Plasma-Assisted Chemical Vapor Deposition of F-Doped MnO2 Nanostructures on Single Crystal Substrates
Abstract
1. Introduction
2. Experimental Procedure
2.1. Synthesis
2.2. Characterization
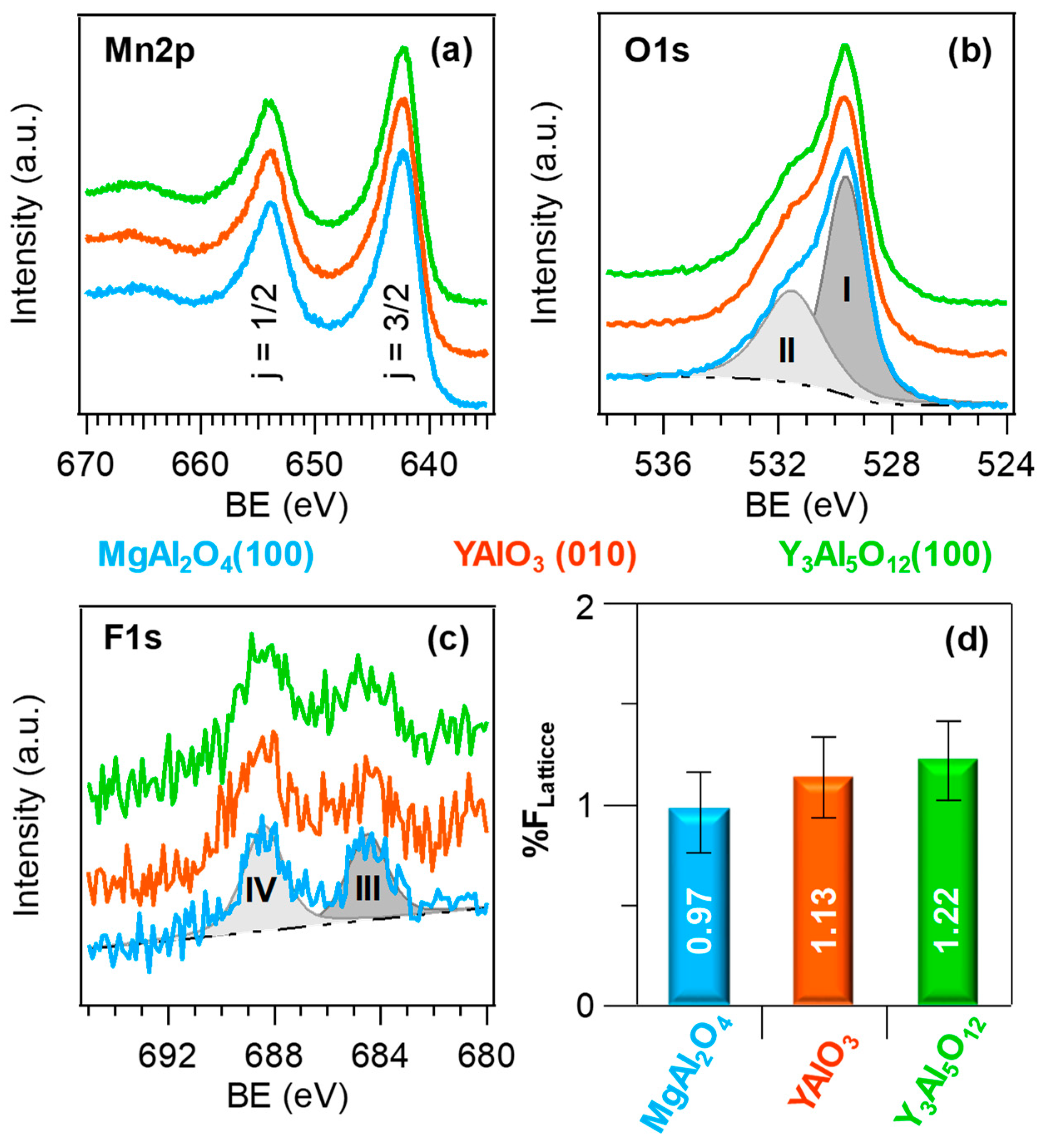
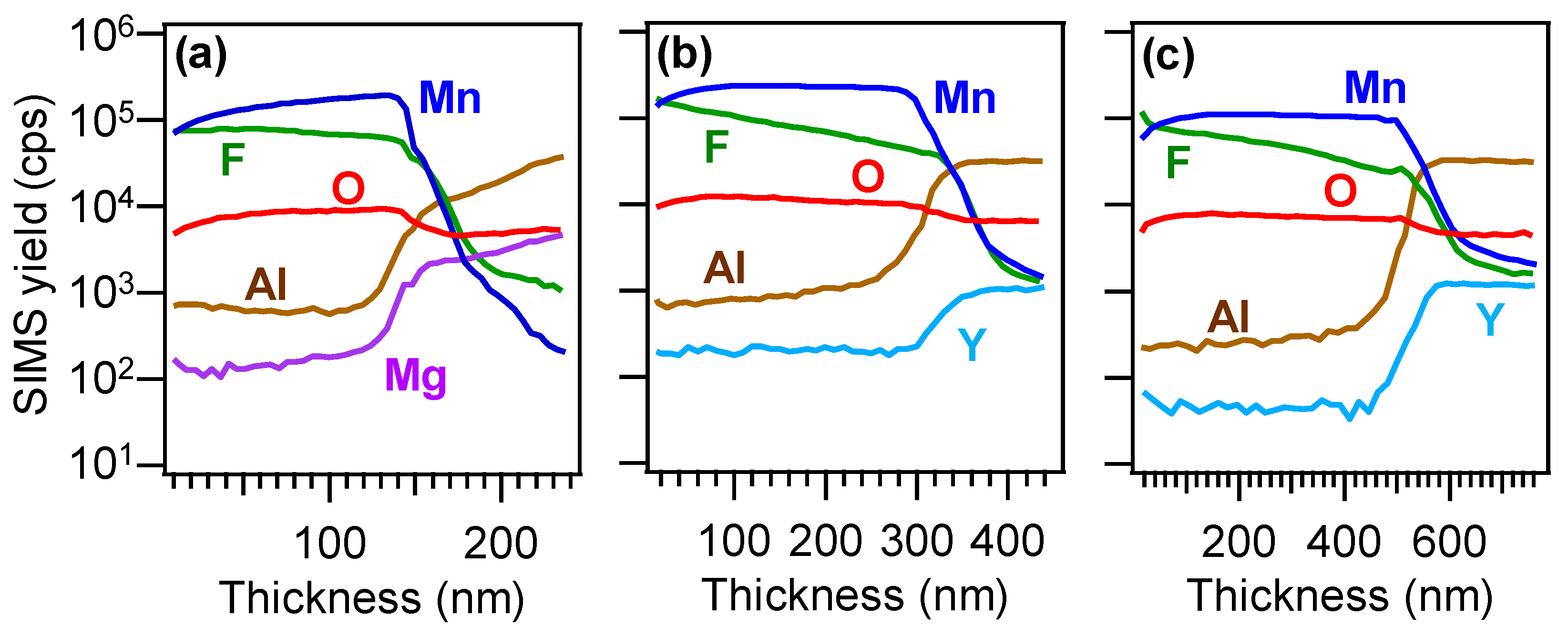
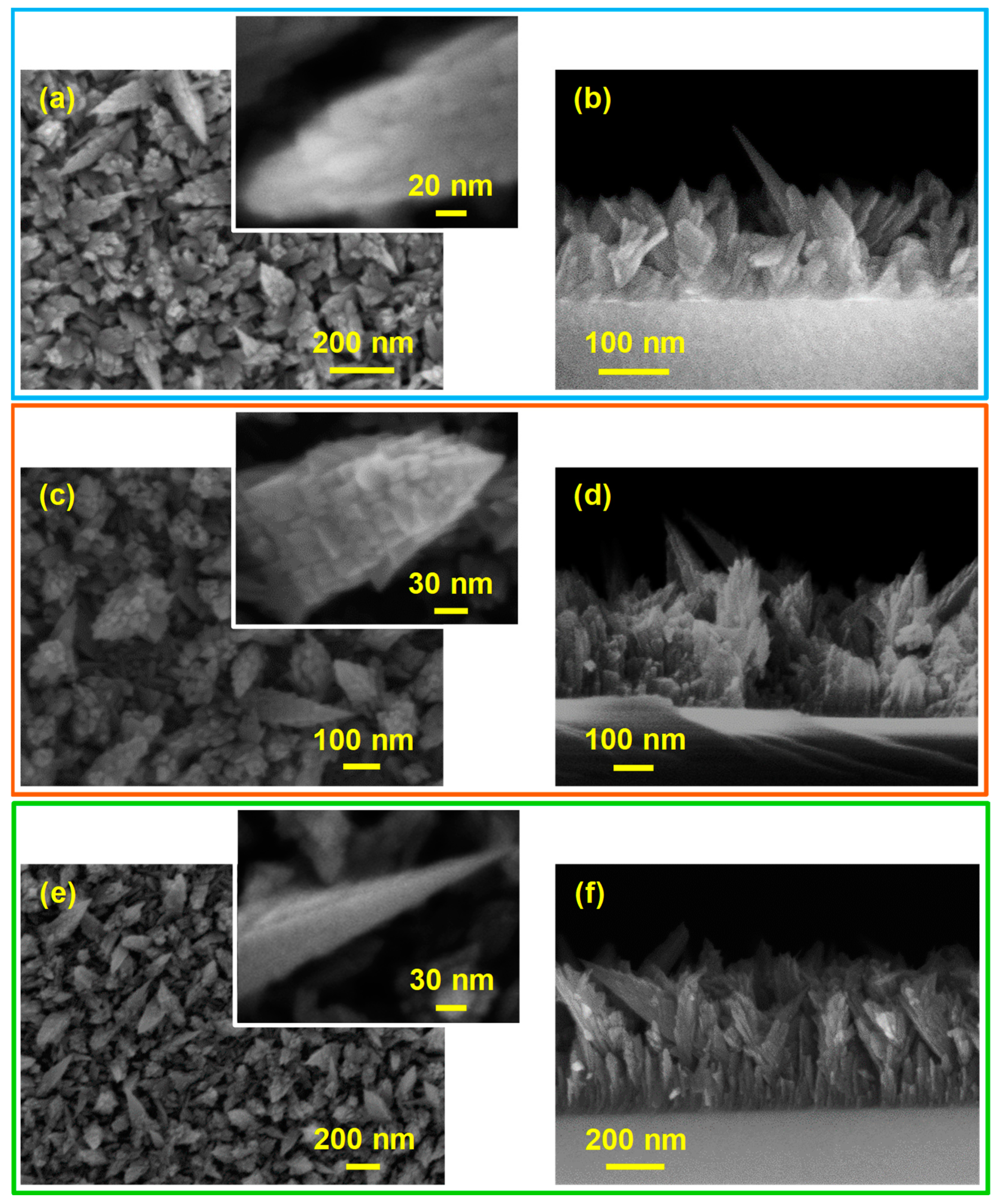
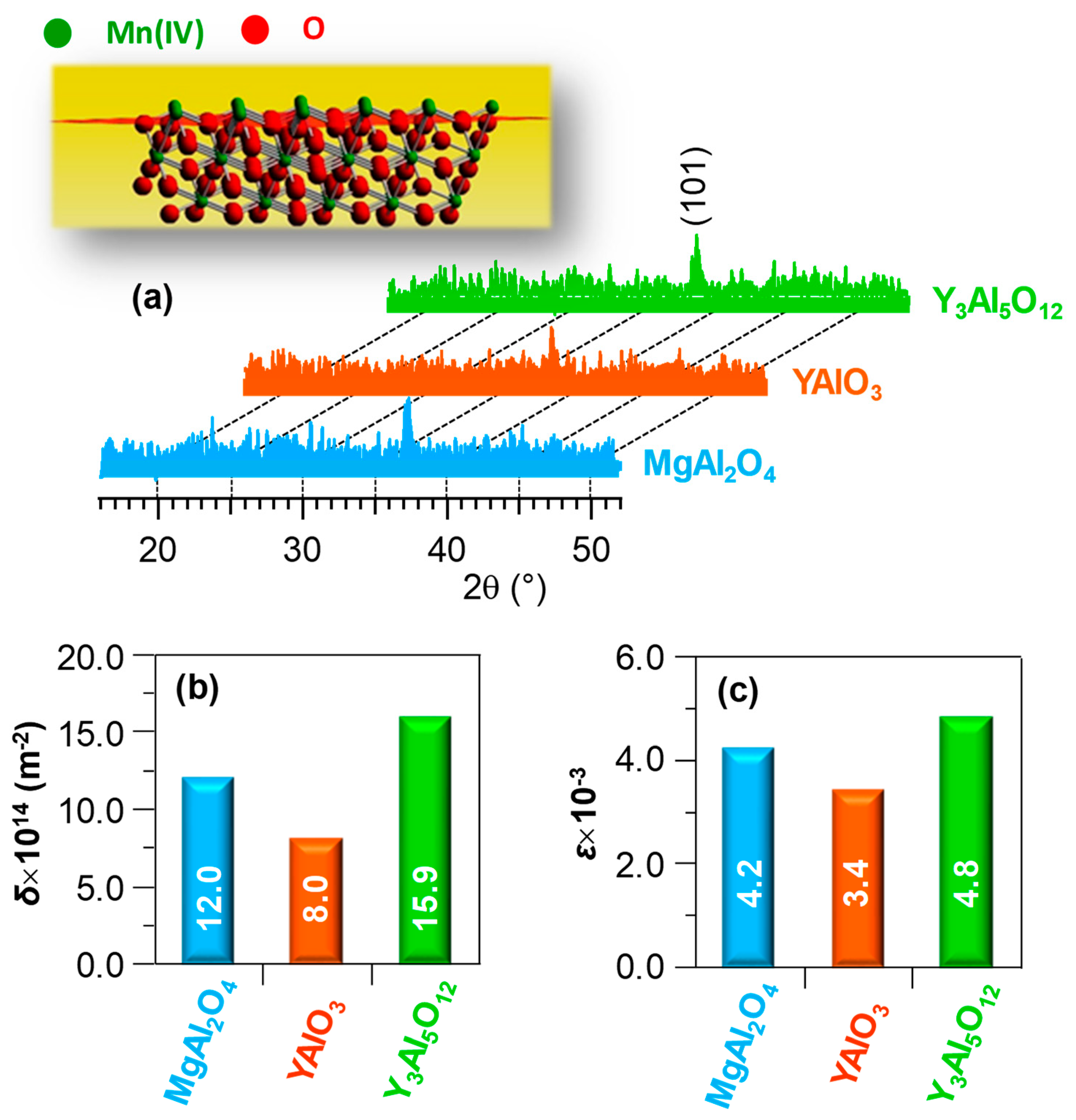
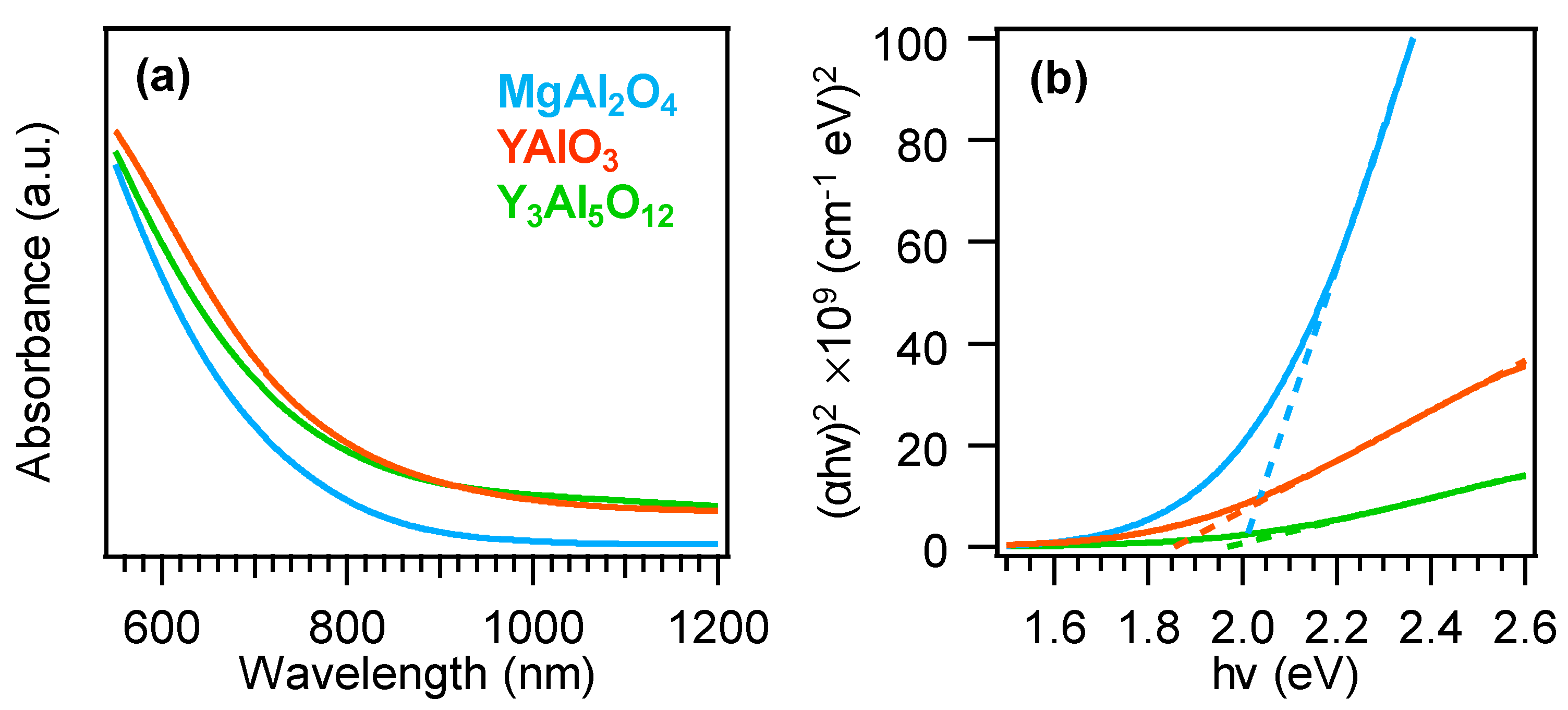
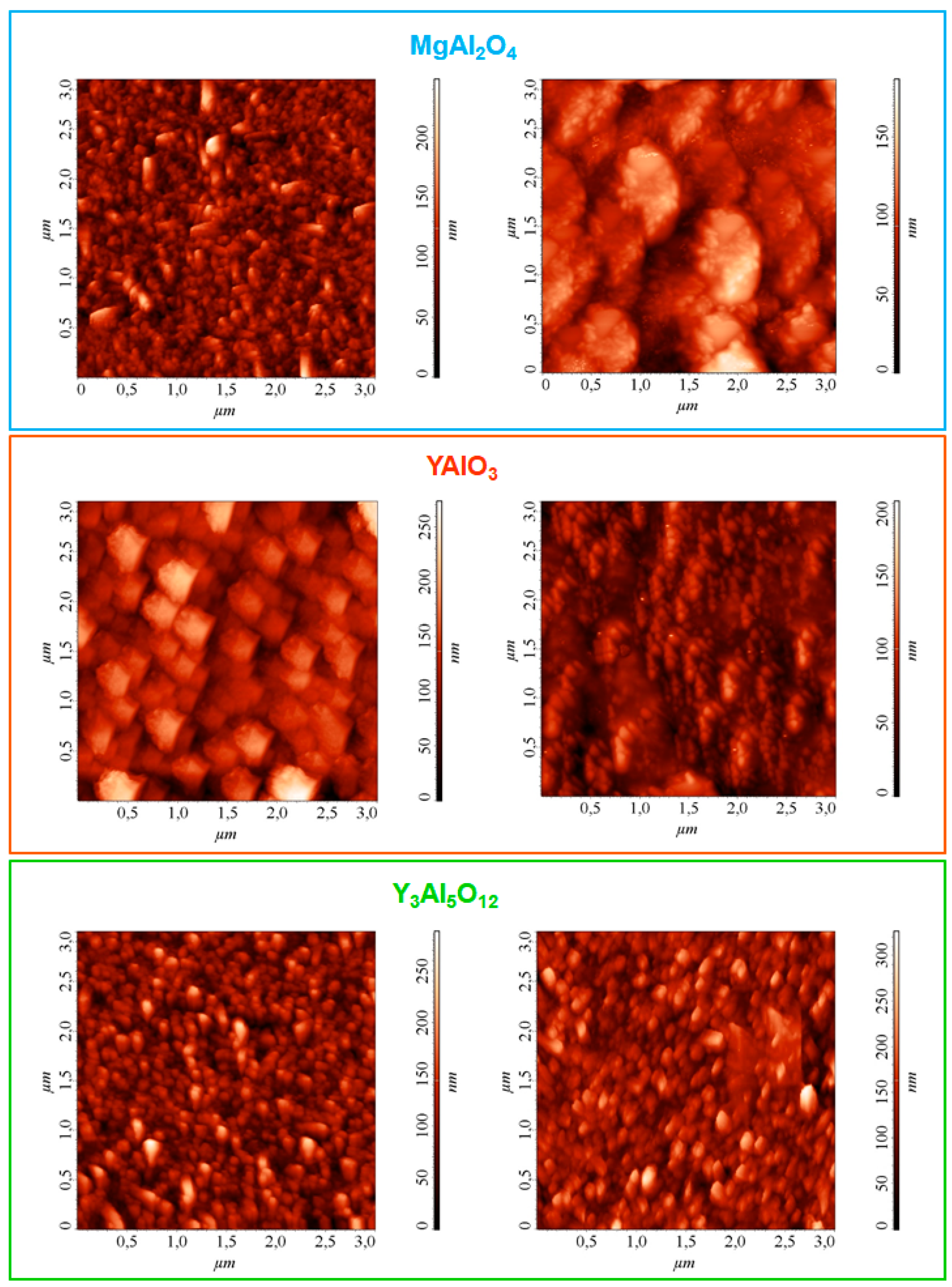
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Note
- Foss, S.; Nilsen, O.; Olsen, A.; Taftø, J. Structure determination of MnO2 films grown on single crystal α-Al2O3 substrates. Philos. Mag. 2005, 85, 2689–2705. [Google Scholar] [CrossRef]
- Nilsen, O.; Fjellvåg, H.; Kjekshus, A. Growth of manganese oxide thin films by atomic layer deposition. Thin Solid Films 2003, 444, 44–51. [Google Scholar] [CrossRef]
- Ren, L.; Wu, S.; Zhou, W.; Li, S. Epitaxial growth of manganese oxide films on MgAl2O4 (001) substrates and the possible mechanism. J. Cryst. Growth 2014, 389, 55–59. [Google Scholar] [CrossRef]
- Chambers, S.A.; Liang, Y. Growth of β-MnO2 films on TiO2(110) by oxygen plasma assisted molecular beam epitaxy. Surf. Sci. 1999, 420, 123–133. [Google Scholar] [CrossRef]
- Abi-Akl, M.; Tabbal, M.; Kassem, W. Crystalline phase control and growth selectivity of β-MnO2 thin films by remote plasma assisted pulsed laser deposition. Thin Solid Films 2016, 612, 450–455. [Google Scholar] [CrossRef]
- Li, Y.W.; Qiao, Q.; Zhang, J.Z.; Hu, Z.G.; Chu, J.H. Influence of post-annealing on structural, electrical and optical properties of manganese oxide thin films grown by atomic layer deposition. Thin Solid Films 2015, 574, 115–119. [Google Scholar] [CrossRef]
- Moulai, F.; Fellahi, O.; Messaoudi, B.; Hadjersi, T.; Zerroual, L. Electrodeposition of nanostructured γ-MnO2 film for photodegradation of Rhodamine B. Ionics 2018, 24, 2099–2109. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Chen, Z.B.; Abram, D.N.; Jaramillo, T.F. Thin films of sodium birnessite-type MnO2: Optical properties, electronic band structure, and solar photoelectrochemistry. J. Phys. Chem. C 2011, 115, 11830–11838. [Google Scholar] [CrossRef]
- Makhlouf, M.M. Preparation and optical characterization of β-MnO2 nano thin films for application in heterojunction photodiodes. Sens. Actuators A 2018, 279, 145–156. [Google Scholar] [CrossRef]
- Chou, S.L.; Cheng, F.Y.; Chen, J. Electrodeposition synthesis and electrochemical properties of nanostructured γ-MnO2 films. J. Power Sources 2006, 162, 727–734. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, W.; Gan, H.; Wang, J.-W.; Shi, X.-C. Enhancing high-rate capability of MnO2 film electrodeposited on carbon fibers via hydrothermal treatment. Electrochim. Acta 2017, 246, 890–896. [Google Scholar] [CrossRef]
- Wan, X.; Yang, S.; Cai, Z.; He, Q.; Ye, Y.; Xia, Y.; Li, G.; Liu, J. Facile synthesis of MnO2 nanoflowers/N-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.M.; Lokhande, V.C.; Patil, U.M.; Shinde, P.A.; Lokhande, C.D. High performance all-solid-state asymmetric supercapacitor device based on 3D nanospheres of β-MnO2 and nanoflowers of O-SnS. ACS Sustainable Chem. Eng. 2018, 6, 787–802. [Google Scholar] [CrossRef]
- Shinde, P.A.; Lokhande, V.C.; Ji, T.; Lokhande, C.D. Facile synthesis of hierarchical mesoporous weirds-like morphological MnO2 thin films on carbon cloth for high performance supercapacitor application. J. Colloid Interface Sci. 2017, 498, 202–209. [Google Scholar] [CrossRef]
- Balamurugan, S.; Rajalakshmi, A.; Balamurugan, D. Acetaldehyde sensing property of spray deposited β-MnO2 thin films. J. Alloys Compd. 2015, 650, 863–870. [Google Scholar] [CrossRef]
- Luo, F.; Song, W.; Yan, C.-H. Enhanced room temperature magnetoresistance effect in oxygen defective β-MnO2 microcrystal. Chem. Phys. Lett. 2006, 431, 337–340. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Kim, Y.-M.; Jung, M.-H.; Jagličić, Z.; Umek, P.; Dolinšek, J. Synthesis, structure and magnetic properties of β-MnO2 nanorods. Nanoscale Res. Lett. 2007, 2, 81. [Google Scholar] [CrossRef]
- Awad, M.A.; Hadia, N.M.A. Towards understanding the morphological, magnetic, optical and electrical properties of MnO2 nanowires for magneto- and optoelectronic applications. J. Mater. Sci. Mater. Electron. 2018, 29, 20695–20702. [Google Scholar] [CrossRef]
- Ramesh, M.; Nagaraja, H.S.; Rao, M.P.; Anandan, S.; Huang, N.M. Fabrication, Characterization and Catalytic Activity of α-MnO2 Nanowires for Dye Degradation of Reactive Black 5. Mater. Lett. 2016, 172, 85–89. [Google Scholar] [CrossRef]
- Yu, X.L.; Wu, S.X.; Liu, Y.J.; Li, S.W. Electronic spectrum of a helically Hund-coupled β-MnO2. Solid State Commun. 2008, 146, 166–168. [Google Scholar] [CrossRef]
- Nilsen, O.; Foss, S.; Fjellvåg, H.; Kjekshus, A. Effect of substrate on the characteristics of manganese(IV) oxide thin films prepared by atomic layer deposition. Thin Solid Films 2004, 468, 65–74. [Google Scholar] [CrossRef]
- Music, D.; Bliem, P.; Geyer, R.W.; Schneider, J.M. Atomistic growth phenomena of reactively sputtered RuO2 and MnO2 thin films. J. Appl. Phys. 2015, 118, 015302. [Google Scholar] [CrossRef]
- Barreca, D.; Gri, F.; Gasparotto, A.; Carraro, G.; Bigiani, L.; Altantzis, T.; Žener, B.; Lavrenčič Štangar, U.; Alessi, B.; Padmanaban, D.B.; et al. Multi-Functional MnO2 nanomaterials for photo-activated applications by a plasma-assisted fabrication route. Nanoscale 2019, 11, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; Chen, J.; Wu, S.X.; Liu, Y.J.; Li, S.W. Polarized Raman scattering in helimagnetic β-MnO2. J. Raman Spectrosc. 2008, 39, 1440–1443. [Google Scholar] [CrossRef]
- Xing, X.J.; Yu, Y.P.; Xu, L.M.; Wu, S.X.; Li, S.W. Magnetic properties of β-MnO2 thin films grown by plasma-assisted molecular beam epitaxy. J. Phys. Chem. C 2008, 112, 15526–15531. [Google Scholar] [CrossRef]
- Guo, L.W.; Peng, D.L.; Makino, H.; Hanada, T.; Hong, S.K.; Sumiyama, K.; Yao, T.; Inaba, K. Structural characteristics and magnetic properties of λ-MnO2 films grown by plasma-assisted molecular beam epitaxy. J. Appl. Phys. 2001, 90, 351–354. [Google Scholar] [CrossRef]
- Guo, L.W.; Makino, H.; Ko, H.J.; Chen, Y.F.; Hanada, T.; Peng, D.L.; Inaba, K.; Yao, T. Structural characteristic and magnetic properties of Mn oxide films grown by plasma-assisted MBE. J. Cryst. Growth 2001, 227–228, 955–959. [Google Scholar] [CrossRef]
- Cui, J.; Ji, T.; Nie, T.; Lv, Y.; Yang, S.; Yang, X.; Jiang, Z.; Zou, J. Growth and memory effect of Er-stabilized β-MnO2 films grown on Si substrates. Mater. Res. Express 2014, 1, 036302. [Google Scholar] [CrossRef]
- Guo, C.; Ma, H.; Zhang, Q.; Li, M.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Nano MnO2 radially grown on lignin-based carbon fiber by one-step solution reaction for supercapacitors with high performance. Nanomaterials 2020, 10, 594. [Google Scholar] [CrossRef]
- Nayak, D.; Ghosh, S.; Adyam, V. Thin film manganese oxide polymorphs as anode for sodium-ion batteries: An electrochemical and DFT based study. Mater. Chem. Phys. 2018, 217, 82–89. [Google Scholar] [CrossRef]
- Nieminen, H.-E.; Miikkulainen, V.; Settipani, D.; Simonelli, L.; Hönicke, P.; Zech, C.; Kayser, Y.; Beckhoff, B.; Honkanen, A.-P.; Heikkilä, M.J.; et al. Intercalation of lithium ions from gaseous precursors into β-MnO2 thin films deposited by atomic layer deposition. J. Phys. Chem. C 2019, 123, 15802–15814. [Google Scholar] [CrossRef]
- Mattelaer, F.; Bosserez, T.; Ronge, J.; Martens, J.A.; Dendooven, J.; Detavernier, C. Manganese oxide films with controlled oxidation state for water splitting devices through a combination of atomic layer deposition and post-deposition annealing. RSC Adv. 2016, 6, 98337–98343. [Google Scholar] [CrossRef]
- Zhu, T.; He, Z.; Zhang, G.; Lu, Y.; Lin, C.; Chen, Y.; Guo, H. Effect of low magnetic fields on the morphology and electrochemical properties of MnO2 films on nickel foams. J. Alloys Compd. 2015, 644, 186–192. [Google Scholar] [CrossRef]
- Barreca, D.; Gri, F.; Gasparotto, A.; Altantzis, T.; Gombac, V.; Fornasiero, P.; Maccato, C. Insights into the plasma-assisted fabrication and nanoscopic investigation of tailored MnO2 nanomaterials. Inorg. Chem. 2018, 57, 14564–14573. [Google Scholar] [CrossRef] [PubMed]
- Regulski, M.; Przeniosło, R.; Sosnowska, I.; Hoffmann, J.U. Incommensurate magnetic structure of β-MnO2. Phys. Rev. B 2003, 68, 172401. [Google Scholar] [CrossRef]
- Ilton, E.S.; Droubay, T.C.; Chaka, A.M.; Kovarik, L.; Varga, T.; Arey, B.W.; Kerisit, S.N. Epitaxial single-crystal thin films of MnxTi1−xO2−δ grown on (rutile)TiO2 substrates with pulsed laser deposition: Experiment and theory. Surf. Sci. 2015, 632, 185–194. [Google Scholar] [CrossRef]
- Regulski, M.; Przeniosło, R.; Sosnowska, I.; Hoffmann, J.-U. Short and long range magnetic ordering in β-MnO2-A temperature study -. J. Phys. Soc. Jpn. 2004, 73, 3444–3447. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Jin, X.; Han, Y.; Lin, Y.; Lei, Z.; Wang, S.; Qin, L.; Jiao, S.; Cao, R. A hollow-structured manganese oxide cathode for stable Zn-MnO2 batteries. Nanomaterials 2018, 8, 301. [Google Scholar] [CrossRef]
- Carraro, G.; Gasparotto, A.; Maccato, C.; Bontempi, E.; Lebedev, O.I.; Turner, S.; Sada, C.; Depero, L.E.; Van Tendeloo, G.; Barreca, D. Fluorine doped Fe2O3 nanostructures by a one-pot plasma-assisted strategy. RSC Adv. 2013, 3, 23762–23768. [Google Scholar] [CrossRef]
- Gasparotto, A.; Barreca, D.; Bekermann, D.; Devi, A.; Fischer, R.A.; Fornasiero, P.; Gombac, V.; Lebedev, O.I.; Maccato, C.; Montini, T.; et al. F-doped Co3O4 photocatalysts for sustainable H2 generation from water/ethanol. J. Am. Chem. Soc. 2011, 133, 19362–19365. [Google Scholar] [CrossRef]
- Carraro, G.; Gasparotto, A.; Maccato, C.; Bontempi, E.; Lebedev, O.I.; Sada, C.; Turner, S.; Van Tendeloo, G.; Barreca, D. Rational synthesis of F-doped iron oxides on Al2O3(0001) single crystals. RSC Adv. 2014, 4, 52140–52146. [Google Scholar] [CrossRef]
- Bigiani, L.; Maccato, C.; Gasparotto, A.; Sada, C.; Barreca, D. Structure and properties of Mn3O4 thin films grown on single crystal substrates by chemical vapor deposition. Mater. Chem. Phys. 2019, 223, 591–596. [Google Scholar] [CrossRef]
- Yan, D.; Yan, P.X.; Cheng, S.; Chen, J.T.; Zhuo, R.F.; Feng, J.J.; Zhang, G.A. Fabrication, in-depth characterization, and formation mechanism of crystalline porous birnessite MnO2 film with amorphous bottom layers by hydrothermal method. Cryst. Growth Des. 2009, 9, 218–222. [Google Scholar] [CrossRef]
- Barreca, D.; Carraro, G.; Fois, E.; Gasparotto, A.; Gri, F.; Seraglia, R.; Wilken, M.; Venzo, A.; Devi, A.; Tabacchi, G.; et al. Manganese(II) molecular sources for plasma-assisted CVD of Mn oxides and fluorides: From precursors to growth process. J. Phys. Chem. C 2018, 122, 1367–1375. [Google Scholar] [CrossRef]
- Barreca, D.; Devi, A.; Fischer, R.A.; Bekermann, D.; Gasparotto, A.; Gavagnin, M.; Maccato, C.; Tondello, E.; Bontempi, E.; Depero, L.E.; et al. Strongly oriented Co3O4 thin films on MgO(100) and MgAl2O4(100) substrates by PE-CVD. CrystEngComm 2011, 13, 3670–3673. [Google Scholar] [CrossRef]
- Maccato, C.; Bigiani, L.; Carraro, G.; Gasparotto, A.; Seraglia, R.; Kim, J.; Devi, A.; Tabacchi, G.; Fois, E.; Pace, G.; et al. Molecular engineering of MnII diamine diketonate precursors for the vapor deposition of manganese oxide nanostructures. Chem. Eur. J. 2017, 23, 17954–17963. [Google Scholar] [CrossRef]
- Hu, S.; Cazorla, C.; Xiang, F.; Ma, H.; Wang, J.; Wang, J.; Wang, X.; Ulrich, C.; Chen, L.; Seidel, J. Strain control of giant magnetic anisotropy in metallic perovskite SrCoO3−δ thin films. ACS Appl. Mater. Interfaces 2018, 10, 22348–22355. [Google Scholar] [CrossRef]
- Carraro, G.; Peeters, D.; Gasparotto, A.; Maccato, C.; Bontempi, E.; Barreca, D. Fe2O3 nanostructures on SrTiO3(111) by chemical vapor deposition: Growth and characterization. Mater. Lett. 2014, 136, 141–145. [Google Scholar] [CrossRef]
- Rastei, M.V.; Pierron-Bohnes, V.; Toulemon, D.; Bouillet, C.; Kákay, A.; Hertel, R.; Tetsi, E.; Begin-Colin, S.; Pichon, B.P. Defect-driven magnetization configuration of isolated linear assemblies of iron oxide nanoparticles. Adv. Funct. Mater. 2019, 29, 1903927. [Google Scholar] [CrossRef]
- Krivcov, A.; Ehrler, J.; Fuhrmann, M.; Junkers, T.; Möbius, H. Influence of dielectric layer thickness and roughness on topographic effects in magnetic force microscopy. Beilstein J. Nanotechnol. 2019, 10, 1056–1064. [Google Scholar] [CrossRef]
- Available online: https://xpspeak.software.informer.com/4.1/ (accessed on 20 February 2020).
- Available online: http://imagej.nih.gov/ij/ (accessed on 2 April 2020).
- Sarilmaz, A.; Ozel, F.; Aljabour, A.; Rauf Khaskheli, A.; Kus, M. Effect of doping on thin film solar cell efficiency based on ZnMn2O4 nanocrystals. Mater. Today Proc. 2019, 18, 1861–1867. [Google Scholar] [CrossRef]
- JCPDS card No. 024-0735 (2000) (accessed on 29 April 2020).
- Divagar, M.; Sriramprabha, R.; Ponpandian, N.; Viswanathan, C. Highly selective and sensitive electrochemical detection of dopamine with hydrothermally prepared β-MnO2 nanostructures. Mater. Sci. Semicond. Process. 2018, 83, 216–223. [Google Scholar] [CrossRef]
- Quiroz, H.P.; Galíndez, E.F.; Dussan, A. Ferromagnetic-like behavior of Co doped TiO2 flexible thin films fabricated via co-sputtering for spintronic applications. Heliyon 2020, 6, e03338. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, H.P.; Dussan, A. Synthesis temperature dependence on magnetic properties of cobalt doped TiO2 thin films for spintronic applications. Appl. Surf. Sci. 2019, 484, 688–691. [Google Scholar] [CrossRef]
- Tiberto, P.; Barrera, G.; Celegato, F.; Coisson, M.; Olivetti, E.S.; Vinai, F. Microstructural evolution and magnetic properties in Fe50Pd50 sputtered thin films submitted to post-deposition annealing. J. Alloys Compd. 2014, 615, S236–S241. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigiani, L.; Maccato, C.; Gasparotto, A.; Sada, C.; Bontempi, E.; Barreca, D. Plasma-Assisted Chemical Vapor Deposition of F-Doped MnO2 Nanostructures on Single Crystal Substrates. Nanomaterials 2020, 10, 1335. https://doi.org/10.3390/nano10071335
Bigiani L, Maccato C, Gasparotto A, Sada C, Bontempi E, Barreca D. Plasma-Assisted Chemical Vapor Deposition of F-Doped MnO2 Nanostructures on Single Crystal Substrates. Nanomaterials. 2020; 10(7):1335. https://doi.org/10.3390/nano10071335
Chicago/Turabian StyleBigiani, Lorenzo, Chiara Maccato, Alberto Gasparotto, Cinzia Sada, Elza Bontempi, and Davide Barreca. 2020. "Plasma-Assisted Chemical Vapor Deposition of F-Doped MnO2 Nanostructures on Single Crystal Substrates" Nanomaterials 10, no. 7: 1335. https://doi.org/10.3390/nano10071335
APA StyleBigiani, L., Maccato, C., Gasparotto, A., Sada, C., Bontempi, E., & Barreca, D. (2020). Plasma-Assisted Chemical Vapor Deposition of F-Doped MnO2 Nanostructures on Single Crystal Substrates. Nanomaterials, 10(7), 1335. https://doi.org/10.3390/nano10071335