Surface States of (100) O-Terminated Diamond: Towards Other 1 × 1:O Reconstruction Models
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. C1s Spectra Comparison
3.2. O-Terminated Diamond C1s Spectra Deconvolution
3.3. O1s Spectra Comparison
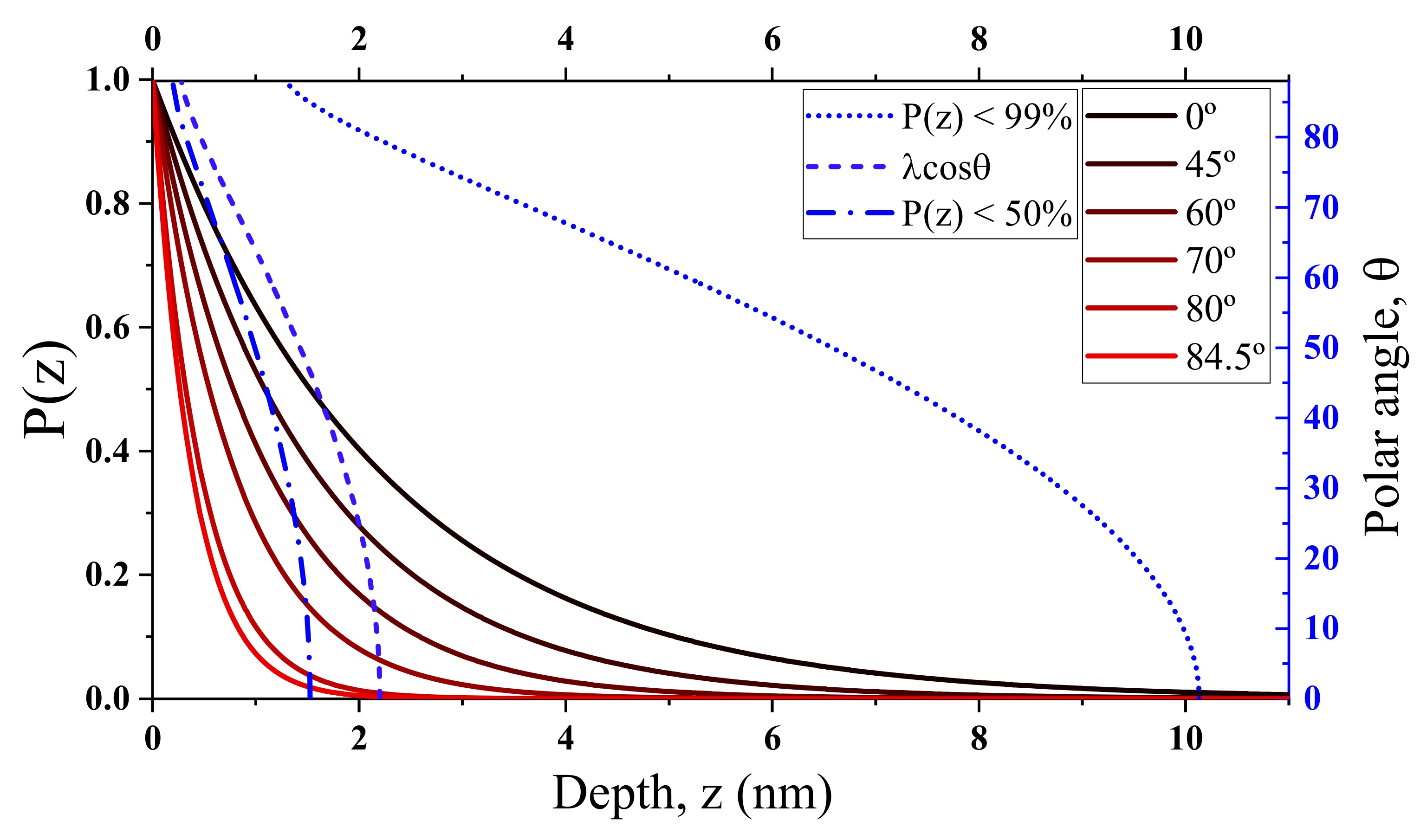
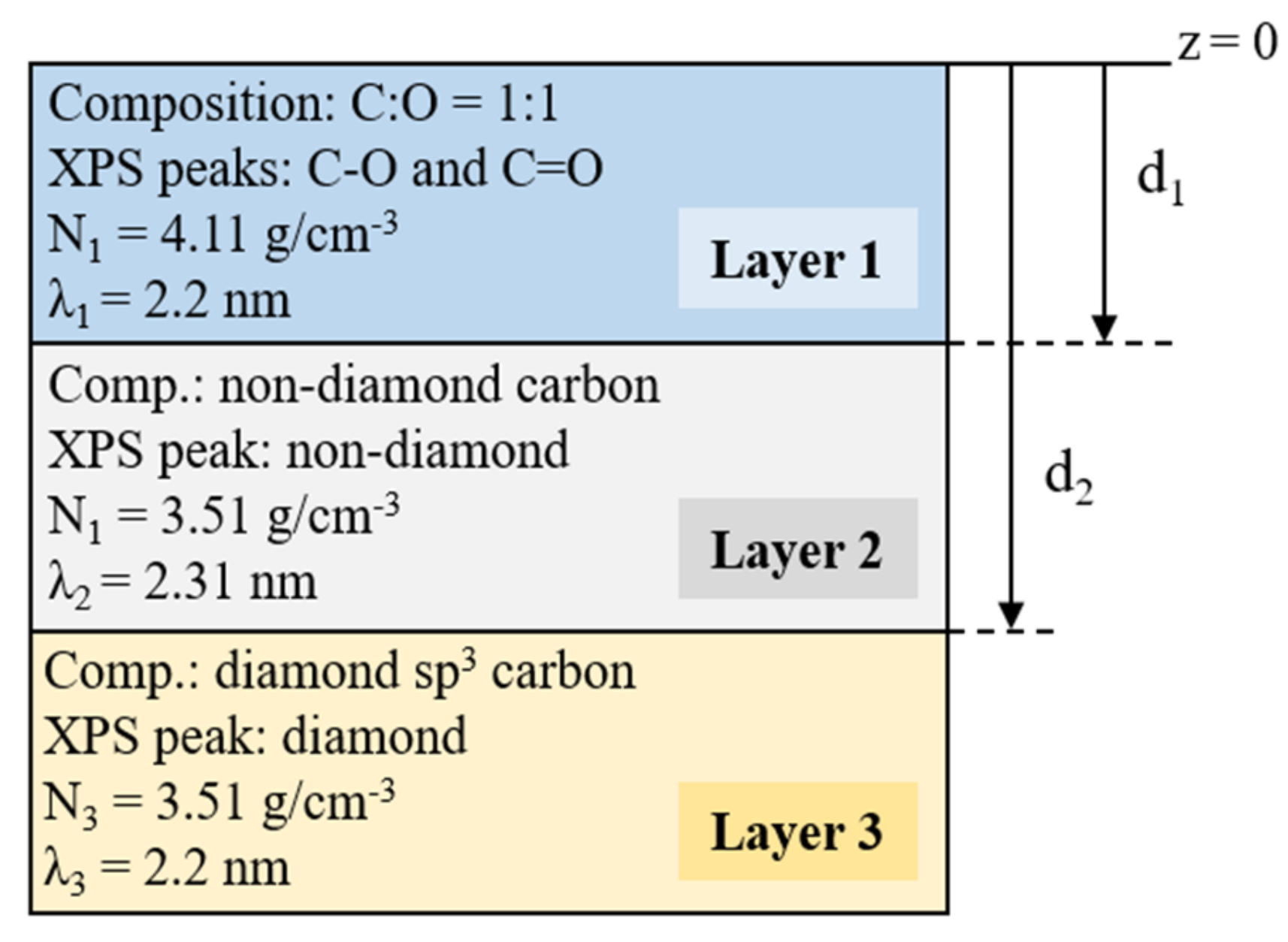
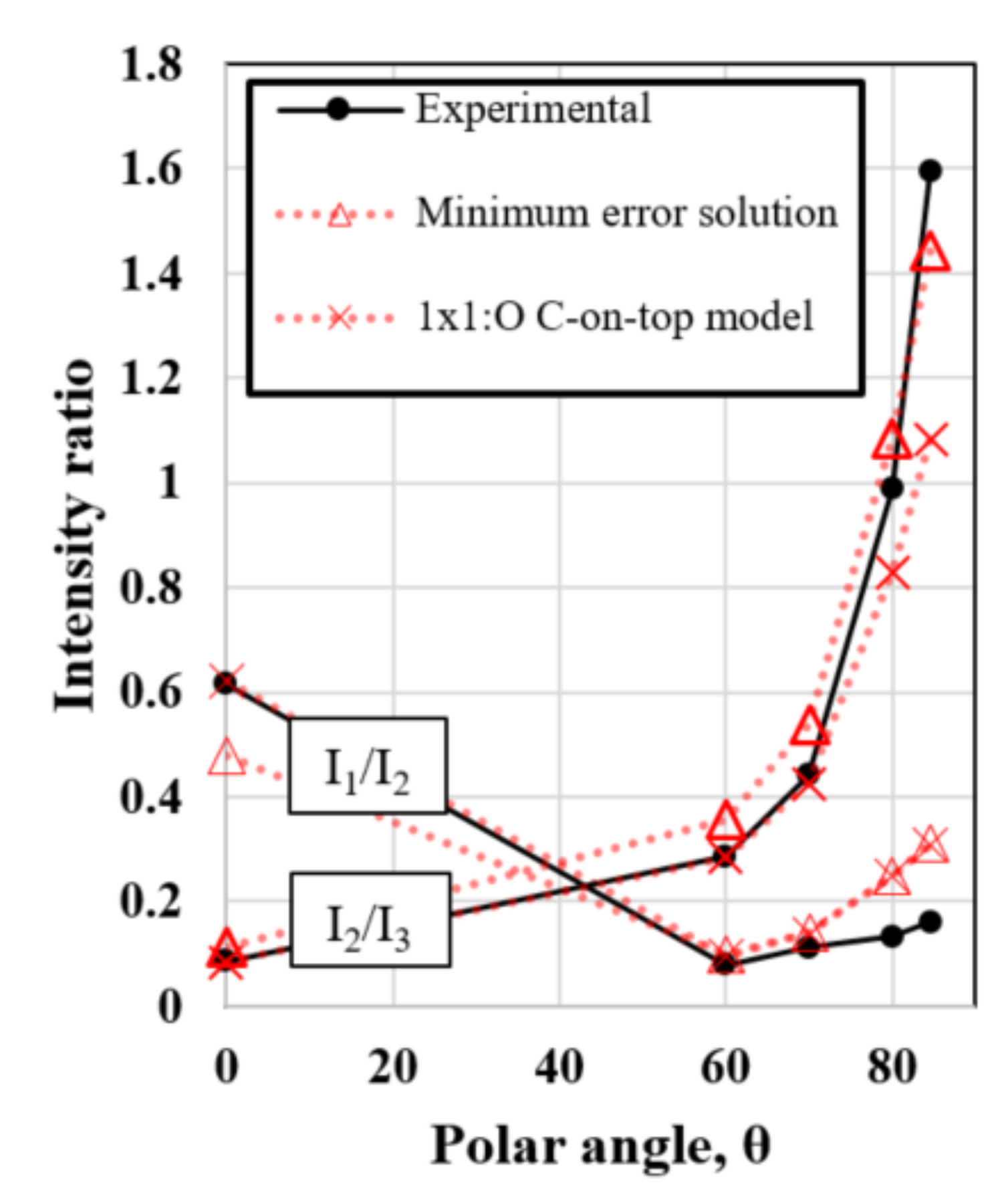
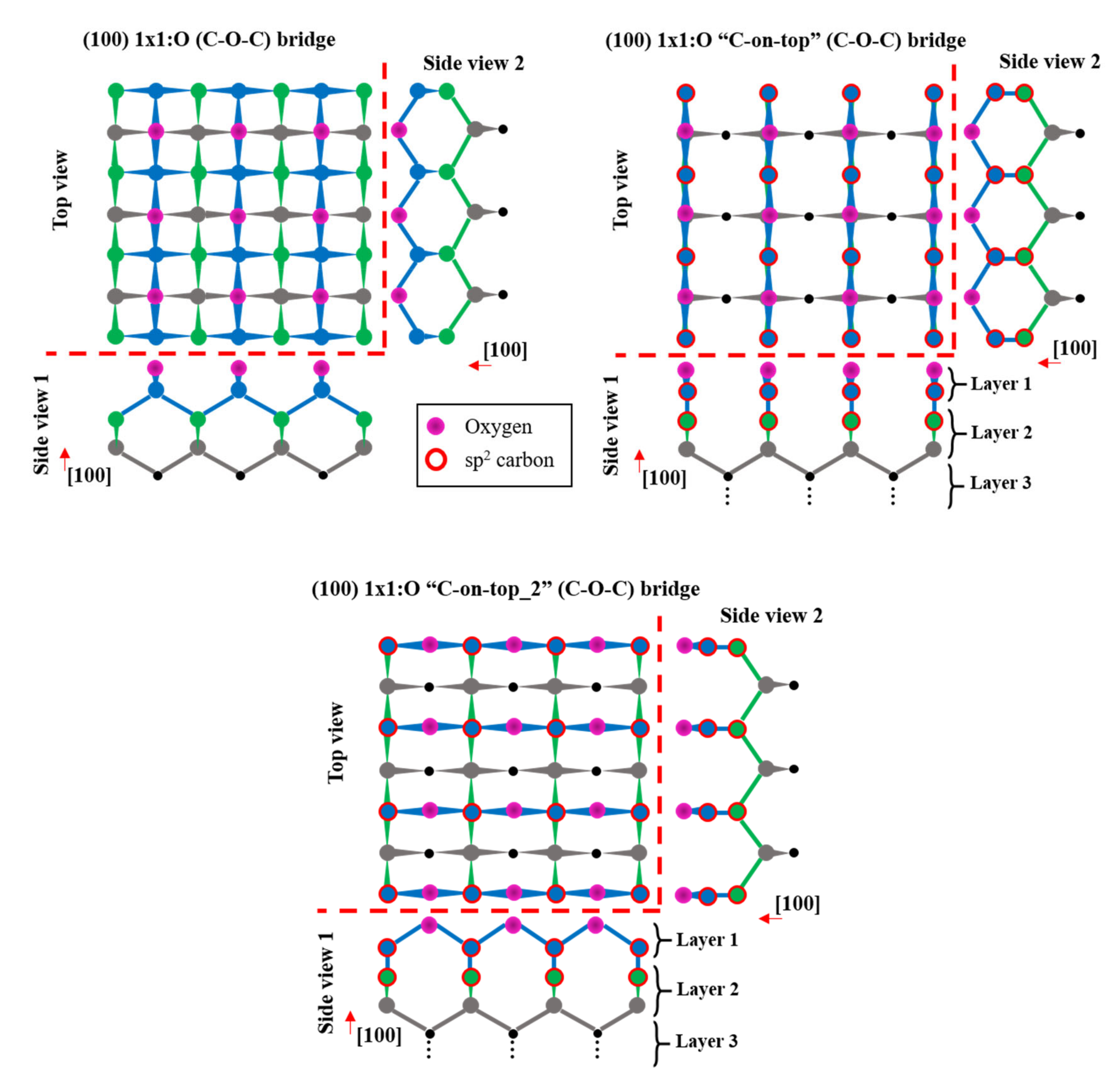
3.4. Surface Model and Quantification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balmer, R.S.; Friel, I.; Woollard, S.M.; Wort, C.J.; Scarsbrook, G.A.; Coe, S.E.; El-Hajj, H.; Kaiser, A.; Denisenko, A.; Kohn, E.; et al. Unlocking diamond’s potential as an electronic material. Philos. Trans. A Math. Phys. Eng. Sci. 2008, 366, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, A.; Kohn, E. Diamond power devices. Concepts and limits. Diam. Relat. Mater. 2005, 14, 491–498. [Google Scholar] [CrossRef]
- Kawarada, H. Hydrogen-terminated diamond surfaces and interfaces. Surf. Sci. Rep. 1996, 26, 205–259. [Google Scholar] [CrossRef]
- Ristein, J. Surface science of diamond: Familiar and amazing. Surf. Sci. 2006, 600, 3677–3689. [Google Scholar] [CrossRef]
- Edmonds, M.T.; Pakes, C.I.; Ley, L. Self-consistent solution of the Schrödinger-Poisson equations for hydrogen-terminated diamond. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 1–10. [Google Scholar] [CrossRef]
- Winn, M.; Rassinger, M.; Hafner, J. Atomic and electronic structure of the diamond (100) surface: Reconstructions and rearrangements at high hydrogen coverage. Phys. Rev. B Condens. Matter Mater. Phys. 1997, 55, 5364–5375. [Google Scholar] [CrossRef]
- Krainsky, I.L.; Asnin, V.M. Negative electron affinity mechanism for diamond surfaces. Appl. Phys. Lett. 1998, 72, 2574–2576. [Google Scholar] [CrossRef]
- Stallcup, R.E.; Villarreal, L.M.; Lim, S.C.; Akwani, I.; Aviles, A.F.; Perez, J.M. Atomic structure of the diamond (100) surface studied using scanning tunneling microscopy. J. Vac. Sci. Technol. B Microelectron Nanom. Struct. 1996, 14, 929–932. [Google Scholar] [CrossRef]
- Navas, J.; Araujo, D.; Piñero, J.C.; Sánchez-Coronilla, A.; Blanco, E.; Villar, P.; Alcántara, R.; Montserrat, J.; Florentin, M.; Eon, D.; et al. Oxygen termination of homoepitaxial diamond surface by ozone and chemical methods: An experimental and theoretical perspective. Appl. Surf. Sci. 2018, 433, 408–418. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, J.K.; Lee, J.L.; Baik, Y.J. Pretreatment effects by aqua-regia solution on field emission of diamond film. Appl. Phys. Lett. 2000, 76, 3694–3696. [Google Scholar] [CrossRef]
- Scharpf, J.; Denisenko, A.; Pietzka, C.; Kohn, E. Effect of surface defects by RF oxygen plasma on the electrical properties of thin boron-doped diamond layers in electrolyte. Diam. Relat. Mater. 2011, 20, 1250–1254. [Google Scholar] [CrossRef]
- Teraji, T.; Koizumi, S.; Koide, Y. Ohmic contact for p -type diamond without postannealing. J. Appl. Phys. 2008, 104, 10–13. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Wang, X.; Zhang, M.; Wang, H. Barrier Heights of Au on Diamond with Different Terminations Determined by X-ray Photoelectron Spectroscopy. Coatings 2017, 7, 88. [Google Scholar]
- Thomas, R.E.; Rudder, R.A.; Markunas, R.J. Thermal desorption from hydrogenated and oxygenated diamond (100) surfaces. J. Vac. Sci. Technol. A Vac. Surf. Film. 1992, 10, 2451–2457. [Google Scholar] [CrossRef]
- Loh, K.P.; Xie, X.N.; Lim, Y.H.; Teo, E.J.; Zheng, J.C.; Ando, T. Surface oxygenation studies on (1 0 0)-oriented diamond using an atom beam source and local anodic oxidation. Surf. Sci. 2002, 505, 93–114. [Google Scholar] [CrossRef]
- Maier, F.; Ristein, J.; Ley, L. Electron affinity of plasma-hydrogenated and chemically oxidized diamond (100) surfaces. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 64, 1–7. [Google Scholar] [CrossRef]
- Wang, M.; Simon, N.; Decorse-Pascanut, C.; Bouttemy, M.; Etcheberry, A.; Li, M.; Boukherroub, R.; Szunerits, S. Comparison of the chemical composition of boron-doped diamond surfaces upon different oxidation processes. Electrochim. Acta 2009, 54, 5818–5824. [Google Scholar] [CrossRef]
- Pehrsson, P.E.; Mercer, T.W.; Chaney, J.A. Oxidation of the hydrogenated diamond (100) surface. Surf. Sci. 2002, 497, 13–28. [Google Scholar] [CrossRef]
- Skokov, S.; Weiner, B.; Frenklach, M. Molecular-dynamics study of oxygenated (100) diamond surfaces. Phys. Rev. B 1994, 49, 11374. [Google Scholar] [CrossRef]
- Zheng, J.C.; Xie, X.N.; Wee, A.T.S.; Loh, K.P. Oxygen-induced surface state on diamond (100). Diam. Relat. Mater. 2001, 10, 500–505. [Google Scholar] [CrossRef]
- Koinkar, P.M.; Patil, P.P.; More, M.A.; Tondare, V.N.; Joag, D.S. Field emission studies of CVD diamond thin films: Effect of acid treatment. Vacuum 2003, 72, 321–326. [Google Scholar] [CrossRef]
- Evans, T.; Phaal, C. The kinetics of the diamond-oxygen reaction. Conf. Carbon 1961, 5, 147–153. [Google Scholar]
- Davies, G.; Evans, T. Graphitization of diamond at zero pressure and at a high pressure. Proc. R. Soc. London. A Math. Phys. Sci. 1972, 328, 413–427. [Google Scholar]
- Khmelnitsky, R.A.; Gippius, A.A. Transformation of diamond to graphite under heat treatment at low pressure. Phase Transit. 2014, 87, 175–192. [Google Scholar] [CrossRef]
- Butenko, Y.V.; Kuznetsov, V.L.; Chuvilin, A.L.; Kolomiichuk, V.N.; Stankus, S.V.; Khairulin, R.A.; Segall, B. Kinetics of the graphitization of dispersed diamonds at “low” temperatures. J. Appl. Phys. 2000, 88, 4380–4388. [Google Scholar] [CrossRef]
- Petit, T.; Arnault, J.C.; Girard, H.A.; Sennour, M.; Bergonzo, P. Early stages of surface graphitization on nanodiamond probed by x-ray photoelectron spectroscopy. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 84, 1–5. [Google Scholar] [CrossRef]
- Joshi, A.; Nimmagadda, R.; Herrington, J. Oxidation kinetics of diamond, graphite, and chemical vapor deposited diamond films by thermal gravimetry. J. Vac. Sci. Technol. A Vac. Surf. Film. 1990, 8, 2137–2142. [Google Scholar] [CrossRef]
- Pehrsson, P.E.; Mercer, T.W. Oxidation of heated diamond C(100):H surfaces. Surf. Sci. 2000, 460, 74–90. [Google Scholar] [CrossRef]
- Alba, G.; Eon, D.; Villar, M.P.; Alcántara, R.; Chicot, G.; Cañas, J.; Letellier, J.; Pernot, J.; Araujo, D. H-Terminated Diamond Surface Band Bending Characterization by Angle-Resolved XPS. Surfaces 2020, 3, 61–71. [Google Scholar] [CrossRef]
- Cañas, J.; Alba, G.; Leinen, D.; Lloret, F.; Gutierrez, M.; Eon, D.; Pernot, J.; Gheeraert, E.; Araujo, D. Diamond/γ-alumina band offset determination by XPS. Appl. Surf. Sci. 2020, 146301. [Google Scholar] [CrossRef]
- Kono, S.; Teraji, T.; Kodama, H.; Ichikawa, K.; Ohnishi, S.; Sawabe, A. Direct determination of the barrier height of Ti-based ohmic contact on p-type diamond (001). Diam. Relat. Mater. 2015, 60, 117–122. [Google Scholar] [CrossRef]
- Kubovic, M.; Kasu, M. Enhancement and stabilization of hole concentration of hydrogen-terminated diamond surface using ozone adsorbates. Jpn. J. Appl. Phys. 2010, 49, 11–14. [Google Scholar] [CrossRef]
- Gao, X.; Liu, L.; Qi, D.; Chen, S.; Wee, A.T.; Ouyang, T.; Loh, K.P.; Yu, X.; Moser, H.O. Water-induced negative electron affinity on diamond (100). J. Phys. Chem. C 2008, 112, 2487–2491. [Google Scholar] [CrossRef]
- Kono, S.; Kageura, T.; Hayashi, Y.; Ri, S.G.; Teraji, T.; Takeuchi, D.; Ogura, M.; Kodama, H.; Sawabe, A.; Inaba, M.; et al. Carbon 1s X-ray photoelectron spectra of realistic samples of hydrogen-terminated and oxygen-terminated CVD diamond (111) and (001). Diam. Relat. Mater. 2019, 93, 105–130. [Google Scholar] [CrossRef]
- Denisenko, A.; Romanyuk, A.; Pietzka, C.; Scharpf, J.; Kohn, E. Electronic surface barrier properties of fluorine-terminated boron-doped diamond in electrolytes. Surf. Sci. 2011, 605, 632–637. [Google Scholar] [CrossRef]
- Pietzka, C.; Denisenko, A.; Romanyuk, A.; Schäfer, P.J.; Kibler, L.A.; Scharpf, J.; Kohn, E. Electronic surface barrier properties of boron-doped diamond oxidized by plasma treatment. Diam. Relat. Mater. 2010, 19, 213–216. [Google Scholar] [CrossRef]
- John, P.; Polwart, N.; Troupe, C.E.; Wilson, J.I. The oxidation of diamond: The geometry and stretching frequency of carbonyl on the (100) surface. J. Am. Chem. Soc. 2003, 125, 6600–6601. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Kono, S.; Saitou, T.; Kawata, H.; Goto, T.; Kakefuda, Y.; Komeda, T. Characteristic energy band values and electron attenuation length of a chemical-vapor-deposition diamond (0 0 1) 2 × 1 surface. Surf. Sci. 2009, 603, 860–866. [Google Scholar] [CrossRef]
- Tougaard, P.; Simund, S. Elastic and inelastic scattering of electrons reflected from solids: Effects on energy spectra. Phys. Rev. B 1982, 25, 4452–4466. [Google Scholar] [CrossRef]
- Maier, F.; Riedel, M.; Mantel, B.; Ristein, J.; Ley, L. Origin of Surface Conductivity in Diamond. Phys. Rev. Lett. 2000, 85, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Kono, S.; Saito, T.; Kang, S.H.; Jung, W.Y.; Kim, B.Y.; Kawata, H.; Goto, T.; Kakefuda, Y.; Yeom, H.W. Band diagram for chemical vapor deposition diamond surface conductive layer: Presence of downward band bending due to shallow acceptors. Surf. Sci. 2010, 604, 1148–1165. [Google Scholar] [CrossRef]
- Torrengo, S.; Canteri, R.; Dell’Anna, R.; Minati, L.; Pasquarelli, A.; Speranza, G. XPS and ToF-SIMS investigation of nanocrystalline diamond oxidized surfaces. Appl. Surf. Sci. 2013, 276, 101–111. [Google Scholar] [CrossRef]
- Hontoria-Lucas, C.; López-Peinado, A.J.; López-González, J.d.; Rojas-Cervantes, M.L.; Martín-Aranda, R.M. Study of oxygen-containing groups in a series of graphite oxides: Physical and chemical characterization. Carbon N. Y. 1995, 33, 1585–1592. [Google Scholar] [CrossRef]
- Titantah, J.T.; Lamoen, D. Sp3/sp2 characterization of carbon materials from first-principles calculations: X-ray photoelectron versus high energy electron energy-loss spectroscopy techniques. Carbon N. Y. 2005, 43, 1311–1316. [Google Scholar] [CrossRef]
- Sette, F.; Wertheim, G.K.; Ma, Y.; Meigs, G.; Modesti, S.; Chen, C.T. Lifetime and screening of the C 1s photoemission in graphite. Phys. Rev. B 1990, 41, 9766–9770. [Google Scholar] [CrossRef]
- Stacey, A.; Dontschuk, N.; Chou, J.P.; Broadway, D.A.; Schenk, A.K.; Sear, M.J.; Tetienne, J.P.; Hoffman, A.; Prawer, S.; Pakes, C.I.; et al. Evidence for Primal sp 2 Defects at the Diamond Surface: Candidates for Electron Trapping and Noise Sources. Adv. Mater. Interfaces 2019, 6, 1–8. [Google Scholar] [CrossRef]
- de Theije, F.K.; Reedijk, M.F.; Arsic, J.; van Enckevort, W.J.P.; Vlieg, E. Atomic structure of diamond (111) surfaces etched in oxygen water vapor. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 64, 1–7. [Google Scholar] [CrossRef]
- Bobrov, K.; Mayne, A.; Comtet, G.; Dujardin, G.; Hellner, L.; Hoffman, A. Atomic-scale visualization and surface electronic structure of the hydrogenated diamond C(100)-(2×1):H surface. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 68, 195416. [Google Scholar] [CrossRef]
- Fadley, C.S. Instrumentation for surface studies: XPS angular distributions. J. Electron Spectros. Relat. Phenom. 1974, 5, 725–754. [Google Scholar] [CrossRef]
- Bernstein, R.W.; Grepstad, J.K. XPS intensity analysis for assessment of thickness and composition of thin overlayer films: Application to chemically etched GaAs (100) Surfaces. Surf. Interface Anal. 1989, 14, 109–114. [Google Scholar] [CrossRef]
- Kunz, C.; Cowie, B.C.; Drube, W.; Lee, T.L.; Thiess, S.; Wild, C.; Zegenhagen, J. Relative electron inelastic mean free paths for diamond and graphite at 8 keV and intrinsic contributions to the energy-loss. J. Electron Spectros. Relat. Phenom. 2009, 173, 29–39. [Google Scholar] [CrossRef]
- Shinotsuka, H.; Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. X. Data for 41 elemental solids over the 50eV to 200keV range with the relativistic full Penn algorithm. Surf. Interface Anal. 2015, 47, 871–888. [Google Scholar] [CrossRef]
- Nakamura, J.; Ito, T. Oxidization process of CVD diamond (1 0 0):H 2 × 1 surfaces. Appl. Surf. Sci. 2005, 244, 301–304. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alba, G.; Villar, M.P.; Alcántara, R.; Navas, J.; Araujo, D. Surface States of (100) O-Terminated Diamond: Towards Other 1 × 1:O Reconstruction Models. Nanomaterials 2020, 10, 1193. https://doi.org/10.3390/nano10061193
Alba G, Villar MP, Alcántara R, Navas J, Araujo D. Surface States of (100) O-Terminated Diamond: Towards Other 1 × 1:O Reconstruction Models. Nanomaterials. 2020; 10(6):1193. https://doi.org/10.3390/nano10061193
Chicago/Turabian StyleAlba, Gonzalo, M. Pilar Villar, Rodrigo Alcántara, Javier Navas, and Daniel Araujo. 2020. "Surface States of (100) O-Terminated Diamond: Towards Other 1 × 1:O Reconstruction Models" Nanomaterials 10, no. 6: 1193. https://doi.org/10.3390/nano10061193
APA StyleAlba, G., Villar, M. P., Alcántara, R., Navas, J., & Araujo, D. (2020). Surface States of (100) O-Terminated Diamond: Towards Other 1 × 1:O Reconstruction Models. Nanomaterials, 10(6), 1193. https://doi.org/10.3390/nano10061193







