Enhancing the Performance of Aqueous Solution-Processed Cu2ZnSn(S,Se)4 Photovoltaic Materials by Mn2+ Substitution
Abstract
1. Introduction
2. Experiments
2.1. CMZTSSe Thin Film Preparation
2.2. Solar Cell Fabrication
2.3. Thin Film and Device Characterization
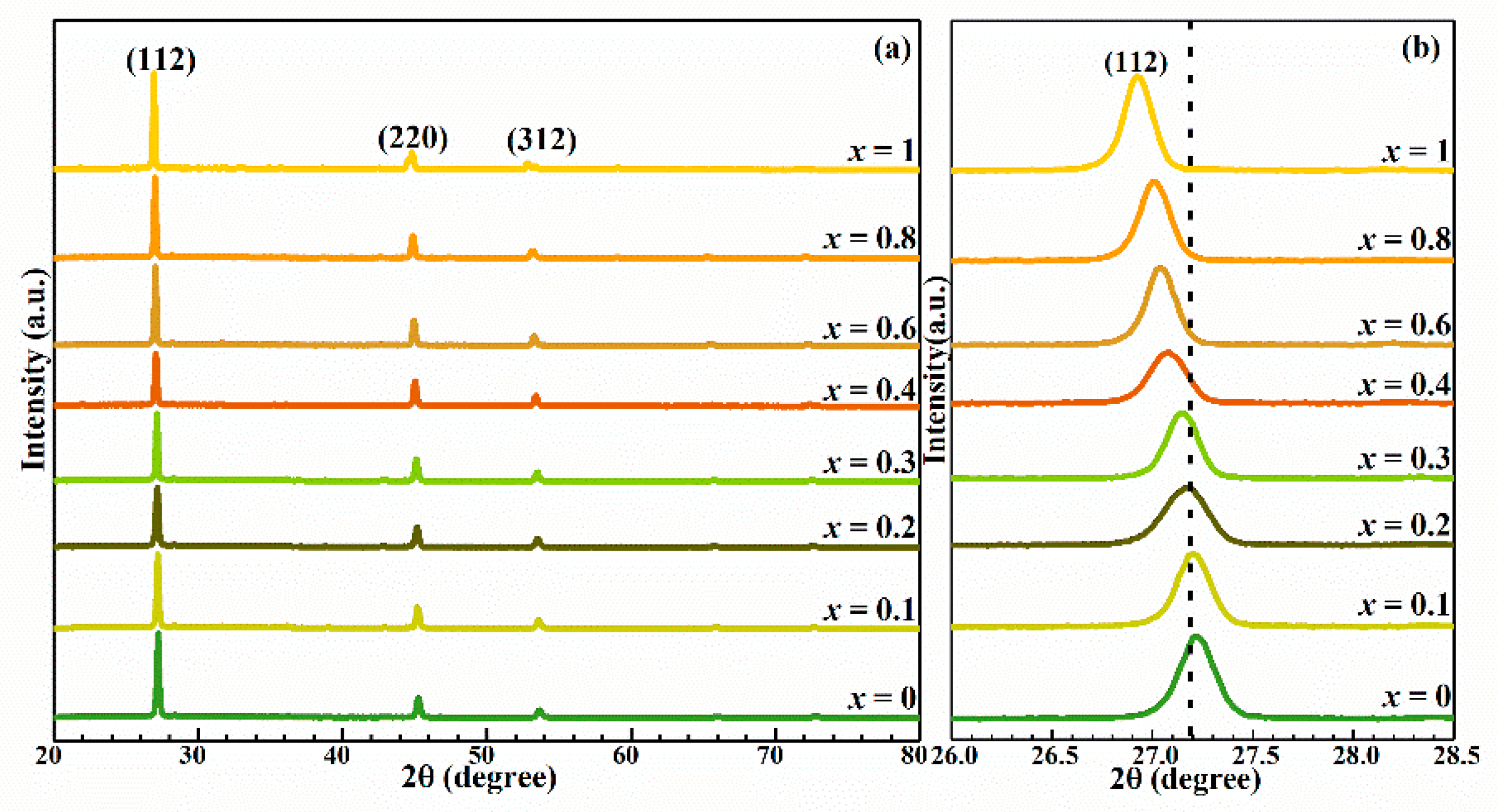
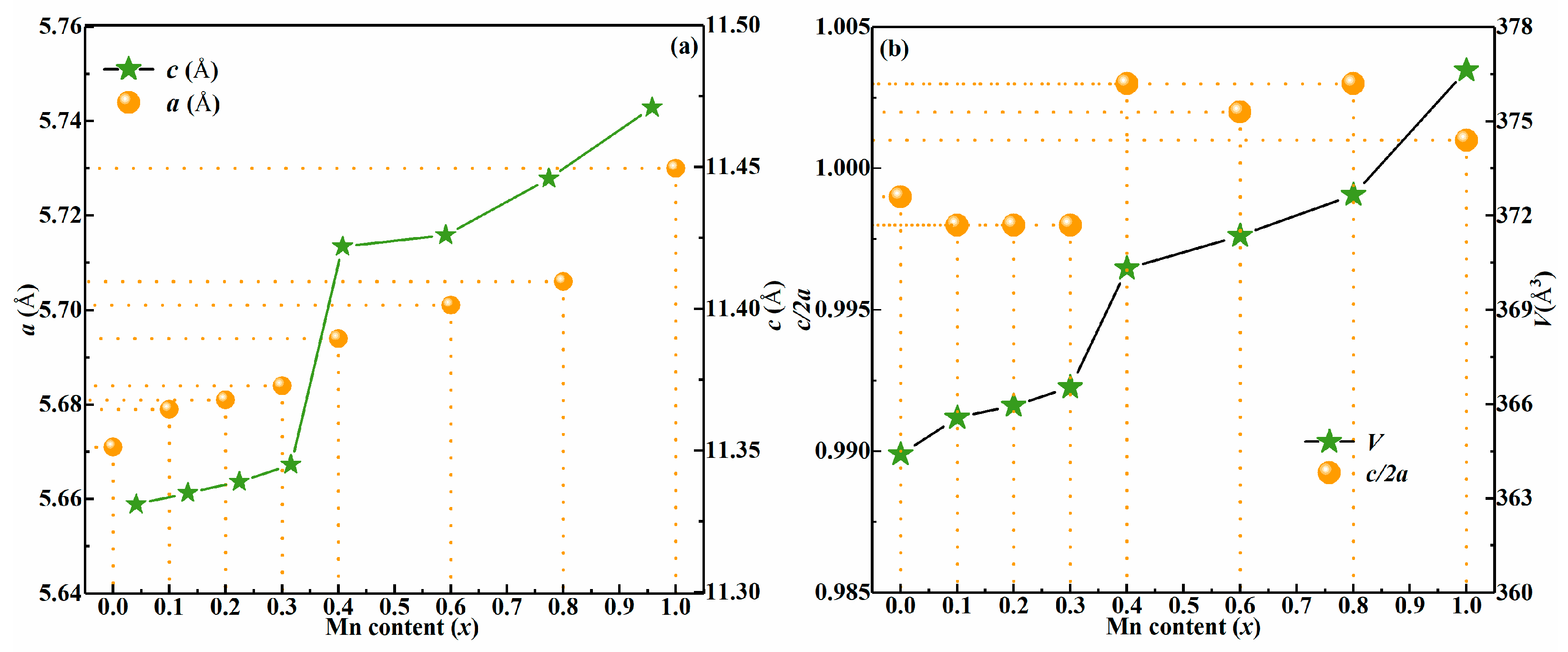
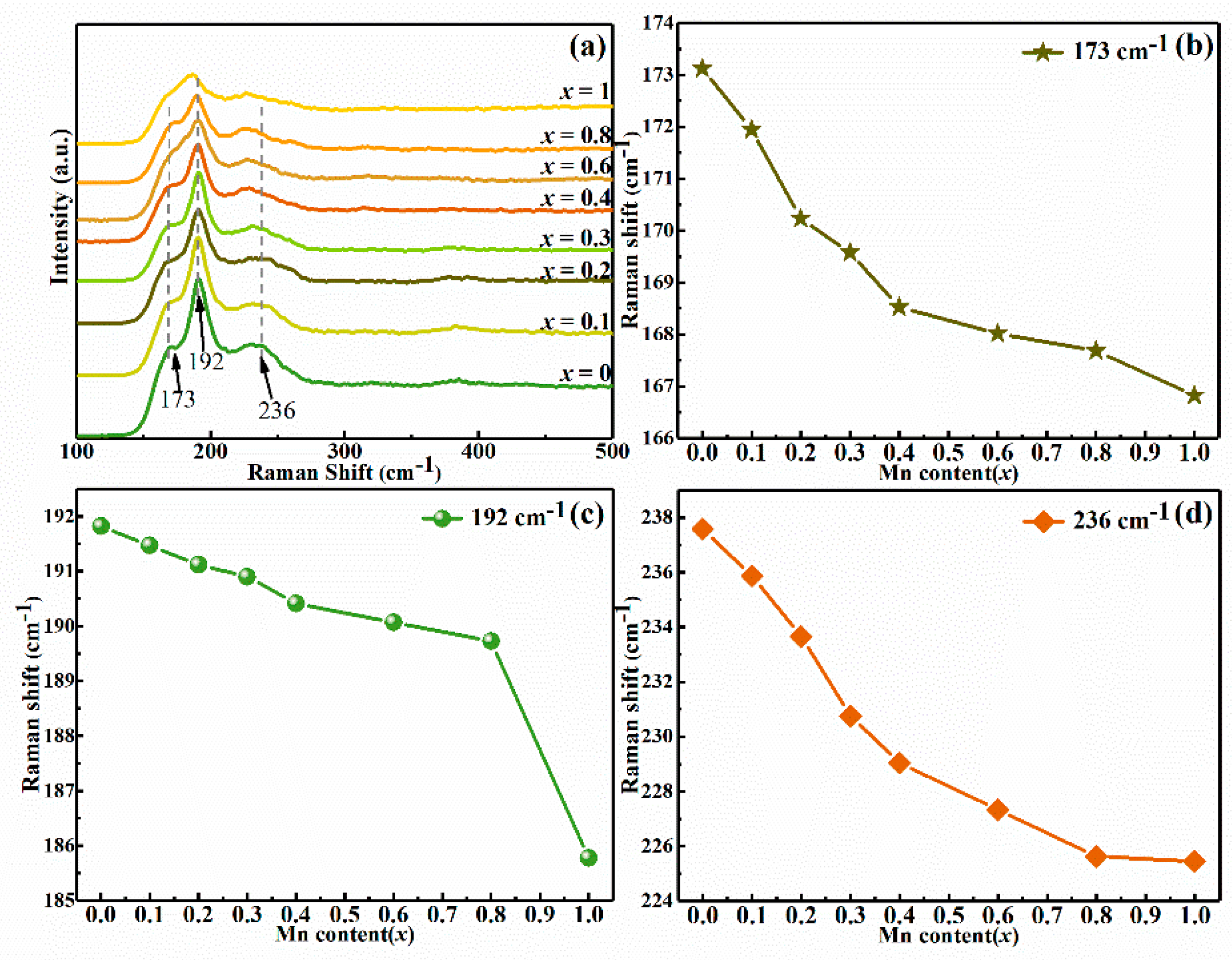
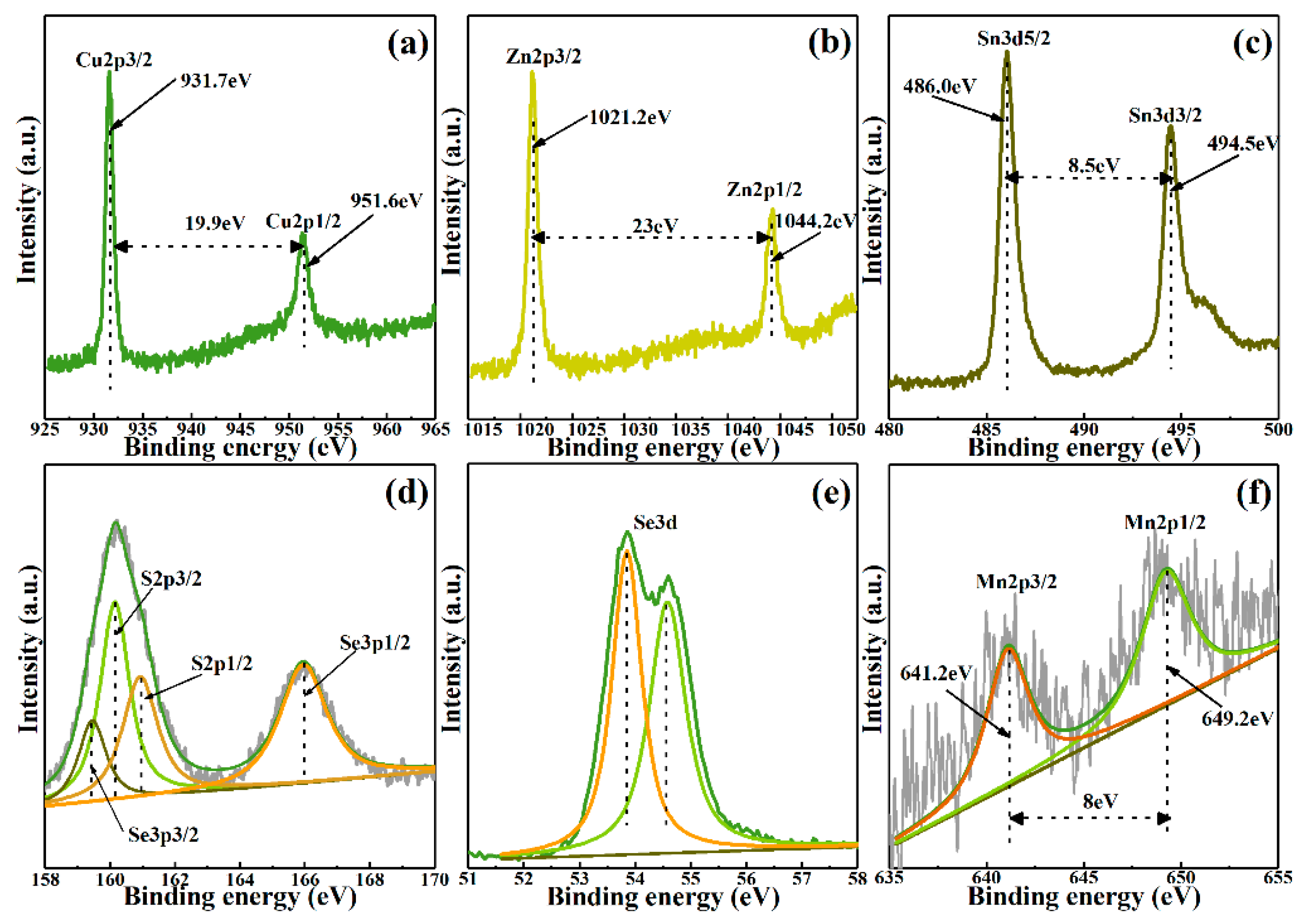
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ito, K.; Nakazawa, T. Electrical and optical properties of stannite-type quaternary semiconductor thin films. Jpn. J. Appl. Phys. 1988, 27, 2094–2097. [Google Scholar] [CrossRef]
- Zhou, H.; Hsu, W.-C.; Duan, H.-S.; Bob, B.; Yang, W.; Song, T.-B.; Hsu, C.-J.; Yang, Y. CZTS nanocrystals: A promising approach for next generation thin film photovoltaics. Energy Environ. Sci. 2013, 6, 2822. [Google Scholar] [CrossRef]
- Kumar, M.; Dubey, A.; Adhikari, N.; Venkatesan, S.; Qiao, Q. Strategic review of secondary phases, defects and defect-complexes in kesterite CZTS–Se solar cells. Energy Environ. Sci. 2015, 8, 3134–3159. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device characteristics of CZTSSe thin-film solar cells with 12.6% efficiency. Adv. Energy Mater. 2013, 4, 1301465. [Google Scholar] [CrossRef]
- Kim, J.; Hiroi, H.; Todorov, T.K.; Gunawan, O.; Kuwahara, M.; Gokmen, T.; Nair, D.; Hopstaken, M.; Shin, B.; Lee, Y.S.; et al. High efficiency Cu2ZnSn(S,Se)4 solar cells by applying a double In2S3/CdS emitter. Adv. Mater. 2014, 26, 7427–7431. [Google Scholar] [CrossRef]
- Jackson, P.; Wuerz, R.; Hariskos, D.; Lotter, E.; Witte, W.; Powalla, M. Effects of heavy alkali elements in Cu(In,Ga)Se2 solar cells with efficiencies up to 22.6%. Phys. Status Solidi RRL 2016, 10, 583–586. [Google Scholar] [CrossRef]
- Huang, W.-C.; Wei, S.-Y.; Cai, C.-H.; Ho, W.-H.; Lai, C.-H. The role of Ag in aqueous solution processed (Ag,Cu)2ZnSn(S,Se)4 kesterite solar cells: Antisite defect elimination and importance of Na passivation. J. Mater. Chem. A 2018, 6, 15170–15181. [Google Scholar] [CrossRef]
- Schorr, S. The crystal structure of kesterite type compounds: A neutron and X-ray diffraction study. Sol. Energy Mater. Sol. Cells 2011, 95, 1482–1488. [Google Scholar] [CrossRef]
- Gokmen, T.; Gunawan, O.; Todorov, T.K.; Mitzi, D.B. Band tailing and efficiency limitation in kesterite solar cells. Appl. Phys. Lett. 2013, 103, 103506. [Google Scholar] [CrossRef]
- Yuan, Z.-K.; Chen, S.; Xiang, H.; Gong, X.-G.; Walsh, A.; Park, J.-S.; Repins, I.; Wei, S.-H. Engineering solar cell absorbers by exploring the band alignment and defect disparity: The case of Cu- and Ag-based kesterite compounds. Adv. Funct. Mater. 2015, 25, 6733–6743. [Google Scholar] [CrossRef]
- Su, Z.; Tan, J.M.R.; Li, X.; Zeng, X.; Batabyal, S.K.; Wong, L.H. Cation substitution of solution-processed Cu2ZnSnS4 thin film solar cell with over 9% efficiency. Adv. Energy Mater. 2015, 5, 1500682. [Google Scholar] [CrossRef]
- Hadke, S.; Levcenko, S.; Gautam, G.S.; Hages, C.J.; Márquez, J.A.; Izquierdo-Roca, V.; Carter, E.A.; Unold, T.; Wong, L.H. Suppressed deep traps and bandgap fluctuations in Cu2CdSnS4 solar cells with ≈8% efficiency. Adv. Energy Mater. 2019, 9, 1902509. [Google Scholar] [CrossRef]
- Hages, C.J.; Koeper, M.J.; Agrawal, R. Optoelectronic and material properties of nanocrystal-based CZTSe absorbers with Ag-alloying. Sol. Energy Mater. Sol. Cells 2016, 145, 342–348. [Google Scholar] [CrossRef]
- Wu, Y.; Sui, Y.; He, W.; Zeng, F.; Wang, Z.; Wang, F.; Yao, B.; Yang, L. Substitution of Ag for Cu in Cu2ZnSn(S,Se)4: Toward wide band gap absorbers with low antisite defects for thin film solar cells. Nanomaterials 2020, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Gershon, T.; Sardashti, K.; Gunawan, O.; Mankad, R.; Singh, S.; Lee, Y.S.; Ott, J.A.; Kummel, A.; Haight, R. Photovoltaic device with over 5% efficiency based on an n-type Ag2ZnSnSe4 absorber. Adv. Energy Mater. 2016, 6, 1601182. [Google Scholar] [CrossRef]
- Wu, Y.J.; Sui, Y.R.; Zhang, Y.; Wang, Z.W.; Lv, S.Q.; Wei, M.B.; Sun, Y.F.; Yao, B.; Liu, X.Y.; Yang, L.L. Bandgap engineering of Cu2InxZn1−xSn(S,Se)4 alloy films for photovoltaic applications. Ceram. Int. 2018, 44, 1942–1950. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, D.; Sui, Y.; Wu, Y.; Wang, Z.; Yang, L.; Wang, F.; Lv, S.; Yao, B. Synthesis and investigation of environmental protection and earth-abundant kesterite Cu2MgxZn1-xSn(S,Se)4 thin films for solar cells. Ceram. Int. 2018, 44, 15249–15255. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, L.; Pan, D. New insight of Li-doped Cu2ZnSn(S,Se)4 thin films: Li-induced Na diffusion from soda lime glass by a cation-exchange reaction. ACS Appl. Mater. Interfaces 2017, 9, 23878–23883. [Google Scholar] [CrossRef]
- Li, J.; Wang, N.; Li, X.; Zeng, Y.; Zhang, Y. Cation substitution in earth-abundant kesterite photovoltaic materials. Adv. Sci. 2018, 5, 1700744. [Google Scholar] [CrossRef]
- Ozel, F. Earth-abundant quaternary semiconductor Cu2MSnS4 (M = Fe, Co, Ni and Mn) nanofibers: Fabrication, characterization and band gap arrangement. J. Alloys Compd. 2016, 657, 157–162. [Google Scholar] [CrossRef]
- Berg, D.M.; Arasimowicz, M.; Djemour, R.; Gütay, L.; Siebentritt, S.; Schorr, S.; Fontané, X.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Dale, P. Discrimination and detection limits of secondary phases in Cu2ZnSnS4 using X-ray diffraction and Raman spectroscopy. Thin Solid Films 2014, 569, 113–123. [Google Scholar] [CrossRef]
- Lin, X.; Kavalakkatt, J.; Kornhuber, K.; Levcenko, S.; Lux-Steiner, M.C.; Ennaoui, A. Structural and optical properties of Cu2ZnSnS4 thin film absorbers from ZnS and Cu3SnS4 nanoparticle precursors. Thin Solid Films 2013, 535, 10–13. [Google Scholar] [CrossRef]
- Khadka, D.B.; Kim, J. Band gap engineering of alloyed Cu2ZnGexSn1–xQ4 (Q = S, Se) films for solar cell. J. Phys. Chem. C 2015, 119, 1706–1713. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Zhang, P.; Yuan, X.; Huang, F.; Zhang, W. Structural properties and quasiparticle band structures of Cu-based quaternary semiconductors for photovoltaic applications. J. Appl. Phys. 2012, 111, 63709. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.-H. Electronic structure and stability of quaternary chalcogenide semiconductors derived from cation cross-substitution of II-VI and I-III-VI2 compounds. Phys. Rev. B 2009, 79, 165211. [Google Scholar] [CrossRef]
- Van Embden, J.; Chesman, A.S.R.; Della Gaspera, E.; Duffy, N.W.; Watkins, S.; Jasieniak, J. Cu2ZnSnS4xSe4(1–x) solar cells from polar nanocrystal inks. J. Am. Chem. Soc. 2014, 136, 5237–5240. [Google Scholar] [CrossRef]
- Rondiya, S.; Wadnerkar, N.; Jadhav, Y.; Jadkar, S.; Haram, S.K.; Kabir, M. Structural, electronic, and optical properties of Cu2NiSnS4: A combined experimental and theoretical study toward photovoltaic applications. Chem. Mater. 2017, 29, 3133–3142. [Google Scholar] [CrossRef]
- Calderón, C.; Gordillo, G.; Becerra, R.; Bartolo-Pérez, P. XPS analysis and characterization of thin films Cu2ZnSnS4 grown using a novel solution based route. Mater. Sci. Semicond. Process. 2015, 39, 492–498. [Google Scholar] [CrossRef]
- Tsega, M.; Dejene, F.; Kuo, D.-H. Morphological evolution and structural properties of Cu2ZnSn(S,Se)4 thin films deposited from single ceramic target by a one-step sputtering process and selenization without H2Se. J. Alloys Compd. 2015, 642, 140–147. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, Y.; Sui, Y.; He, W.; Wang, Z.; Yang, L.; Wang, F.; Yao, B. Investigation on the selenization treatment of kesterite Cu2Mg0.2Zn0.8Sn(S,Se)4 films for solar cell. Nanomaterials 2019, 9, 946. [Google Scholar] [CrossRef]
- Liu, W.; Guo, B.L.; Zhang, F.M.; Mak, C.L.; Wu, X.S.; Wong, K.H. Facile hydrothermal synthesis of hydrotropic Cu2ZnSnS4 nanocrystalquantum dots: Band-gap engineering and phonon confinement effect. J. Mater. Chem. A 2013, 1, 3182. [Google Scholar] [CrossRef]
- Jiang, Y.; Yao, B.; Li, Y.-F.; Ding, Z.; Luan, H.; Jia, J.; Li, Y.; Shi, K.; Sui, Y.; Zhang, B. Structure, optical and electrical properties of (Cu1-xAgx)2ZnSn(S,Se)4 alloy thin films for photovoltaic application. Mater. Sci. Semicond. Process. 2018, 81, 54–59. [Google Scholar] [CrossRef]
- Guan, X.; Ge, X.; Wang, C.; Wang, F.; Liu, X.; Cao, J.; Fan, L.; Yang, L. Effects of Mn, Cl co-doping on the structure and photoluminescence properties of novel walnut-shape MAPb0.95Mn0.05I3-xClx films. Ceram. Int. 2019, 45, 468–473. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, Y.; Zhang, Y.; Wang, F.; Gao, Y.; Lv, S.; Wang, Z.; Sun, Y.; Wei, M.; Yao, B.; et al. Synthesis of simple, low cost and benign sol–gel Cu2InxZn1−xSnS4 alloy thin films: Influence of different rapid thermal annealing conditions and their photovoltaic solar cells. RSC Adv. 2018, 8, 9038–9048. [Google Scholar] [CrossRef]
- Lie, S.; Sandi, M.I.; Tay, Y.F.; Li, W.; Tan, J.M.R.; Bishop, D.M.; Gunawan, O.; Wong, L.H. Improving the charge separation and collection at the buffer/absorber interface by double-layered Mn-substituted CZTS. Sol. Energy Mater. Sol. Cells 2018, 185, 351–358. [Google Scholar] [CrossRef]
- Li, Y.F.; Yao, B.; Lu, Y.M.; Wei, Z.P.; Gai, Y.Q.; Zheng, C.J.; Zhang, Z.Z.; Li, B.H.; Shen, D.Z.; Fan, X.W.; et al. Realization of p-type conduction in undoped MgxZn1−xO thin films by controlling Mg content. Appl. Phys. Lett. 2007, 91, 232115. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.-H. Crystal and electronic band structure of Cu2ZnSnX4 (X = S and Se) photovoltaic absorbers: First-principles insights. Appl. Phys. Lett. 2009, 94, 41903. [Google Scholar] [CrossRef]
- Fukushima, T.; Yamauchi, K.; Picozzi, S. Magnetically induced ferroelectricity in Cu2MnSnS4 and Cu2MnSnSe4. Phys. Rev. B 2010, 82, 82. [Google Scholar] [CrossRef]
- Song, Y.; Yao, B.; Li, Y.-F.; Ding, Z.; Liu, R.-J.; Sui, Y.; Zhang, L.; Zhang, Z.; Zhao, H. Improving the back electrode interface quality of Cu2ZnSn(S,Se)4 thin-film solar cells using a novel CuAlO2 buffer layer. ACS Appl. Energy Mater. 2019, 2, 2230–2237. [Google Scholar] [CrossRef]
- Jo, E.; Gil Gang, M.; Shim, H.; Suryawanshi, M.P.; Ghopade, U.; Kim, J.H. 8% efficiency Cu2ZnSn(S,Se)4 (CZTSSe) thin film solar cells on flexible and lightweight molybdenum foil substrates. ACS Appl. Mater. Interfaces 2019, 11, 23118–23124. [Google Scholar] [CrossRef]
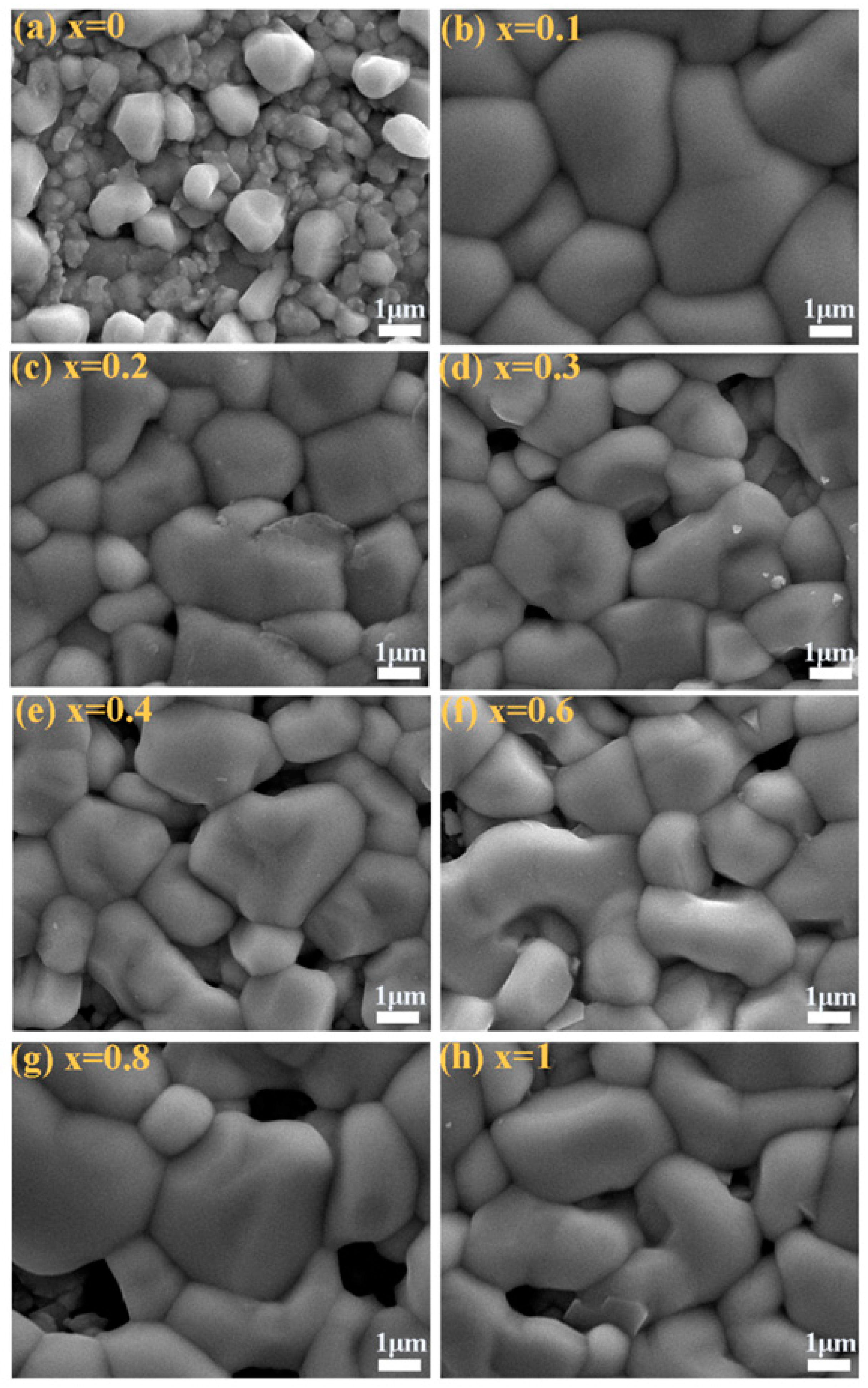
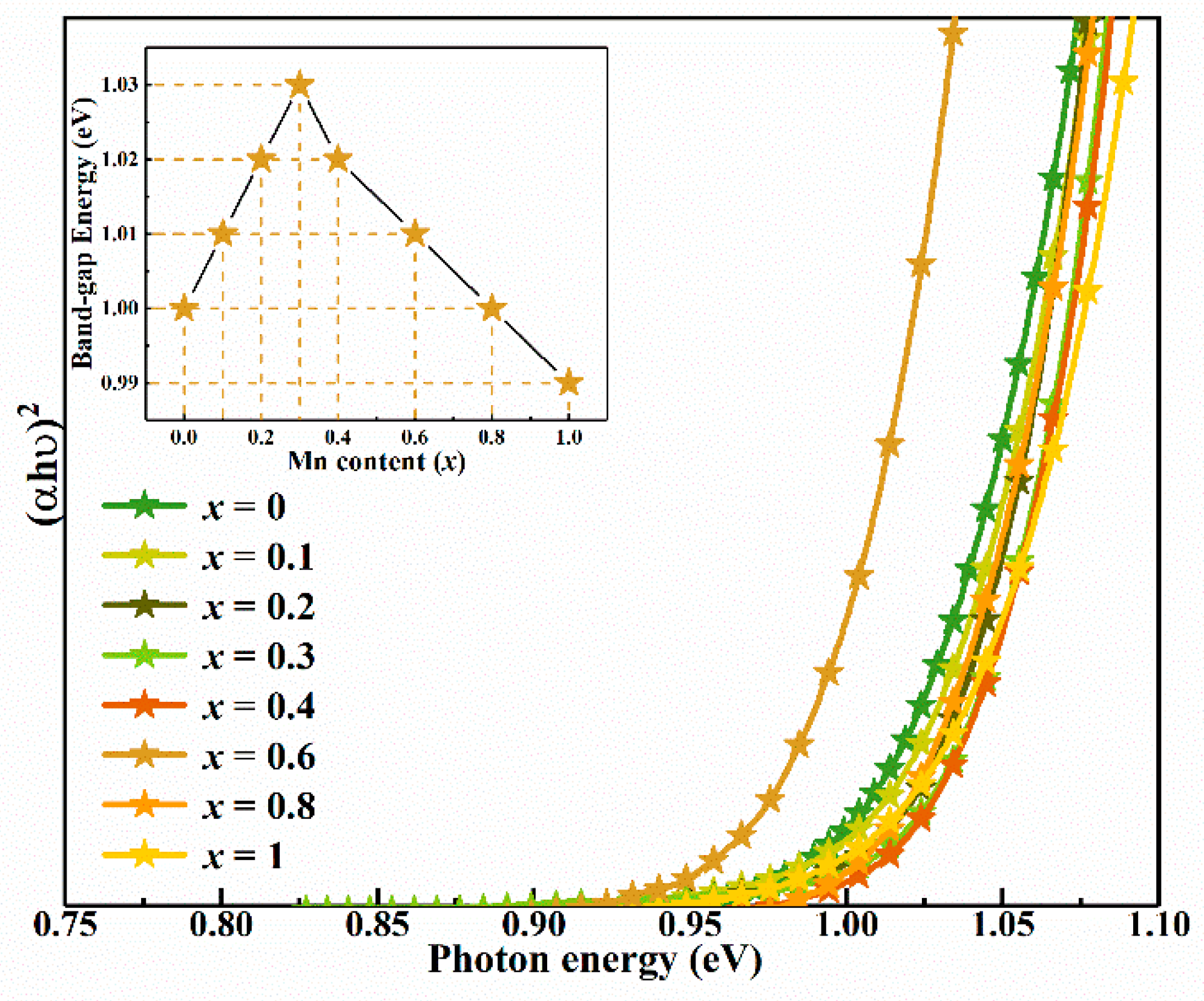
| Sample | Cu (% at) | Mn (% at) | Zn (% at) | Sn (% at) | S (% at) | Se (% at) | Cu/(Zn + Mn + Sn) | (Mn + Zn)/Sn | Mn/(Mn + Zn) |
|---|---|---|---|---|---|---|---|---|---|
| x = 0 | 23.49 | 0 | 15.64 | 11.48 | 2.88 | 46.51 | 0.87 | 1.36 | 0 |
| x = 0.1 | 24.01 | 1.30 | 14.79 | 11.32 | 2.42 | 46.16 | 0.88 | 1.42 | 0.08 |
| x = 0.2 | 24.23 | 2.22 | 13.36 | 11.53 | 2.63 | 46.03 | 0.89 | 1.35 | 0.14 |
| x = 0.3 | 24.34 | 3.26 | 11.67 | 11.53 | 2.66 | 46.56 | 0.91 | 1.29 | 0.22 |
| x = 0.4 | 24.24 | 4.64 | 10.38 | 11.59 | 3.72 | 45.43 | 0.91 | 1.29 | 0.31 |
| x = 0.6 | 25.97 | 6.23 | 7.61 | 11.18 | 3.72 | 45.28 | 1.04 | 1.23 | 0.45 |
| x = 0.8 | 26.38 | 9.33 | 3.82 | 11.14 | 3.02 | 46.32 | 1.08 | 1.18 | 0.71 |
| x = 1 | 27.50 | 12.34 | 0 | 11.31 | 2.90 | 45.98 | 1.16 | 1.09 | 1 |
| Samples | Resistivity (Ω·cm) | Carrier Concentration (cm−3) | Mobility (cm2∙V−1∙s−1) | Type |
|---|---|---|---|---|
| x = 0 | 5.3 × 101 | 4.2 × 1016 | 2.8 | p |
| x = 0.1 | 8.7 × 101 | 2.3 × 1016 | 3.2 | p |
| x = 0.2 | 8.6 × 101 | 4.6 × 1016 | 1.6 | p |
| x = 0.3 | 6.4 × 101 | 6.3 × 1016 | 1.5 | p |
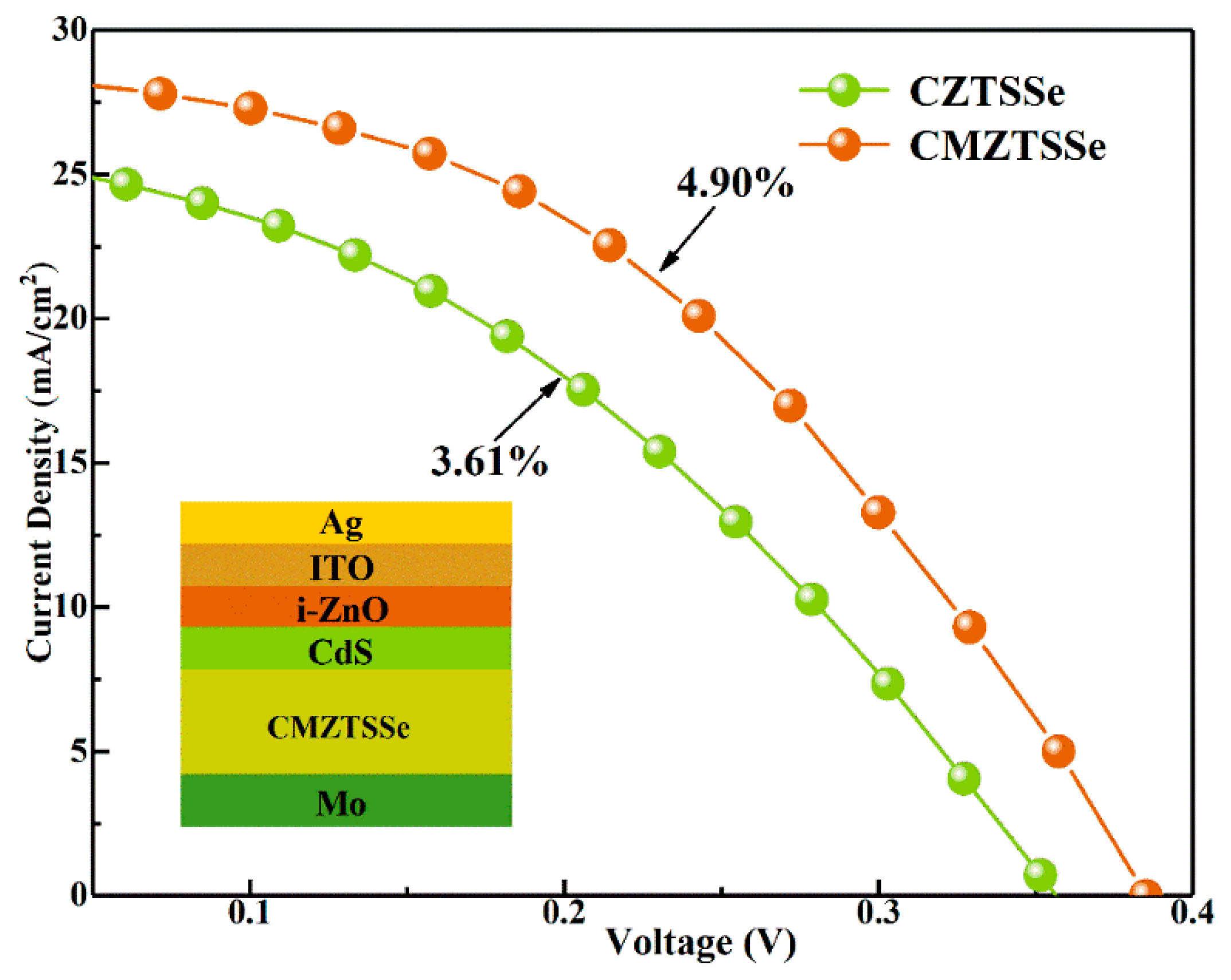
| Device | Active Area | VOC (mV) | JSC (mA/cm2) | FF (%) | PCE (%) | Rs (Ω∙cm2) | Rsh (Ω∙cm2) |
|---|---|---|---|---|---|---|---|
| CZTSSe | 0.19 cm2 | 356 | 25.77 | 39.35 | 3.61 | 2.9 | 353.1 |
| CMZTSSe (x = 0.1) | 0.19 cm2 | 385 | 28.64 | 44.40 | 4.90 | 1.5 | 366.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Sui, Y.; Zeng, F.; Wang, Z.; Wang, F.; Yao, B.; Yang, L. Enhancing the Performance of Aqueous Solution-Processed Cu2ZnSn(S,Se)4 Photovoltaic Materials by Mn2+ Substitution. Nanomaterials 2020, 10, 1250. https://doi.org/10.3390/nano10071250
He W, Sui Y, Zeng F, Wang Z, Wang F, Yao B, Yang L. Enhancing the Performance of Aqueous Solution-Processed Cu2ZnSn(S,Se)4 Photovoltaic Materials by Mn2+ Substitution. Nanomaterials. 2020; 10(7):1250. https://doi.org/10.3390/nano10071250
Chicago/Turabian StyleHe, Wenjie, Yingrui Sui, Fancong Zeng, Zhanwu Wang, Fengyou Wang, Bin Yao, and Lili Yang. 2020. "Enhancing the Performance of Aqueous Solution-Processed Cu2ZnSn(S,Se)4 Photovoltaic Materials by Mn2+ Substitution" Nanomaterials 10, no. 7: 1250. https://doi.org/10.3390/nano10071250
APA StyleHe, W., Sui, Y., Zeng, F., Wang, Z., Wang, F., Yao, B., & Yang, L. (2020). Enhancing the Performance of Aqueous Solution-Processed Cu2ZnSn(S,Se)4 Photovoltaic Materials by Mn2+ Substitution. Nanomaterials, 10(7), 1250. https://doi.org/10.3390/nano10071250




