Inkjet Printing Humidity Sensing Pattern Based on Self-Organizing Polystyrene Spheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Characterization
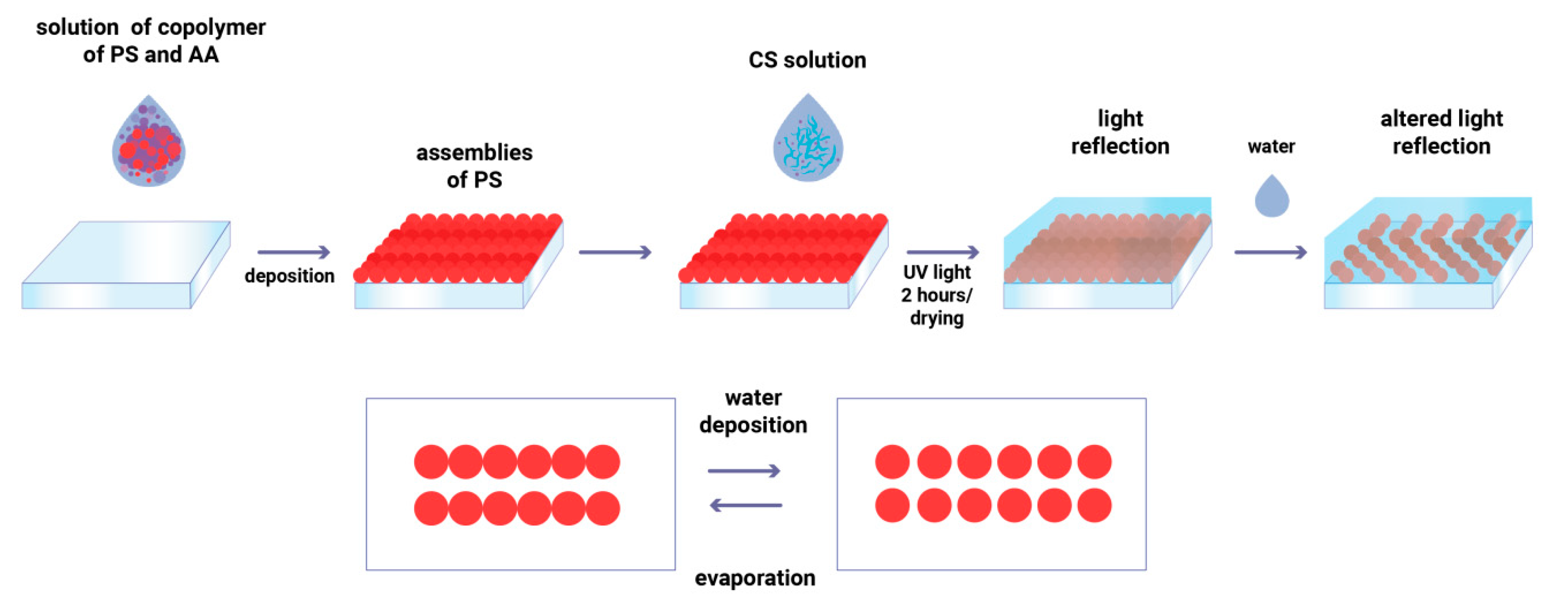
2.3. Preparation of Inks and Printing
2.4. Preparation of Substrates
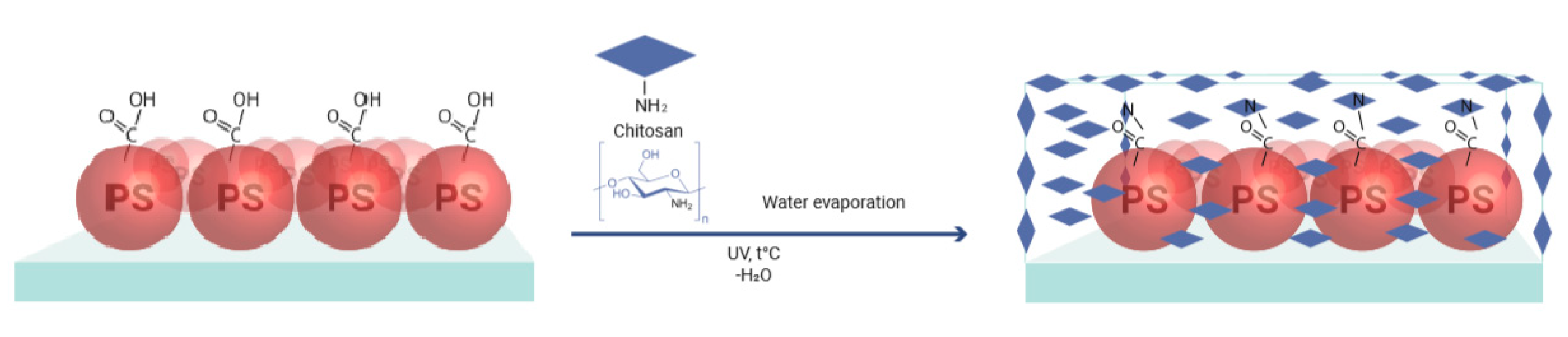
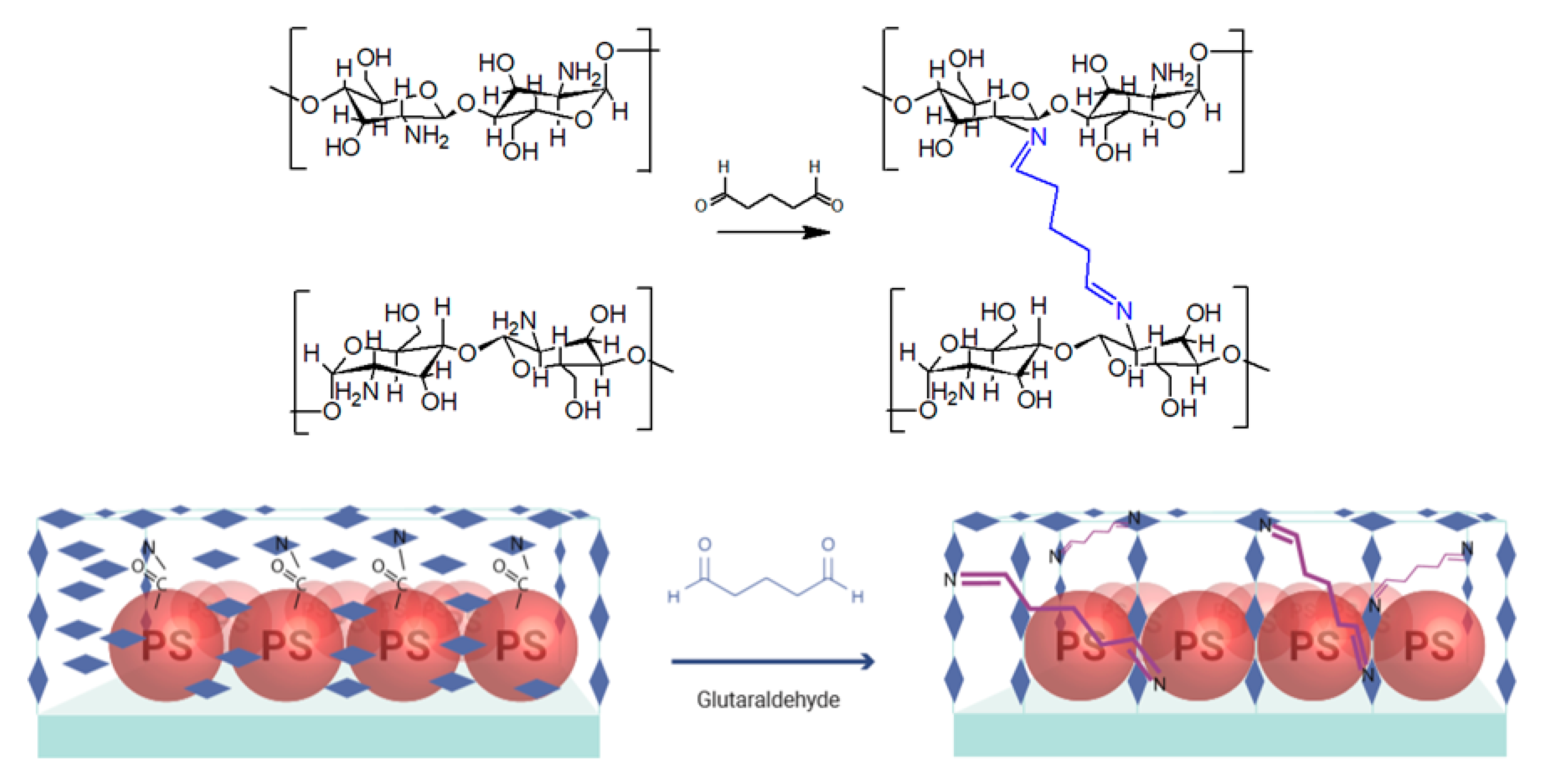
2.5. Preparation of Hydrogel Coating
2.6. Study of Optical Properties of Photonic Coating
3. Results and Discussion
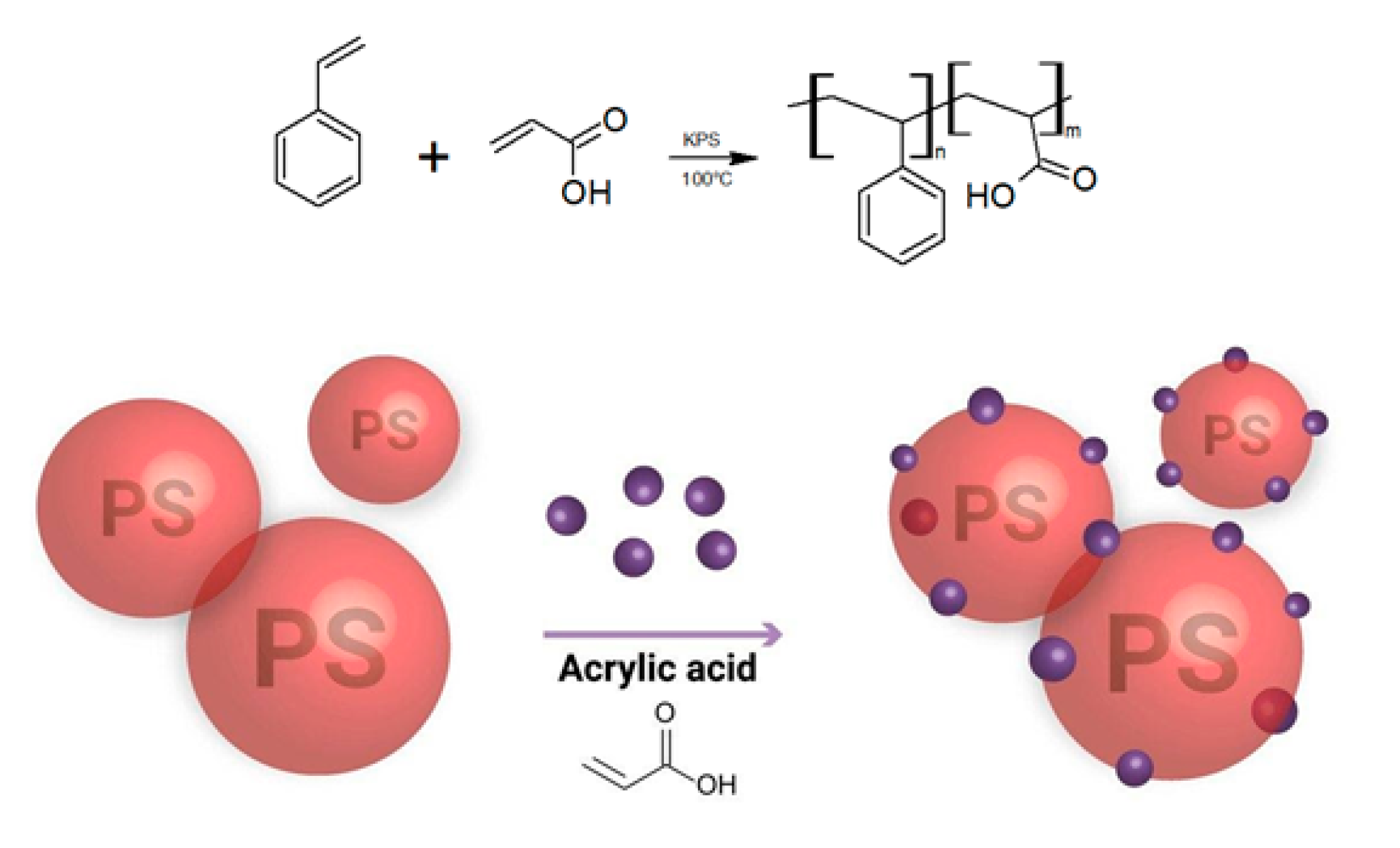
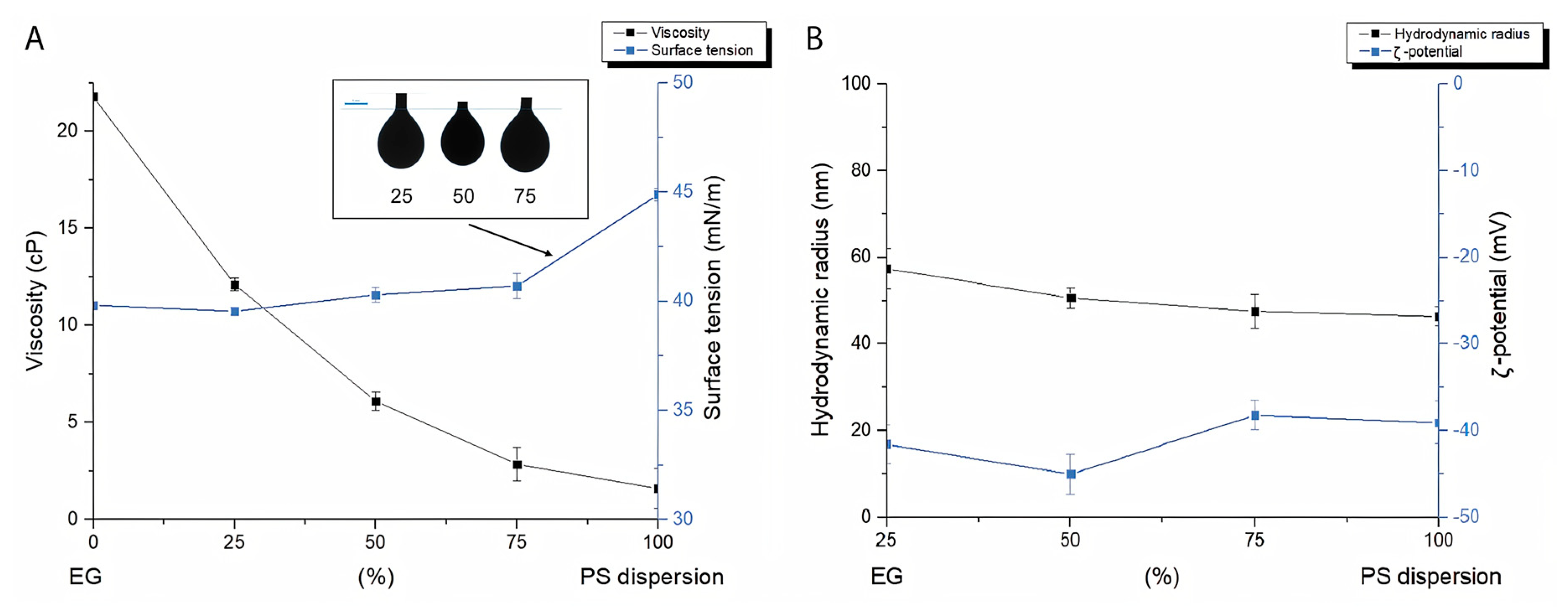
3.1. Ink Formulation
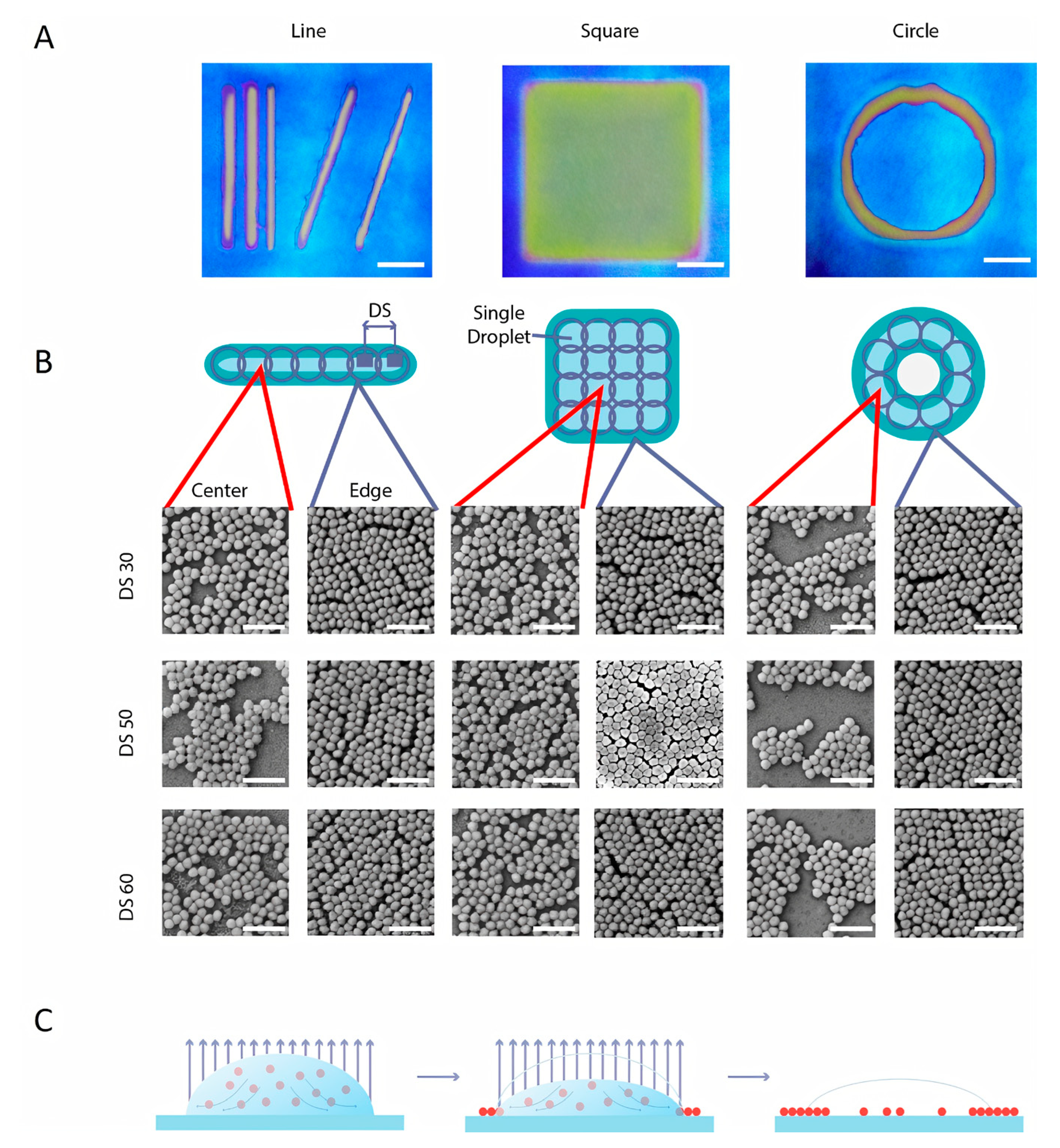
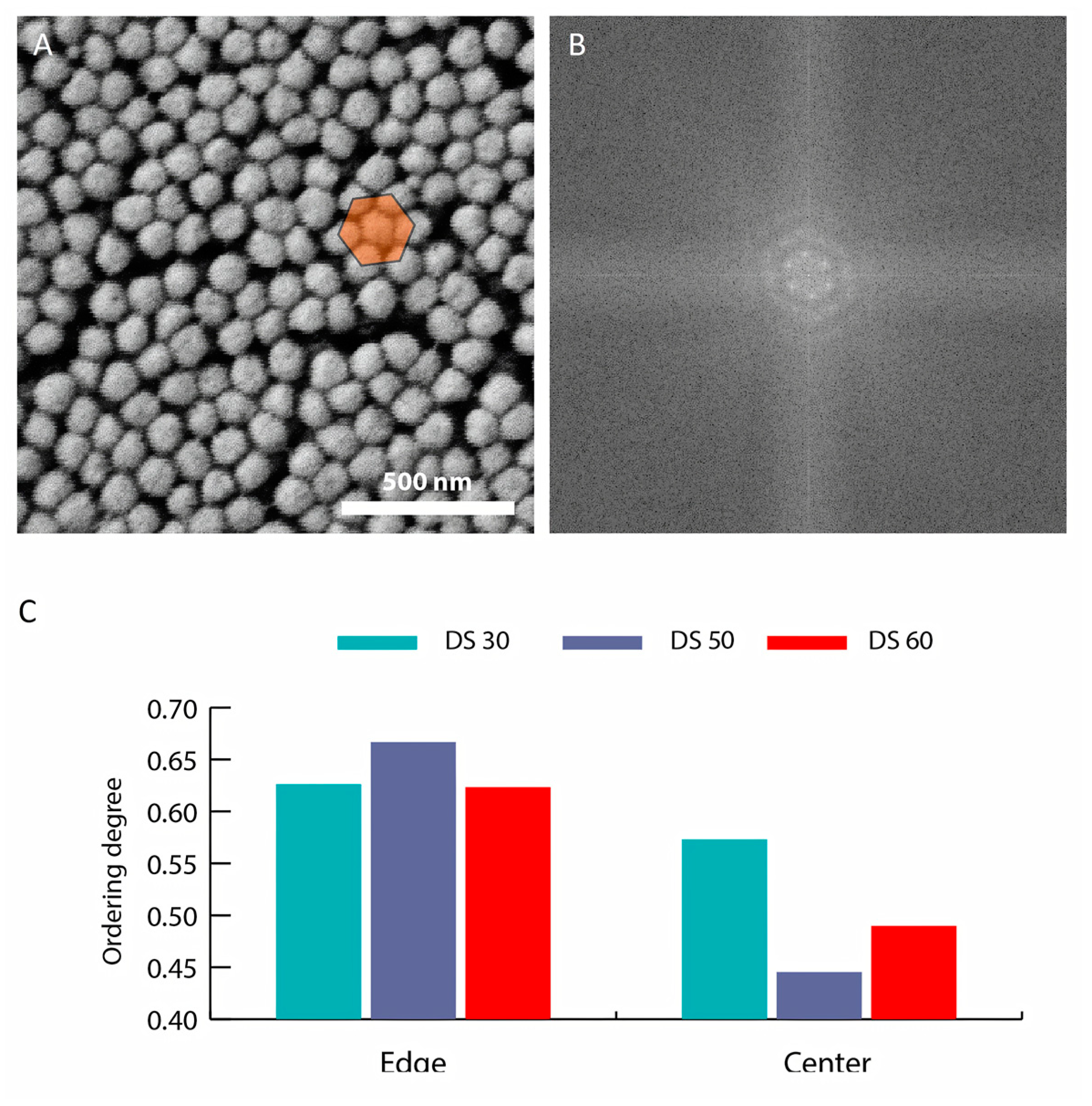
3.2. Inkjet Printing Parameters
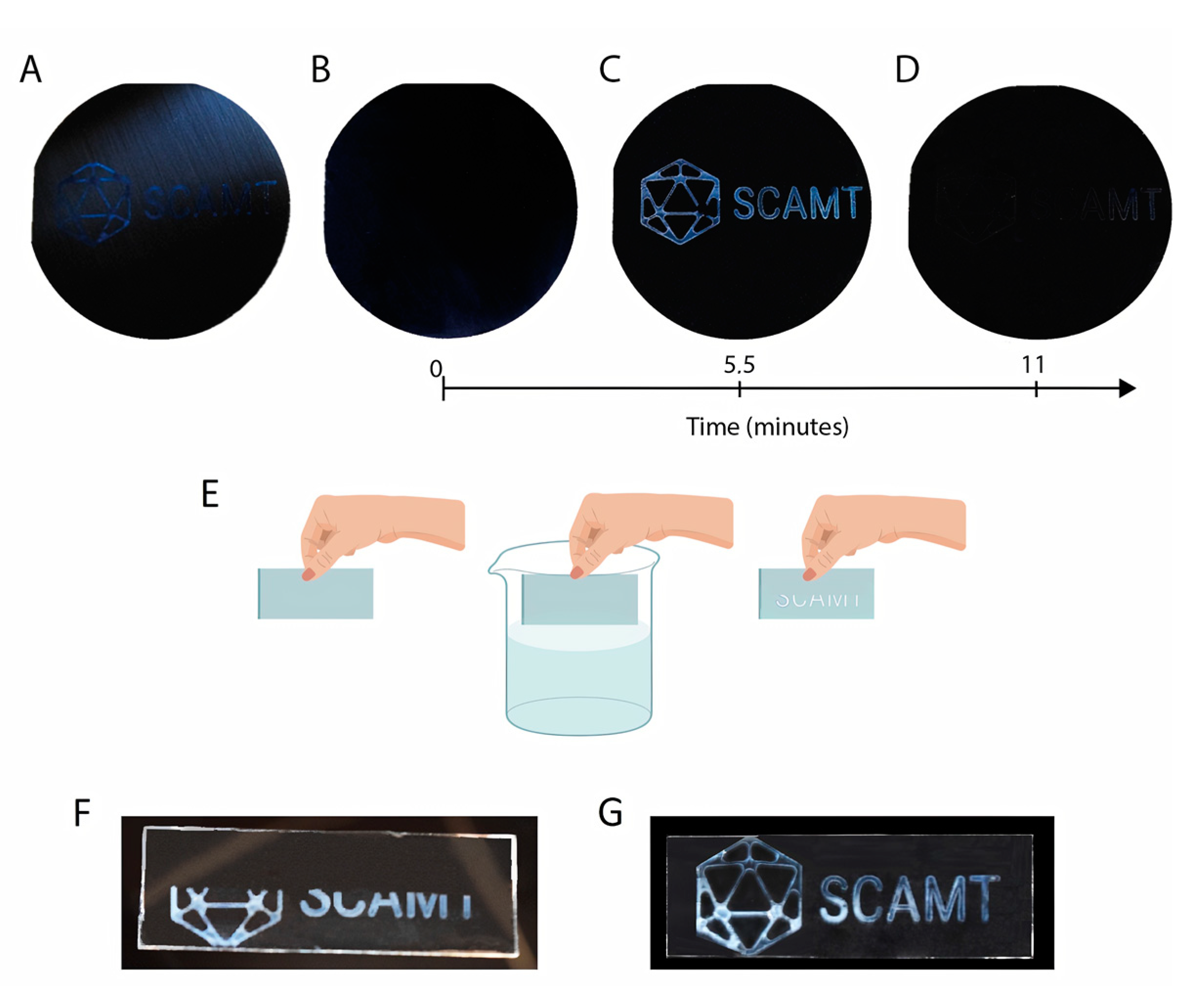
3.3. Hydrogel Formation on the Substrate
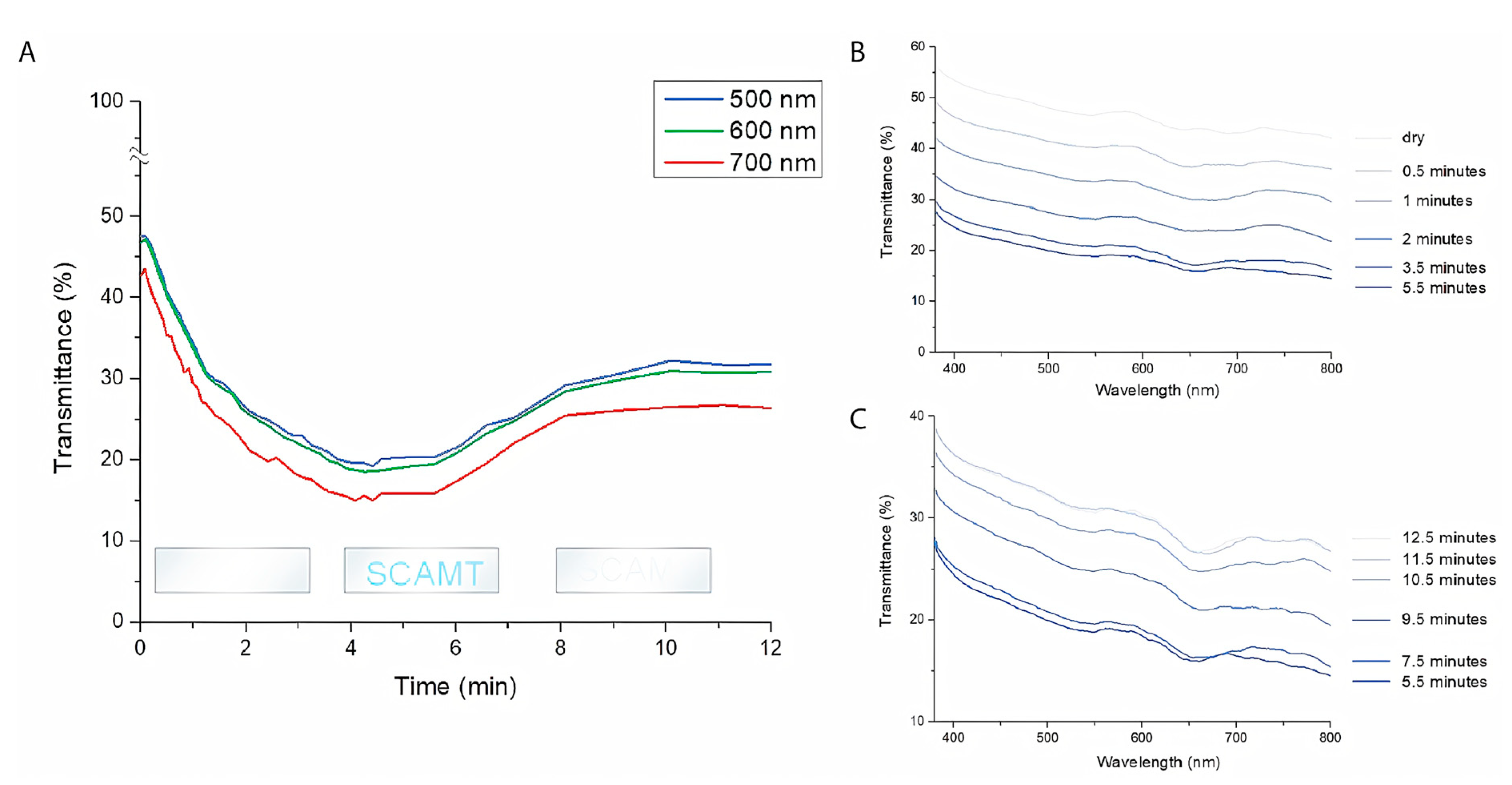
3.4. Optical Humidity Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stein, A.; Schroden, R.C. Colloidal crystal templating of three-dimensionally ordered macroporous solids: Materials for photonics and beyond. Curr. Opin. Solid State Mater. Sci. 2001, 5, 553–564. [Google Scholar] [CrossRef]
- Inan, H.; Poyraz, M.; Inci, F.; Lifson, M.A.; Baday, M.; Cunningham, B.T.; Demirci, U. Photonic crystals: Emerging biosensors and their promise for point-of-care applications. Chem. Soc. Rev. 2017, 46, 366–388. [Google Scholar] [CrossRef] [PubMed]
- Ruda, H.E.; Matsuura, N. Nano-Engineered Tunable Photonic Crystals. In Springer Handbook of Electronic and Photonic Materials; Kasap, S., Capper, P., Eds.; Springer International Publishing: New York, NY, USA, 2017; p. 1. [Google Scholar] [CrossRef]
- Ma, H.; Cui, J.; Chen, J.; Hao, J. Self-Organized Polymer Nanocomposite Inverse Opal Films with Combined Optical Properties. Chem. Eur. J. 2011, 17, 655–660. [Google Scholar] [CrossRef]
- Miklyaev, Y.V.; Meisel, D.C.; Blanco, A.; Von Freymann, G.; Busch, K.; Koch, W.; Enkrich, C.; Deubel, M.; Wegener, M. Three-dimensional face-centered-cubic photonic crystal templates by laser holography: Fabrication, optical characterization, and band-structure calculations. Appl. Phys. Lett. 2003, 82, 1284–1286. [Google Scholar] [CrossRef]
- Hou, J.; Li, M.; Song, Y. Recent advances in colloidal photonic crystal sensors: Materials, structures and analysis methods. Nano Today 2018, 22, 132–144. [Google Scholar] [CrossRef]
- Mathew, J.; Semenova, Y.; Farrell, G. Effect of coating thickness on the sensitivity of a humidity sensor based on an Agarose coated photonic crystal fiber interferometer. Opt. Express 2013, 21, 6313. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Asher, S.A. Photonic Crystal Chemical Sensors: pH and Ionic Strength. J. Am. Chem. Soc. 2000, 122, 9534–9537. [Google Scholar] [CrossRef]
- Huang, R.; Chen, D.; Jiang, M. Polymeric core-shell stars with a novel fluorescent, cross-linked and swollen core: Their efficient one-step preparation, further self-assembly into superparticles and application as a chemosensor. J. Mater. Chem. 2010, 20, 9988. [Google Scholar] [CrossRef]
- Du, X.; Lei, N.-Y.; Chiu, H.-M.; Ge, X.; Zhang, Z.; Hon-Wah Lam, M. Non-invasive in vivo imaging of the ionic regimes along the gastrointestinal tract of a freshwater vertebrate model organism (Japanese medaka) using responsive photonic crystal beads. J. Mater. Chem. B 2013, 1, 1535. [Google Scholar] [CrossRef]
- Nam, H.; Song, K.; Ha, D.; Kim, T. Inkjet Printing Based Mono-layered Photonic Crystal Patterning for Anti-counterfeiting Structural Colors. Sci. Rep. 2016, 6, 30885. [Google Scholar] [CrossRef]
- Wang, C.; Yap, F.L.; Zhang, Y. Micropatterning of polystyrene nanoparticles and its bioapplications. Colloids Surf. B Biointerfaces 2005, 46, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-T.; Wang, L.; Lamont, D.N.; Velankar, S.S.; Asher, S.A. Fabrication of Large-Area Two-Dimensional Colloidal Crystals. Angew. Chem. Int. Ed. 2012, 51, 6117–6120. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, T.; Li, L.; Zhang, Z.; Wu, T. Water as a colorful ink: Transparent, rewritable photonic coatings based on colloidal crystals embedded in chitosan hydrogel. J. Mater. Chem. C 2015, 3, 3542–3546. [Google Scholar] [CrossRef]
- Du, X.; Lei, N.-Y.; Hu, P.; Lei, Z.; Ong, D.H.-C.; Ge, X.; Zhang, L.; Lam, M.H.-W. In vivo imaging of the morphology and changes in pH along the gastrointestinal tract of Japanese medaka by photonic band-gap hydrogel microspheres. Anal. Chim. Acta 2013, 787, 193–202. [Google Scholar] [CrossRef]
- Shieh, J.-Y.; Kuo, J.-Y.; Weng, H.-P.; Yu, H.H. Preparation and Evaluation of the Bioinspired PS/PDMS Photochromic Films by the Self-Assembly Dip–Drawing Method. Langmuir 2013, 29, 667–672. [Google Scholar] [CrossRef]
- Gu, Z.-Z.; Fujishima, A.; Sato, O. Fabrication of High-Quality Opal Films with Controllable Thickness. Chem. Mater. 2002, 14, 760–765. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Z.; Li, F.; Zhou, H.; Song, Y. Emerging Progress of Inkjet Technology in Printing Optical Materials. Adv. Opt. Mater. 2016, 4, 1915–1932. [Google Scholar] [CrossRef]
- Keller, K.; Yakovlev, A.V.; Grachova, E.V.; Vinogradov, A.V. Inkjet Printing of Multicolor Daylight Visible Opal Holography. Adv. Funct. Mater. 2018, 28, 1706903. [Google Scholar] [CrossRef]
- Kolchanov, D.S.; Slabov, V.S.; Keller, K.; Sergeeva, E.; Zhukov, M.V.; Drozdov, A.S.; Vinogradov, A.V. Sol–gel magnetite inks for inkjet printing. J. Mater. Chem. C 2019, 7, 6426–6432. [Google Scholar] [CrossRef]
- Zakharzhevskii, M.; Drozdov, A.S.; Kolchanov, D.S.; Shkodenko, L.; Vinogradov, V.V. Test-System for Bacteria Sensing Based on Peroxidase-Like Activity of Inkjet-Printed Magnetite Nanoparticles. Nanomaterials 2020, 10, 313. [Google Scholar] [CrossRef]
- Nayak, L.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. A review on inkjet printing of nanoparticle inks for flexible electronics. J. Mater. Chem. C 2019, 7, 8771–8795. [Google Scholar] [CrossRef]
- Bugakova, D.; Slabov, V.; Sergeeva, E.; Zhukov, M.; Vinogradov, A.V. Comprehensive characterization of TiO2 inks and their application for inkjet printing of microstructures. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124146. [Google Scholar] [CrossRef]
- Zhan, Z.; An, J.; Wei, Y.; Tran, V.T.; Du, H. Inkjet-printed optoelectronics. Nanoscale 2017, 9, 965–993. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Zhong, J.; Hong, Y.; Duan, Z.; Lin, M.; Xu, F. Inkjet printing of upconversion nanoparticles for anti-counterfeit applications. Nanoscale 2015, 7, 4423–4431. [Google Scholar] [CrossRef]
- Al-Milaji, K.N.; Secondo, R.R.; Ng, T.N.; Kinse, N.; Zhao, H. Interfacial Self-Assembly of Colloidal Nanoparticles in Dual-Droplet Inkjet Printing. Adv. Mater. Interfaces 2018, 5, 1701561. [Google Scholar] [CrossRef]
- Minemawari, H.; Yamada, T.; Matsui, H.; Tsutsumi, J.Y.; Haas, S.; Chiba, R.; Kumai, R.; Hasegawa, T. Inkjet printing of single-crystal films. Nature 2011, 475, 364–367. [Google Scholar] [CrossRef]
- You, M.; Lin, M.; Wang, S.; Wang, X.; Zhang, G.; Hong, Y.; Dong, Y.; Jin, G.; Xu, F. Three-dimensional quick response code based on inkjet printing of upconversion fluorescent nanoparticles for drug anti-counterfeiting. Nanoscale 2016, 8, 10096–10104. [Google Scholar] [CrossRef]
- Derby, B. Inkjet Printing of Functional and Structural Materials: Fluid Property Requirements, Feature Stability, and Resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Conway, J.H.; Sloane, N.J.A. Sphere Packings, Lattices and Groups; Springer: New York, NY, USA, 1999; Volume 290. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neterebskaia, V.O.; Goncharenko, A.O.; Morozova, S.M.; Kolchanov, D.S.; Vinogradov, A.V. Inkjet Printing Humidity Sensing Pattern Based on Self-Organizing Polystyrene Spheres. Nanomaterials 2020, 10, 1538. https://doi.org/10.3390/nano10081538
Neterebskaia VO, Goncharenko AO, Morozova SM, Kolchanov DS, Vinogradov AV. Inkjet Printing Humidity Sensing Pattern Based on Self-Organizing Polystyrene Spheres. Nanomaterials. 2020; 10(8):1538. https://doi.org/10.3390/nano10081538
Chicago/Turabian StyleNeterebskaia, Valeriia O., Anna O. Goncharenko, Sofia M. Morozova, Denis S. Kolchanov, and Alexandr V. Vinogradov. 2020. "Inkjet Printing Humidity Sensing Pattern Based on Self-Organizing Polystyrene Spheres" Nanomaterials 10, no. 8: 1538. https://doi.org/10.3390/nano10081538
APA StyleNeterebskaia, V. O., Goncharenko, A. O., Morozova, S. M., Kolchanov, D. S., & Vinogradov, A. V. (2020). Inkjet Printing Humidity Sensing Pattern Based on Self-Organizing Polystyrene Spheres. Nanomaterials, 10(8), 1538. https://doi.org/10.3390/nano10081538