Magnetic Porous Controlled Fe3O4–Chitosan Nanostructure: An Ecofriendly Adsorbent for Efficient Removal of Azo Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Chitosan/Fe3O4 NPs
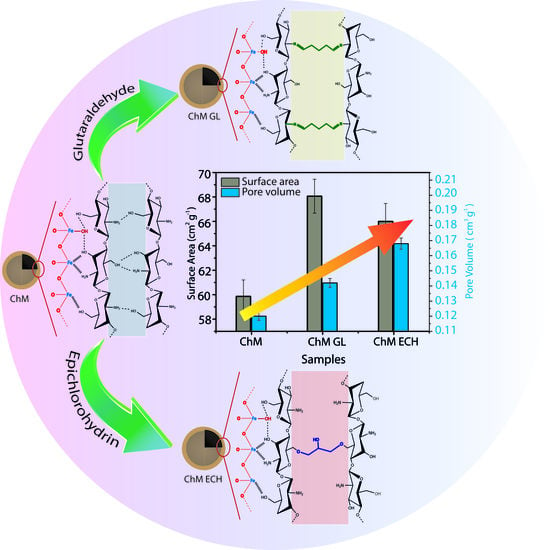
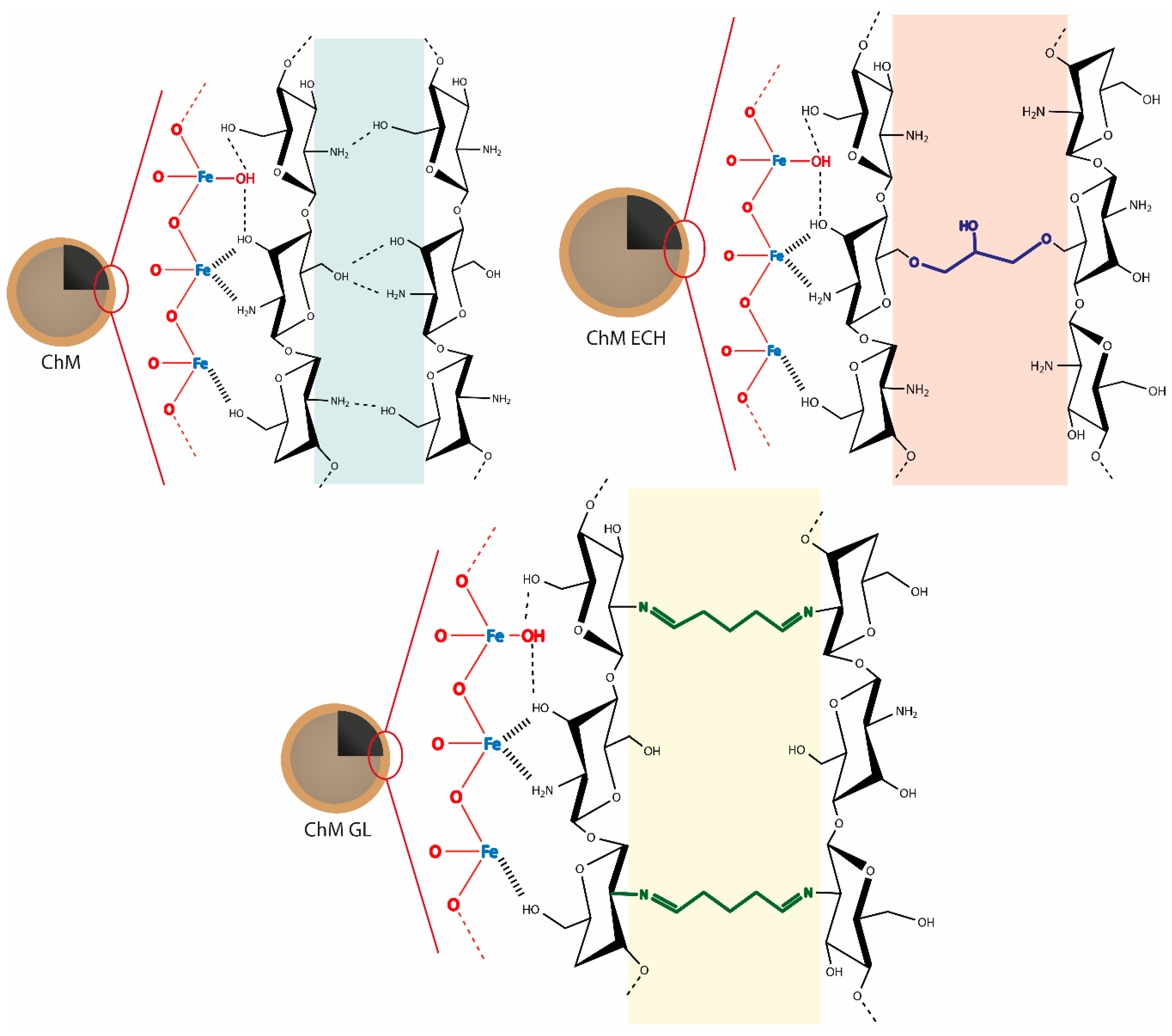
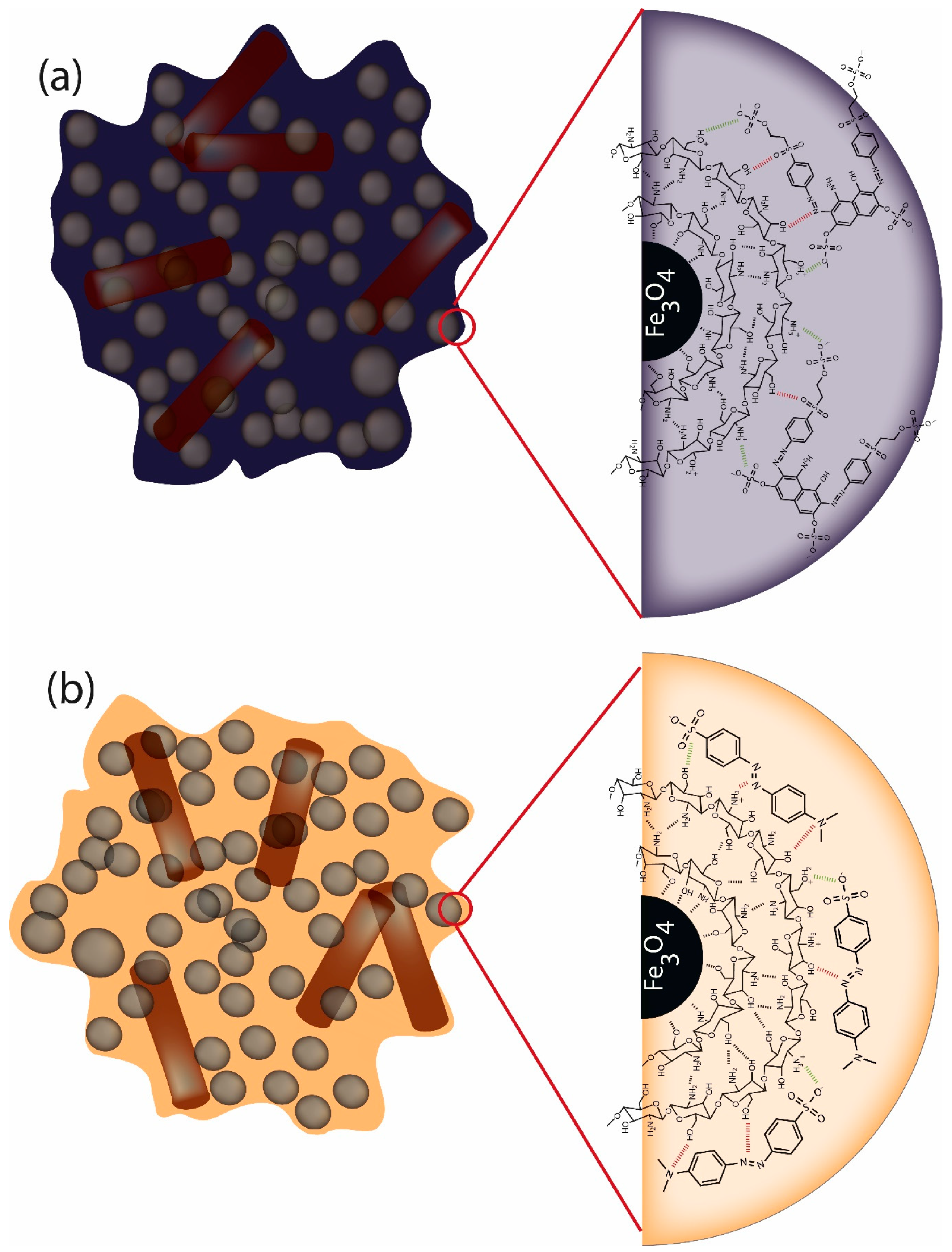
2.3. Preparation of Surface-Modified Chitosan/Fe3O4 NPs
2.4. Characterization Methods
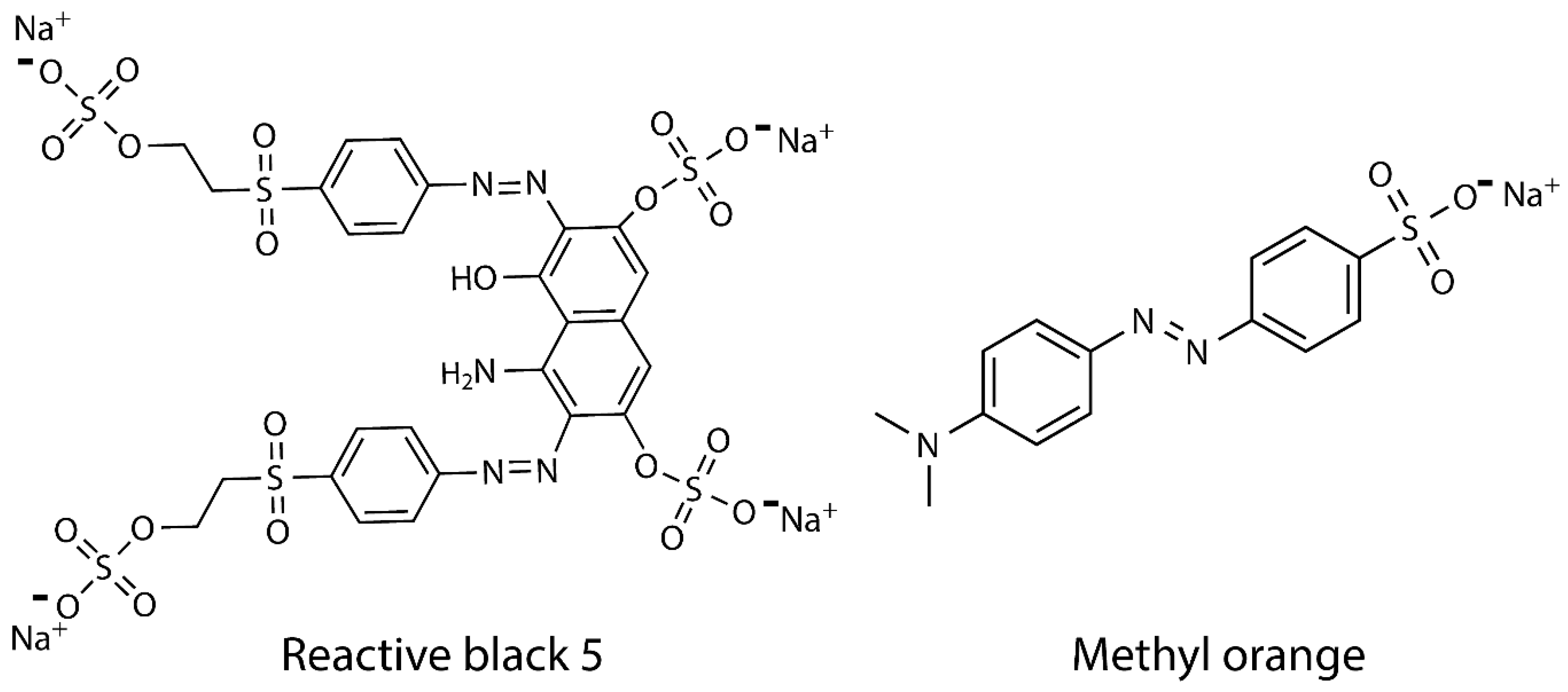
2.5. Adsorption Assay
2.5.1. pH Effect
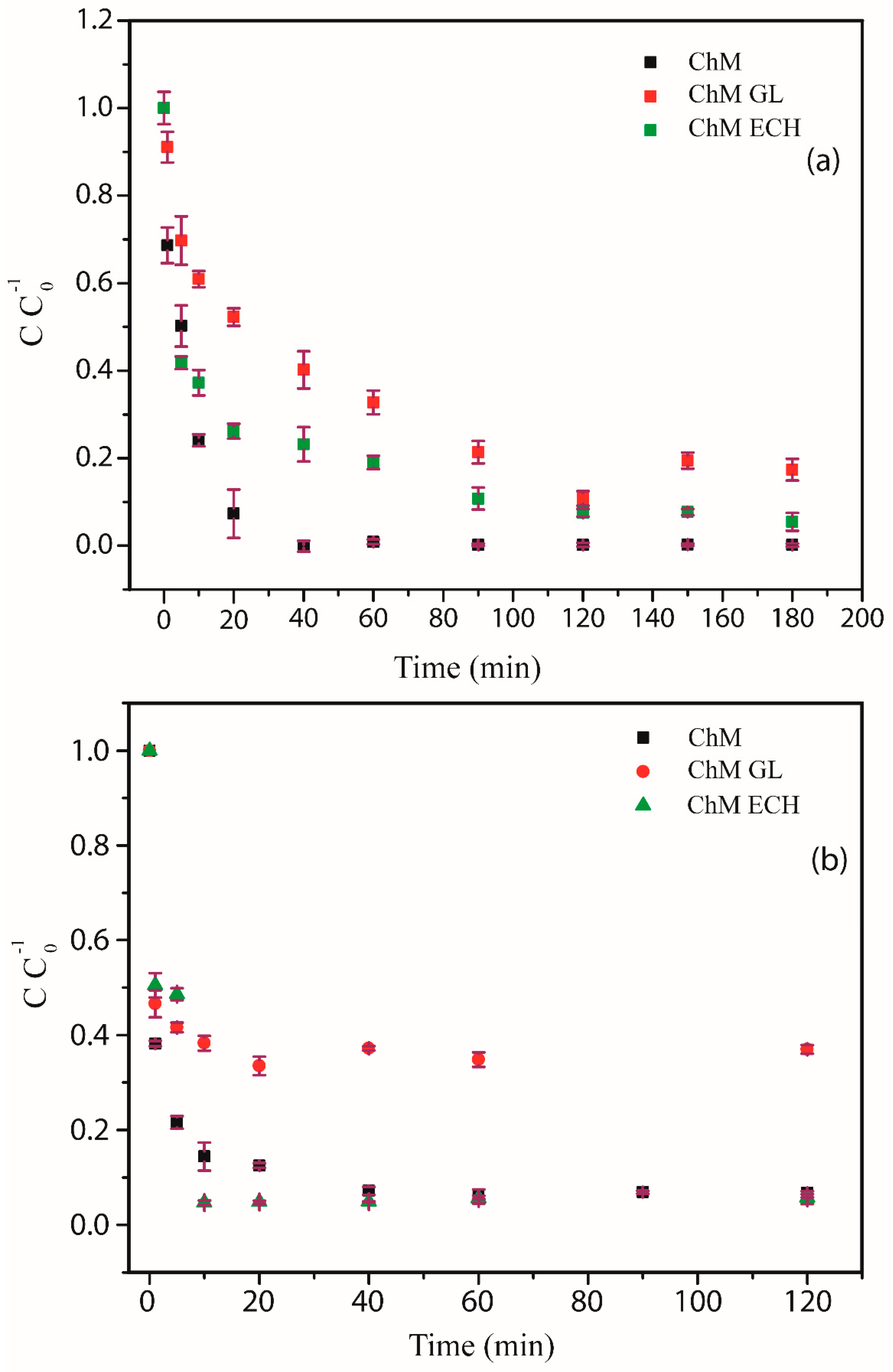
2.5.2. Adsorption Kinetics
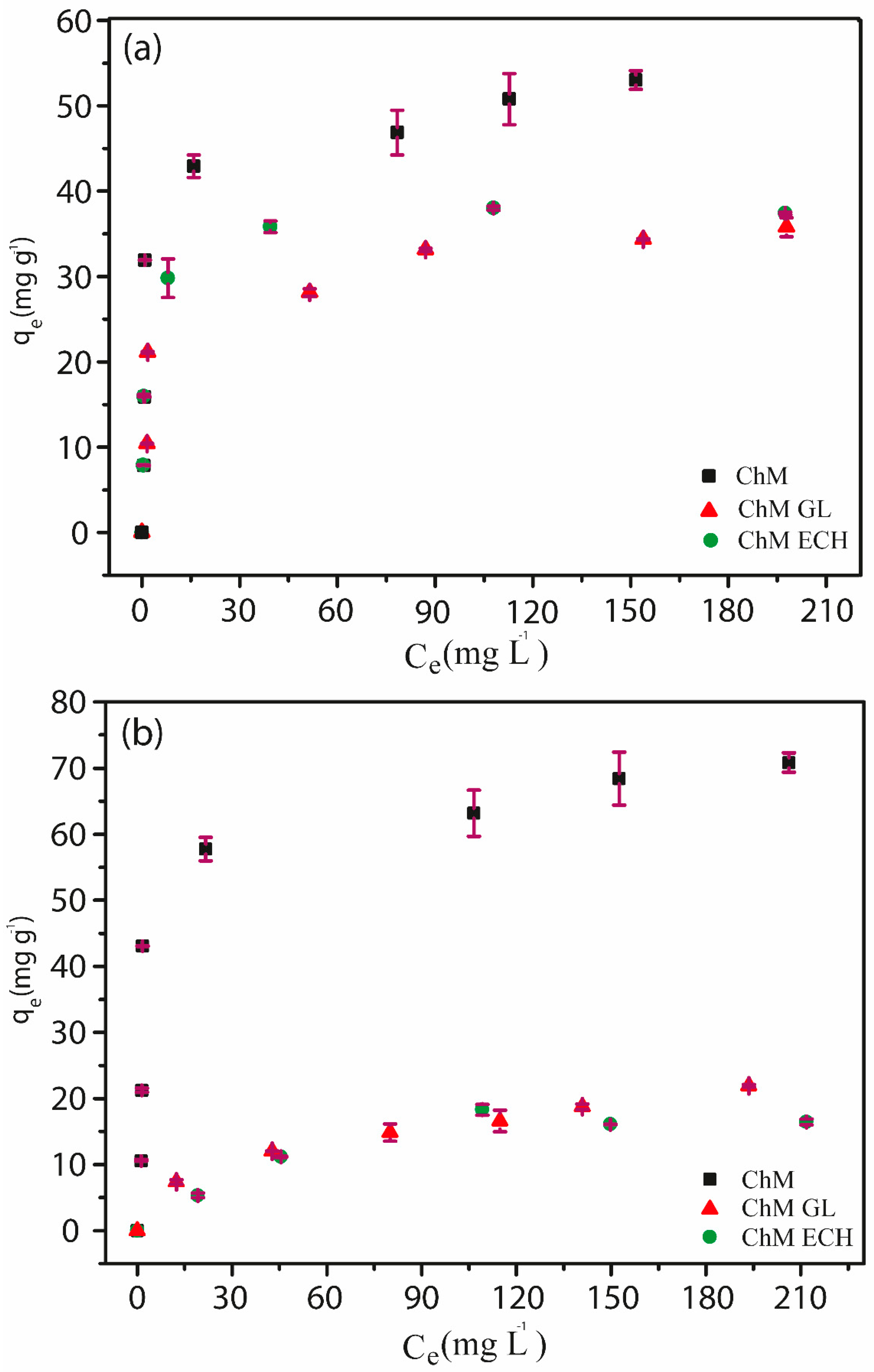
2.5.3. Adsorption Isotherms
3. Results and Discussion
3.1. Structure and Morphology Analyses
3.1.1. X-ray Powder Diffraction
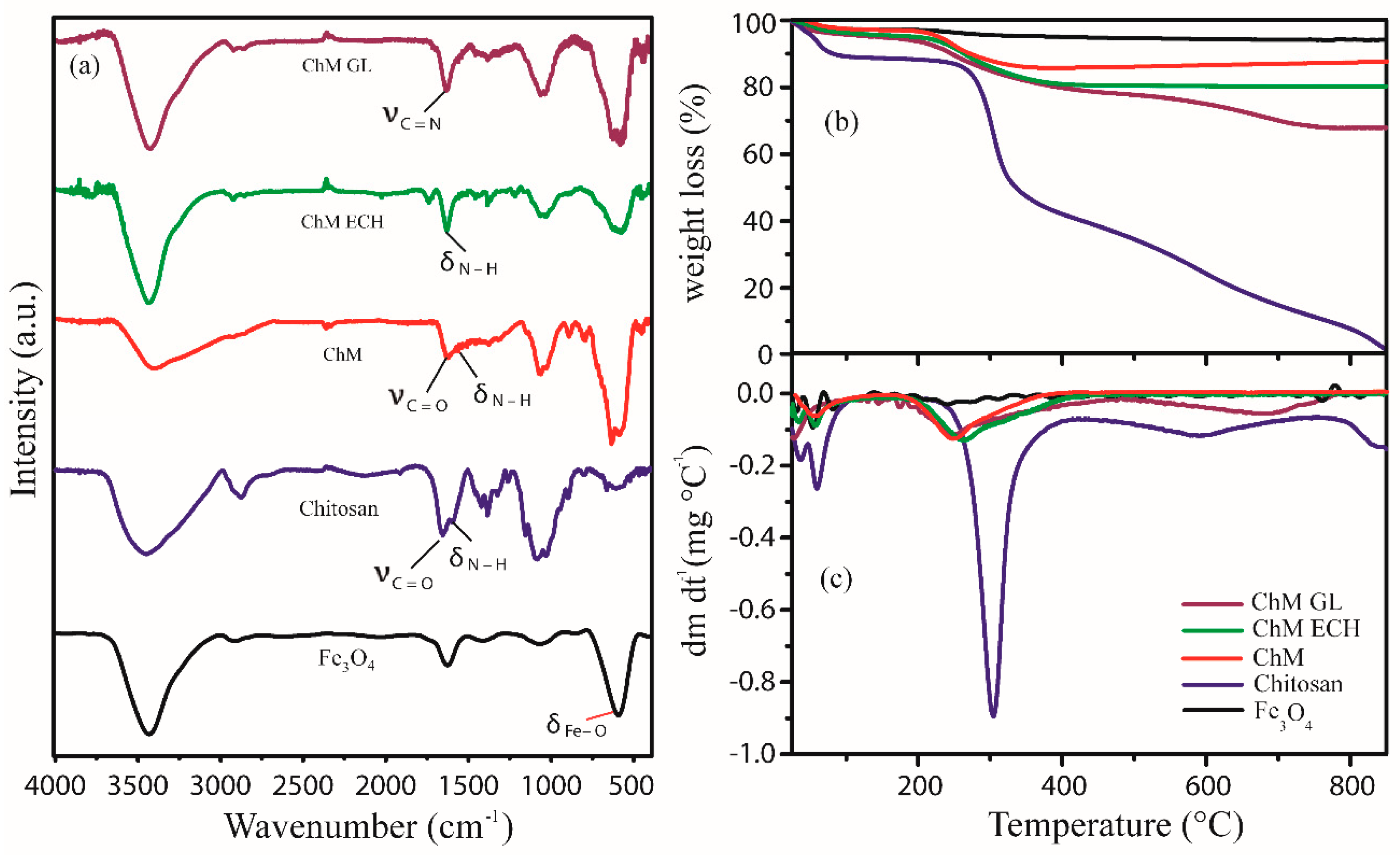
3.1.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.1.3. TGA–DTG
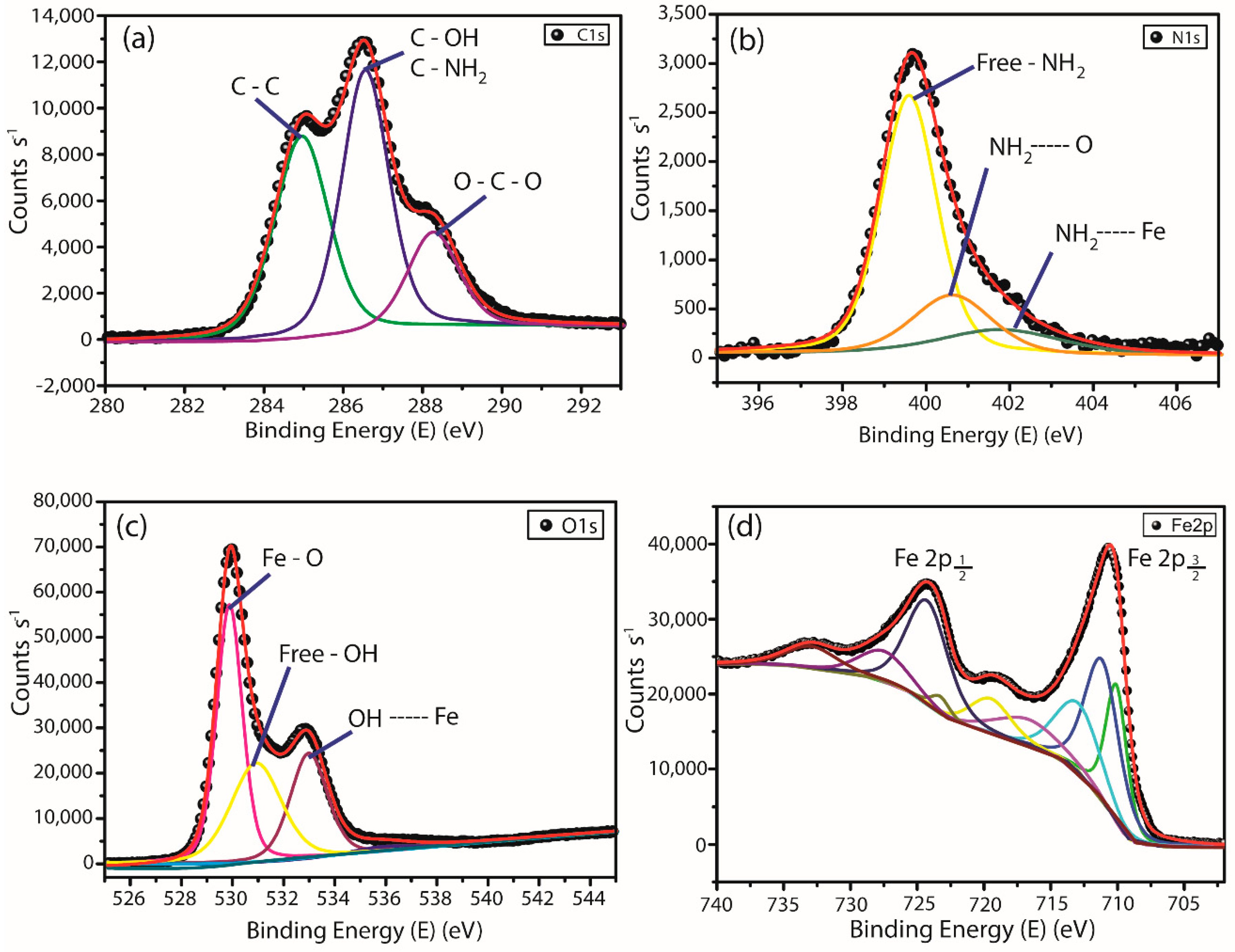
3.1.4. X-ray Photoelectron Spectroscopy (XPS)
3.1.5. TEM
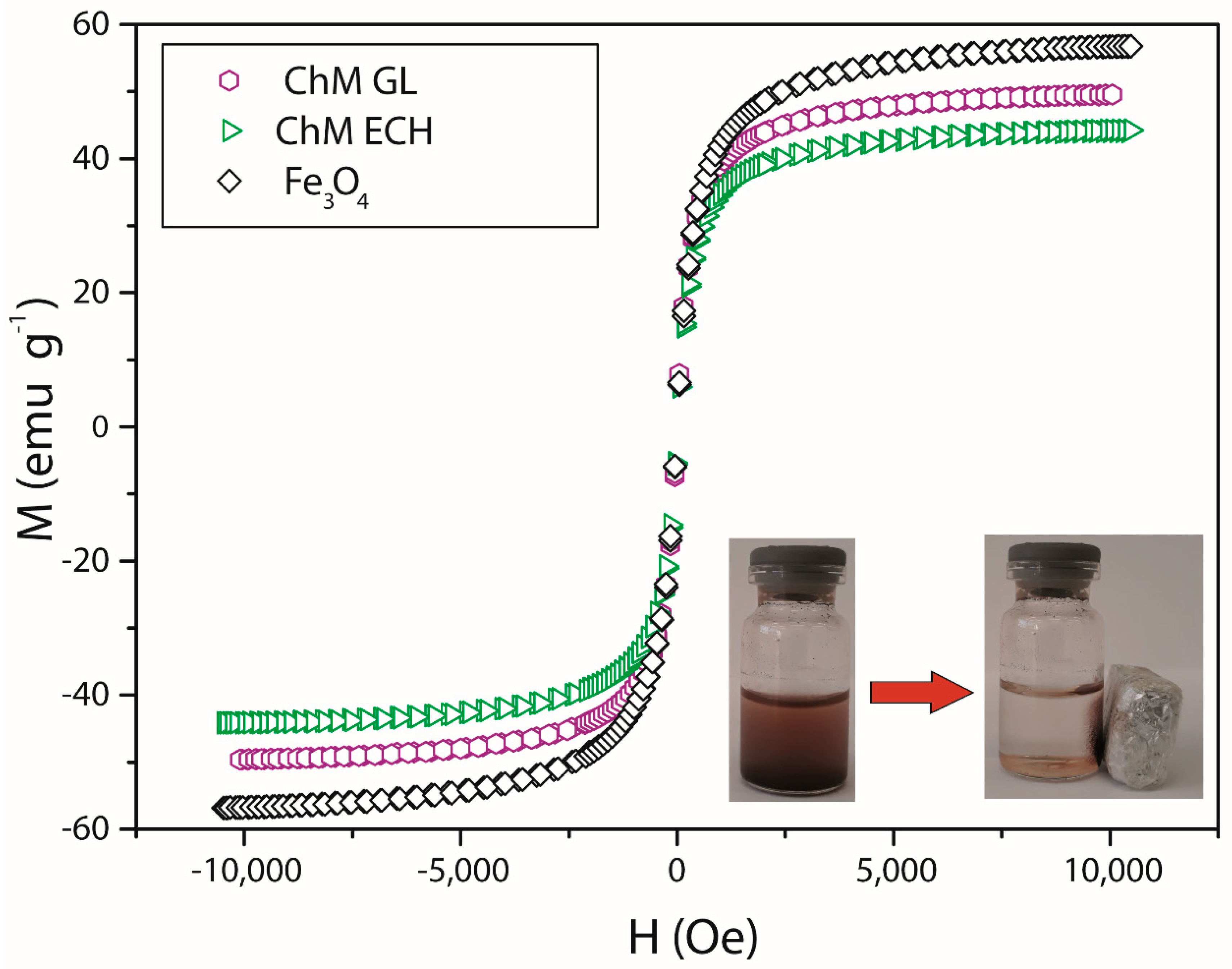
3.2. Magnetic Property
3.3. N2 Adsorption–Desorption
3.4. Adsorption Evaluation
3.4.1. pH Effect
3.4.2. Effect of Contact Time
3.4.3. Adsorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Semião, M.A.; Haminiuk, C.W.I.; Maciel, G.M. Residual diatomaceous earth as a potential and cost effective biosorbent of the azo textile dye Reactive Blue 160. J. Environ. Chem. Eng. 2020, 8, 103617. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2019, 276, 105–114. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Kalaamani, P.; Porkodi, K.; Varadarajan, P.R.; Subburaam, C.V. Adsorption of dissolved Reactive red dye from aqueous phase onto activated carbon prepared from agricultural waste. Bioresour. Technol. 2006, 97, 1618–1625. [Google Scholar] [CrossRef]
- Cervellino, A.; Frison, R.; Cernuto, G.; Guagliardi, A.; Masciocchi, N. Lattice parameters and site occupancy factors of magnetite-maghemite core-shell nanoparticles. A critical study. J. Appl. Crystallogr. 2014, 47, 1755–1761. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Briguglio, S.; Mattiussi, M.; Gelao, V.; Cabras, I.; Zorzenon, L.; Trovarelli, A.; Goi, D. Enhanced ibuprofen removal by heterogeneous-fenton process over Cu/ZrO2 and Fe/ZrO2 catalysts. J. Environ. Chem. Eng. 2020, 8, 103586. [Google Scholar] [CrossRef]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Oliveira, M.C.; Florêncio, M.H. Visible light photocatalytic degradation of amitriptyline using cobalt doped titanate nanowires: Kinetics and characterization of transformation products. J. Environ. Chem. Eng. 2020, 8, 103585. [Google Scholar] [CrossRef]
- Zahrim, A.Y.; Dexter, Z.D.; Joseph, C.G.; Hilal, N. Effective coagulation-flocculation treatment of highly polluted palm oil mill biogas plant wastewater using dual coagulants: Decolourisation, kinetics and phytotoxicity studies. J. Water Process Eng. 2017, 16, 258–269. [Google Scholar] [CrossRef]
- Du, X.; Qu, F.-S.; Liang, H.; Li, K.; Bai, L.-M.; Li, G.-B. Control of submerged hollow fiber membrane fouling caused by fine particles in photocatalytic membrane reactors using bubbly flow: Shear stress and particle forces analysis. Sep. Purif. Technol. 2017, 172, 130–139. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, H.; Li, W.; Li, J.; Xu, R.; Zhang, K.; He, G.; Shearing, P.R.; Brett, D.J.L. Core–shell TiO2@C ultralong nanotubes with enhanced adsorption of antibiotics. J. Mater. Chem. A 2019, 7, 19081–19086. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Jiang, H.; Lin, R.; Wang, Z.; Wu, J.; He, G.; Shearing, P.R.; Brett, D.J.L. ZIF-8-Derived hollow carbon for efficient adsorption of antibiotics. Nanomaterials 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, A.; Bhat, A.H.; Naeem, A.; Isa, M.H.; Danish, M. High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int. J. Biol. Macromol. 2018, 107, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kaur, H.; Sharma, M.; Sahore, V. A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ. Monit. Assess. 2011, 183, 151–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, L.; Ma, Y.; Yang, W. Super-adsorbent material based on functional polymer particles with a multilevel porous structure. NPG Asia Mater. 2016, 8, e301. [Google Scholar] [CrossRef]
- Min, L.-L.; Zhong, L.-B.; Zheng, Y.-M.; Liu, Q.; Yuan, Z.-H.; Yang, L.-M. Functionalized chitosan electrospun nanofiber for effective removal of trace arsenate from water. Sci. Rep. 2016, 6, 32480. [Google Scholar] [CrossRef]
- Demarchi, C.A.; Campos, M.; Rodrigues, C.A. Adsorption of textile dye Reactive Red 120 by the chitosan–Fe(III)-crosslinked: Batch and fixed-bed studies. J. Environ. Chem. Eng. 2013, 1, 1350–1358. [Google Scholar] [CrossRef]
- Chen, L.; Hao, H.; Zhang, W.; Shao, Z. Adsorption mechanism of copper ions in aqueous solution by chitosan–carboxymethyl starch composites. J. Appl. Polym. Sci. 2020, 137, 48636. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y.; Zhao, X.; Ji, Q.; Xia, Y.; Ma, X. Robust, recoverable poly(N,N-dimethylacrylamide)-based hydrogels crosslinked by vinylated chitosan with recyclable adsorbability for acid red. J. Appl. Polym. Sci. 2019, 136, 47473. [Google Scholar] [CrossRef]
- Rusmin, R.; Sarkar, B.; Liu, Y.; McClure, S.; Naidu, R. Structural evolution of chitosan–palygorskite composites and removal of aqueous lead by composite beads. Appl. Surf. Sci. 2015, 353, 363–375. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Xu, X.; Gao, B.-Y.; Yue, Q.-Y.; Zhong, Q.-Q. Preparation and utilization of wheat straw bearing amine groups for the sorption of acid and reactive dyes from aqueous solutions. J. Hazard. Mater. 2010, 182, 1–9. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, L.; Chen, C.; Shi, X.; Cao, Q.; Gao, L. In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction–precipitation method and their application in adsorption of reactive azo dye. Powder Technol. 2014, 260, 90–97. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Kołodyńska, D.; Kozioł, M.; Gorbyk, P.P. Gd-DTPA Adsorption on chitosan/magnetite nanocomposites. Nanoscale Rese. Lett. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Sureshkumar, V.; Kiruba Daniel, S.C.G.; Ruckmani, K.; Sivakumar, M. Fabrication of chitosan–magnetite nanocomposite strip for chromium removal. Appl. Nanosci. 2016, 6, 277–285. [Google Scholar] [CrossRef]
- Freire, T.M.; Dutra, L.M.U.; Queiroz, D.C.; Ricardo, N.M.P.S.; Barreto, K.; Denardin, J.C.; Wurm, F.R.; Sousa, C.P.; Correia, A.N.; de Lima-Neto, P.; et al. Fast ultrasound assisted synthesis of chitosan-based magnetite nanocomposites as a modified electrode sensor. Carbohydr. Polym. 2016, 151, 760–769. [Google Scholar] [CrossRef]
- Lagrergen, S. Zur theorie der sogenannten adsorption gelöster stoffe kungliga svenska vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S. Absorption of Heavy Metals from Waste Streams by Peat; University of Birmingham: Birmingham, UK, 1995. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Colloid & Capillary Chemistry; Methuen & Co. Ltd.: London, UK, 1926. [Google Scholar]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Tempkin, M.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327. [Google Scholar]
- Zaheer, Z.; Al-Asfar, A.; Aazam, E.S. Adsorption of methyl red on biogenic Ag@Fe nanocomposite adsorbent: Isotherms, kinetics and mechanisms. J. Mol. Liq. 2019, 283, 287–298. [Google Scholar] [CrossRef]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. equilibrium studies for acid dye adsorption onto chitosan. Langmuir 2003, 19, 7888–7894. [Google Scholar] [CrossRef]
- Mou, Y.; Yang, H.; Xu, Z. Morphology, surface layer evolution, and structure–dye adsorption relationship of porous Fe3O4 MNPs prepared by solvothermal/gas generation process. ACS Sustain. Chem. Eng. 2017, 5, 2339–2349. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.S.; Alves, F.J.; Silva, E.C.S.; Andrade, M.A.S. A novel and efficient epoxy/chitosan cement slurry for use in severe acidic environments of oil wells—Structural characterization and kinetic modeling. J. Hazard. Mater. 2012, 213–214, 109–116. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Y.-A.; Zhu, Q.-J.; Ye, C. Chitosan in molecularly-imprinted polymers: Current and future prospects. Int. J. Mol. Sci. 2015, 16, 18328–18347. [Google Scholar] [CrossRef]
- Rajar, K.; Karakus, B.; Koc, K.; Alveroglu, E. One pot synthesis and characterization of Fe3O4 nanorod-PNIPA nanogel composite for protein adsorption. Mate. Sci. Eng. C 2016, 68, 59–64. [Google Scholar] [CrossRef]
- Nikiforov, V.N.; Ignatenko, A.N.; Irkhin, V.Y. Size and surface effects on the magnetism of magnetite and maghemite nanoparticles. J. Exp. Theor. Phys. 2017, 124, 304–310. [Google Scholar] [CrossRef]
- Cui, J.-J.; Niu, C.-G.; Wang, X.-Y.; Zeng, G.-M. Facile preparation of magnetic chitosan modified with thiosemicarbazide for adsorption of copper ions from aqueous solution. J. Appl. Polym. Sci. 2017, 134, 44528. [Google Scholar] [CrossRef]
- Soares, P.P.; Barcellos, G.S.; Petzhold, C.L.; Lavayen, V. Iron oxide nanoparticles modified with oleic acid: Vibrational and phase determination. J. Phys. Chem. Solids 2016, 99, 111–118. [Google Scholar] [CrossRef]
- Tian, F.; Liu, Y.; Hu, K.; Zhao, B. The depolymerization mechanism of chitosan by hydrogen peroxide. J. Mate. Sci. 2003, 38, 4709–4712. [Google Scholar] [CrossRef]
- Gutha, Y.; Zhang, Y.; Zhang, W.; Jiao, X. Magnetic-epichlorohydrin crosslinked chitosan schiff’s base (m-ECCSB) as a novel adsorbent for the removal of Cu(II) ions from aqueous environment. Int. J. Biol. Macromol. 2017, 97, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Monier, M.; Ayad, D.M.; Abdel-Latif, D.A. Adsorption of Cu(II), Cd(II) and Ni(II) ions by cross-linked magnetic chitosan-2-aminopyridine glyoxal Schiff’s base. Colloids Surf. B Biointerfaces 2012, 94, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.C.; Ravi, N. A magnetic study of an Fe−Chitosan complex and its relevance to other biomolecules. Biomacromolecules 2000, 1, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Zhou, Y.; Jia, D. In situ mineralization of magnetite nanoparticles in chitosan hydrogel. Nanoscale Res. Lett. 2009, 4, 1041. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Adelagun, R.O.A.; Ahmad, A.L.; Unuabonah, E.I.; Bello, H.A. Preparation of magnetic, macro-reticulated cross-linked chitosan for tetracycline removal from aquatic systems. Colloids Surf. B Biointerfaces 2014, 117, 51–59. [Google Scholar] [CrossRef]
- Neto, C.G.T.; Giacometti, J.A.; Job, A.E.; Ferreira, F.C.; Fonseca, J.L.C.; Pereira, M.R. Thermal analysis of chitosan based networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Marques, J.S.; Chagas, J.A.O.D.; Fonseca, J.L.C.; Pereira, M.R. Comparing homogeneous and heterogeneous routes for ionic crosslinking of chitosan membranes. React. Funct. Polym. 2016, 103, 156–161. [Google Scholar] [CrossRef]
- Rueda, D.R.; Secall, T.; Bayer, R.K. Differences in the interaction of water with starch and chitosan films as revealed by infrared spectroscopy and differential scanning calorimetry. Carbohydr. Polym. 1999, 40, 49–56. [Google Scholar] [CrossRef]
- Kumari, M.; Pittman, C.U.; Mohan, D. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H. Thermal stability of magnetic nanoparticles coated by blends of modified chitosan and poly(quaternary ammonium) salt. J. Therm. Anal. Calorim. 2015, 119, 499–506. [Google Scholar] [CrossRef]
- Poon, L.; Wilson, L.D.; Headley, J.V. Chitosan-glutaraldehyde copolymers and their sorption properties. Carbohydr. Polym. 2014, 109, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, J.; Hou, Y. Fe3O4 nanostructures: Synthesis, growth mechanism, properties and applications. Chem. Commun. 2011, 47, 5130–5141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Li, S.; Zhou, W.; Chu, H.; Chen, W.; Li, I.L.; Tang, Z. Single crystalline trigonal selenium nanotubes and nanowires synthesized by sonochemical process. Cryst. Growth Des. 2005, 5, 911–916. [Google Scholar] [CrossRef]
- Neto, D.M.A.; Freire, R.M.; Gallo, J.; Freire, T.M.; Queiroz, D.C.; Ricardo, N.M.P.S.; Vasconcelos, I.F.; Mele, G.; Carbone, L.; Mazzetto, S.E.; et al. Rapid sonochemical approach produces functionalized Fe3O4 nanoparticles with excellent magnetic, colloidal, and relaxivity properties for MRI application. J. Phys. Chem. C 2017, 121, 24206–24222. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, O.N.; Singh, K. Shape and size-dependent magnetic properties of Fe3O4 nanoparticles synthesized using piperidine. Nanoscale Res. Lett. 2017, 12, 298. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdallah, S. Facile controllable hydrothermal route for a porous CoMn2O4 nanostructure: Synthesis, characterization, and textile dye removal from aqueous media. RSC Adv. 2016, 6, 84050–84067. [Google Scholar] [CrossRef]
- Zhou, Z.; Lin, S.; Yue, T.; Lee, T.-C. Adsorption of food dyes from aqueous solution by glutaraldehyde cross-linked magnetic chitosan nanoparticles. J. Food Eng. 2014, 126, 133–141. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of ionic and covalent crosslinking agents on properties of chitosan beads and sorption effectiveness of Reactive Black 5 dye. React. Funct. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Yarramuthi, V.; Kim, D.-S. Methyl orange removal from aqueous solution using goethite, chitosan beads and goethite impregnated with chitosan beads. J. Mol. Liq. 2017, 240, 329–339. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Kousy, S.; El-Shorbagy, H.G.; El-Ghaffar, M.A.A. Comparison between the removal of Reactive Black 5 from aqueous solutions by 3-amino-1,2,4 triazole,5-thiol and melamine grafted chitosan prepared through four different routes. J. Environ. Chem. Eng. 2016, 4, 733–745. [Google Scholar] [CrossRef]
- Aguiar, J.E.; de Oliveira, J.C.A.; Silvino, P.F.G.; Neto, J.A.; Silva, I.J.; Lucena, S.M.P. Correlation between PSD and adsorption of anionic dyes with different molecular weigths on activated carbon. Colloids Surf. A Physicochem. Eng. Asp. 2016, 496, 125–131. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Li, G.; Liu, Y.; Huang, J.; Yang, D.-P. Hydrothermal synthesis of 3D hollow porous Fe3O4 microspheres towards catalytic removal of organic pollutants. Nanoscale Res. Lett. 2014, 9, 648. [Google Scholar] [CrossRef][Green Version]
- Zhou, L.; Jin, J.; Liu, Z.; Liang, X.; Shang, C. Adsorption of acid dyes from aqueous solutions by the ethylenediamine-modified magnetic chitosan nanoparticles. J. Hazard. Mater. 2011, 185, 1045–1052. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Park, S.-S.; Cho, S.-Y. Adsorption characteristics of Reactive Black 5 onto chitosan beads cross-linked with epichlorohydrin. J. Ind. Eng. Chem. 2012, 18, 1458–1464. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K. Relating interactions of dye molecules with chitosan to adsorption kinetic data. Langmuir 2010, 26, 9617–9626. [Google Scholar] [CrossRef]
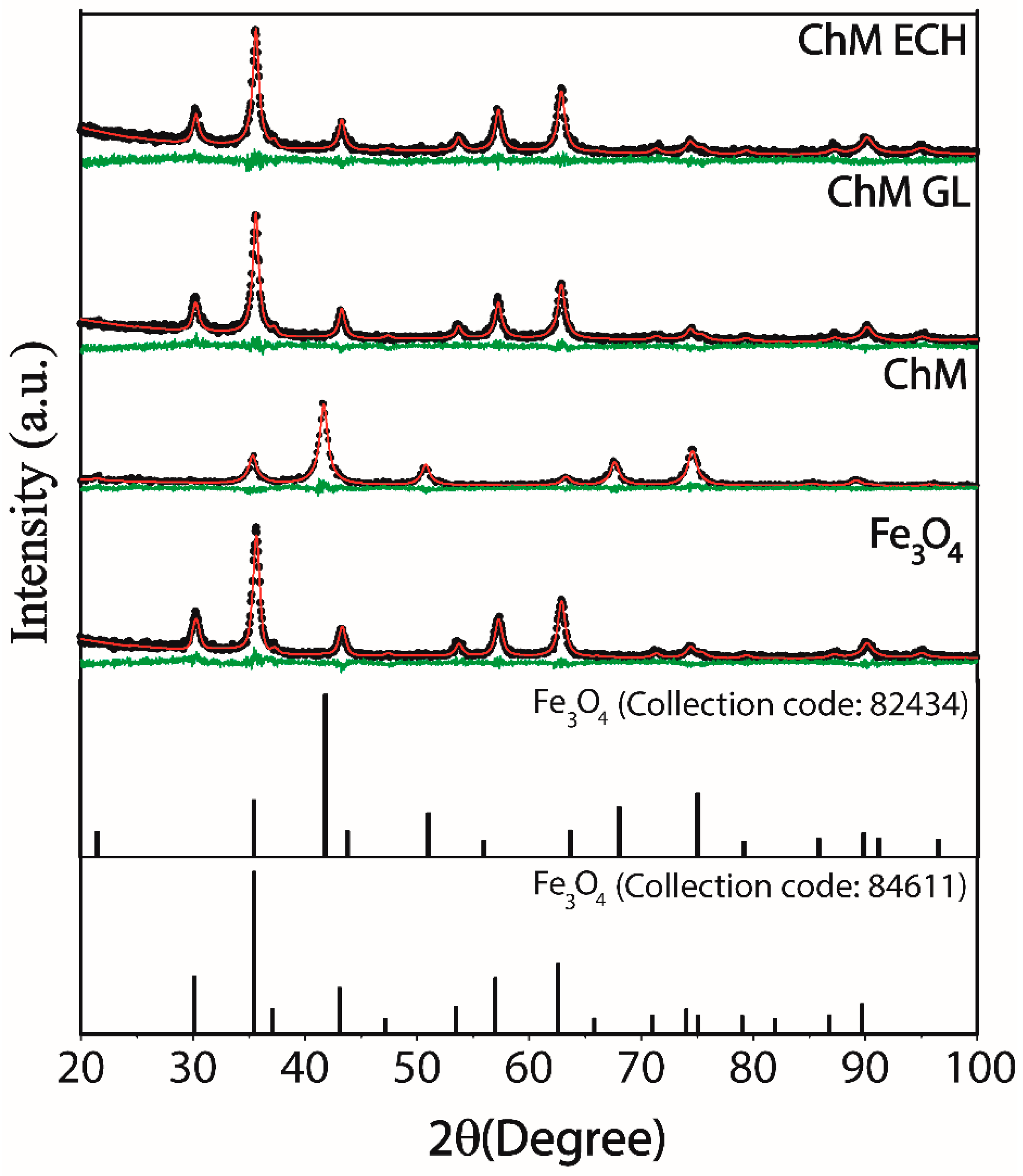
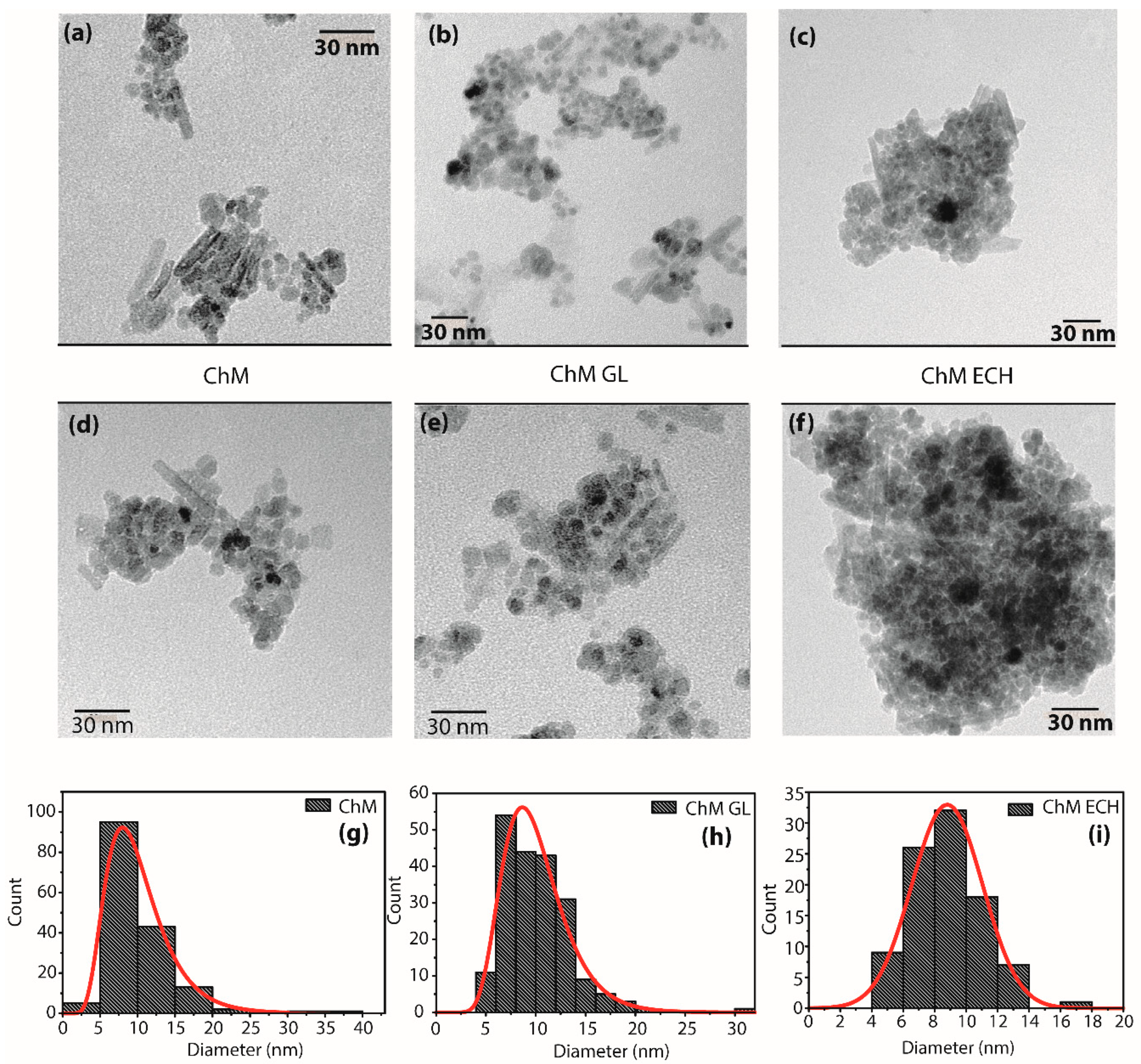


| Samples | Rwp (%) | χ2 | a, b and c (Å) | D (nm) |
|---|---|---|---|---|
| Fe3O4 | 11.73 | 1.04 | 8.3625 | 11.60 ± 0.11 |
| ChM | 14.75 | 0.94 | 8.3655 | 13.33 ± 0.47 |
| ChM GL | 12.39 | 1.14 | 8.3533 | 11.92 ± 0.16 |
| ChM ECH | 11.80 | 1.02 | 8.3548 | 11.74 ± 1.73 |
| Element | Binding Energy (eV) | Atomic Fraction (%) | Assignments |
|---|---|---|---|
| C 1s | 284.9 | 34.36 | C–C |
| 286.5 | C–OH; C–NH2 | ||
| 288.2 | O–C–O | ||
| N 1s | 399.6 | 3.88 | NH2 |
| 400.6 | NH2---O | ||
| 402.0 | NH2---Fe | ||
| O 1s | 529.8 | 51.7 | Fe–O |
| 530.9 | –OH | ||
| 533.0 | –OH---Fe | ||
| Fe 2p3/2 | 710.1 | 10.6 | Fe2+ Oct |
| 711.3 | Fe3+ Thd | ||
| 713.3 | Fe3+ Oct | ||
| Fe 2p1/2 | 723.4 | Fe2+ Oct | |
| 724.4 | Fe3+ Oct | ||
| 727.7 | Fe3+ Thd |
| Reactive Black 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Pseudo-first-order | Pseudo-second–order | Intraparticle diffusion | ||||||
| R2 | qe (mg g−1) | k1 (min−1) | R2 | qe (mg g−1) | k2 (g mg−1 min−1) | Kp (g mg−1 t−0.5) | C | R2 | |
| ChM | 0.9770 | 33.420 | 0.14675 | 0.9998 | 33.200 | 0.01180 | 7.7541 | 1.4071 | 0.9682 |
| ChM GL | 0.9714 | 23.816 | 0.4779 | 0.9954 | 24.485 | 0.00270 | 1.7490 | 4.759 | 0.9976 |
| ChM ECH | 0.9532 | 12.062 | 0.00673 | 0.9870 | 35.211 | 0.00206 | 0.6260 | 19.949 | 0.9856 |
| Methyl orange | |||||||||
| ChM | 0.9828 | 8.682 | 0.1045 | 0.9999 | 27.762 | 0.03858 | 3.2495 | 15.2461 | 0.9890 |
| ChM GL | – | – | – | 0.9994 | 18.175 | −0.05491 | 2.0206 | 13.5764 | 0.9786 |
| ChM ECH | 0.9798 | 15.807 | 0.8833 | 0.9990 | 26.055 | 0.02207 | 4.2461 | 8.5636 | 0.8749 |
| Dye | Reactive Black 5 | Methyl Orange | ||||||
|---|---|---|---|---|---|---|---|---|
| Langmuir | Adj. R2 | qm (mg g−1) | Ka (L mg−1) | Adj. R2 | qm (mg g−1) | Ka (L mg−1) | ||
| ChM | 0.913 | 50.004 | 0.820 | 0.903 | 68.004 | 0.402 | ||
| ChM ECH | 0.978 | 36.944 | 0.932 | 0.989 | 20.386 | 0.0229 | ||
| ChM GL | 0.923 | 33.630 | 0.523 | 0.964 | 24.647 | 0.0231 | ||
| Freundlich | Adj. R2 | Kf (L g−1) | n | Adj. R2 | Kf (L g−1) | n | ||
| ChM | 0.873 | 21.438 | 5.390 | 0.861 | 25.652 | 5.071 | ||
| ChM ECH | 0.907 | 17.346 | 6.053 | 0.957 | 2.248 | 2.617 | ||
| ChM GL | 0.938 | 14.531 | 5.774 | 0.996 | 2.633 | 2.515 | ||
| Redlich–Peterson | Adj. R2 | Krp (L mg−1) | Arp (L mg−1)β | β | Adj. R2 | Krp (L mg−1) | Arp (L mg−1)β | β |
| ChM | 0.900 | 47.918 | 1.167 | 0.955 | 0.886 | 30.964 | 0.545 | 0.962 |
| ChM ECH | 0.984 | 40.674 | 1.302 | 0.960 | 0.973 | 0.876 | 0.154 | 0.776 |
| ChM GL | 0.926 | 48.866 | 2.699 | 0.871 | 0.986 | 1.053 | 0.154 | 0.768 |
| Temkin | Adj. R2 | KT (L mg−1) | B (J mol−1) | Adj. R2 | KT (L mg−1) | B (J mol−1) | ||
| ChM | 0.899 | 25.003 | 6.4895 | 0.887 | 10.636 | 9.3778 | ||
| ChM ECH | 0.953 | 35.964 | 4.6139 | 0.983 | 0.194 | 4.6397 | ||
| ChM GL | 0.950 | 25.201 | 4.1678 | 0.978 | 0.299 | 4.9853 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, T.M.; Fechine, L.M.U.D.; Queiroz, D.C.; Freire, R.M.; Denardin, J.C.; Ricardo, N.M.P.S.; Rodrigues, T.N.B.; Gondim, D.R.; Junior, I.J.S.; Fechine, P.B.A. Magnetic Porous Controlled Fe3O4–Chitosan Nanostructure: An Ecofriendly Adsorbent for Efficient Removal of Azo Dyes. Nanomaterials 2020, 10, 1194. https://doi.org/10.3390/nano10061194
Freire TM, Fechine LMUD, Queiroz DC, Freire RM, Denardin JC, Ricardo NMPS, Rodrigues TNB, Gondim DR, Junior IJS, Fechine PBA. Magnetic Porous Controlled Fe3O4–Chitosan Nanostructure: An Ecofriendly Adsorbent for Efficient Removal of Azo Dyes. Nanomaterials. 2020; 10(6):1194. https://doi.org/10.3390/nano10061194
Chicago/Turabian StyleFreire, Tiago M., Lillian M. U. D. Fechine, Danilo C. Queiroz, Rafael M. Freire, Juliano C. Denardin, Nágila M. P. S. Ricardo, Thaina N. B. Rodrigues, Diego R. Gondim, Ivanildo J. S. Junior, and Pierre B. A. Fechine. 2020. "Magnetic Porous Controlled Fe3O4–Chitosan Nanostructure: An Ecofriendly Adsorbent for Efficient Removal of Azo Dyes" Nanomaterials 10, no. 6: 1194. https://doi.org/10.3390/nano10061194
APA StyleFreire, T. M., Fechine, L. M. U. D., Queiroz, D. C., Freire, R. M., Denardin, J. C., Ricardo, N. M. P. S., Rodrigues, T. N. B., Gondim, D. R., Junior, I. J. S., & Fechine, P. B. A. (2020). Magnetic Porous Controlled Fe3O4–Chitosan Nanostructure: An Ecofriendly Adsorbent for Efficient Removal of Azo Dyes. Nanomaterials, 10(6), 1194. https://doi.org/10.3390/nano10061194