Magneto-Optical Nanostructures for Viral Sensing
Abstract
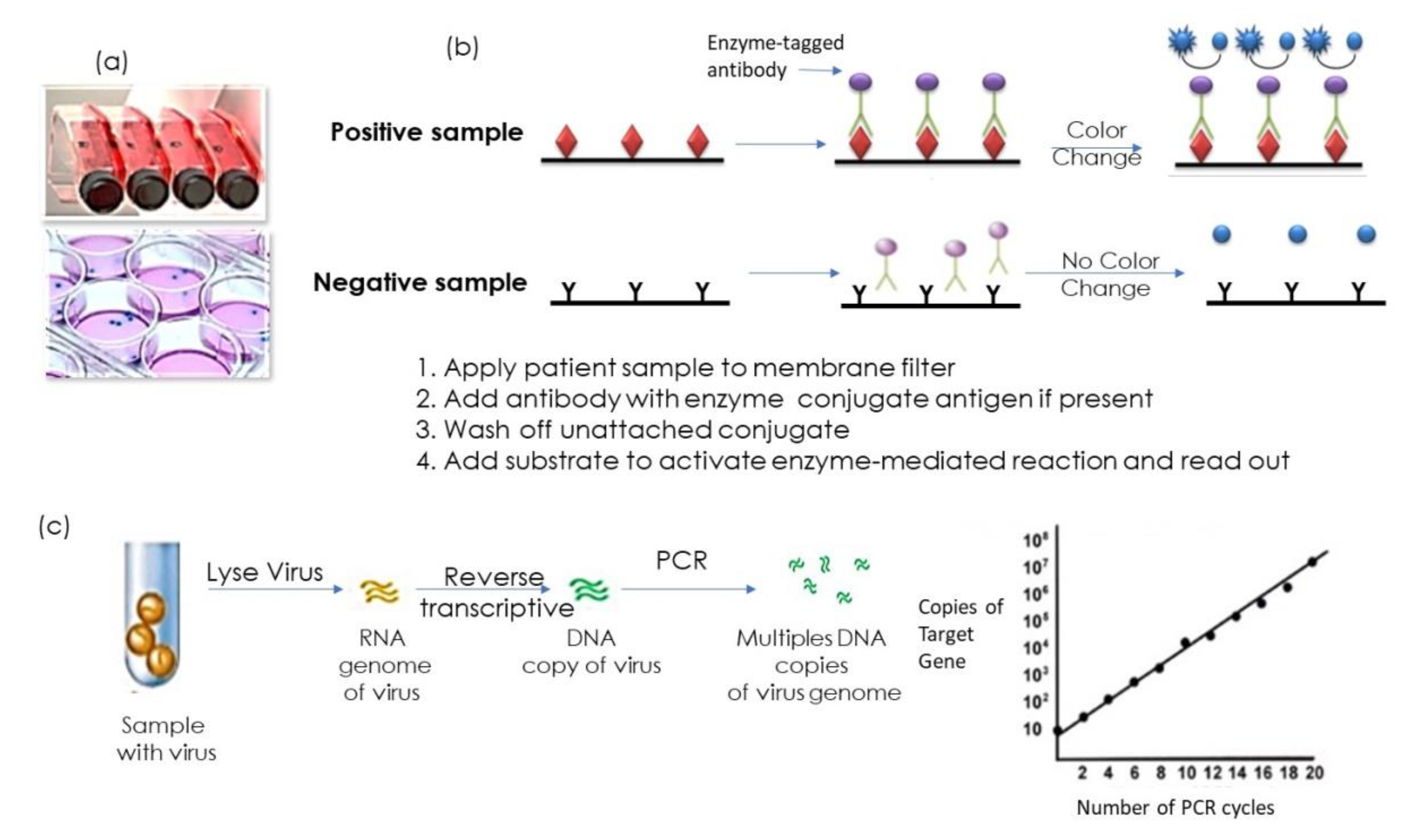
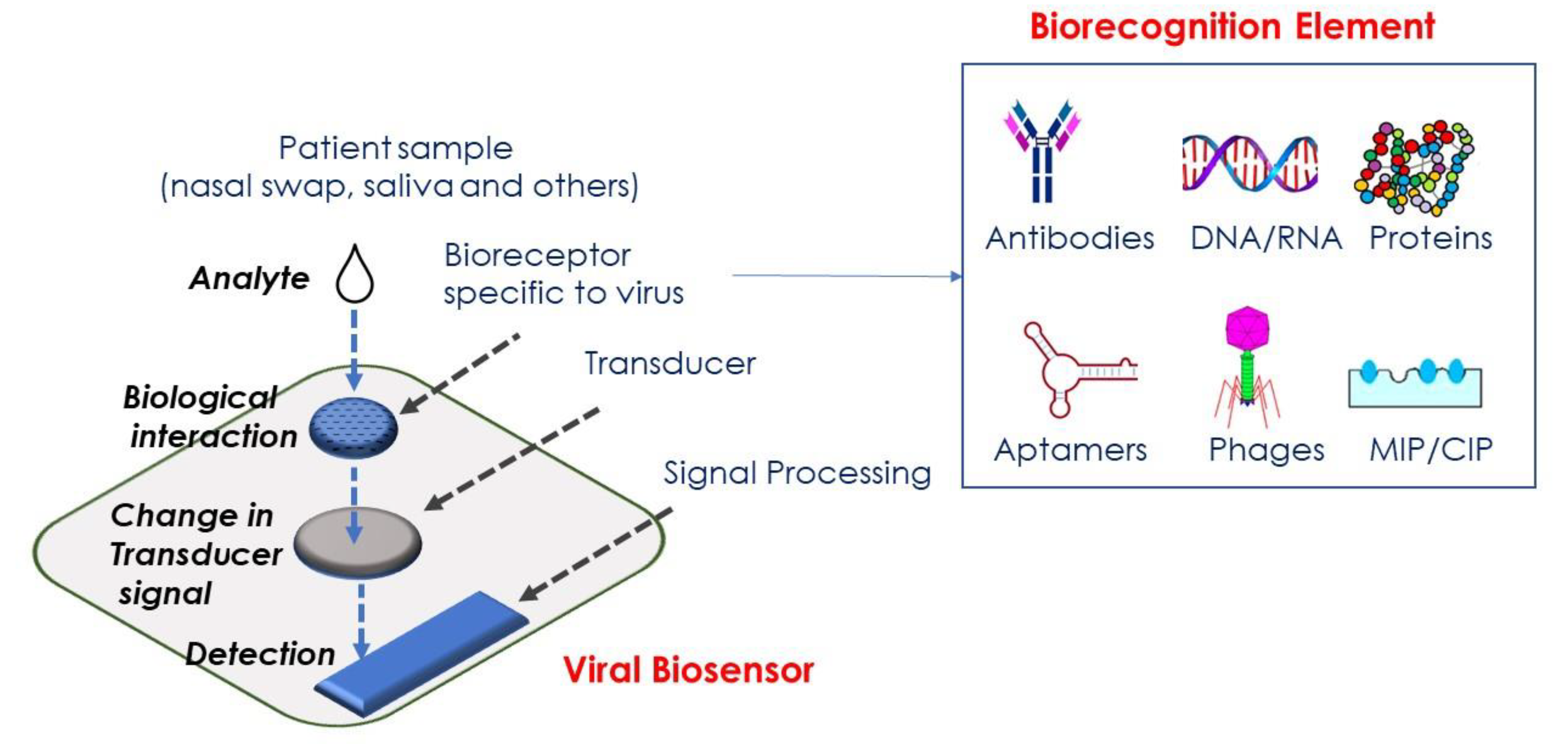
:1. Introduction
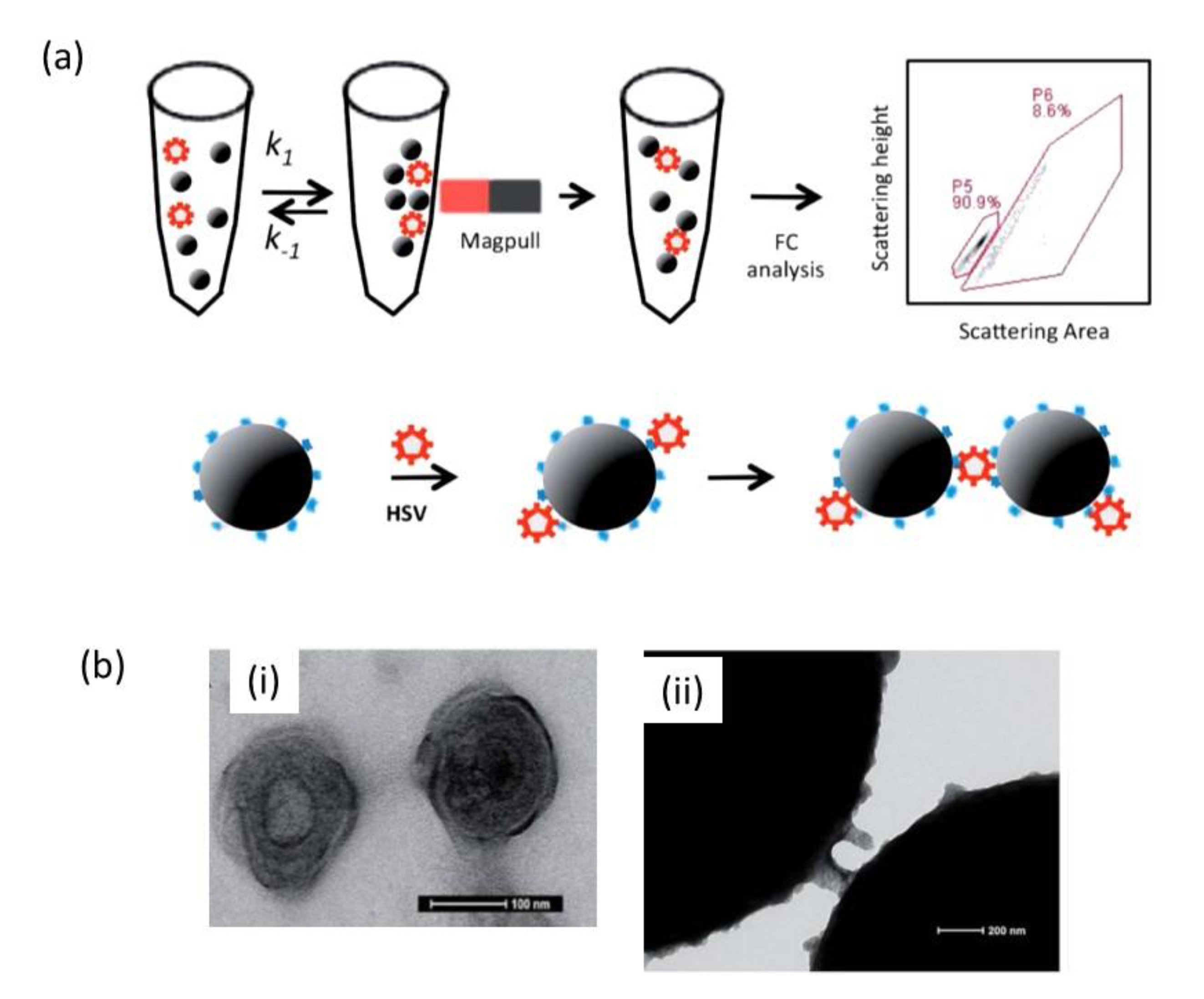
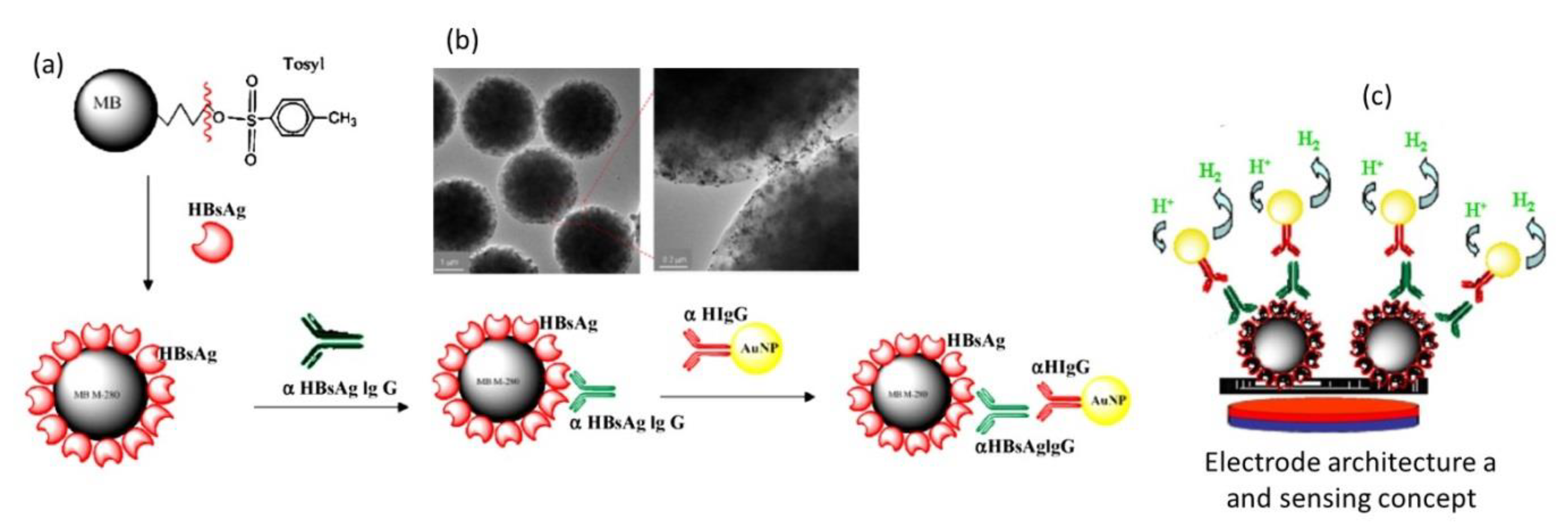
2. Magnetic Nanoparticles for Viral Sensing
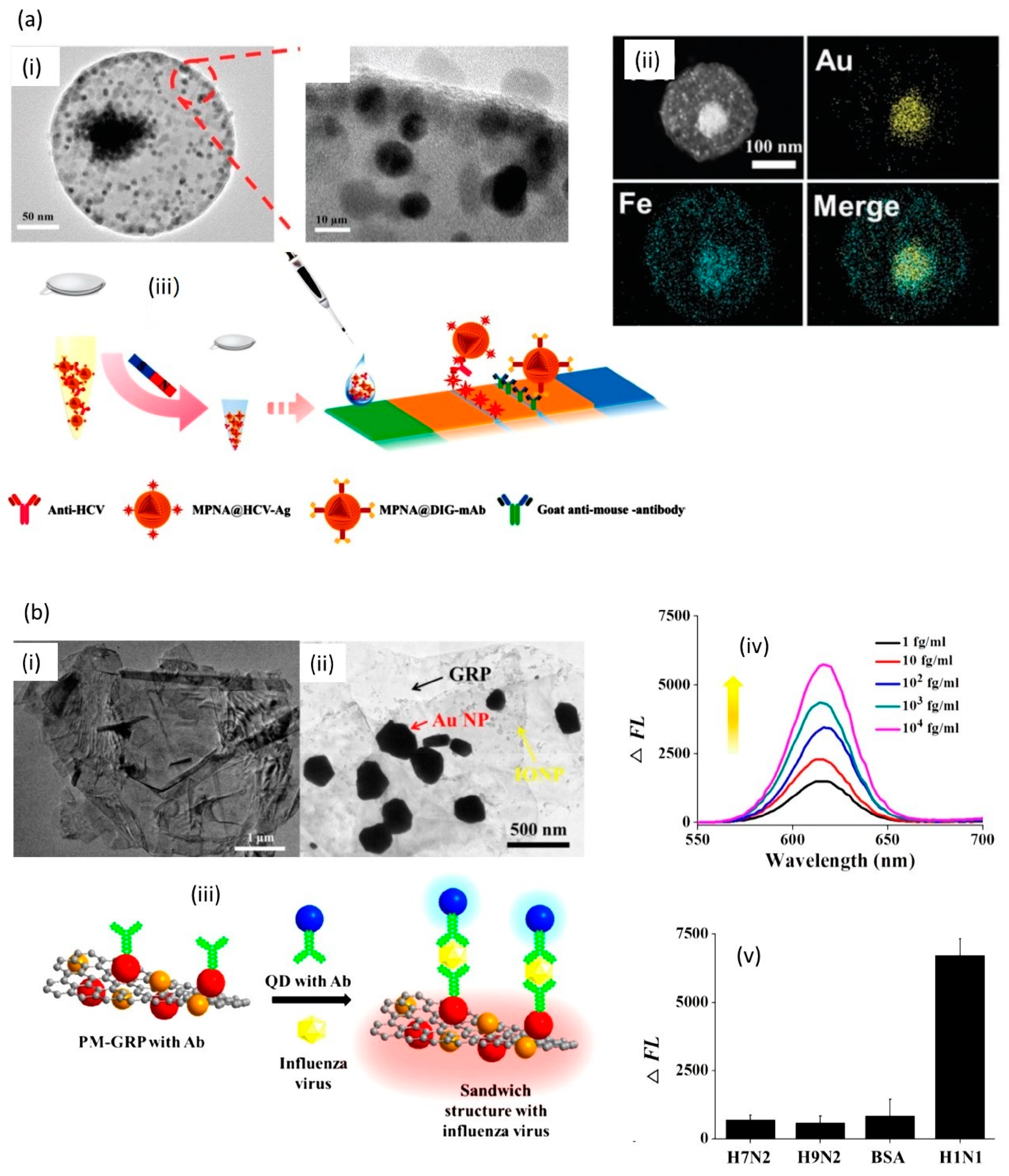
3. Magneto-Plasmonic Nanoparticles for Sensing
4. Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kievits, T.; van Gemen, B.; van Strijp, D.; Schukkink, R.; Dircks, M.; Adriaanse, H.; Malek, L.; Sooknanan, R.; Lens, P. NASBA Isothermal Enzymatic in Vitro Nucleic Acid Amplification Optimized for the Diagnosis of HIV-1 Infection. J. Virol. Methods 1991, 35, 273–286. [Google Scholar] [CrossRef]
- Voller, A.; Bartlett, A.; Bidwell, D.E.; Clark, M.F.; Adams, A.N. The Detection of Viruses by Enzyme-Linked Immunosorbent Assay (ELISA) Free. J. Gen. Virol. 1976, 33. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; Emergy, L.; Munday, P.E.; Moulsdale, M.; Brown, D.W.G. A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. J. Med. Virol. 1998, 55, 177–183. [Google Scholar] [CrossRef]
- Verano, L.; Michalski, F.J. Comparison of a direct antigen enzyme immunoassay, Herpchek, with cell culture for detection of herpes simplex virus from clinical specimens. J. Clin. Microbiol. 1995, 33, 1378–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, M.; Reynolds, K.S.; Aldous, W.; Hickman, M. Comparison of Light-Cycler PCR, Enzyme Immunoassay, and Tissue Culture for Detection of Herpes Simplex Virus. Diag. Microbiol. Infect. Dis. 2001, 40, 107. [Google Scholar] [CrossRef]
- Watzinger, F.; Ebner, K.; Lion, T. Detection and monitoring of virus infections by real-time PCR. Mol. Asp. Med. 2006, 27, 254–298. [Google Scholar] [CrossRef]
- Steininger, C.; Kundi, M.; Aberle, S.W.; Aberle, J.H.; Popow-Kraupp, T. Effectiveness of Reverse Transcription-PCR, Virus Isolation, and Enzyme-Linked Immunosorbent Assay for Diagnosis of Influenza A Virus Infection in Different Age Groups. J. Clin. Microbiol. 2002, 40, 2051–2056. [Google Scholar] [CrossRef] [Green Version]
- Caygill, R.L.; Blair, G.E.; Millner, P.A. A review on viral biosensors to detect human pathogens. Anal. Chim. Acta 2010, 681, 8–15. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Takemura, K.; Park, E.Y. Plasmonic/magnetic graphene-based magnetofluoro-immunosensing platform for virus detection. Sens. Actuators B 2018, 276, 254–261. [Google Scholar] [CrossRef]
- de la Escosura-Muñiz, A.; Maltez-da Costa, M.; Sánchez-Espinel, C.; Díaz-Freitas, B.; Fernández-Suarez, J.; González-Fernández, Á.; Merkoçi, A. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosens. Bioelectron. 2010, 26, 1710–1714. [Google Scholar] [CrossRef] [PubMed]
- Chekin, F.; Bagg, K.; Subramanian, P.; Jijie, R.; Singh, S.K.; Kurungot, S.; Boukherroub, R.; Szunerits, S. Nucleic aptamer modified porous reduced graphene oxide/MoS2 based electrodes for viral detection: Application to human papillomavirus (HPV). Sens. Actuators B 2018, 262, 991–1000. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detectionc. ACS Nano 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babamiri, B.; Salimi, A.; Hallaj, R. A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens. Bioelectron. 2018, 117, 332–339. [Google Scholar] [CrossRef]
- Birnbaumer, G.M.; Lieberzeit, P.A.; Richter, L.; Schirhagl, R.; Milnera, M.; Dickert, F.L.; Bailey, A.; Ertl, P. Detection of viruses with molecularly imprinted polymers integrated on a microfluidic biochip using contact-less dielectric microsensors. Lab. Chip 2009, 9, 3549–3556. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.-F.; Fields, C.; Muzard, J.; Liauchuk, V.; Carr, M.; Hall, W.; Lee, G.U. Rapid, Highly Sensitive Detection of Herpes Simplex virus-1 Using Multiple Antigenic Peptide-Coated Superparamagnetic Beads. Analyst 2014, 139, 6126–6134. [Google Scholar] [CrossRef] [Green Version]
- Hassen, W.M.; Chaix, C.; Abdelghani, A.; Bessueille, F.; Leonard, D.; Jaffrezic-Renault, N. An impedimetric DNA sensor based on functionalized magnetic nanoparticles for HIV and HBV detection. Sens. Actuators B 2008, 134, 755–760. [Google Scholar] [CrossRef]
- Ilkhani, H.; Farhad, S. A novel electrochemical DNA biosensor for Ebola virus detection. Anal. Biochem. 2018, 557, 151–155. [Google Scholar] [CrossRef]
- Kaushik, A.; Yndart, A.; Kumar, S.; Jayant, R.D.; Vashist, A.; Brown, A.N.; Li, C.-Z.; Nair, M. A sensitive electrochemical immunosensor for label-free detection of Zika-virus protein. Sci. Rep. 2018, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Morita, M.; Takemura, K.; Park, E.Y. A multi-functional gold/iron-oxide nanoparticle-CNT hybrid nanomaterial as virus DNA sensing platform. Biosens. Bioelectron. 2018, 102, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.D.; Van Hieu, N.; Chien, N.D.; Le, A.-T.; Tuan, M.A. DNA sensor development based on multi-wall carbon nanotubes for label-free influenza virus (type A) detection. J. Immunol. Methods 2009, 350, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Toh, C.-S. Impedimetric DNA biosensor based on a nanoporous alumina membrane for the detection of the specific oligonucleotide sequence of dengue virus. Sensors 2013, 13, 7774–7785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiee, H.; Lidstone, E.A.; Jahangir, M.; Inci, F.; Hanhauser, E.; Henrich, T.J.; Kuritzkes, D.R.; Cunningham, B.T.; Demirci, U. Nanostructured optical photonic crystal biosensor for HIV viral load measurement. Sci. Rep. 2014, 4, 4116. [Google Scholar] [CrossRef] [Green Version]
- Zengin, A.; Tamer, U.; Caykara, T. SERS detection of hepatitis B virus DNA in a temperature-responsive sandwich-hybridization assay. J. Raman Spectrosc. 2017, 48, 668–672. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.; Tran, V.T.; Lee, D.K.; Ahmed, S.R.; Hong, J.C.; Lee, J.; Park, E.Y.; Lee, J. Magnetic nanozyme-linked immunosorbent assay for ultrasensitive influenza A virus detection. ACS Appl. Mater. Interfaces 2018, 10, 12534–12543. [Google Scholar] [CrossRef]
- Cai, H.; Parks, J.; Wall, T.; Stott, M.; Stambaugh, A.; Alfson, K.; Griffiths, A.; Mathies, R.; Carrion, R.; Patterson, J. Optofluidic analysis system for amplification-free, direct detection of Ebola infection. Sci. Rep. 2015, 5, 14494. [Google Scholar] [CrossRef]
- Song, J.; Mauk, M.G.; Hackett, B.A.; Cherry, S.; Bau, H.H.; Liu, C. Instrument-free point-of-care molecular detection of Zika virus. Anal. Chem. 2016, 88, 7289–7294. [Google Scholar] [CrossRef] [Green Version]
- Ashiba, H.; Sugiyama, Y.; Wang, X.; Shirato, H.; Higo-Moriguchi, K.; Taniguchi, K.; Ohki, Y.; Fujimaki, M. Detection of norovirus virus-like particles using a surface plasmon resonance-assisted fluoroimmunosensor optimized for quantum dot fluorescent labels. Biosens. Bioelectron. 2017, 93, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Rong, Z.; Wang, J.; Xiao, R.; Wang, S. A fluorescent aptasensor for H5N1 influenza virus detection based-on the core–shell nanoparticles metal-enhanced fluorescence (MEF). Biosens. Bioelectron. 2015, 66, 527–532. [Google Scholar] [CrossRef]
- Jahanshahi, P.; Zalnezhad, E.; Sekaran, S.D.; Adikan, F.R.M. Rapid immunoglobulin M-based dengue diagnostic test using surface plasmon resonance biosensor. Sci. Rep. 2014, 4, 3851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inan, H.; Wang, S.; Inci, F.; Baday, M.; Zangar, R.; Kesiraju, S.; Anderson, K.S.; Cunningham, B.T.; Demirci, U. Isolation, detection, and quantification of cancer biomarkers in HPV-associated malignancies. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusoff, A.H.; Salimi, M.N.; Jamlos, M.F. A review: Synthetic strategy control of magnetite nanoparticles production. Adv. Nano. Res. 2018, 6, 1. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Mazur, M.; Barras, A.; Kuncser, V.; Galatanu, A.; Zaitzev, V.; Turcheniuk, K.V.; Woisel, P.; Lyskawa, J.; Laure, W.; Siriwardena, A.; et al. Iron oxide magnetic nanoparticles with versatile surface functions based on dopamine anchors. Nanoscale 2013, 5, 2692–2702. [Google Scholar] [CrossRef]
- Muzard, J.; Platt, M.; Lee, G.U. M13 Bacteriophage-Activated Superparamagnetic Beads for Affinity Separation. Small 2012, 8, 2403–2411. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.M.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef] [Green Version]
- Tada, D.B.; Vono, L.L.R.; Duarte, E.L.; Itri, R.; Kiyohara, P.K.; Baptista, M.S.; Rossi, L.M. Methylene Blue-Containing Silica-Coated Magnetic Particles: A Potential Magnetic Carrier for Photodynamic Therapy. Langmuir 2007, 23, 8194–8199. [Google Scholar] [CrossRef]
- Chang, W.-S. Rapid detection of dengue virus in serum using magnetic separation and fluorescence detection. Analyst 2008, 133, 233. [Google Scholar] [CrossRef]
- Perez, J.M.; Simeone, F.J.; Saeki, Y.; Josephson, L.; Weissleder, R. Viral-Induced Self-Assembly of Magnetic Nanoparticles Allows the Detection of Viral Particles in Biological Media. J. Am. Chem. Soc. 2003, 125, 34. [Google Scholar] [CrossRef]
- Armijos-Jaramillo, V.; Yeager, J.; Muslin, C.; Perez-Castillo, Y. SARS-CoV-2, an evolutionary perspective of interaction with human ACE2 reveals undiscovered amino acids necessary for complex stability. Evol. Appl. 2020. [Google Scholar] [CrossRef] [Green Version]
- Norway turns silica-coated iron oxide nanoparticles insto 150.000 COVID-19 tests per week. Available online: https://statnano.com/news/67573/Norway-Turns-Silica-coated-Iron-Oxide-Nanoparticles-into-150-000-COVID-19-Tests-Per-Week (accessed on 1 June 2020).
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2011, 34, 257. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Sensing using localised surface plasmon resonance sensors. Chem. Commun. 2012, 48, 8999–9010. [Google Scholar] [CrossRef]
- Goon, I.Y.; Lai, L.M.; Lim, M.; Amal, R.; Gooding, J.J. ‘Dispersible Electrodes’: A Solution to Slow Response Times of Sensitive Sensors. Chem. Commun. 2010, 46, 8821–8823. [Google Scholar] [CrossRef]
- Takemura, K.; Lee, J.; Suzuki, T.; Hara, T.; Abe, F.; Park, E.Y. Ultrasensitive detection of norovirus using a magnetofluoroimmunoassay based on synergic properties of gold/magnetic nanoparticle hybrid nanocomposites and quantum dots. Sens. Actuators B 2019, 296, 126672. [Google Scholar] [CrossRef]
- Chuah, K.; Leao, M.H.; Goon, I.Y.; Parker, S.G.; Amal, R.; Gooding, J.J. Ultrasensitive electrochemical detection of prostate-specific antigen (PSA) using gold-coated magnetic nanoparticles as ‘dispersible electrodes’. Chem. Commun. 2012, 48, 3503. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, B.C.; Kim, Y.J.; Lee, J.H.; Koo, T.M.; Ko, M.J.; Kim, Y.K. Design of Magnetic-Plasmonic Nanoparticle Assemblies via Interface Engineering of Plasmonic Shells for Targeted Cancer Cell Imaging and Separation. Small 2020. [Google Scholar] [CrossRef] [PubMed]
- Mo, A.H.; Landon, P.B.; Gomez, K.S.; Kang, H.; Lee, J.; Zhang, C.; Janetanakit, W.; Sant, V.; Lu, T.; Colburn, D.A. Magnetically-responsive silica–gold nanobowls for targeted delivery and SERS-based sensing. Nanoscale 2016, 8, 11840–11850. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, M.; Cuya Huaman, J.L.; Matsumoto, T.; Suzuki, K.; Miyamura, H.; Balachandran, J. Magneto-Plasmonic Co@ Pt@ Au Nanocrystals for Biosensing and Therapeutics. ACS Appl. Nano Mater. 2019. [Google Scholar] [CrossRef]
- Hao, L.; Leng, Y.; Zeng, L.; Chen, X.; Chen, J.; Duan, H.; Huang, X.; Xiong, Y.; Chen, X. Core–Shell-Heterostructured Magnetic–Plasmonic Nanoassemblies with Highly Retained Magnetic–Plasmonic Activities for Ultrasensitive Bioanalysis in Complex Matrix. Adv. Sci. 2020, 7, 1902433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touahir, L.; GAlopin, E.; Boukherroub, R.; Gouget-Laemmel, A.C.; Chazalviel, J.-N.; Ozanam, F.; Szunerits, S. Localized surface plasmon-enhanced fluorescence spectroscopy for highly-sensitive real-time detection of DNA hybridization. Biosens. Bioelectron. 2010, 25, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, O.; Park, E.Y. Bright Luminescent Optically Engineered Core/Alloyed Shell Quantum Dots: An Ultrasensitive Signal Transducer for Dengue Virus RNA via Localized Surface Plasmon Resonance-Induced Hairpin Hybridization. J. Mater. Chem. B 2017, 28, 3047–3058. [Google Scholar] [CrossRef] [PubMed]
- Mashhadizadeh, M.H.; Talemi, R.P. Synergistic effect of magnetite and gold nanoparticles onto the response of a label-free impedimetric hepatitis B virus DNA biosensor. Mater. Sci. Eng. C 2016, 59, 773–781. [Google Scholar] [CrossRef]
- Hong, W.-E.; Hsu, I.-L.; Huang, S.-Y.; Lee, C.-W.; Ko, H.; Tsai, P.-J.; Shieh, D.-B.; Huang, C.-C. Assembled growth of 3D Fe 3 O 4@ Au nanoparticles for efficient photothermal ablation and SERS detection of microorganisms. J. Mater. Chem. B 2018, 6, 5689–5697. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Wang, C.; Rong, Z.; Ding, H.; Li, H.; Li, S.; Shao, N.; Dong, P.; Xiao, R. Facile synthesis of Au-coated magnetic nanoparticles and their application in bacteria detection via a SERS method. ACS Appl. Mater. Interfaces 2016, 8, 19958–19967. [Google Scholar] [CrossRef]
- Pang, Y.; Wan, N.; Shi, L.; Wang, C.; Sun, Z.; Xiao, R.; Wang, S. Dual-recognition surface-enhanced Raman scattering (SERS) biosensor for pathogenic bacteria detection by using vancomycin-SERS tags and aptamer-Fe3O4@ Au. Anal. Chim. Acta 2019, 1077, 288–296. [Google Scholar] [CrossRef]
- Zou, F.; Wang, X.; Qi, F.; Koh, K.; Lee, J.; Zhou, H.; Chen, H. Magneto-plamonic nanoparticles enhanced surface plasmon resonance TB sensor based on recombinant gold binding antibody. Sens. Actuators B 2017, 250, 356–363. [Google Scholar] [CrossRef]
- Rawal, R.; Chawla, S.; Pundir, C.S. An electrochemical sulfite biosensor based on gold coated magnetic nanoparticles modified gold electrode. Biosens. Bioelectron. 2012, 31, 144–150. [Google Scholar] [CrossRef]
- Pham, T.T.-H.; Sim, S.J. Electrochemical analysis of gold-coated magnetic nanoparticles for detecting immunological interaction. J. Nanopart. Res. 2010, 12, 227–235. [Google Scholar] [CrossRef]
| Sensor Type | Virus | Surface Ligand | LoD 1 | Linear Range | Ref. |
|---|---|---|---|---|---|
| Electrochemical | HIV-1 | MIP of HIV aptamer | 0.3 fM | 3 fM–0.3 nM | [15] |
| Electrochemical | HIV, HBV | streptavidin | 50 pM | 2.53–50.60 nM/mL | [18] |
| Electrochemical | Ebola | biotinylated-DNA | 4.7 nM | - | [19] |
| Electrochemical | Zika | envelope protein antibody | <10 pM | 10 pM–1 nM | [20] |
| Electrochemical | Norovirus | DNA | 8.8 pM | 1 pM–10 nM | [21] |
| Electrochemical | Influenza A | DNA | 0.5 nM | 1–10 nM | [22] |
| Electrochemical | Dengue | DNA | 2.7 pM | 10 pM–10 µM | [23] |
| Electrochemical | HPV 16E7 | RNA aptamer | 0.1 ng/mL | 0.2–2 ng/mL | [13] |
| Electrochemical | HRV | MIP | - | 3 µg/mL–3 g/mL | [16] |
| Electrical | SARS-CoV-2 | antibody | 2.42 × 102 copies/mL | 102–104 copies/mL | [10] |
| Optical | HIV-1 | Glycoprotein-120 antibody | 105 copies/mL | 104–108 copies/mL | [24] |
| Optical | HVC | DNA stand | 0.1 fM | 0.1 fM–6 µM | [25] |
| Optical | Influenza A | anti-HA | 5 pg/mL | 5 ag/mL–5 µg/mL | [26] |
| Optical | Ebola | DNA | 0.2 pfu/mL | 0.21–1.05 × 10−5 pfu/mL | [27] |
| Optical | Zika | envelope protein | 5 pfu/mL | 5–500 pfu/mL | [28] |
| Optical | Norovirus | anti-norovirus antibody | 0.01 ng/mL | 0.01–100 ng/mL | [29] |
| Optical | Influenza H5N1 | aptamer | 3.5 ng/mL | 2–200 ng/mL | [30] |
| Optical | Dengue | antigen | / | / | [31] |
| Optical | HPV 16E7 | anti-HPV 16E7 | 2.87 ng/mL | 0.021–15 ng/mL | [32] |
| Sensor Type | Analyte | Particle Type | Limit of Detection | Linear Range | Ref. |
|---|---|---|---|---|---|
| Optical (fluorescence) | Norovirus | Au/Fe3O4 and CdSeS QDs | 0.48 pg/mL | 1 pg/mL–5 ng/mL | [46] |
| Optical | HCV | Au/Fe3O4 | 0.24 pg/mL | 0.24–120 pg/mL | [51] |
| Electrochemistry | HBV | Fe3O4 and Au NPs | - | - | [12] |
| Optical (fluorescence) | Influenza H1N1 | Au/Fe3O4 decorated graphene | 7.27 fg/mL | 10–104 fg/mL | [11] |
| Electrochemistry | HBV | Fe3O4 and Au NPs | 83 pM | 8 3 pM–64 µM | [54] |
| SERS | E. Coli | Au/Fe3O4 | 105 cfu/mL | / | [55] |
| SERS | S. Aureus | MnFe2O4@Au | 10 cfu/mL | 101–105 cfu/mL | [56] |
| SERS | S. Aureus | Au/Fe3O4 | 3 cfu/mL | 10–107 cfu/mL | [57] |
| SPR | Tuberculosis | Au/Fe3O4 | 0.1 ng/mL | 0.1–100 ng/mL | [58] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szunerits, S.; Nait Saada, T.; Meziane, D.; Boukherroub, R. Magneto-Optical Nanostructures for Viral Sensing. Nanomaterials 2020, 10, 1271. https://doi.org/10.3390/nano10071271
Szunerits S, Nait Saada T, Meziane D, Boukherroub R. Magneto-Optical Nanostructures for Viral Sensing. Nanomaterials. 2020; 10(7):1271. https://doi.org/10.3390/nano10071271
Chicago/Turabian StyleSzunerits, Sabine, Tamazouzt Nait Saada, Dalila Meziane, and Rabah Boukherroub. 2020. "Magneto-Optical Nanostructures for Viral Sensing" Nanomaterials 10, no. 7: 1271. https://doi.org/10.3390/nano10071271
APA StyleSzunerits, S., Nait Saada, T., Meziane, D., & Boukherroub, R. (2020). Magneto-Optical Nanostructures for Viral Sensing. Nanomaterials, 10(7), 1271. https://doi.org/10.3390/nano10071271







