Cu/CuO Composite Track-Etched Membranes for Catalytic Decomposition of Nitrophenols and Removal of As(III)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electroless Synthesis of Composite Membranes
2.3. Thermal Annealing
2.4. Composite Characterization
2.5. Catalytic Performance of Cu/CuO/PET Composite Membrane
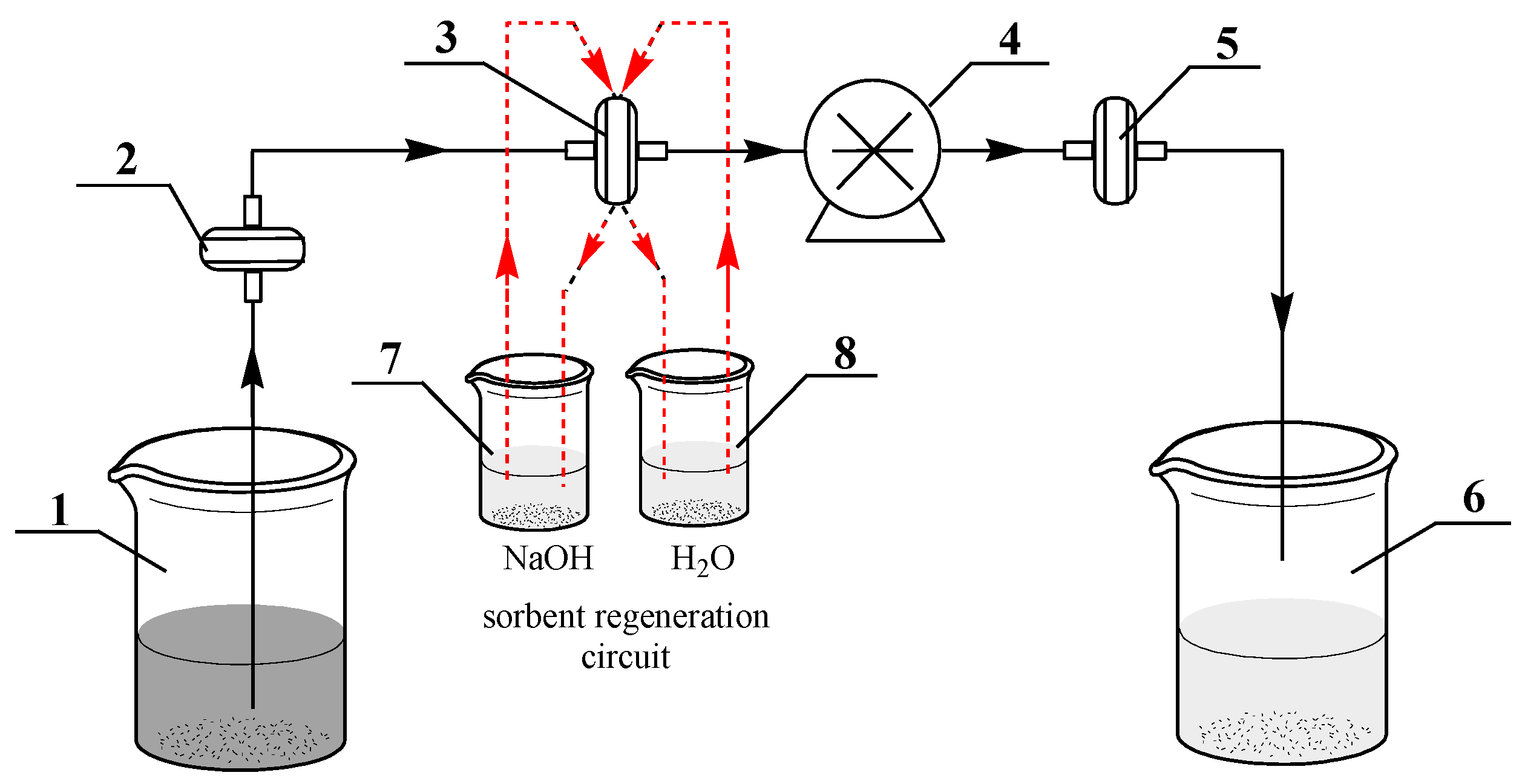
2.6. Adsorption of As (III) in Cross-Flow Mode
3. Results
3.1. Synthesis and Thermal Annealing of Cu/PET Composites
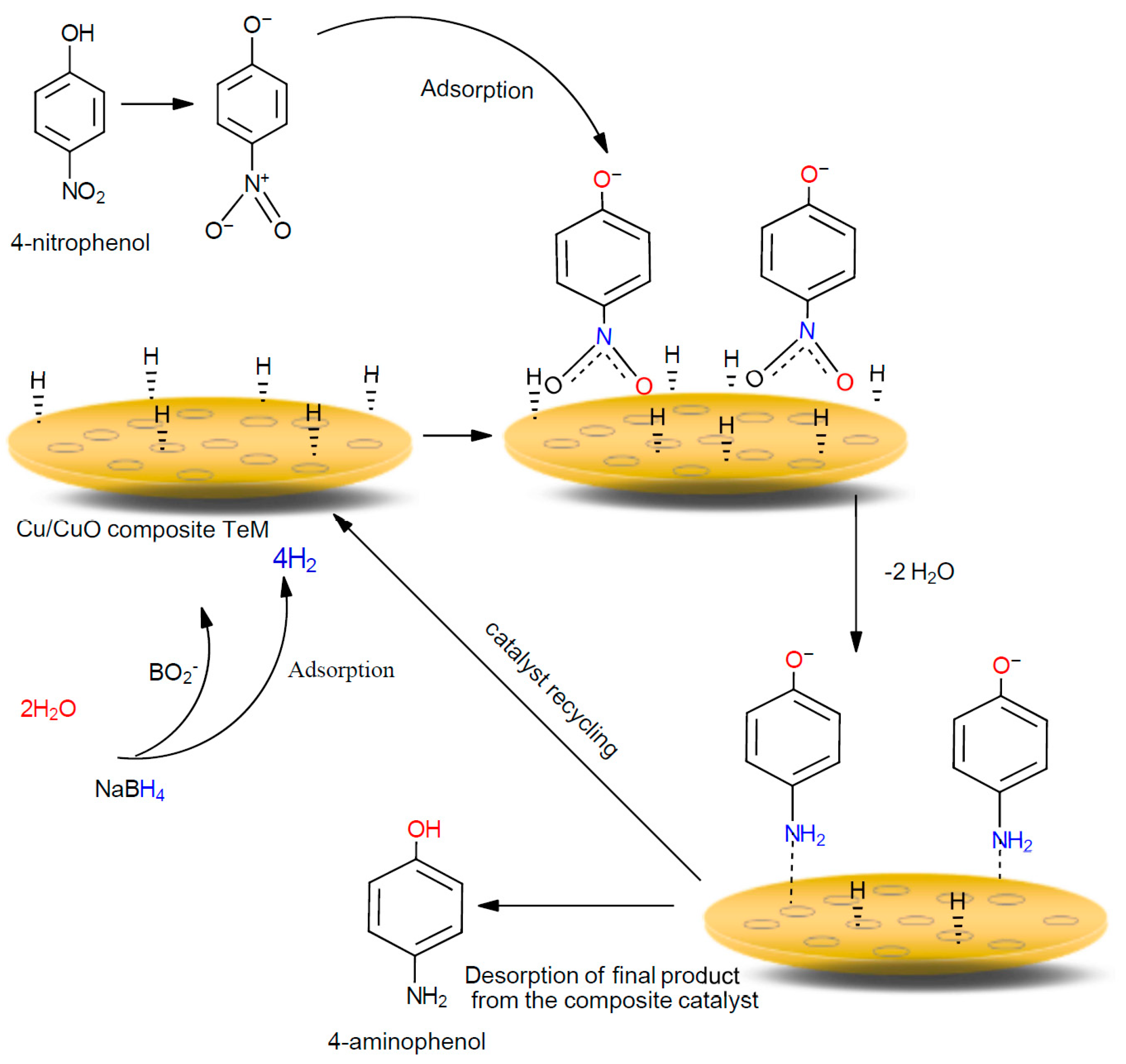
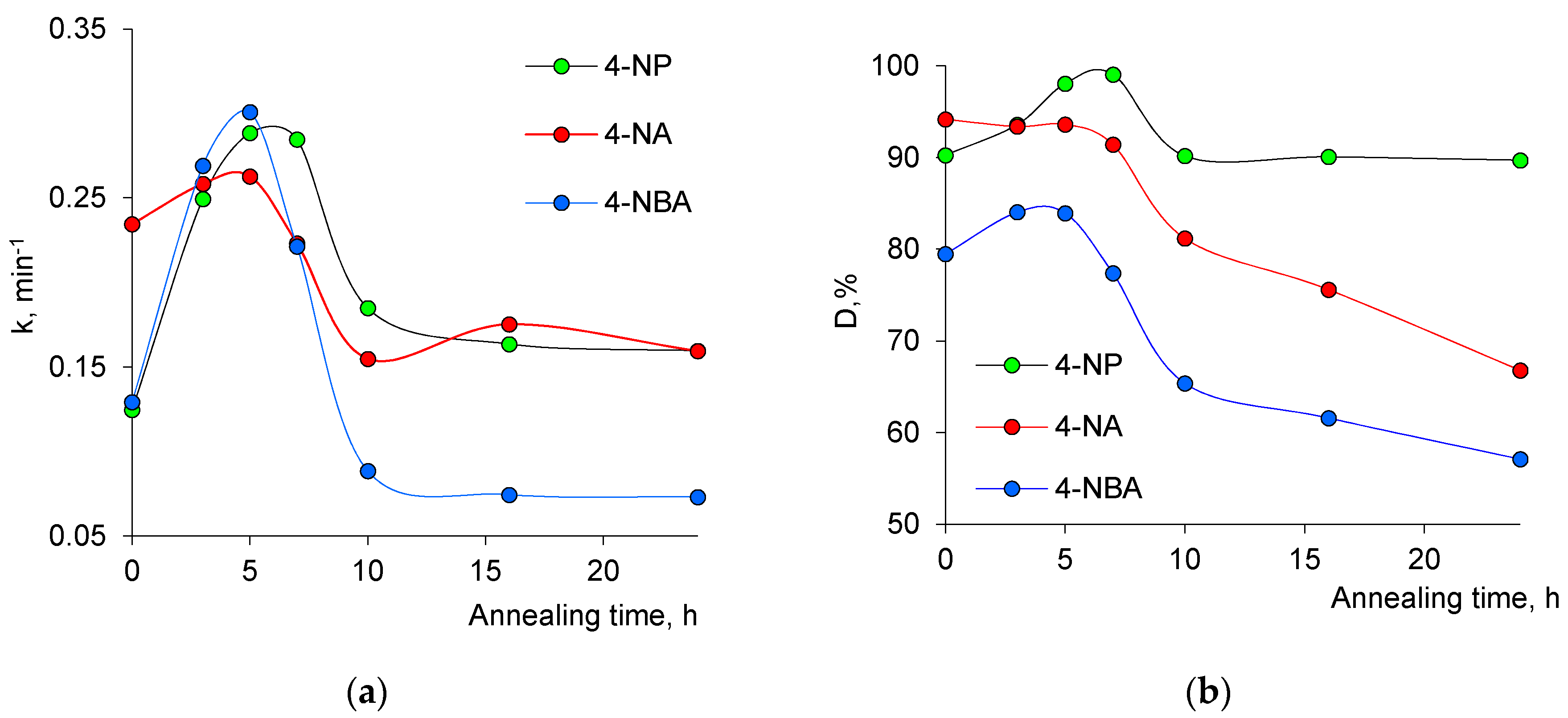
3.2. Assessment of Catalytic Activity
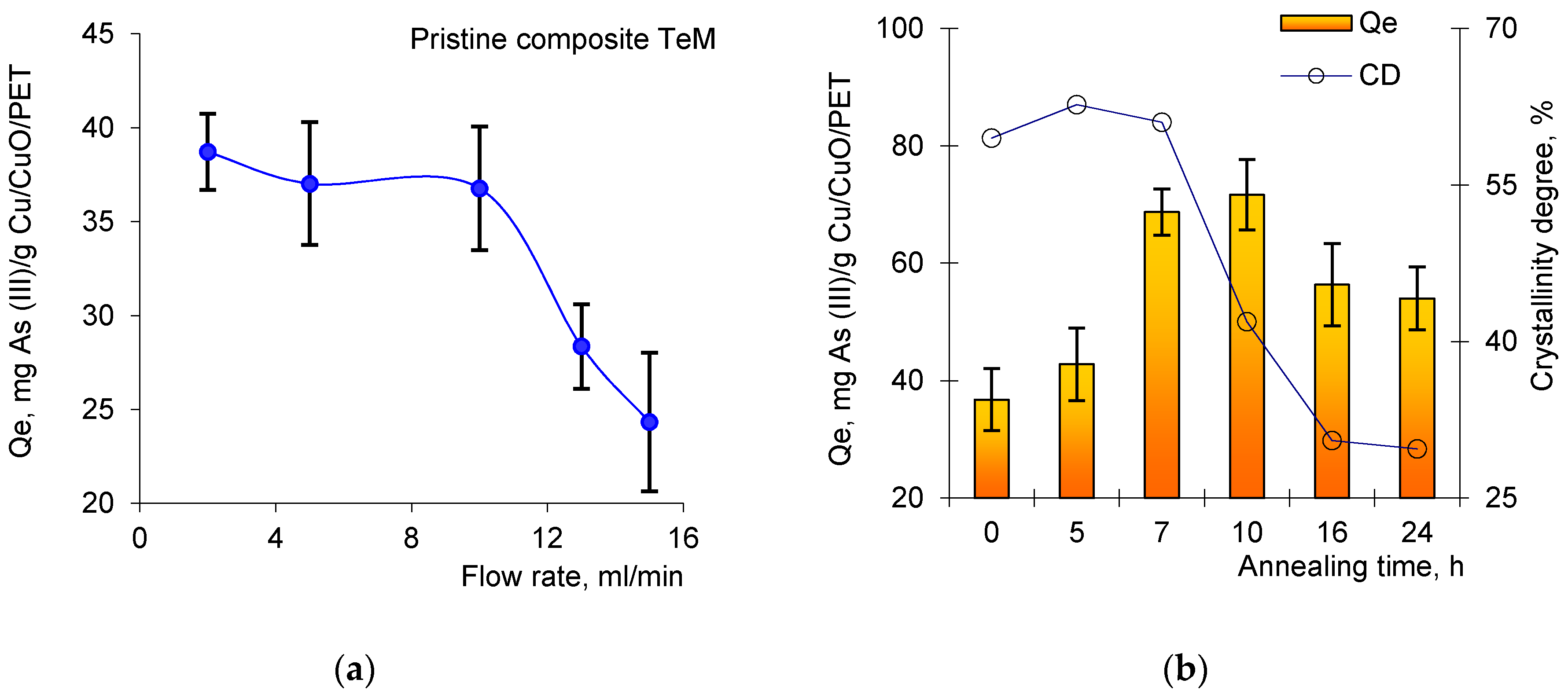
3.3. Flow-Through Removal of As(III)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Gu, A.; Wang, G.; Zhang, X.; Fang, B. Synthesis of CuO nanoflower and its application as a H2O2 sensor. Bull. Mater. Sci. 2010, 33, 17–20. [Google Scholar] [CrossRef]
- Muench, F.; Sun, L.; Kottakkat, T.; Antoni, M.; Schaefer, S.; Kunz, U.; Molina-Luna, L.; Duerrschnabel, M.; Kleebe, H.-J.; Ayata, S.; et al. Free-Standing Networks of Core-Shell Metal and Metal Oxide Nanotubes for Glucose Sensing. ACS Appl. Mater. Interfaces 2017, 9, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Liu, X.; Wu, J.; Li, M.; Gu, J.; Liu, H.; Fang, B. Different CuO Nanostructures: Synthesis, Characterization, and Applications for Glucose Sensors. J. Phys. Chem. C 2008, 112, 16845–16849. [Google Scholar] [CrossRef]
- Chen, J.; Wang, K.; Hartman, L.; Zhou, W. H2S Detection by Vertically Aligned CuO Nanowire Array Sensors. J. Phys. Chem. C 2008, 112, 16017–16021. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Gopalakrishnan, N. Facile synthesis of low dimensional CuO nanostructures and their gas sensing applications. Cryst. Res. Technol. 2016, 51, 145–153. [Google Scholar] [CrossRef]
- Luo, L.-B.; Wang, X.-H.; Xie, C.; Li, Z.-J.; Lu, R.; Yang, X.-B.; Lu, J. One-dimensional CuO nanowire: Synthesis, electrical, and optoelectronic devices application. Nanoscale Res. Lett. 2014, 9, 637. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Sow, C.H.; Thong, J.T.L. Enhanced field emission from CuO nanowire arrays by in situ laser irradiation. J. Appl. Phys. 2007, 102, 114302. [Google Scholar] [CrossRef]
- Grigore, M.; Biscu, E.; Holban, A.; Gestal, M.; Grumezescu, A. Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Mohan Mantravadi, K.; Nageswara Rao, G.V.S. Strategies to synthesise copper oxide nanoparticles and their bio applications—A review. Mater. Sci. Technol. 2018, 34, 2214–2222. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Hedberg, Y.; Tornberg, M.; De Battice, L.; Svedhem, S.; Wallinder, I.O. Cell membrane damage and protein interaction induced by copper containing nanoparticles—Importance of the metal release process. Toxicology 2013, 313, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ojha, N.K.; Zyryanov, G.V.; Majee, A.; Charushin, V.N.; Chupakhin, O.N.; Santra, S. Copper nanoparticles as inexpensive and efficient catalyst: A valuable contribution in organic synthesis. Coord. Chem. Rev. 2017, 353, 1–57. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.F.; Mohamed, A.T.; El-Shall, M.S.; Alqaradawi, S.Y.; Aljaber, A.S. Tailoring the reducibility and catalytic activity of CuO nanoparticles for low temperature CO oxidation. RSC Adv. 2018, 8, 19499–19511. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.M.; Lee, T.H.; Cha, J.H.; Choi, J.-W.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Copper oxide–graphene oxide nanocomposite: Efficient catalyst for hydrogenation of nitroaromatics in water. Nano Converg. 2019, 6, 6. [Google Scholar] [CrossRef]
- Wang, D.; Song, C.; Lv, X.; Wang, Y. Design of preparation parameters for commendable photocatalytic properties in CuO nanostructures. Appl. Phys. A 2016, 122, 1020. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Shi, L.; Gao, M. Surfactant-free Synthesis of CuO with Controllable Morphologies and Enhanced Photocatalytic Property. Nanoscale Res. Lett. 2016, 11, 125. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, H.; Lee, W.-J.; Bae, J.-S. Effects of ammonia in the synthesis of copper (II) oxide nanostructures grown via microwave chemical bath deposition. Surf. Coat. Technol. 2018, 334, 438–443. [Google Scholar] [CrossRef]
- Abdelmounaïm, C.; Amara, Z.; Maha, A.; Mustapha, D. Effects of molarity on structural, optical, morphological and CO2 gas sensing properties of nanostructured copper oxide films deposited by spray pyrolysis. Mater. Sci. Semicond. Process. 2016, 43, 214–221. [Google Scholar] [CrossRef]
- Kenzhina, I.E.; Zdorovets, M.V.; Kozlovskiy, A.L.; Kadyrzhanov, K.K. Synthesis and properties of Cu/CuO nanostructures obtained by electrochemical deposition. Mater. Res. Express 2018, 5, 035052. [Google Scholar] [CrossRef]
- Chen, L.; Shet, S.; Tang, H.; Wang, H.; Deutsch, T.; Yan, Y.; Turner, J.; Al-Jassim, M. Electrochemical deposition of copper oxide nanowires for photoelectrochemical applications. J. Mater. Chem. 2010, 20, 6962. [Google Scholar] [CrossRef]
- Papadimitropoulos, G.; Vourdas, N.; Vamvakas, V.E.; Davazoglou, D. Deposition and characterization of copper oxide thin films. J. Phys. Conf. Ser. 2005, 10, 182–185. [Google Scholar] [CrossRef]
- Lee, S.K.; Hsu, H.C.; Tuan, W.H. Oxidation behavior of copper at a temperature below 300 °C and the methodology for passivation. Mater. Res. 2016, 19, 51–56. [Google Scholar] [CrossRef]
- Gao, W.; Gong, H.; He, J.; Thomas, A.; Chan, L.; Li, S. Oxidation behaviour of Cu thin films on Si wafer at 175–400 °C. Mater. Lett. 2001, 51, 78–84. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Shlimas, D.I.; Zdorovets, M.V.; Mashentseva, A.A.; Kadyrzhanov, K.K. Thermal annealing-induced modification of the structure and electrical conductivity of metallic nanotubes embedded in PET track-etched membranes. Chem. Pap. 2018, 72, 173–180. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Shlimas, D.I.; Mashentseva, A.A.; Zdorovets, M.V.; Kadyrzhanov, K.K. Effect of thermal annealing on the structural and conducting properties of zinc nanotubes synthesized in the matrix of track-etched membranes. Pet. Chem. 2016, 56, 330–334. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zdorovets, M.; Kadyrzhanov, K.; Korolkov, I.; Rusakov, V.; Nikolaevich, L.; Fesenko, O.; Budnyk, O.; Yakimchuk, D.; Shumskaya, A.; et al. FeCo nanotubes: Possible tool for targeted delivery of drugs and proteins. Appl. Nanosci. 2019, 9, 1091–1099. [Google Scholar] [CrossRef]
- Stohr, T.; Fischer, A.; Muench, F.; Antoni, M.; Wollstadt, S.; Lohaus, C.; Kunz, U.; Clemens, O.; Klein, A.; Ensinger, W. Electroless Nanoplating of Pd−Pt Alloy Nanotube Networks: Catalysts with Full Compositional Control for the Methanol Oxidation Reaction. Chem. Chem. 2020, 7, 855–864. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Korolkov, I.V.; Kalkabay, G.; Ibragimova, M.A.; Ibrayeva, A.D.; Zdorovets, M.V.; Mikulich, V.S.; Yakimchuk, D.V.; Shumskaya, A.E.; Kaniukov, E.Y. Comprehensive Study of Ni Nanotubes for Bioapplications: From Synthesis to Payloads Attaching. J. Nanomater. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Mashentseva, A.A. Effect of the Oxidative Modification and Activation of Templates Based on Poly(ethylene terephthalate) Track-Etched Membranes on the Electroless Deposition of Copper and the Catalytic Properties of Composite Membranes. Pet. Chem. 2019, 59, 1337–1344. [Google Scholar] [CrossRef]
- Yeszhanov, A.B.; Mashentseva, A.A.; Korolkov, I.V.; Gorin, Y.G.; Kozlovskiy, A.L.; Zdorovets, M.V. Copper nanotube composite membrane as a catalyst in Mannich reaction. Chem. Pap. 2018, 72, 3189–3194. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Zdorovets, M.V. Accelerated electron-induced regeneration of the catalytic properties of composite membranes with embedded copper nanotubes. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 472, 53–58. [Google Scholar] [CrossRef]
- Martinson, C.A.; Reddy, K.J. Adsorption of arsenic(III) and arsenic(V) by cupric oxide nanoparticles. J. Colloid Interface Sci. 2009, 336, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Farghali, A.A.; Bahgat, M.; Enaiet Allah, A.; Khedr, M.H. Adsorption of Pb(II) ions from aqueous solutions using copper oxide nanostructures. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 61–71. [Google Scholar] [CrossRef]
- Hassan, K.H.; Jarullah, A.A.; Saadi, S.K. Synthesis of Copper Oxide Nanoparticle as an Adsorbent for Removal of Cd ( II ) and Ni ( II ) Ions from Binary System. Int. J. Appl. Environ. Sci. 2017, 12, 1841–1861. [Google Scholar]
- Egirani, D.E.; Poyi, N.R.; Wessey, N. Synthesis of a copper(II) oxide–montmorillonite composite for lead removal. Int. J. Miner. Metall. Mater. 2019, 26, 803–810. [Google Scholar] [CrossRef]
- Luan, H.; Zhang, Q.; Cheng, G.; Huang, H. As(III) Removal from Drinking Water by Carbon Nanotube Membranes with Magnetron-Sputtered Copper: Performance and Mechanisms. ACS Appl. Mater. Interfaces 2018, 10, 20467–20477. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Kozlovskiy, A.L.; Turapbay, K.O.; Temir, A.M.; Seytbaev, A.S.; Zdorovets, M.V. Determination of Optimal Conditions for Electoless Synthesis of Copper Nanotubes in the Polymer Matrix. Russ. J. Gen. Chem. 2018, 88, 1213–1218. [Google Scholar] [CrossRef]
- Borgekov, D.; Mashentseva, A.; Kislitsin, S.; Kozlovskiy, A.; Russakova, A.; Zdorovets, M. Temperature Dependent Catalytic Activity of Ag/PET Ion-Track Membranes Composites. Acta Phys. Pol. A 2015, 128, 871–875. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Gorin, Y.G.; Kozlovskiy, A.L.; Zdorovets, M.V.; Zhidkov, I.S.; Cholach, S.O. Electron/gamma radiation-induced synthesis and catalytic activity of gold nanoparticles supported on track-etched poly(ethylene terephthalate) membranes. Mater. Chem. Phys. 2018, 217, 31–39. [Google Scholar] [CrossRef]
- Barsbay, M.; Kavaklı, P.A.; Tilki, S.; Kavaklı, C.; Güven, O. Porous cellulosic adsorbent for the removal of Cd (II), Pb(II) and Cu(II) ions from aqueous media. Radiat. Phys. Chem. 2018, 142, 70–76. [Google Scholar] [CrossRef]
- Muench, F.; Schaefer, S.; Hagelüken, L.; Molina-Luna, L.; Duerrschnabel, M.; Kleebe, H.J.; Brötz, J.; Vaskevich, A.; Rubinstein, I.; Ensinger, W. Template-Free Electroless Plating of Gold Nanowires: Direct Surface Functionalization with Shape-Selective Nanostructures for Electrochemical Applications. ACS Appl. Mater. Interfaces 2017, 9, 31142–31152. [Google Scholar] [CrossRef] [PubMed]
- Shacham-Diamand, Y.; Osaka, T.; Okinaka, Y.; Sugiyama, A.; Dubin, V. 30 years of electroless plating for semiconductor and polymer micro-systems. Microelectron. Eng. 2015, 132, 35–45. [Google Scholar] [CrossRef]
- Krulik, G.A. Tin-Palladium Catalysts for Electroless Plating. Platin. Met. Rev. 1982, 26, 58–64. [Google Scholar]
- Szunerits, S.; Thouin, L. Microelectrode Arrays. In Handbook of Electrochemistry; Zoski, S.C., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 391–428. [Google Scholar]
- Stohr, T.; Brötz, J.; Oezaslan, M.; Muench, F. Dual Metastability in Electroless Plating: Complex Inertness Enabling the Deposition of Composition-Tunable Platinum Copper Alloy Nanostructures. Chem. A Eur. J. 2020, 26, 3030–3033. [Google Scholar] [CrossRef]
- Yakimov, I.; Zaloga, A.; Dubinin, P.; Bezrukova, O.; Samoilo, A.; Burakov, S.; Semenkin, E.; Semenkina, M.; Andruschenko, E. Application of Evolutionary Rietveld Method Based XRD Phase Analysis and a Self-Configuring Genetic Algorithm to the Inspection of Electrolyte Composition in Aluminum Electrolysis Baths. Crystals 2018, 8, 402. [Google Scholar] [CrossRef]
- Zhao, P.; Lu, L.; Liu, X.; De la Torre, A.; Cheng, X. Error Analysis and Correction for Quantitative Phase Analysis Based on Rietveld-Internal Standard Method: Whether the Minor Phases Can Be Ignored? Crystals 2018, 8, 110. [Google Scholar] [CrossRef]
- Abrosimova, G.E.; Aronin, A.S.; Kholstinina, N.N. On the determination of the volume fraction of the crystalline phase in amorphous-crystalline alloys. Phys. Solid State 2010, 52, 445–451. [Google Scholar] [CrossRef]
- Zdorovets, M.V.; Kozlovskiy, A.L. Investigation of phase transformations and corrosion resistance in Co/CoCo2O4 nanowires and their potential use as a basis for lithium-ion batteries. Sci. Rep. 2019, 9, 16646. [Google Scholar] [CrossRef]
- Das, G.; Tran, T.; Yoon, H.H. Spherulitic copper-copper oxide nanostructure-based highly sensitive nonenzymatic glucose sensor. Int. J. Nanomed. 2015, 10, 165–178. [Google Scholar] [CrossRef]
- Sınmazçelik, T.; Yılmaz, T. Thermal aging effects on mechanical and tribological performance of PEEK and short fiber reinforced PEEK composites. Mater. Des. 2007, 28, 641–648. [Google Scholar] [CrossRef]
- Viswanath, V.; Maity, S.; Bochinski, J.R.; Clarke, L.I.; Gorga, R.E. Thermal Annealing of Polymer Nanocomposites via Photothermal Heating: Effects on Crystallinity and Spherulite Morphology. Macromolecules 2013, 46, 8596–8607. [Google Scholar] [CrossRef]
- Khelchand Singh, N.; Rajkumari, R. Effect of Annealing on Metal-Oxide Nanocluster. In Concepts of Semiconductor Photocatalysis; IntechOpen: London, UK, 2019. [Google Scholar]
- DeAlba-Montero, I.; Guajardo-Pacheco, J.; Morales-Sánchez, E.; Araujo-Martínez, R.; Loredo-Becerra, G.M.; Martínez-Castañón, G.-A.; Ruiz, F.; Compeán Jasso, M.E. Antimicrobial Properties of Copper Nanoparticles and Amino Acid Chelated Copper Nanoparticles Produced by Using a Soya Extract. Bioinorg. Chem. Appl. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Korolkov, I.V.; Güven, O.; Mashentseva, A.A.; Atıcı, A.B.; Gorin, Y.G.; Zdorovets, M.V.; Taltenov, A.A. Radiation induced deposition of copper nanoparticles inside the nanochannels of poly(acrylic acid)-grafted poly(ethylene terephthalate) track-etched membranes. Radiat. Phys. Chem. 2017, 130, 480–487. [Google Scholar] [CrossRef]
- Jeong, S.; Woo, K.; Kim, D.; Lim, S.; Kim, J.S.; Shin, H.; Xia, Y.; Moon, J. Controlling the Thickness of the Surface Oxide Layer on Cu Nanoparticles for the Fabrication of Conductive Structures by Ink-Jet Printing. Adv. Funct. Mater. 2008, 18, 679–686. [Google Scholar] [CrossRef]
- Kuşçuoğlu, C.K.; Güner, H.; Söylemez, M.A.; Güven, O.; Barsbay, M. A smartphone-based colorimetric PET sensor platform with molecular recognition via thermally initiated RAFT-mediated graft copolymerization. Sens. Actuators B Chem. 2019, 296. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; Van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330. [Google Scholar] [CrossRef]
- Espinós, J.P.; Morales, J.; Barranco, A.; Caballero, A.; Holgado, J.P.; González-Elipe, A.R. Interface Effects for Cu, CuO, and Cu2O Deposited on SiO2 and ZrO2. XPS Determination of the Valence State of Copper in Cu/SiO2 and Cu/ZrO2 Catalysts. J. Phys. Chem. B 2002, 106, 6921–6929. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface Oxidation and Reduction of CuO and Cu2O Studied Using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Miller, A.C.; Simmons, G.W. Copper by XPS. Surf. Sci. Spectra 1993, 2, 55–60. [Google Scholar] [CrossRef]
- Vasquez, R.P. CuO by XPS. Surf. Sci. Spectra 1998, 5, 262–266. [Google Scholar] [CrossRef]
- Venezia, A.M. X-ray photoelectron spectroscopy (XPS) for catalysts characterization. Catal. Today 2003, 77, 359–370. [Google Scholar] [CrossRef]
- Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Recent Developments in the Reduction of Aromatic and Aliphatic Nitro Compounds to Amines. Org. Process. Res. Dev. 2018, 22, 430–445. [Google Scholar] [CrossRef]
- Ju, K.-S.; Parales, R.E. Nitroaromatic Compounds, from Synthesis to Biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Nandanwar, S.U.; Chakraborty, M. Synthesis of colloidal CuO/γ-Al2O3 by microemulsion and its catalytic reduction of aromatic nitro compounds. Cuihua Xuebao/Chin. J. Catal. 2012, 33, 1532–1541. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, H.; Zhang, W.; Lai, B.; Yao, G. Removal of nitrophenols and their derivatives by chemical redox: A review. Chem. Eng. J. 2019, 359, 13–31. [Google Scholar] [CrossRef]
- Pozun, Z.D.; Rodenbusch, S.E.; Keller, E.; Tran, K.; Tang, W.; Stevenson, K.J.; Henkelman, G. A systematic investigation of p -nitrophenol reduction by bimetallic dendrimer encapsulated nanoparticles. J. Phys. Chem. C 2013, 117, 7598–7604. [Google Scholar] [CrossRef]
- Guo, P.; Tang, L.; Tang, J.; Zeng, G.; Huang, B.; Dong, H.; Zhang, Y.; Zhou, Y.; Deng, Y.; Ma, L.; et al. Catalytic reduction–adsorption for removal of p-nitrophenol and its conversion p-aminophenol from water by gold nanoparticles supported on oxidized mesoporous carbon. J. Colloid Interface Sci. 2016, 469, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, R.; Heravi, M.M.; Asadi, S.; Nazari, N.; Nabid, M.R. Recently Used Nanocatalysts in Reduction of Nitroarenes. Curr. Org. Chem. 2016, 20, 696–734. [Google Scholar] [CrossRef]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.M.; Choi, J.-W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent Advances in the Nanocatalyst-Assisted NaBH 4 Reduction of Nitroaromatics in Water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Asharani, I.V.; Thirumalai, D.; Sivakumar, A. Dendrimer encapsulated Silver nanoparticles as novel catalysts for reduction of aromatic nitro compounds. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Zdorovets, M. V Catalytic Activity of Composite Track-Etched Membranes Based on Copper Nanotubes in Flow and Static Modes. Pet. Chem. 2019, 59, 552–557. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Shlimas, D.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Russakova, A.V.; Kassymzhanov, M.; Borisenko, A.N. Electron Beam Induced Enhancement of the Catalytic Properties of Ion-Track Membranes Supported Copper Nanotubes in the Reaction of the P-Nitrophenol Reduction. Catalysts 2019, 9, 737. [Google Scholar] [CrossRef]
- Muench, F.; Rauber, M.; Stegmann, C.; Lauterbach, S.; Kunz, U.; Kleebe, H.-J.; Ensinger, W. Ligand-optimized electroless synthesis of silver nanotubes and their activity in the reduction of 4-nitrophenol. Nanotechnology 2011, 22, 415602. [Google Scholar] [CrossRef]
- Thawarkar, S.R.; Thombare, B.; Munde, B.S.; Khupse, N.D. Kinetic investigation for the catalytic reduction of nitrophenol using ionic liquid stabilized gold nanoparticles. RSC Adv. 2018, 8, 38384–38390. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef]
- Kästner, C.; Thünemann, A.F. Catalytic Reduction of 4-Nitrophenol Using Silver Nanoparticles with Adjustable Activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef]
- Iben Ayad, A.; Luart, D.; Ould Dris, A.; Guénin, E. Kinetic Analysis of 4-Nitrophenol Reduction by “Water-Soluble” Palladium Nanoparticles. Nanomaterials 2020, 10, 1169. [Google Scholar] [CrossRef]
- Zuo, Y.; Song, J.-M.; Niu, H.-L.; Mao, C.-J.; Zhang, S.-Y.; Shen, Y.-H. Synthesis of TiO2 -loaded Co 0.85 Se thin films with heterostructure and their enhanced catalytic activity for p-nitrophenol reduction and hydrazine hydrate decomposition. Nanotechnology 2016, 27, 145701. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhu, H.; Chen, C.; Huang, G.; Chen, Q. Insights into the reduction of 4-nitrophenol to 4-aminophenol on catalysts. Chem. Phys. Lett. 2017, 684, 148–152. [Google Scholar] [CrossRef]
- Murzin, D.Y. Nanokinetics for nanocatalysis. Catal. Sci. Technol. 2011, 1, 380. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Zaen, R.; Oktiani, R. Correlation between crystallite size and photocatalytic performance of micrometer-sized monoclinic WO3 particles. Arab. J. Chem. 2020, 13, 1283–1296. [Google Scholar] [CrossRef]
- Tong, D.-G.; Chu, W.; Luo, Y.-Y.; Ji, X.-Y.; He, Y. Effect of crystallinity on the catalytic performance of amorphous Co–B particles prepared from cobalt nitrate and potassium borohydride in the cinnamaldehyde hydrogenation. J. Mol. Catal. A Chem. 2007, 265, 195–204. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Suslova, E.V.; Egorov, A.V.; Maslakov, K.I.; Savilov, S.V.; Lunin, V.V. Effect of Co crystallinity on Co/CNT catalytic activity in CO/CO2 hydrogenation and CO disproportionation. Appl. Surf. Sci. 2016, 372, 100–107. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R.; Sukmafitri, A.; Zaen, R. Amorphous content on the photocatalytic performance of micrometer-sized tungsten trioxide particles. Arab. J. Chem. 2020, 13, 2912–2924. [Google Scholar] [CrossRef]
- Park, J.; Dattatraya Saratale, G.; Cho, S.-K.; Bae, S. Synergistic effect of Cu loading on Fe sites of fly ash for enhanced catalytic reduction of nitrophenol. Sci. Total Environ. 2020, 705, 134544. [Google Scholar] [CrossRef]
- Verma, A.D.; Mandal, R.K.; Sinha, I. Kinetics of p-Nitrophenol Reduction Catalyzed by PVP Stabilized Copper Nanoparticles. Catal. Lett. 2015, 145, 1885–1892. [Google Scholar] [CrossRef]
- Tamuly, C.; Saikia, I.; Hazarika, M.; Das, M.R. Reduction of aromatic nitro compounds catalyzed by biogenic CuO nanoparticles. RSC Adv. 2014, 4, 53229–53236. [Google Scholar] [CrossRef]
- Duan, Z.; Ma, G.; Zhang, W. Preparation of Copper Nanoparticles and Catalytic Properties for the Reduction of Aromatic Nitro Compounds. Bull. Korean Chem. Soc. 2012, 33, 4003–4006. [Google Scholar] [CrossRef]
- Vinod Kumar, V.; Dharani, A.; Mariappan, M.; Anthony, S.P. Synthesis of CuO and Cu2O nano/microparticles from a single precursor: Effect of temperature on CuO/Cu2O formation and morphology dependent nitroarene reduction. RSC Adv. 2016, 6, 85083–85090. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Encyclopedia of Materials: Science and Technology, 1st ed.; Jürgen Buschow, K.H., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Pergamon: Oxford, UK, 2001; ISBN 978-0-08-043152-9. [Google Scholar]
- Shimada, H. Support effect on the catalytic activity and properties of sulfided molybdenum catalysts. J. Catal. 1988, 110, 275–284. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Kozlovskiy, A.L.; Zdorovets, M. V Influence of deposition temperature on the structure and catalytic properties of the copper nanotubes composite membranes. Mater. Res. Express 2018, 5, 065041. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U. Shikha Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nicomel, N.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef]
- World Health Organization. Arsenic in Drinking-Water. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Reddy, K.J.; McDonald, K.J.; King, H. A novel arsenic removal process for water using cupric oxide nanoparticles. J. Colloid Interface Sci. 2013, 397, 96–102. [Google Scholar] [CrossRef]
- Reddy, K.J.; Roth, T.R. Arsenic Removal from Natural Groundwater Using Cupric Oxide. Ground Water 2013, 51, 83–91. [Google Scholar] [CrossRef]
- McDonald, K.J.; Reynolds, B.; Reddy, K.J. Intrinsic properties of cupric oxide nanoparticles enable effective filtration of arsenic from water. Sci. Rep. 2015, 5, 11110. [Google Scholar] [CrossRef]
- Agrawal, S.; Singh, N.B. Removal of arsenic from aqueous solution by an adsorbent nickel ferrite-polyaniline nanocomposite. Indian J. Chem. Technol. 2016, 23, 374–383. [Google Scholar]
- Roy, E.; Patra, S.; Madhuri, R.; Sharma, P.K. A single solution for arsenite and arsenate removal from drinking water using cysteine@ZnS:TiO2 nanoparticle modified molecularly imprinted biofouling-resistant filtration membrane. Chem. Eng. J. 2016, 304, 259–270. [Google Scholar] [CrossRef]
- Babaee, Y.; Mulligan, C.N.; Rahaman, M.S. Removal of arsenic (III) and arsenic (V) from aqueous solutions through adsorption by Fe/Cu nanoparticles. J. Chem. Technol. Biotechnol. 2018, 93, 63–71. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation: A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Wong, W.; Wong, H.Y.; Badruzzaman, A.B.M.; Goh, H.H.; Zaman, M. Recent advances in exploitation of nanomaterial for arsenic removal from water: A review. Nanotechnology 2017, 28, 042001. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Naushad, M.; Chaudhry, S.A. Promising prospects of nanomaterials for arsenic water remediation: A comprehensive review. Process. Saf. Environ. Prot. 2019, 126, 60–97. [Google Scholar] [CrossRef]
- Zeng, L.; Wan, B.; Huang, R.; Yan, Y.; Wang, X.; Tan, W.; Liu, F. Catalytic oxidation of arsenite and reaction pathways on the surface of CuO nanoparticles at a wide range of pHs. Geochem. Trans. 2018, 19, 12. [Google Scholar] [CrossRef]

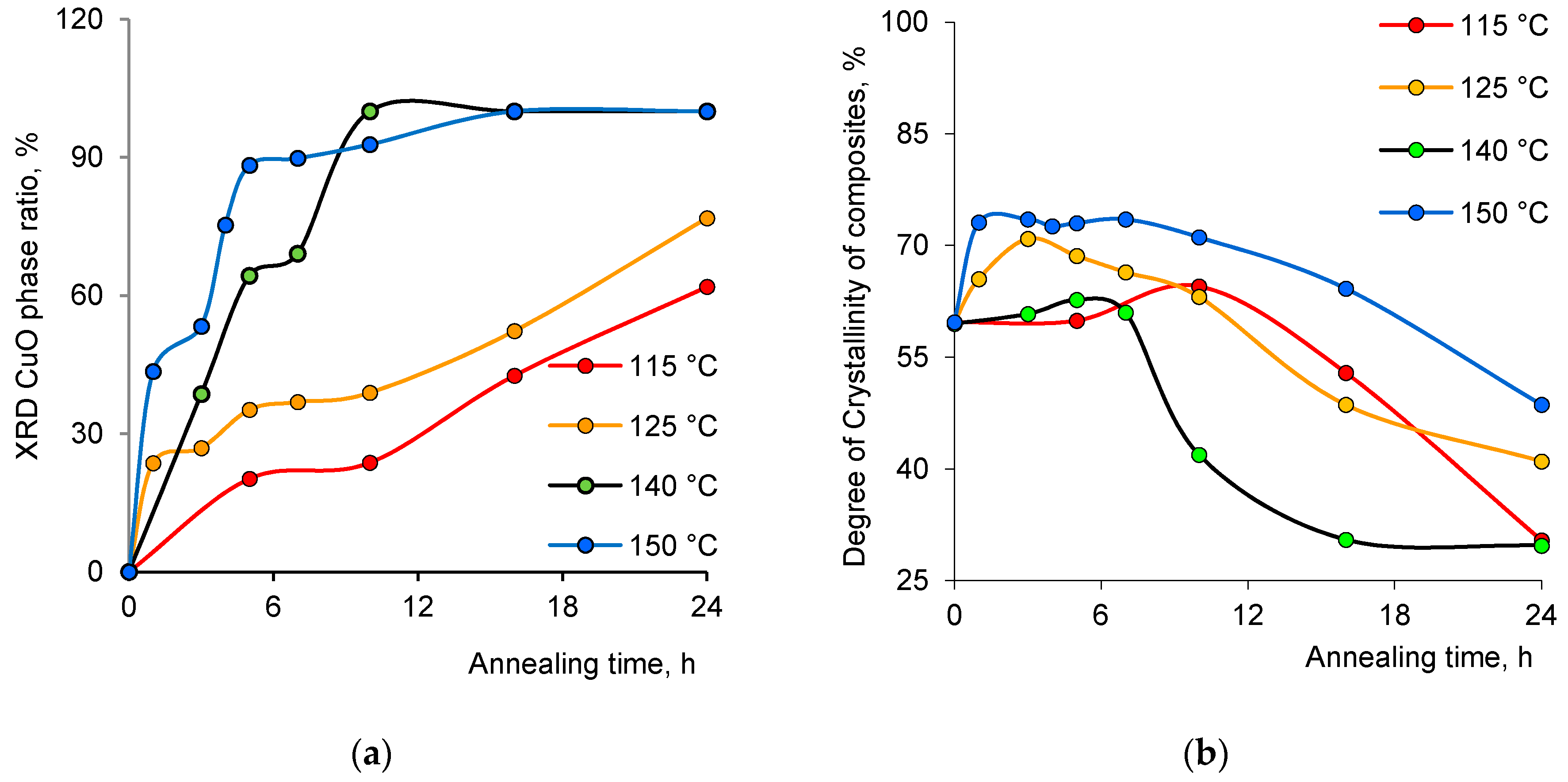


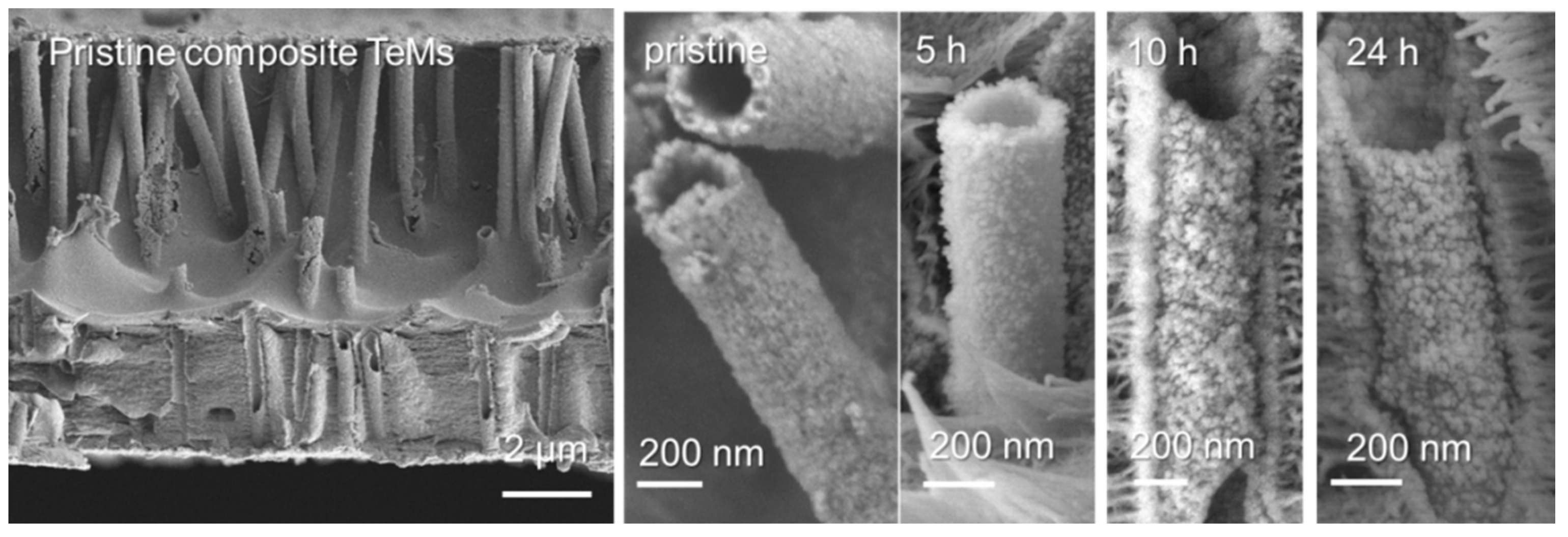
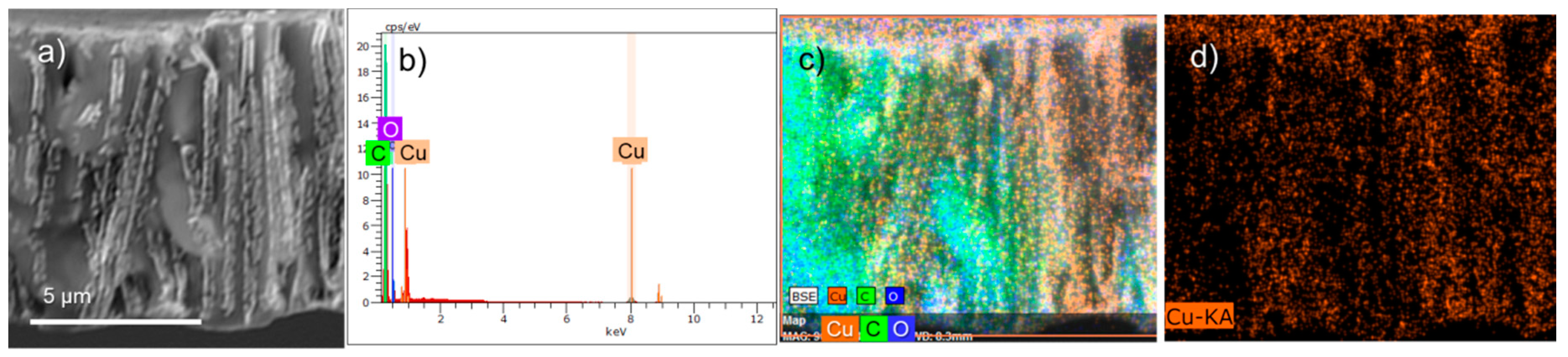
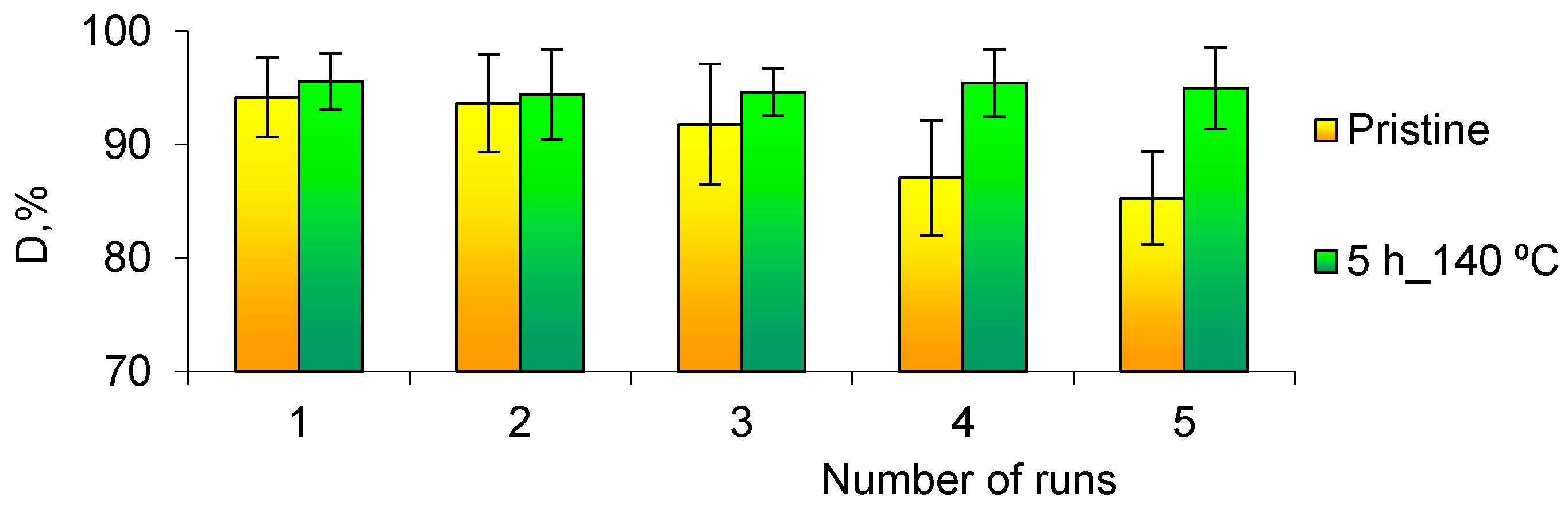
| Annealing Time, h | Phase | hkl a | 2θ° | d b, Å | L c, nm | a d, Å | DC e, % | FWHM f | Phase Ratio, % |
|---|---|---|---|---|---|---|---|---|---|
| 0 | Cu | 111 | 43.48 | 2.08 | 9.87 | 3.592 | 59.5 | 0.96 | 72.5 |
| CuO | 111 | 36.84 | 2.44 | 13.67 | 4.241 | 0.68 | 27.5 | ||
| 3 | Cu | 111 | 43.41 | 2.08 | 11.71 | 3.595 | 60.8 | 0.81 | 61.4 |
| CuO | 111 | 36.47 | 2.46 | 8.50 | 4.255 | 1.09 | 38.6 | ||
| 5 | Cu | 111 | 43.33 | 2.09 | 12.89 | 3.613 | 62.7 | 0.74 | 35.7 |
| CuO | 111 | 36.84 | 2.44 | 8.40 | 4.236 | 1.11 | 64.3 | ||
| 7 | Cu | 111 | 43.26 | 2.09 | 18.33 | 3.627 | 61.0 | 0.52 | 30.9 |
| CuO | 111 | 36.55 | 2.46 | 7.65 | 4.238 | 1.22 | 69.1 | ||
| 10 | CuO | 111 | 36.47 | 2.46 | 4.99 | 4.229 | 41.9 | 1.86 | ~100 |
| 16 | CuO | 111 | 36.77 | 2.44 | 6.91 | 4.235 | 30.5 | 1.35 | ~100 |
| 24 | CuO | 111 | 36.47 | 2.46 | 9.50 | 4.246 | 29.7 | 0.98 | ~100 |
| Decomposed Pollutant Reacted | Annealing Time, h/CuO Ratio From XRD, % | |||
|---|---|---|---|---|
| 0/27.5 | 5/64.3 | 10/100 | 24/100 | |
| 4-NP | 36.97 | 39.95 | 26.90 | 37.92 |
| 4-NA | 42.55 | 13.39 | 21.86 | 22.18 |
| 4-NBA | 56.18 | 52.30 | 34.82 | 25.25 |
| Loaded Catalyst | Studied Aromatic Nitro Compound | Nanocatalyst Efficiency | Ref. | |||
|---|---|---|---|---|---|---|
| Type | Amount | k, min−1 | Ea, kJ/mol | D, % | ||
| 0.25 wt% Cu/water-washed coal fly ash | 400 mg/L | 4-NP | 0.41 | - | - | [90] |
| Cu NPs (9 nm) | 0.0017 mg/L | 4-NP | 0.26 | 45.8 | - | [91] |
| CuO NPs | 1 mol % CuO | 4-NP | - | - | 96.0 | [92] |
| Cu NPs | 10 mol % Cu | 4-NP | - | - | 66.0 | [93] |
| 4-NBA | - | - | 92.0 | |||
| CuO nano/microparticles | 5.0 mg/L | 4-NP | 0.21 | - | - | [94] |
| CuO/γ-Al2O3 NPs | 50 mg | 4-NP | 0.17 | 27.4 | - | [67] |
| Cu/PET TeMs | 3.2 mg | 4-NP | 0.52 | 28.3 | 83.8 | [30] |
| Cu/CuO/PET TeMs (5 h, 140 °C) | 3.2 mg (2 × 2 cm) | 4-NP | 0.29 | 39.9 | 98.1 | This study |
| 4-NA | 0.26 | 13.4 | 90.6 | |||
| 4-NBA | 0.30 | 52.3 | 83.9 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashentseva, A.A.; Barsbay, M.; Zdorovets, M.V.; Zheltov, D.A.; Güven, O. Cu/CuO Composite Track-Etched Membranes for Catalytic Decomposition of Nitrophenols and Removal of As(III). Nanomaterials 2020, 10, 1552. https://doi.org/10.3390/nano10081552
Mashentseva AA, Barsbay M, Zdorovets MV, Zheltov DA, Güven O. Cu/CuO Composite Track-Etched Membranes for Catalytic Decomposition of Nitrophenols and Removal of As(III). Nanomaterials. 2020; 10(8):1552. https://doi.org/10.3390/nano10081552
Chicago/Turabian StyleMashentseva, Anastassiya A., Murat Barsbay, Maxim V. Zdorovets, Dmitriy A. Zheltov, and Olgun Güven. 2020. "Cu/CuO Composite Track-Etched Membranes for Catalytic Decomposition of Nitrophenols and Removal of As(III)" Nanomaterials 10, no. 8: 1552. https://doi.org/10.3390/nano10081552
APA StyleMashentseva, A. A., Barsbay, M., Zdorovets, M. V., Zheltov, D. A., & Güven, O. (2020). Cu/CuO Composite Track-Etched Membranes for Catalytic Decomposition of Nitrophenols and Removal of As(III). Nanomaterials, 10(8), 1552. https://doi.org/10.3390/nano10081552







