhalimov, E.V.; Ershov, B.G.; Krysanov, E.Y.; Kustov, L.M. Ecotoxicity of different-shaped silver nanoparticles: Case of zebrafish embryos. J. Hazard. Mater. 2018, 347, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mallik, D.; Nayar, S. Comparative Study of Biomimetic Iron Oxides Synthesized Using Microwave Induced and Conventional Method. IEEE Trans. Magn. 2011, 47, 1647–1652. [Google Scholar] [CrossRef]
- Obaidat, I.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Sciancalepore, C.; Bondioli, F.; Manfredini, T.; Gualtieri, A. Quantitative phase analysis and microstructure characterization of magnetite nanocrystals obtained by microwave assisted non-hydrolytic sol–gel synthesis. Mater. Charact. 2015, 100, 88–97. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Belin, T.; Guigue-Millot, N.; Caillot, T.; Aymes, D.; Niepce, J. Influence of Grain Size, Oxygen Stoichiometry, and Synthesis Conditions on the γ-Fe2O3 Vacancies Ordering and Lattice Parameters. J. Solid State Chem. 2002, 163, 459–465. [Google Scholar] [CrossRef]
- Hu, L.; Percheron, A.; Chaumont, D.; Brachais, C.-H. Microwave-assisted one-step hydrothermal synthesis of pure iron oxide nanoparticles: Magnetite, maghemite and hematite. J. Sol-Gel Sci. Technol. 2011, 60, 198–205. [Google Scholar] [CrossRef]
- Chen, Y.H. Thermal properties of nanocrystalline goethite, magnetite, and maghemite. J. Alloys Compd. 2013, 553, 194–198. [Google Scholar] [CrossRef]
- Alp, E.; Aydogan, N. A comparative study: Synthesis of superparamagnetic iron oxide nanoparticles in air and N2 atmosphere. Colloids Surf. A 2016, 510, 205–212. [Google Scholar] [CrossRef]
- Aparicio, F.; Escalada, J.P.; De Gerónimo, E.; Aparicio, V.C.; García Einschlag, F.S.; Magnacca, G.; Carlos, L.; Mártire, D.O. Carbamazepine Degradation Mediated by Light in the Presence of Humic Substances-Coated Magnetite Nanoparticles. Nanomaterials 2019, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Y.; Zhou, Y.; Su, Z.; Liu, B.; Li, G.; Jiang, T. Adsorption-desorption characteristics and mechanisms of Pb(II) on natural vanadium, titanium-bearing magnetite-humic acid magnetic adsorbent. Powder Technol. 2019, 344, 947–958. [Google Scholar] [CrossRef]
- Cîrcu, M.; Nan, A.; Borodi, G.; Liebscher, J.; Turcu, R. Refinement of Magnetite Nanoparticles by Coating with Organic Stabilizers. Nanomaterials 2016, 6, 228. [Google Scholar] [CrossRef]
- Kandasamy, G.; Surendran, S.; Chakrabarty, A.; Kale, S.N.; Maity, D. Facile synthesis of novel hydrophilic and carboxyl-amine functionalized superparamagnetic iron oxide nanoparticles for biomedical applications. RSC Adv. 2016, 6, 99948–99959. [Google Scholar] [CrossRef]
- Patsula, V.; Kosinová, L.; Lovrić, M.; Ferhatovic Hamzić, L.; Rabyk, M.; Konefal, R.; Paruzel, A.; Šlouf, M.; Herynek, V.; Gajović, S.; et al. Superparamagnetic Fe3O4 Nanoparticles: Synthesis by Thermal Decomposition of Iron(III) Glucuronate and Application in Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2016, 8, 7238–7247. [Google Scholar] [CrossRef]
- Guardia, P.; Labarta, A.; Batlle, X. Tuning the Size, the Shape, and the Magnetic Properties of Iron Oxide Nanoparticles. J. Phys. Chem. C 2011, 115, 390–396. [Google Scholar] [CrossRef]
- Salafranca, J.; Gazquez, J.; Pérez, N.; Labarta, A.; Pantelides, S.T.; Pennycook, S.J.; Batlle, X.; Varela, M. Surfactant Organic Molecules Restore Magnetism in Metal-Oxide Nanoparticle Surfaces. Nano Lett. 2012, 12, 2499–2503. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Qin, X.; Liu, F.; Wang, G.; Huang, G. Adsorption of humic acid from aqueous solution by hematite: Effects of pH and ionic strength. Environ. Earth Sci. 2015, 73, 4011–4017. [Google Scholar] [CrossRef]
- Vega-Chacón, J.; Arbeláez, M.I.A.; Jorge, J.H.; Marques, R.F.C.; Jafelicci, M. pH-responsive poly(aspartic acid) hydrogel-coated magnetite nanoparticles for biomedical applications. Mater. Sci. Eng. C 2017, 77, 366–373. [Google Scholar] [CrossRef]
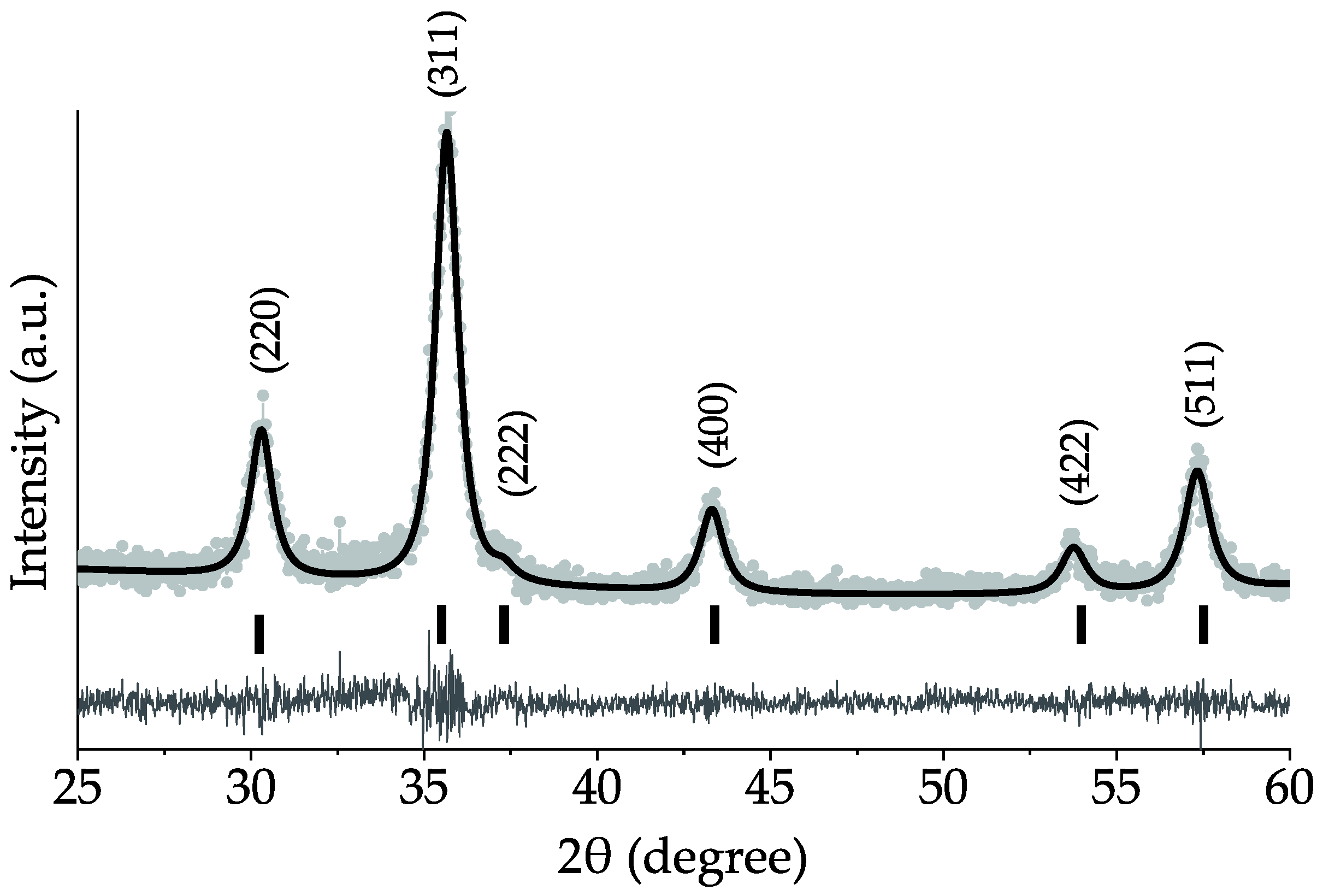
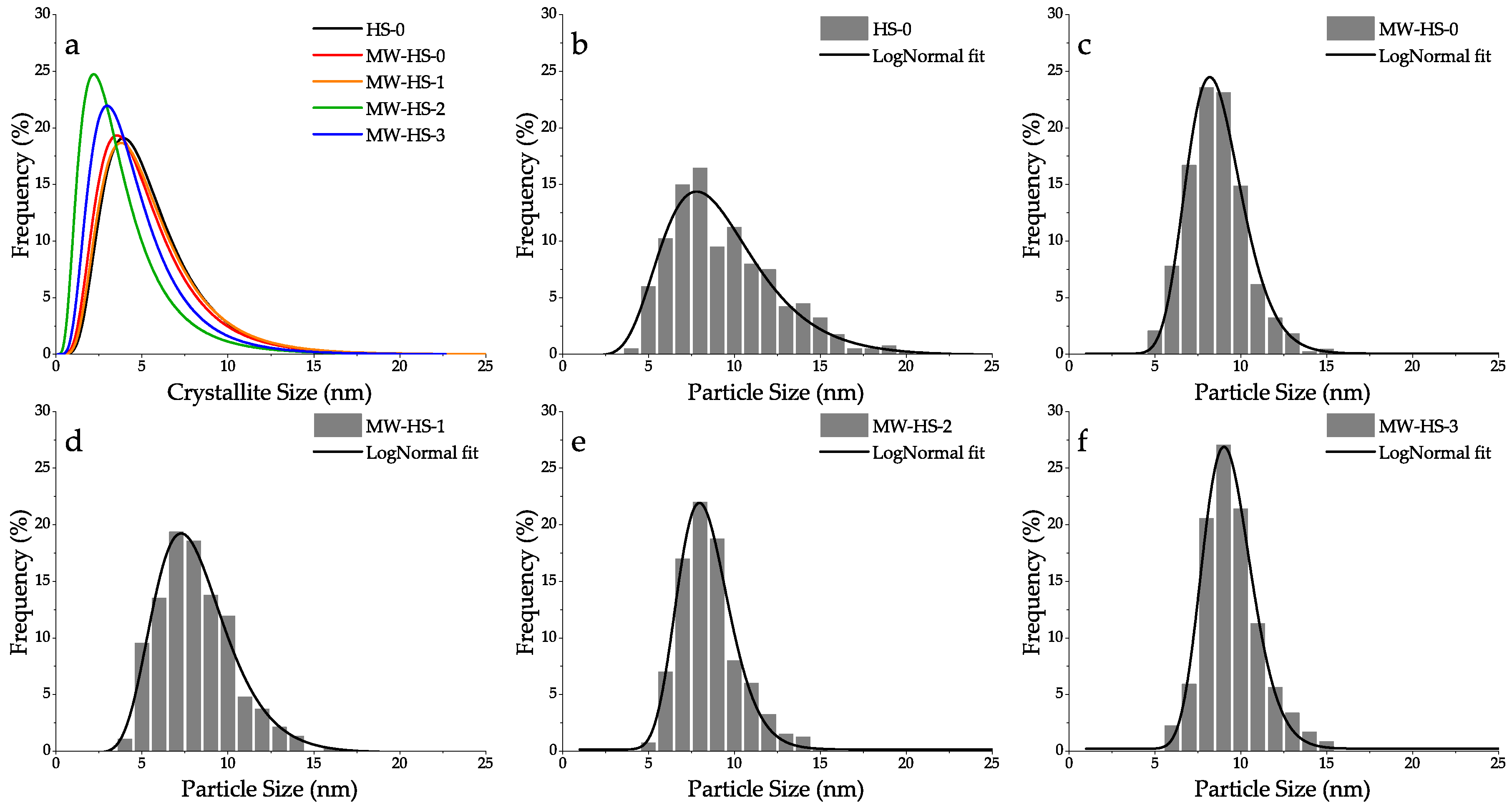


| Sample | Synthesis Method | Use of Potassium Humate | Volume Weighted Crystallite Size, WPPM (nm) | Lattice Parameter (nm) | Average Particle Size, TEM (nm) |
|---|---|---|---|---|---|
| HS-0 | thermal | not used | 8.3 | 0.8426 | 9.3 ± 3.3 |
| MW-HS-0 | microwave | not used | 8.6 | 0.8429 | 8.7 ± 1.7 |
| MW-HS-1 | microwave | after precipitation | 8.7 | 0.8429 | 8.2 ± 2.3 |
| MW-HS-2 | microwave | before precipitation | 8.8 | 0.8431 | 8.4 ± 1.6 |
| MW-HS-3 | microwave | before salts dissolution | 7.9 | 0.8455 | 9.4 ± 1.5 |
| Sample | Saturation Magnetization (emu g−1) | Point of Zero Charge (pH) | Average Hydrodynamic Diameter (nm) |
|---|---|---|---|
| HS-0 | 58 | 8.33 ± 0.04 | 35.7 ± 12.0 |
| MW-HS-0 | 60 | 8.41 ± 0.17 | 52.3 ± 17.2 |
| MW-HS-1 | 80 | 6.44 ± 0.11 | 130.7 ± 36.7 |
| MW-HS-2 | 68 | 7.18 ± 0.36 | 41.5 ± 13.5 |
| MW-HS-3 | 60 | 9.44 ± 0.44 | 34.9 ± 11.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostyukhin, E.M.; Nissenbaum, V.D.; Abkhalimov, E.V.; Kustov, A.L.; Ershov, B.G.; Kustov, L.M. Microwave-Assisted Synthesis of Water-Dispersible Humate-Coated Magnetite Nanoparticles: Relation of Coating Process Parameters to the Properties of Nanoparticles. Nanomaterials 2020, 10, 1558. https://doi.org/10.3390/nano10081558
Kostyukhin EM, Nissenbaum VD, Abkhalimov EV, Kustov AL, Ershov BG, Kustov LM. Microwave-Assisted Synthesis of Water-Dispersible Humate-Coated Magnetite Nanoparticles: Relation of Coating Process Parameters to the Properties of Nanoparticles. Nanomaterials. 2020; 10(8):1558. https://doi.org/10.3390/nano10081558
Chicago/Turabian StyleKostyukhin, Egor M., Vera D. Nissenbaum, Evgeny V. Abkhalimov, Alexander L. Kustov, Boris G. Ershov, and Leonid M. Kustov. 2020. "Microwave-Assisted Synthesis of Water-Dispersible Humate-Coated Magnetite Nanoparticles: Relation of Coating Process Parameters to the Properties of Nanoparticles" Nanomaterials 10, no. 8: 1558. https://doi.org/10.3390/nano10081558
APA StyleKostyukhin, E. M., Nissenbaum, V. D., Abkhalimov, E. V., Kustov, A. L., Ershov, B. G., & Kustov, L. M. (2020). Microwave-Assisted Synthesis of Water-Dispersible Humate-Coated Magnetite Nanoparticles: Relation of Coating Process Parameters to the Properties of Nanoparticles. Nanomaterials, 10(8), 1558. https://doi.org/10.3390/nano10081558