Hot Electrons, Hot Holes, or Both? Tandem Synthesis of Imines Driven by the Plasmonic Excitation in Au/CeO2 Nanorods
Abstract
1. Introduction
2. Experimental Section
2.1. Au Nanoparticles Synthesis
2.2. Au/CeO2 Photocatalyst Synthesis
2.3. Catalyst Characterization
2.4. Photocatalytic Tests
2.5. 31P NMR Measurement of TMP-Adsorbed Samples
2.6. XPS Analysis
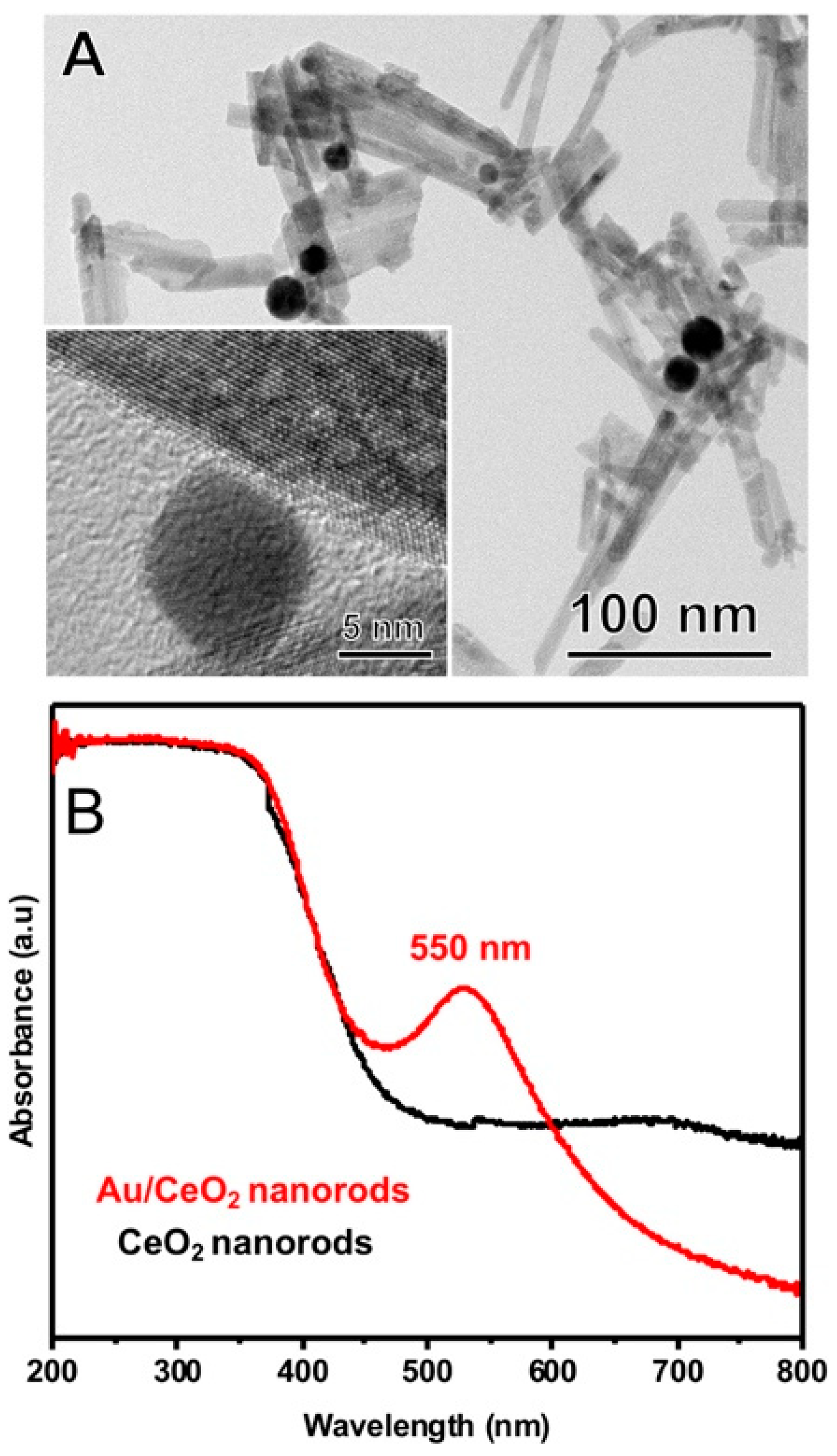
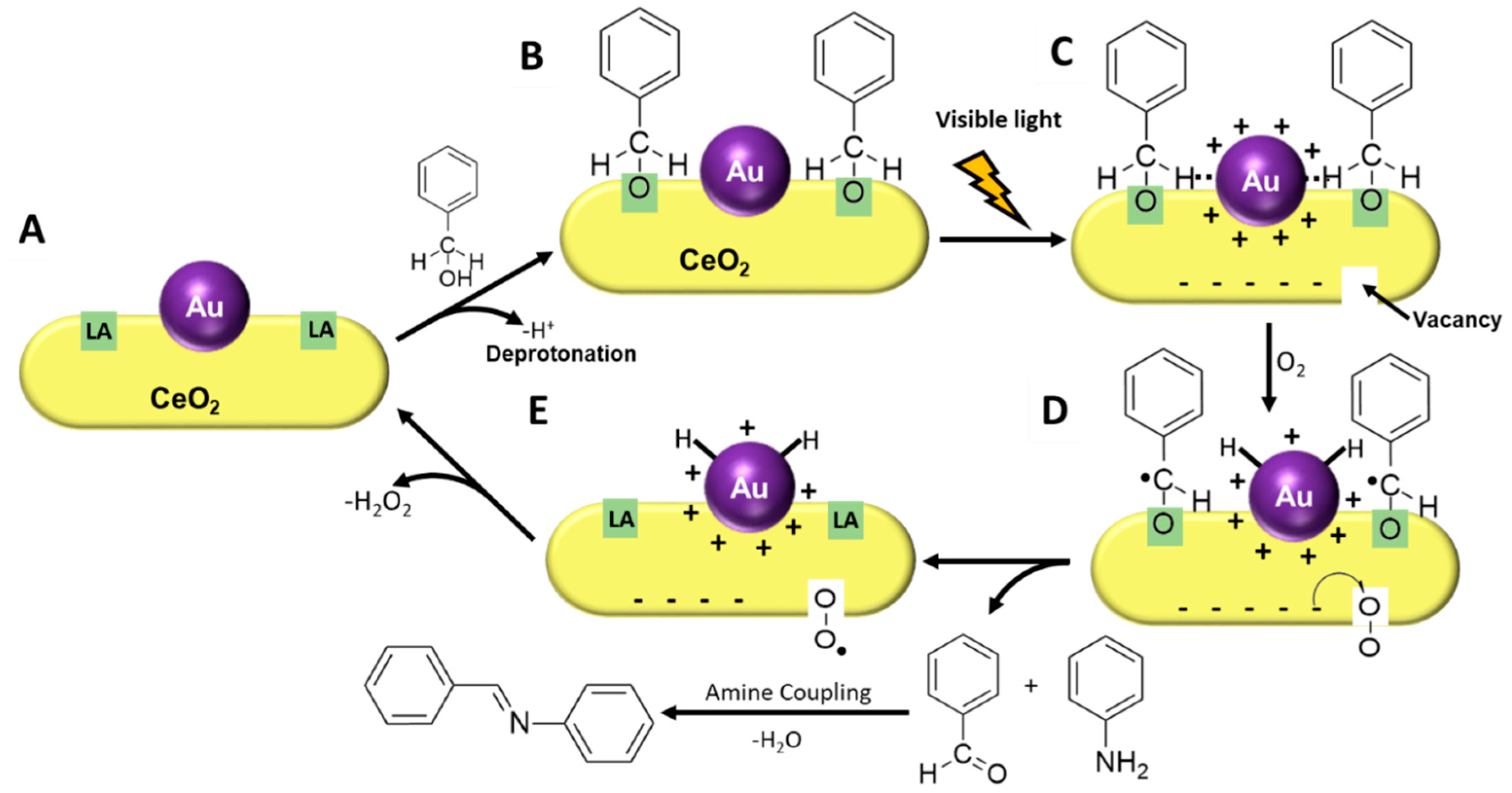
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Li, Q.; Xu, D. Recent Progress in Semiconductor-Based Nanocomposite Photocatalysts for Solar-to-Chemical Energy Conversion. Adv. Energy Mater. 2017, 7, 1700529. [Google Scholar] [CrossRef]
- Parsons, P.J.; Penkett, C.S.; Shell, A.J. Tandem reactions in organic synthesis: Novel strategies for natural product elaboration and the development of new synthetic methodology. Chem. Rev. 1996, 96, 195–206. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. In Green Chemistry Theory and Practice; Oxford University Press: New York, NY, USA, 1998; pp. 29–56. [Google Scholar]
- Behr, A.; Vorholt, A.J.; Ostrowski, K.A.; Seidensticker, T. Towards resource efficient chemistry: Tandem reactions with renewables. Green Chem. 2014, 16, 982–1006. [Google Scholar] [CrossRef]
- Patil, R.D.; Adimurthy, S. Catalytic methods for imine synthesis. Asian J. Org. Chem. 2013, 2, 726–744. [Google Scholar] [CrossRef]
- Tamura, M.; Tomishige, K. Redox properties of CeO2 at low temperature: The direct synthesis of imines from alcohol and amine. Angew. Chem. Int. Ed. 2015, 54, 864–867. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Wang, J.; Ding, H.; Liu, Q.; Schubert, U.; Rui, Y.; Xu, J. Surface oxygen vacancies dominated CeO2 as efficient catalyst for imine synthesis: Influences of different cerium precursors. Mol. Catal. 2017, 443, 131–138. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Araújo, T.P.; Quiroz, J.; Barbosa, E.M.; Camargo, P.H.C. Understanding plasmonic catalysis with controlled nanomaterials based on catalytic and plasmonic metals. Curr. Opin. Colloid Interface Sci. 2019, 39, 110–122. [Google Scholar]
- Wang, J.; Ando, R.A.; Camargo, P.H.C. Controlling the Selectivity of the Surface Plasmon Resonance Mediated Oxidation of p-Aminothiophenol on Au Nanoparticles by Charge Transfer from UV-excited TiO2. Angew. Chem. Int. Ed. 2015, 54, 6909–6912. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by noble metal nanoparticles: Controlled synthesis for the optimization and understanding of activities. J. Mater. Chem. A 2019, 7, 5857–5874. [Google Scholar] [CrossRef]
- Geonmonond, R.S.; Quiroz, J.; Rocha, G.F.S.R.; Oropeza, F.E.; Rangel, C.J.; Rodrigues, T.S.; Hofmann, J.P.; Hensen, E.J.M.; Ando, R.A.; Camargo, P.H.C. Marrying SPR excitation and metal-support interactions: Unravelling the contribution of active surface species in plasmonic catalysis. Nanoscale 2018, 10, 8560–8568. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Camargo, P.H.C.; Wang, J. Investigating the effect of MnO2 band gap in hybrid MnO2-Au materials over the SPR-mediated activities under visible light. J. Mater. Chem. A 2019, 7, 925–931. [Google Scholar] [CrossRef]
- Wang, J.; de Freitas, I.C.; Alves, T.V.; Ando, R.A.; Fang, Z.; Camargo, P.H.C. On the effect of native SiO2 on Si over the SPR-mediated photocatalytic activities of Au and Ag nanoparticles. Chem. Eur. J. 2017, 23, 7185–7190. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A Gen. 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Coquet, R.; Howard, K.L.; Willock, D.J. Theory and simulation in heterogeneous gold catalysis. Chem. Soc. Rev. 2008, 37, 2046–2076. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal organic frameworks as versatile hosts of Au nanoparticles in heterogeneous catalysis. ACS Catal. 2017, 7, 2896–2919. [Google Scholar] [CrossRef]
- Shahzad, S.A.; Sajid, M.A.; Khan, Z.A.; Canseco-Gonzalez, D. Gold catalysis in organic transformations: A review. Synth. Commun. 2017, 47, 735–755. [Google Scholar] [CrossRef]
- Damato, T.C.; de Oliveira, C.C.S.; Ando, R.M.A.; Camargo, P.H.C. A facile approach to TiO2 colloidal spheres decorated with Au nanoparticles displaying well-defined sizes and uniform dispersion. Langmuir 2013, 29, 1642–1649. [Google Scholar] [CrossRef]
- Si, R.; Flytzani-Stephanopoulos, M. Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water–gas shift reaction. Angew. Chem. Int. Ed. 2008, 47, 2884–2887. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Wang, M.; Lü, J.; Li, L.; Zhang, Z.; Li, M.; Jiang, J.; Wang, F. An investigation of the effects of CeO2 crystal planes on the aerobic oxidative synthesis of imines from alcohols and amines. Chin. J. Catal. 2015, 36, 1623–1630. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709. [Google Scholar] [CrossRef]
- Burroughs, P.; Hamnett, A.; Orchard, A.F.; Thornton, G. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium. J. Chem. Soc. Dalton Trans. 1976, 1686–1698. [Google Scholar] [CrossRef]
- Maslakov, K.I.; Teterin, Y.A.; Popel, A.J.; Teterin, A.Y.; Ivanov, K.E.; Kalmykov, S.N.; Petrov, V.G.; Petrov, P.K.; Farnan, I. XPS study of ion irradiated and unirradiated CeO2 bulk and thin film samples. Appl. Surf. Sci. 2018, 448, 154–162. [Google Scholar] [CrossRef]
- Zhao, K.; Qi, J.; Yin, H.; Wang, Z.; Zhao, S.; Ma, X.; Wan, J.; Chang, L.; Gao, Y.; Yu, R. Efficient water oxidation under visible light by tuning surface defects on ceria nanorods. J. Mater. Chem. A 2015, 3, 20465–20470. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G. Preparation of high oxygen storage capacity and thermally stable ceria-zirconia solid solution. Catal. Sci. Technol. 2016, 6, 897–907. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Petallidou, K.C.; Efstathiou, A.M. The effect of La3+-doping of CeO2 support on the water-gas shift reaction mechanism and kinetics over Pt/Ce1−xLaxO2−δ. Appl. Catal. B Environ. 2013, 136, 225–238. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Tan, Z.; Li, G.; Chou, H.-L.; Li, Y.; Yi, X.; Mahadi, A.H.; Zheng, A.; Tsang, S.C.E.; Peng, Y.-K. Differentiating Surface Ce Species Among CeO2 Facets by Solid-State NMR for Catalytic Correlation. ACS Catal. 2020, 10, 4003–4011. [Google Scholar] [CrossRef]
- Zheng, A.; Liu, S.-B.; Deng, F. 31P NMR chemical shifts of phosphorus probes as reliable and practical acidity scales for solid and liquid catalysts. Chem. Rev. 2017, 117, 12475–12531. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Henkelman, G. CO oxidation mechanism on CeO2-supported Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Hensen, E.J.M. Mechanistic aspects of the water–gas shift reaction on isolated and clustered Au atoms on CeO2 (110): A density functional theory study. ACS Catal. 2014, 4, 1885–1892. [Google Scholar] [CrossRef]
- Abad, A.; Concepción, P.; Corma, A.; García, H. A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew. Chem. Int. Ed. 2005, 44, 4066–4069. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Tomishige, K. Scope and reaction mechanism of CeO2-catalyzed one-pot imine synthesis from alcohols and amines. J. Catal. 2020, 389, 285–296. [Google Scholar] [CrossRef]
- Maldotti, A.; Molinari, A.; Juárez, R.; Garcia, H. Photoinduced reactivity of Au-H intermediates in alcohol oxidation by gold nanoparticles supported on ceria. Chem. Sci. 2011, 2, 1831–1834. [Google Scholar] [CrossRef]
- Conte, M.; Miyamura, H.; Kobayashi, S.; Chechik, V. Spin trapping of Au-H intermediate in the alcohol oxidation by supported and unsupported gold catalysts. J. Am. Chem. Soc. 2009, 131, 7189–7196. [Google Scholar] [CrossRef]
- Li, H.; Qin, F.; Yang, Z.; Cui, X.; Wang, J.; Zhang, L. New reaction pathway induced by plasmon for selective benzyl alcohol oxidation on BiOCl possessing oxygen vacancies. J. Am. Chem. Soc. 2017, 139, 3513–3521. [Google Scholar] [CrossRef]

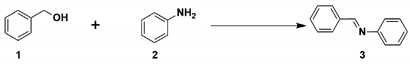
| Entry | Catalyst | Conversion of BnOH (%) | Selectivity (%) | |
|---|---|---|---|---|
| Imine | Other | |||
| 1 a | Au NPs | 2.3 | 100 | 0 |
| (Light) | ||||
| 2 | Nanorods | 62.9 | 96 | 4 |
| (Light) | ||||
| 3 | Nanorods | 62.5 | 97 | 3 |
| (No light) | ||||
| 4 | Au/nanorods | 91.2 | 97 | 3 |
| (Light) | ||||
| 5 | Au/nanorods | 69.5 | 97 | 3 |
| (No light) | ||||
| 6 d | Au/nanorods | 31.7 | 97 | 3 |
| (Light, Ar atmosphere) | ||||
| 7 d | Au/nanorods | 62.1 | 97 | 3 |
| (No light, Ar atmosphere) | ||||
| 8 b | Au/nanorods | 68.7 | 97 | 3 |
| (Light, electron scavenger) | ||||
| 9 c | Au/nanorods | 30.7 | 97 | 3 |
| (Light, hole scavenger) | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, I.F.; Homsi, M.S.; Geonmonond, R.S.; Rocha, G.F.S.R.; Peng, Y.-K.; Silva, I.F.; Quiroz, J.; Camargo, P.H.C. Hot Electrons, Hot Holes, or Both? Tandem Synthesis of Imines Driven by the Plasmonic Excitation in Au/CeO2 Nanorods. Nanomaterials 2020, 10, 1530. https://doi.org/10.3390/nano10081530
Teixeira IF, Homsi MS, Geonmonond RS, Rocha GFSR, Peng Y-K, Silva IF, Quiroz J, Camargo PHC. Hot Electrons, Hot Holes, or Both? Tandem Synthesis of Imines Driven by the Plasmonic Excitation in Au/CeO2 Nanorods. Nanomaterials. 2020; 10(8):1530. https://doi.org/10.3390/nano10081530
Chicago/Turabian StyleTeixeira, Ivo F., Mauricio S. Homsi, Rafael S. Geonmonond, Guilherme F. S. R. Rocha, Yung-Kang Peng, Ingrid F. Silva, Jhon Quiroz, and Pedro H. C. Camargo. 2020. "Hot Electrons, Hot Holes, or Both? Tandem Synthesis of Imines Driven by the Plasmonic Excitation in Au/CeO2 Nanorods" Nanomaterials 10, no. 8: 1530. https://doi.org/10.3390/nano10081530
APA StyleTeixeira, I. F., Homsi, M. S., Geonmonond, R. S., Rocha, G. F. S. R., Peng, Y.-K., Silva, I. F., Quiroz, J., & Camargo, P. H. C. (2020). Hot Electrons, Hot Holes, or Both? Tandem Synthesis of Imines Driven by the Plasmonic Excitation in Au/CeO2 Nanorods. Nanomaterials, 10(8), 1530. https://doi.org/10.3390/nano10081530






