Morphological, Material, and Optical Properties of ZnO/ZnS/CNTs Nanocomposites on SiO2 Substrate
Abstract
1. Introduction
2. Materials and Methods
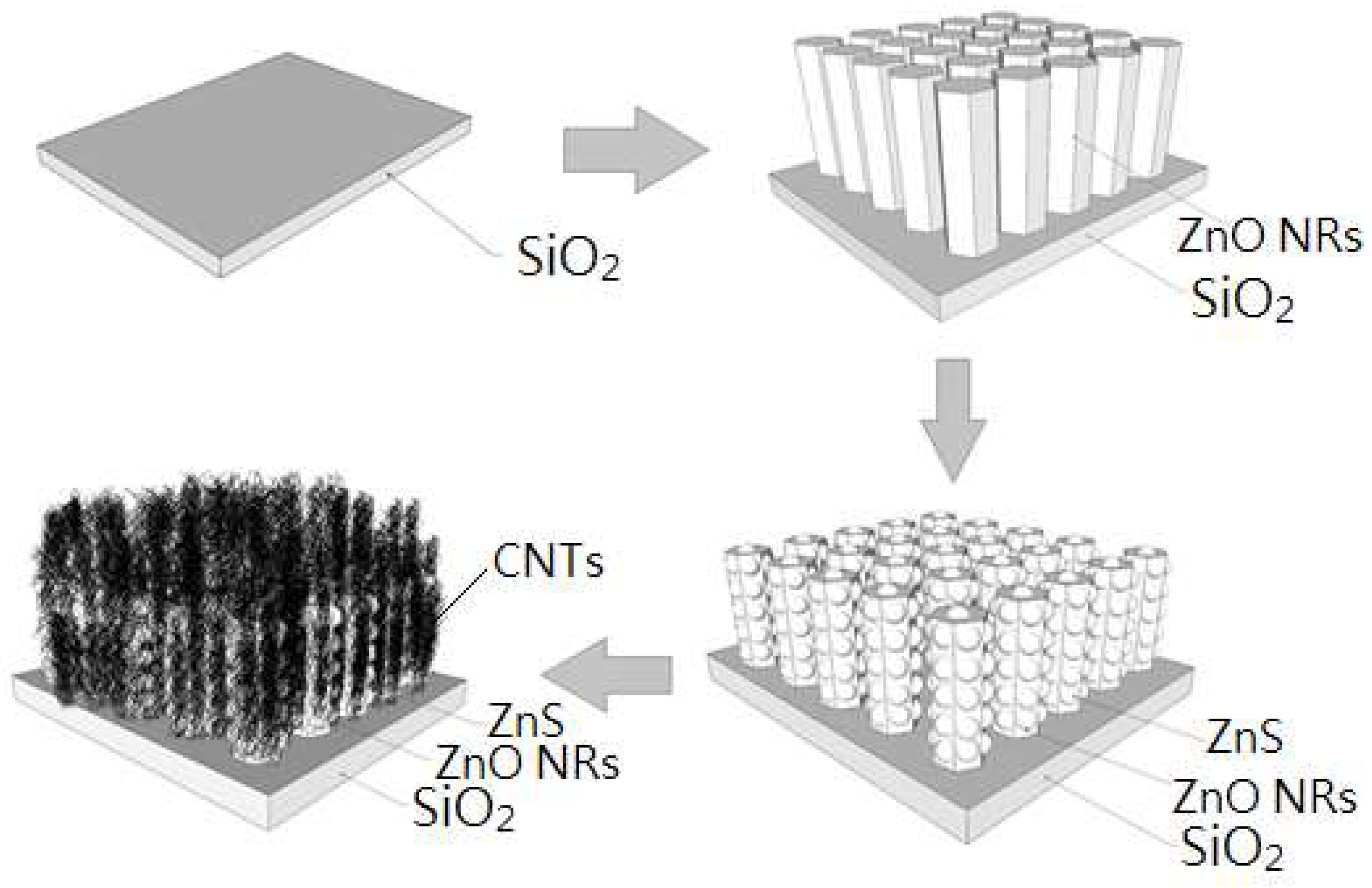
2.1. Preparation of the Seed Layer for ZnO by Sol–Gel Method and Spin Coating
2.2. Synthesis of Array Nanorods by Hydrothermal Method
2.3. Doping Carbon Nanotube (CNT)
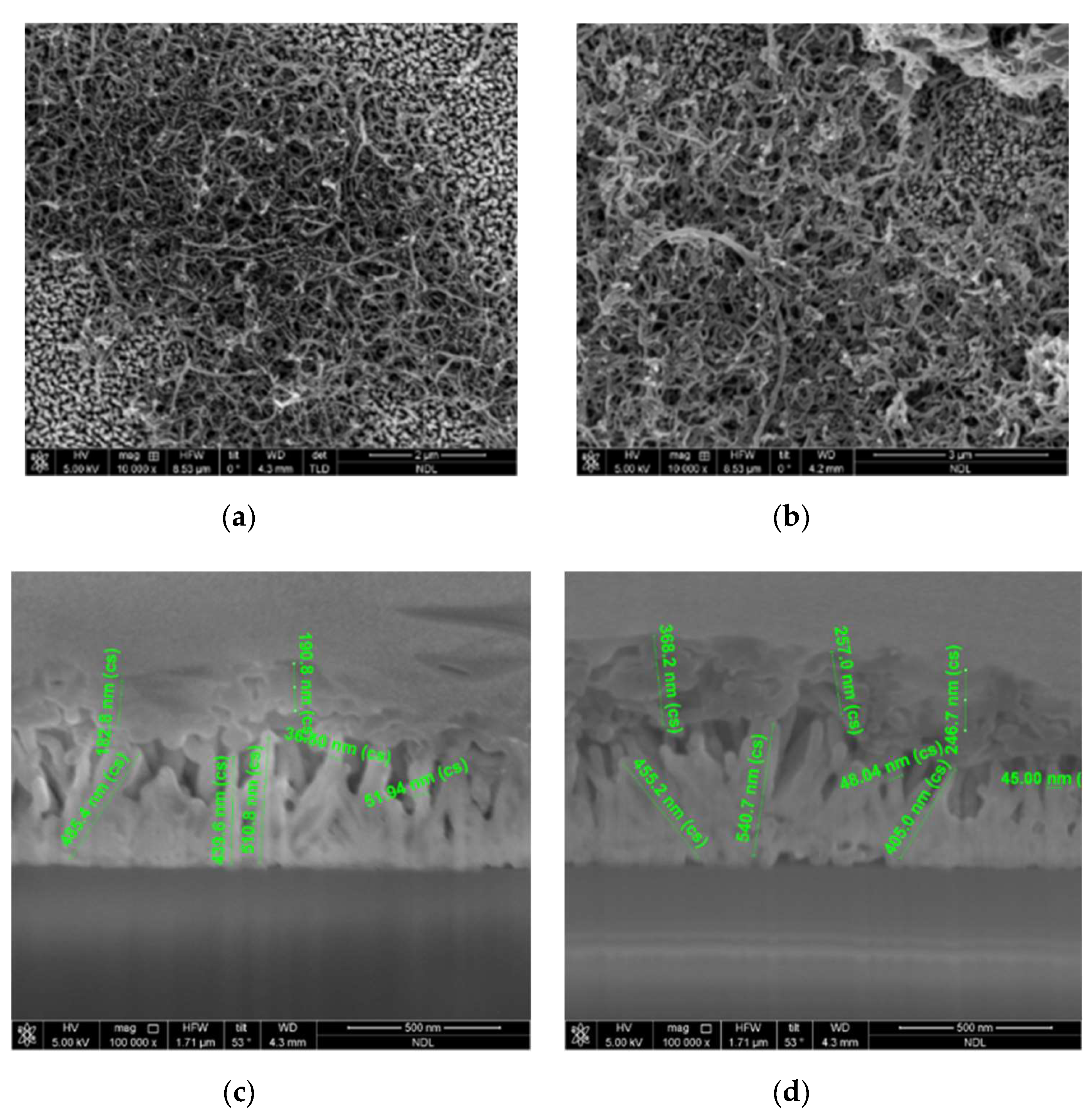
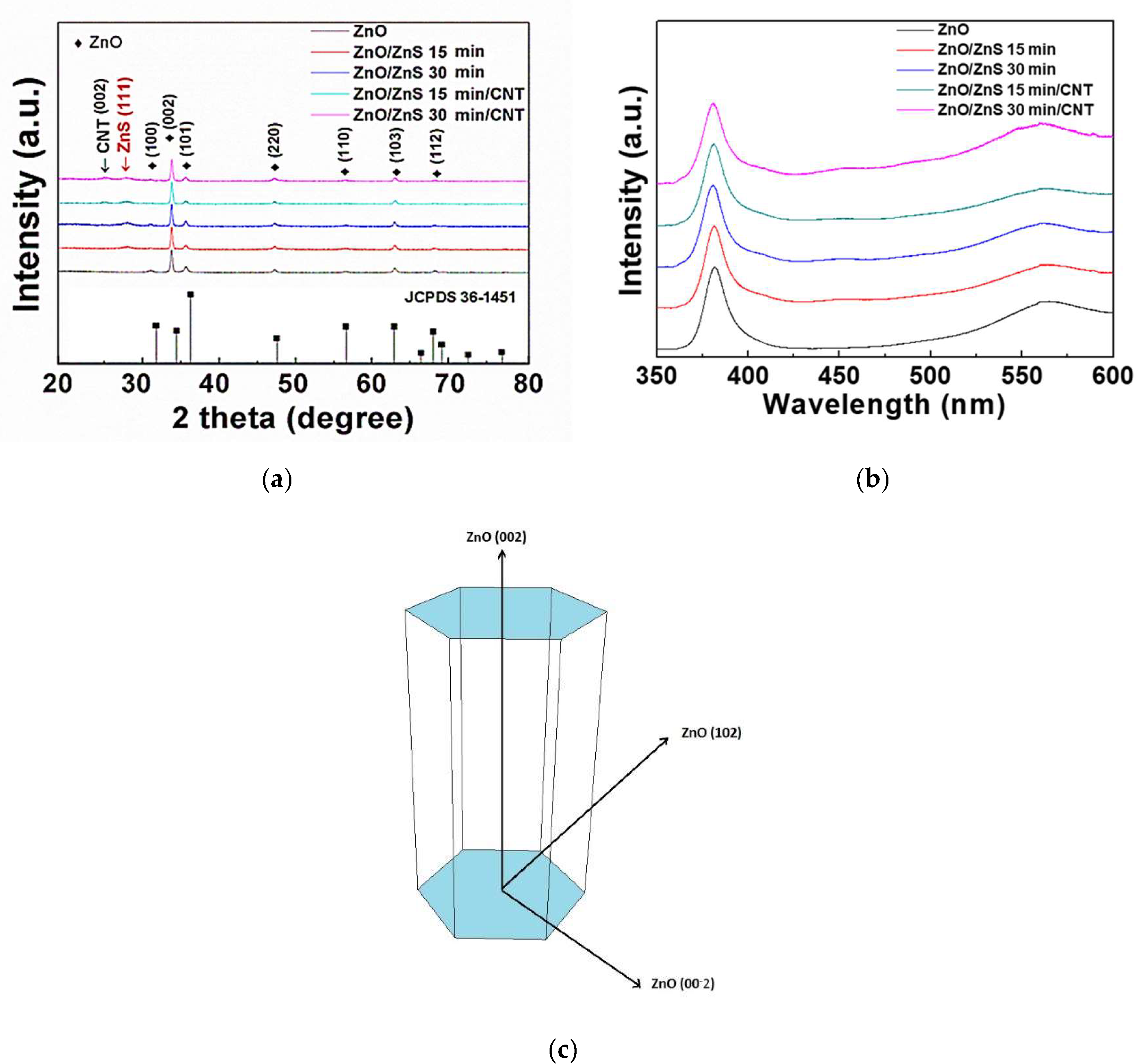
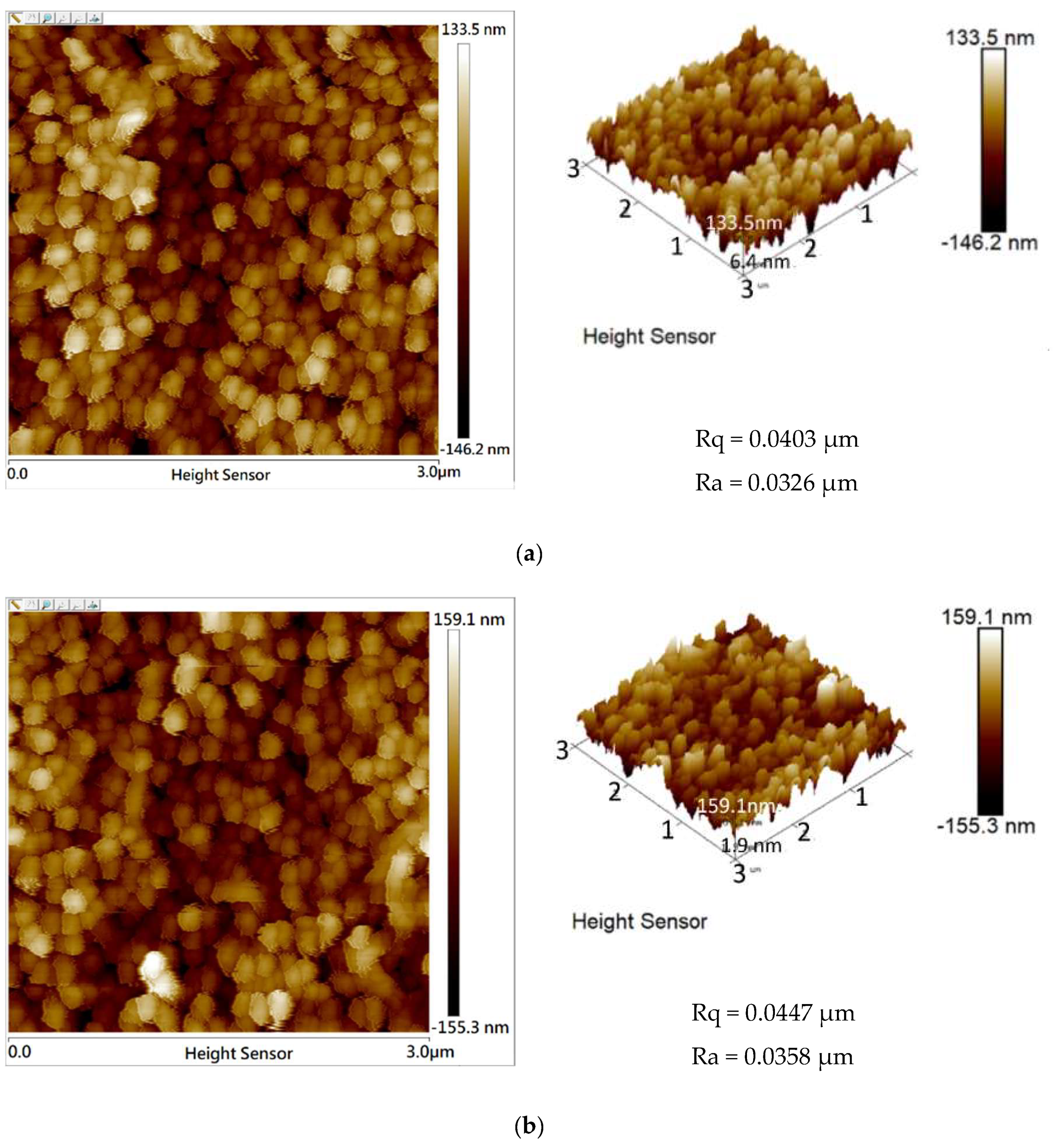
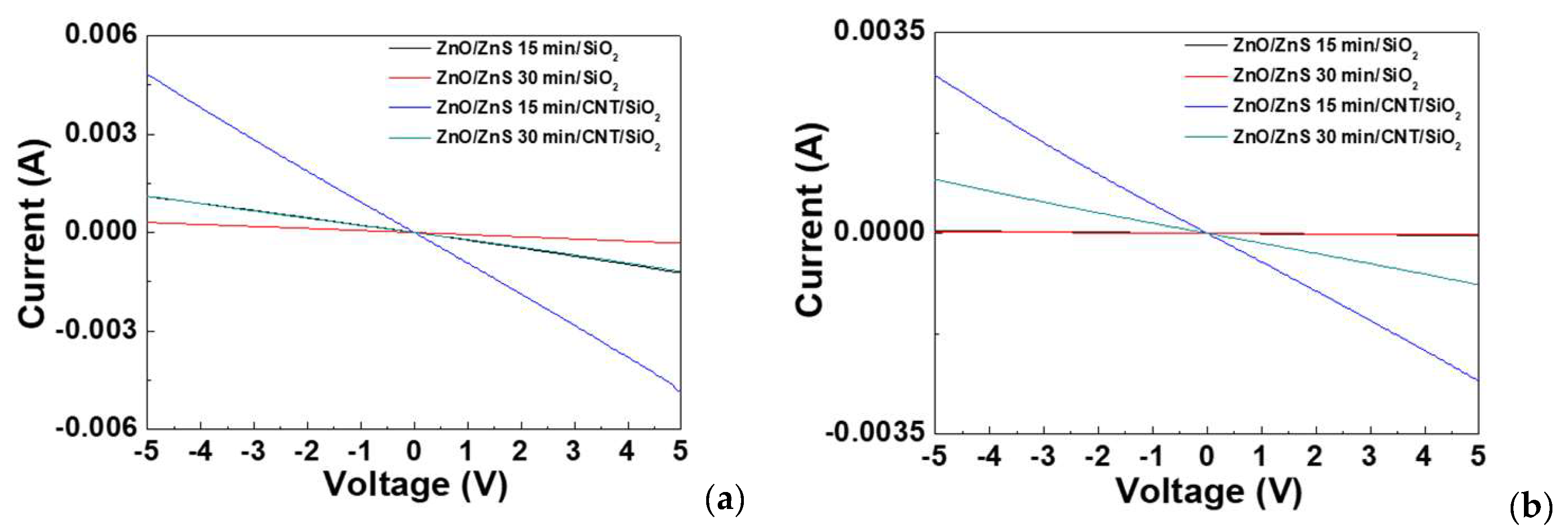
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, X.H.; Zheng, Y.Z.; Bi, S.Q.; Wang, Y.; Tao, X.; Dai, L.; Chen, J.F. Multidimensional ZnO Architecture for Dye-Sensitized Solar Cells with High-Efficiency up to 7.35%. Adv. Energy Mater. 2014, 4, 1301802. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.Z.; Lu, S.; Tao, X.; Che, Y.; Chen, J.F. Visible-light-responsive TiO2-coated ZnO:I nanorod array films with enhanced photoelectrochemical and photocatalytic performance. ACS Appl. Mater. Interfaces 2015, 7, 6093–6101. [Google Scholar] [CrossRef]
- Wang, W.W.; Zhu, Y.J.; Yang, L.X. ZnO–SnO2 Hollow Spheres and Hierarchical Nanosheets: Hydrothermal Preparation, Formation Mechanism, and Photocatalytic Properties. Adv. Funct. Mater. 2007, 17, 59–64. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, N.; Zhang, M.Y.; Zhang, X.T.; Gao, H.; Wen, J. Controllable growth of ZnO-ZnSe heterostructures for visible-light photocatalysis. CrystEngComm 2014, 16, 1201–1206. [Google Scholar] [CrossRef]
- Shuai, X.M.; Shen, W.Z. A Facile Chemical Conversion Synthesis of ZnO/ZnS Core/Shell Nanorods and Diverse Metal Sulfide Nanotubes. J. Phys. Chem. C 2011, 115, 6415–6422. [Google Scholar] [CrossRef]
- Xu, F.; Sun, L. Solution-derived ZnOnanostructures for photoanodes of dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 818–841. [Google Scholar] [CrossRef]
- Sarkar, A.; Singh, A.K.; Khan, G.G.; Sarkar, D.; Mandal, K. TiO2/ZnO core/shell nano-heterostructure arrays as photo-electrodes with enhanced visible light photoelectrochemical performance. RSC Adv. 2014, 4, 55629–55634. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, S.; Lin, Y.; Ren, W.; Cai, H.; Wu, Y.; Zhang, Q.; Pan, N.; Luo, Y.; Wang, X. Improving the photovoltaic performance of solid-state ZnO/CdTe core–shell nanorod array solar cells using a thin CdS interfacial layer. J. Mater. Chem. A 2014, 2, 5675–5681. [Google Scholar] [CrossRef]
- Rouhi, J.; Mamat, M.H.; Ooi, C.H.; Mahmud, S.; Mahmood, M.R. High-performance dye-sensitized solar cells based on morphology-controllable synthesis of ZnO-ZnS heterostructure nanocone photoanodes. PLoS ONE 2015, 10, e0123433. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Chou, T.W.; Xu, C.Y.; Huang, W.C.; Lin, C.F.; Wu, Y.S.; Lin, Y.S.; Chen, H. ZnO/ZnS core-shell nanostructures for hydrogen gas sensing performances. Ceram. Int. 2019, 17751–17757. [Google Scholar] [CrossRef]
- Yu, Y.; Jimmy, C.Y.; Yu, J.G.; Kwok, Y.C.; Che, Y.K.; Zhao, J.C.; Ding, L.; Ge, W.K.; Wong, P.K. Enhancement of photocatalytic activity of mesoporous TiO2 by using carbon nanotubes. Appl. Catal. A Gen. 2005, 289, 186–196. [Google Scholar] [CrossRef]
- Suzuki, S. The Role of Carbon Nanotubes in Enhancement of Photocatalysis. In Syntheses and Applications of Carbon Nanotubes and Their Composites; Saleh, A.T., Ed.; InTech: Rijeka, Croatia, 2013; pp. 479–493. [Google Scholar]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef]
- Meng, Z.-D.; Sarkar, S.; Zhu, L.; Ullah, K.; Ye, S.; Oh, W.-C. Detection of Oxygen Species Generated in the Presence of CNT by Loading ZnS. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2014, 45, 1373–1379. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, H.J.; Lin, T.C.; Weng, W.C.; Chang, Y.C.; Chiu, J.L.; Lin, J.J.; Lin, C.F.; Lin, Y.S.; Chen, H. Incorporation of carbon nanotube and graphene in ZnO nanorods-based hydrogen gas sensor. Ceram. Int. 2018, 44, 12308–12314. [Google Scholar] [CrossRef]
- Schütt, F.; Postica, V.; Adelung, R.; Lupan, O. Single and Networked ZnO–CNT Hybrid Tetrapods for Selective Room-Temperature High-Performance Ammonia Sensors. ACS Appl. Mater. Interfaces 2017, 9, 23107–23118. [Google Scholar] [CrossRef]
- Zhang, R.; Fan, L.; Fang, Y.; Yang, S. Electrochemical route to the preparation of highly dispersed composites of ZnO/carbon nanotubes with significantly enhanced electrochemiluminescence from ZnO. J. Mater. Chem. 2008, 18, 4964–4970. [Google Scholar] [CrossRef]
- Liu, B.; Bando, Y.; Jiang, X.; Li, C.; Fang, X.; Zeng, H.; Terao, T.; Tang, C.; Mitome, M.; Golberg, D. Self-assembled ZnS nanowire arrays: Synthesis, in situ Cu doping and field emission. Nanotechnology 2010, 21, 375601. [Google Scholar] [CrossRef]
- Cheng, C.C.; Cho, C.F.; Chiu, J.L.; Tsai, C.T.; Chen, H. Hydrothermal fabrication and characterization of ZnO/ZnS core-shell structures on white reflective films. Results Phys. 2018, 10, 449–457. [Google Scholar] [CrossRef]
- Feng, S.A.; Zhao, J.H.; Zhu, Z.P. The manufacture of carbon nanotubes decorated with ZnS to enhance the ZnS photocatalytic activity. New Carbon Mater. 2008, 23, 228–234. [Google Scholar] [CrossRef]
- Mustafa, M.K.; Iqbal, Y.; Majeed, U.; Sahdan, M.Z. Effect of precursor’s concentration on structure and morphology of ZnO nanorods synthesized through hydrothermal method on gold surface. In Proceedings of AIP Conference, Solo, Indonesia, 3–5 August 2016; p. 030120. [Google Scholar]
- Okeil, S.; Krausmann, J.; Dönges, I.; Pfleger, S.; Engstler, J.; Schneider, J.J. ZnS/ZnO@CNT and ZnS@CNT nanocomposites by gas phase conversion of ZnO@CNT. A systematic study of their photocatalytic properties. Dalton Trans. 2017, 46, 5189. [Google Scholar] [CrossRef]
- Cheng, C.C.; Weng, W.C.; Lin, H.I.; Chiu, J.L.; Jhao, H.Y.; Liao, Y.T.A.; Yu, C.T.R.; Chen, H. Fabrication and characterization of distinctive ZnO/ZnS core–shell structures on silicon substrates via a hydrothermal method. RSC Adv. 2018, 8, 26341. [Google Scholar] [CrossRef]
- Dutta, M.; Jana, S.; Basak, D. Quenching of Photoluminescence in ZnO QDs Decorating Multiwalled Carbon Nanotubes. ChemPhysChem 2010, 11, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Sahu, P.P.; Bhunia, C.T. High photo sensing performance with electro-optically efficient silicon based ZnO/ZnMgO heterojunction structure. IEEE Sens. J. 2018, 18, 2849089. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.; Wen, J.; Wu, L.; Gao, H.; Zhang, D. Side by side ZnO/ZnS hetero-junction nanocrystal arrays with superior field emission property. CrystEngComm 2013, 15, 1908–1913. [Google Scholar] [CrossRef]
- Ramachandran, R.; Saranya, M.; Kollu, P.; Raghupathy, B.P.; Jeong, S.K.; Grace, A.N. Solvothermal synthesis of Zinc sulfide decorated Graphene (ZnS/G) nanocomposites for novel Supercapacitor electrodes. Electrochim. Acta 2015, 178, 647–657. [Google Scholar] [CrossRef]
- Naeimi, A.; Arabi, A.M.; Gardeshzadeh, A.R.; Afarani, M.S. Study of electrophoretic deposition of ZnS:Ag/CNT composites for luminescent applications. J. Mater. Sci. Mater. Electron. 2014, 25, 1575–1582. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Hung, C.H.; Chan, W.L.; Tsai, S.J.; Lee, Y.S.; Huang, W.C.; Wu, Y.S.; Chen, H. Morphological and crystalline analysis of ZnO/ZnS nanostructures on porous silicon substrate. Vacuum 2020, 109454. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, S.M.; Scholz, R.; Knez, M.; Gösele, U.; Fallert, J.; Kalt, H.; Zacharias, M. Synthesis and optical properties of ZnO and carbon nanotube based coaxial heterostructures. Appl. Phys. Lett. 2008, 93, 103108. [Google Scholar] [CrossRef]
- Ghosh, A.; Chaudhary, D.K.; Biswas, A.; Thangavel, R.; Udayabhanu, G. Solution-processed Cu2XSnS4 (X ¼ Fe, Co, Ni) photo-electrochemical and thin film solar cells on vertically grown ZnO nanorod arrays. RSC Adv. 2016, 6, 115204. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Dai, L. Water-Assisted Growth of Aligned CarbonNanotube–ZnO HeterojunctionArrays. Adv. Mater. 2006, 18, 1740–1744. [Google Scholar] [CrossRef]


| Locations | Weight % | Atomic % | ||||||
|---|---|---|---|---|---|---|---|---|
| Zn | O | S | C | Zn | O | S | C | |
| Spectrum 1 | 0.16 | 15.34 | 0.84 | 83.65 | 0.03 | 12.06 | 0.33 | 87.58 |
| Spectrum 2 | 6.24 | 15.59 | 2.89 | 75.28 | 1.29 | 13.12 | 1.21 | 84.38 |
| Spectrum 3 | 0.01 | 16.08 | 0 | 84.05 | 0.00 | 12.56 | 0 | 87.49 |
| Voltage | Dark Current | Light Current | |
|---|---|---|---|
| ZnO/ZnS 15 min | −2 V | 1.69914 × 10−2 mA | 4.51376 × 10−1 mA |
| ZnO/ZnS 30 min | −2 V | 9.72357 × 10−3 mA | 1.25697 × 10−1 mA |
| ZnO/ZnS 15 min/CNTs | −2 V | 1.03 mA | 1.87 mA |
| ZnO/ZnS 30 min/CNTs | −2 V | 3.5489 × 10−1 mA | 4.34431 × 10−1 mA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.S.; Lin, X.D.; Chan, W.L.; Tsai, S.C.; Liao, W.J.; Wu, Y.C.S.; Chen, H. Morphological, Material, and Optical Properties of ZnO/ZnS/CNTs Nanocomposites on SiO2 Substrate. Nanomaterials 2020, 10, 1521. https://doi.org/10.3390/nano10081521
Tsai YS, Lin XD, Chan WL, Tsai SC, Liao WJ, Wu YCS, Chen H. Morphological, Material, and Optical Properties of ZnO/ZnS/CNTs Nanocomposites on SiO2 Substrate. Nanomaterials. 2020; 10(8):1521. https://doi.org/10.3390/nano10081521
Chicago/Turabian StyleTsai, Yu Sheng, Xin Dai Lin, Wei Lun Chan, Shang Che Tsai, Wei Jen Liao, Yew Chung Sermon Wu, and Hsiang Chen. 2020. "Morphological, Material, and Optical Properties of ZnO/ZnS/CNTs Nanocomposites on SiO2 Substrate" Nanomaterials 10, no. 8: 1521. https://doi.org/10.3390/nano10081521
APA StyleTsai, Y. S., Lin, X. D., Chan, W. L., Tsai, S. C., Liao, W. J., Wu, Y. C. S., & Chen, H. (2020). Morphological, Material, and Optical Properties of ZnO/ZnS/CNTs Nanocomposites on SiO2 Substrate. Nanomaterials, 10(8), 1521. https://doi.org/10.3390/nano10081521





