Nanocomposite Fiber Based on Natural Material for Water Disinfection under Visible Light Irradiation
Abstract
1. Introduction
2. Experimental Section
2.1. Composite Nano-Fiber (CNF) Generation by the Electrospinning Process
2.2. Material Characterization
2.3. Evaluation of the Composite Nanofibers’ Photocatalytic Activity
2.3.1. Methylene Blue Bleaching Test
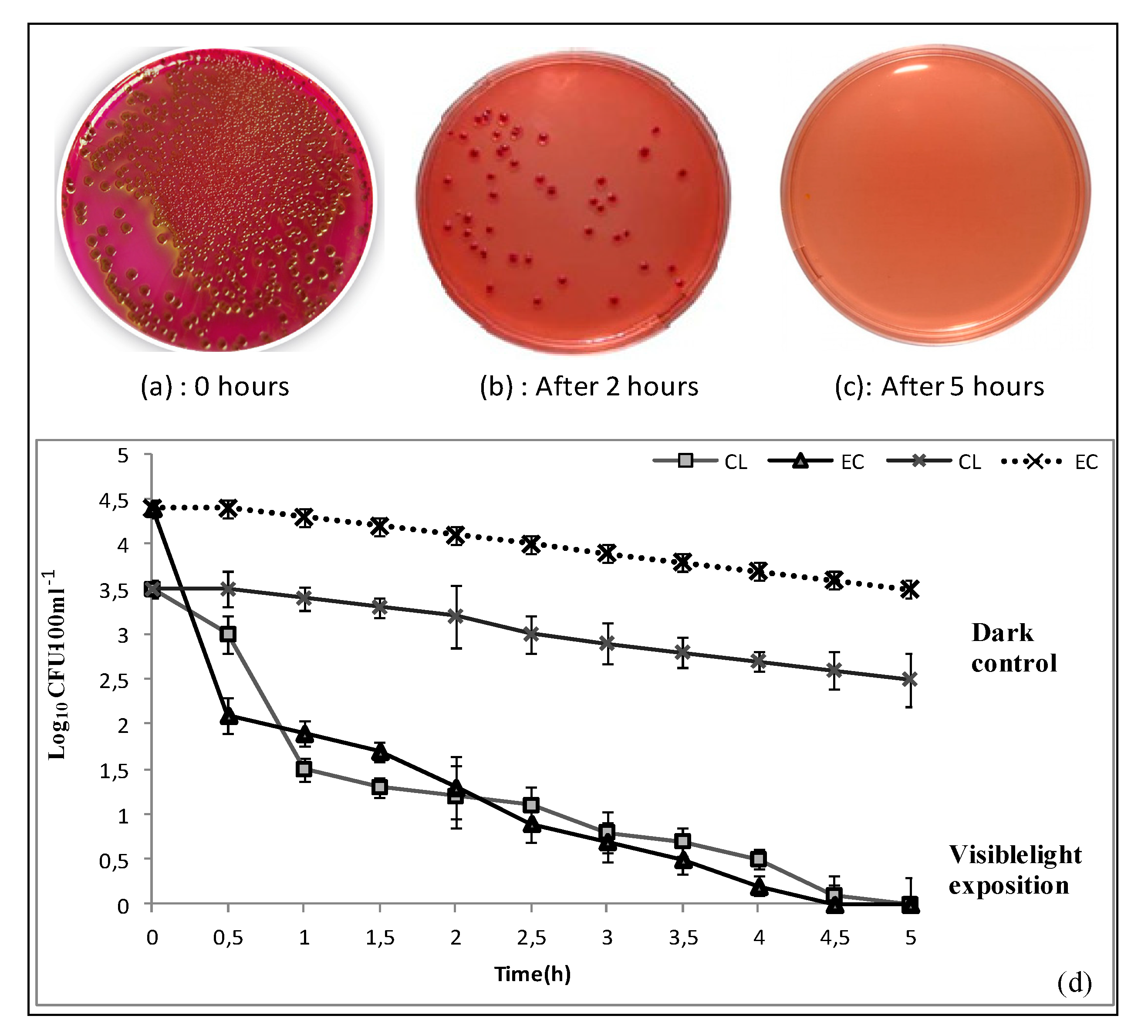
2.3.2. Bacterial Inactivation
3. Results and Discussion
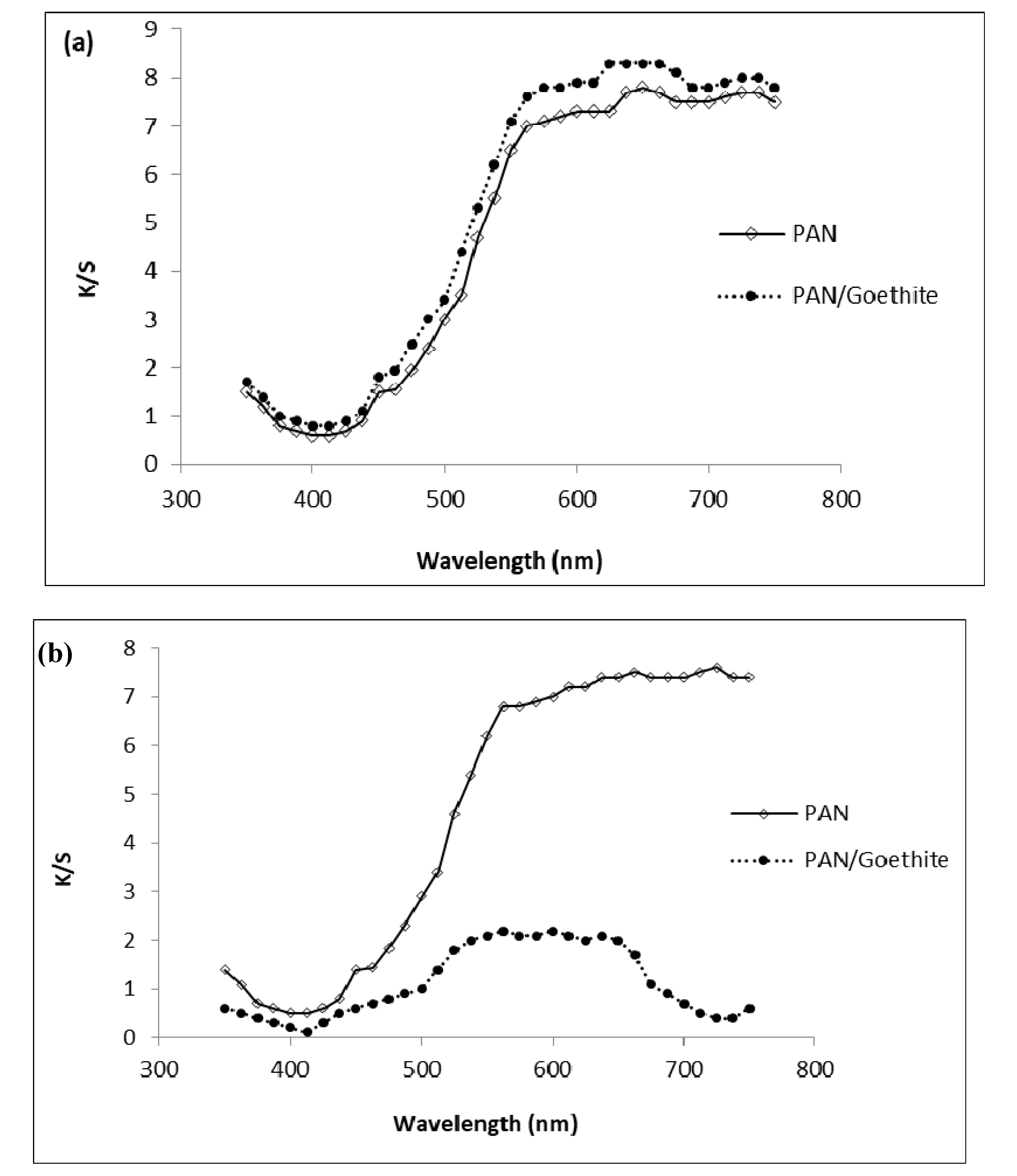
3.1. Nanoparticle and Nanocomposite Membrane Characterization
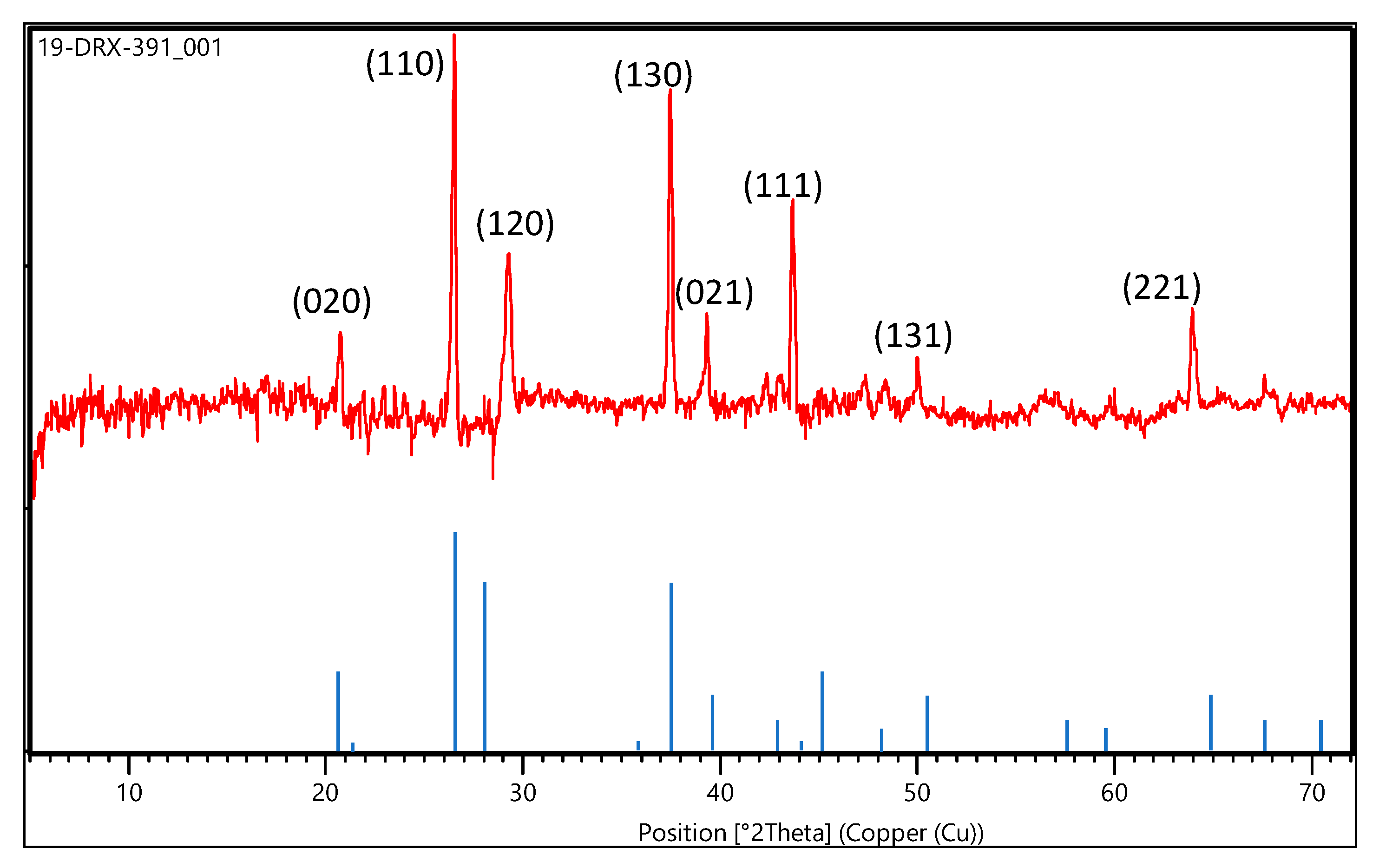
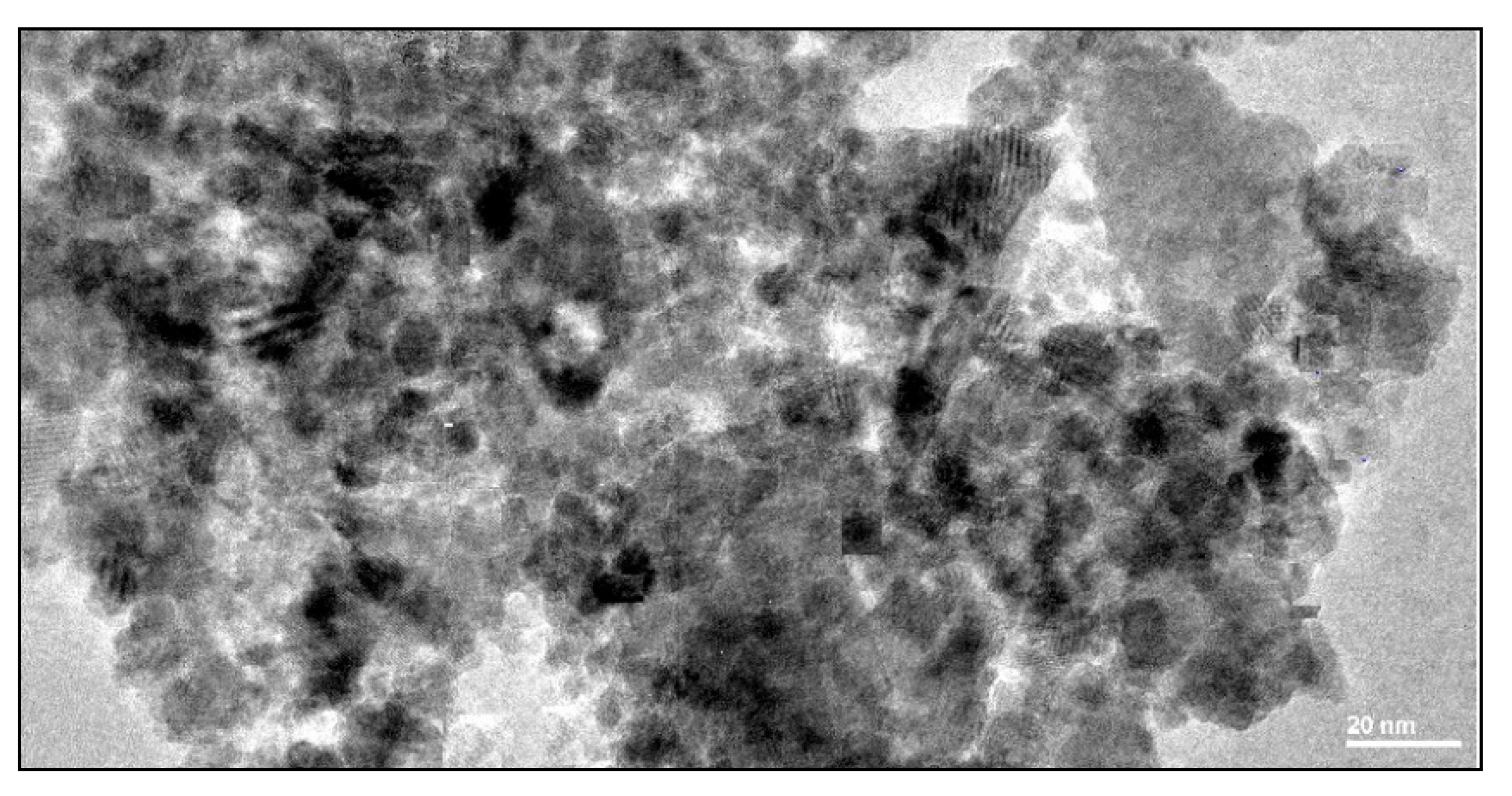
3.1.1. Characterization of Goethite Nanoparticles
3.1.2. Characterization of Goethite Nanocomposite Fiber
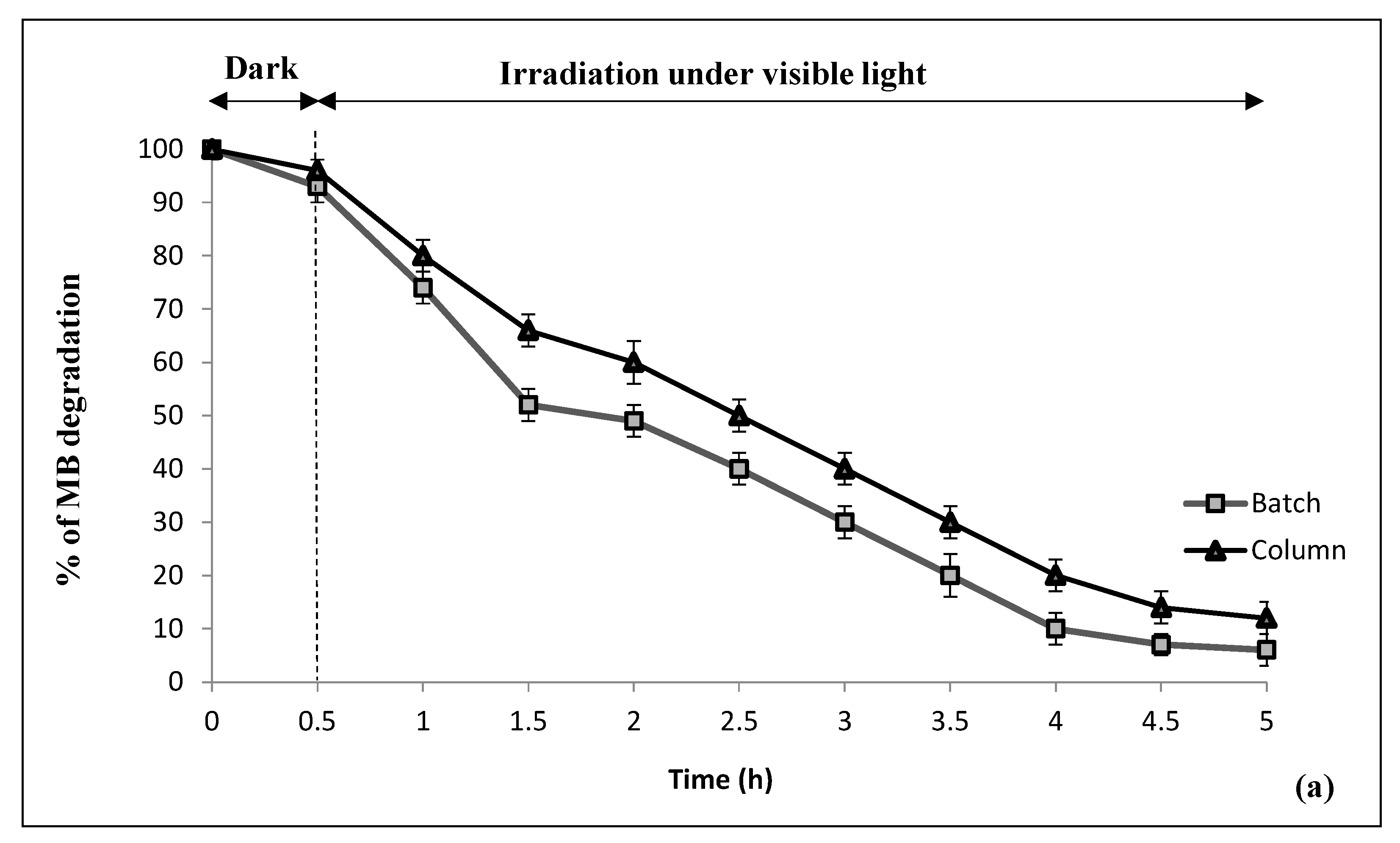
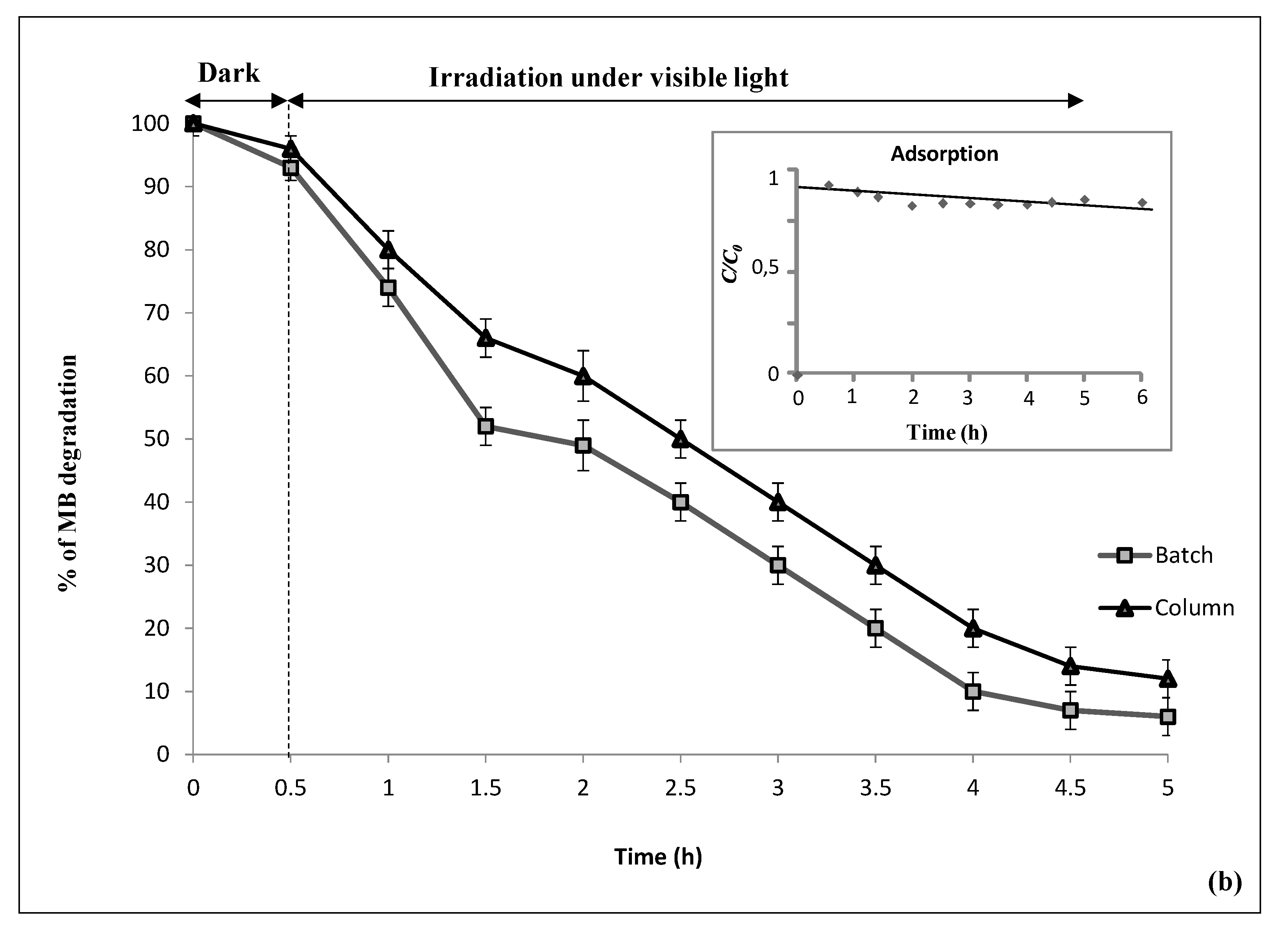
3.2. Methylene Blue Removal
3.2.1. Control Experiments
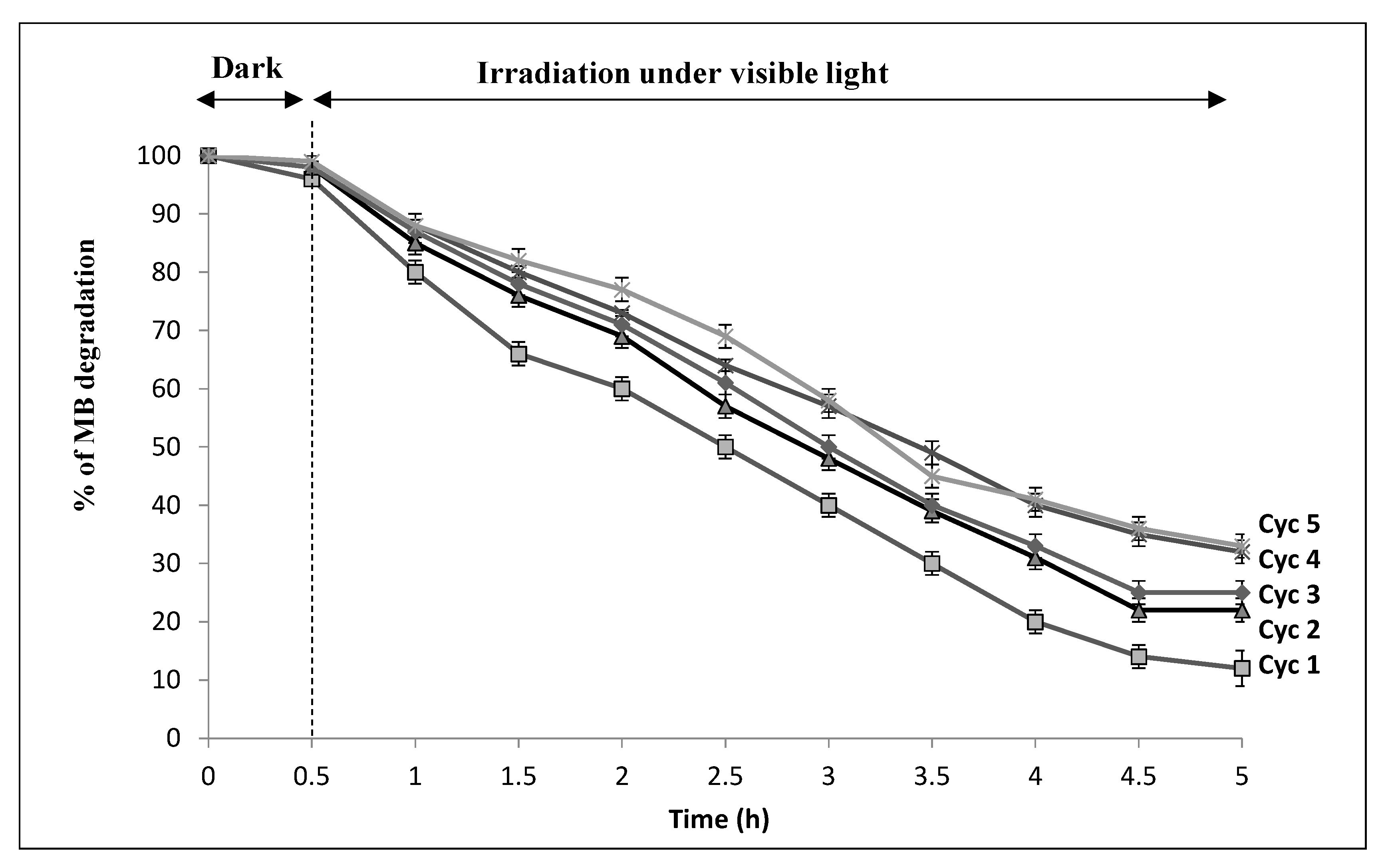
3.2.2. Photocatalytic Decomposition over CNF
3.3. Bacteria Removal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 216, pp. 303–304. [Google Scholar]
- World Health Organization. Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025; The Integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD): Geneva, Switzerland, 2013. [Google Scholar]
- Aziz, F.; Mandi, L.; Boussaid, A.; Boraam, F.; Ouazzani, N. Quality and disinfection trials of consumption water in storage reservoirs for rural area in the Marrakech region (Assif El Mal). J. Water Health 2013, 11, 146–160. [Google Scholar] [CrossRef][Green Version]
- Aziz, F.; Rubio, J.P.; Ouazzani, N.; Dary, M.; Manyani, H.; Morgado, B.R.; Mandi, L. Sanitary impact evaluation of drinking water in storage reservoirs in Moroccan rural area. Saudi J. Biol. Sci. 2017, 24, 767–777. [Google Scholar] [CrossRef][Green Version]
- Wang, L.S.; Hu, H.Y.; Wang, C. Effect of ammonia nitrogen and dissolved organic matter fractions on the genotoxicity of wastewater effluent during chlorine disinfection. Environ. Sci. Technol. 2007, 41, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Legay, C.; Rodriguez, M.J.; Sadiq, R.; Serodes, J.B.; Levallois, P.; Proulx, F. Spatial variations of human health risk associated with exposure to chlorination by-products occurring in drinking water. J. Environ. Manag. 2011, 92, 892–901. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, K.; Joyce, T.; Conroy, R.; Gillespie, J.B.; Elmore-Meegan, M. Solar disinfection of drinking water contained in transparent plastic bottles: Characterizing the bacterial inactivation process. J. Appl. Microbiol. 1998, 84, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.M.C.; Robertson, P.K.J.; Lawton, L.A. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic microorganisms. J. Photochem. Photobiol. A 2005, 175, 51–56. [Google Scholar] [CrossRef]
- Adán, C.; Marugán, J.; Mesones, S.; Casado, C.; Van Grieken, R. Bacterial inactivation and degradation of woorganic molecules by titanium dioxide supported on porous stainless steel photocatalytic membranes. Chem. Eng. J. 2017, 318, 29–38. [Google Scholar] [CrossRef]
- Anca, D.; Visa, M. Simultaneous removal of two industrial dyes by adsorption and photocatalysis on a fly-ash–TiO2composite. J. Photochem. Photobiol. 2015, 306, 21–30. [Google Scholar] [CrossRef]
- Pigeot-Rémy, S.; Simonet, F.; Errazuriz-Cerda, E.; Lazzaroni, J.C.; Atlan, D.; Guillard, C. Photocatalysis and disinfection of water: Identification of potential bacterial targets. Appl. Catal. B Environ. 2011, 104, 390–398. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli: Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Pulgarín. Iron-Oxides and Iron-Citrate as New Photocatalysts in Solar Inactivation of Escherichia Coli in Water: Mechanistic Aspects. Ph.D. Thesis, (No 6684). École Polytechnique Fédérale de Lausanne, Faculté des Sciences de Base, Lausanne, Switzerland, 2015; p. 159. [Google Scholar] [CrossRef]
- Guillard, C.; Baldassare, D.; Duchamp, C.; Ghazzal, M.N.; Daniele, S. Photocatalytic degradation and mineralization of a malodorous compound (dimethyldisulfide) using a continuous flow reactor. Catal. Today 2007, 122, 160–167. [Google Scholar] [CrossRef]
- Daniele, S.; Ghazzal, M.N.; Hubert-Pfalzgraf, L.G.; Duchamp, C.; Guillard, C. Preparations of nano-particles, nano-composites and fibers of ZnO from an amide precursor: Photocatalytic decomposition of (CH3)2S2 in a continuous flow reactor. Mater. Res. Bull. 2006, 41, 2210–2218. [Google Scholar] [CrossRef]
- Chang, T.H. The Reaction Activity Research about Photocatalysis De-Nitrogen Oxides; National Science Council, Executive Yuan: Taipei, Taiwan, 1999; ISBN 88-2113-M-159-001. [Google Scholar]
- Navrotsky, A.; Mazeina, L.; Majzlan, J. Size-driven structural and thermodynamic complexity in iron oxides. Science 2008, 319, 1635–1638. [Google Scholar] [CrossRef]
- Hochella, M.F.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, mineral nanoparticles, and earth systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Sheng, D.N.; Hua, H.L. Degradation mechanism of azo dye CI reactive red 2 by iron powder reduction and photooxidation in aqueous solutions. Chemosphere 2000, 41, 1233–1238. [Google Scholar] [CrossRef]
- Akhavan, O.; Azimirad, R. Photocatalytic property of Fe2O3 nanograin chains coated by TiO2 nanolayer in visible light irradiation. Appl. Catal. A Gen. 2009, 369, 77–82. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Yong, Y.; Pramod Padmanabha, P.; Jaakko, V.I.; Timonen, F.S.; Emami, A.V.; Grzybowski, B.A. Synthesis of Toroidal Gold Nanoparticles Assisted by Soft Templates. Langmuir 2014, 30, 9886–9890. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, W.; Gou, X. a-Fe2O3 nanotubes in gas sensor and lithium–ion battery applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.B. Potentials in synthesizing nanostructured silver particles. Microsyst. Technol. 2017, 23, 4345. [Google Scholar] [CrossRef]
- Uchiyama, H.; Nakanishi, S.; Kozuka, H. Hydrothermal synthesis of nanostructured SnO particles through crystal growth in the presence of gelatin. J. Solid State Chem. 2014, 217, 87–91. [Google Scholar] [CrossRef]
- Tang, B.; Wang, G.; Zhuo, L.; Ge, J.; Cui, L. Facile route to a-FeOOH and a-Fe2O3 nanorods and magnetic property of a-Fe2O3 nanorods. Inorg. Chem. 2006, 45, 5196–5200. [Google Scholar] [CrossRef]
- Zaien, M.; Omar, K.; Hassan, Z. Growth of nanostructured CdO by solid-vapor deposition. Int. J. Phys. Sci. 2011, 6, 4176–4180. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Wang, R.M.; Xu, J.; Chen, J.; Yan, Y.; Narlikar, A.V.; Zhang, H. Synthesis of large arrays of aligned a-Fe2O3 nanowires. Chem. Phys. Lett. 2003, 379, 373–379. [Google Scholar] [CrossRef]
- Hassan, N.A.; Hasnidawani, J.N.; Hassan, N.; Surip, S.N. Synthesis of SiO2 Nanostructures Using Sol-Gel Method. Acta Phys. Pol. A 2016, 129, 842–844. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Gao, L.; Zheng, H.; Ji, M.; Tang, C.; Shen, T.; Zhang, Z. Synthesis of b-FeOOH and a-Fe2O3 nanorods and electrochemical properties of b-FeOOH. J. Mater. Chem. 2004, 14, 905–907. [Google Scholar] [CrossRef]
- Aziz, F.; Ouazzani, N.; Mandi, L.; Mamoun, M.; Uheida, A. Composite nanofibers of polyacrylonitrile/natural clay for decontamination of water containing Pb(II), Cu(II), Zn(II) and pesticides. Sep. Sci. Technol. 2017, 52, 58–70. [Google Scholar] [CrossRef]
- Ghorani, B.; Russell, S.J.; Goswami, P. Controlled morphology and mechanical characterisation of electrospun cellulose acetate fibre webs. Int. J. Polym. Sci. 2013, 12. [Google Scholar] [CrossRef]
- Wang, M.; Yua, J.H.; Hsiehb, A.J.; Rutledge, G.C. Effect of tethering chemistry of cationic surfactants on clay exfoliation, electrospinning and diameter of PMMA/clay nanocomposite fibers. Polymer 2010, 51, 6295–6302. [Google Scholar] [CrossRef]
- Dung, N.T.; Khoa, N.V.; Herrmann, J.M. Photocatalytic degradation of reactive dye RED-3BA in aqueous TiO2 suspension under UV-visible light. Int. J. Photoenergy 2005, 7, 11–15. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Moroccan Standards. Norme Marocaine Homologuée Par Arrêté du Ministre de l’Industrie, du Commerce et de la Mise à Niveau de l’Economie; Service de Normalisation Industrielle Marocaine (SNIMA): Rabat, Morocco, 2006. [Google Scholar]
- Amani-Ghadim, A.R.; Alizadeh, S.; Khodam, F.; Rezvani, Z. Synthesis of rod-like α-FeOOH nanoparticles and its photocatalytic activity in degradation of an azo dye: Empirical kinetic model development. J. Mol. Catal. A Chem. 2015, 408, 60–68. [Google Scholar] [CrossRef]
- Murakami, N.; Matsuo, T.; Tsubota, T.; Ohno, T. Photocatalytic reaction over iron hydroxides: A novel visible-light-responsive photocatalyst. Catal. Commun. 2011, 12, 341–344. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Pierzchała, K.; Sienkiewicz, A.; Forró, L.; Kiwi, J.; Pulgarin, C. Abatement of organics and Escherichia coli by N, S co-doped TiO2 under UV and visible light. Implications of the formation of singlet oxygen (1O2) under visible light. Appl. Catal. B Environ. 2009, 88, 398–406. [Google Scholar] [CrossRef]
- Veréb, G.; Manczinger, L.; Bozsó, G.; Sienkiewicz, A.; Forró, L.; Mogyorósi, K.; Hernádi, K.; Dombi, A. Comparison of the photocatalytic efficiencies of bare and doped rutile and anatase TiO2 photocatalysts under visible light for phenol degradation and E. coli inactivation. Appl. Catal. B Environ. 2013, 129, 566–574. [Google Scholar] [CrossRef]
- Londoño-Calderon, C.L.; Bilovol, V.; Cosio-Castañeda, C.; Gabriela, P.L.; Micheli, S.R.; Pirota, K.R.; Socolovsky, L.M.; Martínez-García, R. Synthesis and Characterization of Iron Oxyhydroxide Nanowires. IEEE Trans. Magn. 2013, 49, 4502–4505. [Google Scholar] [CrossRef]
- Borker, V.; Rajashri, K.; Koyar, R. Comparison of degradation of methylene blue dye by ZnO, doped zno and iron ore rejects. Eur. Chem. Bull. 2014, 3, 520–529. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Liu, W.; Zhang, S. Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nanoheterojunctions. Appl. Surf. Sci. 2017, 405, 60–70. [Google Scholar] [CrossRef]
- Juan, D.; Li, Z.; Shuilin, C. Wash fastness of dyed fabric treated by the sol-gel process. Coloration Technol. 2005, 121, 29–36. [Google Scholar] [CrossRef]
- Venczel, L.V.; Arrowood, M.; Hurd, M.; Sobsey, M.D. Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. Appl. Environ. Microbiol. 1997, 63, 1598–1601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bayne, M.; Fernando, S.; Legg, B.; Zhu, M.; Penn, R.L.; Banfield, J.F. Size-Dependent Bandgap of Nanogoethite. J. Phys. Chem. C 2011, 115, 17704–17710. [Google Scholar] [CrossRef]
- Xu, J.; Sahai, N.; Eggleston, C.M.; Schoonen, M.A.A. Reactive oxygen species at the oxide/water interface: Formation mechanisms and implications for prebiotic chemistry and the origin of life. Earth Planet Sci. Lett. 2013, 363, 156–167. [Google Scholar] [CrossRef]
- Ruales-Lonfat, C.; Barona, J.F.; Benítez, N.; Sienkiewicz, A.; Pulgarín, C. Iron oxides semiconductors are efficient for solar water disinfection: A comparison with photoFenton processes at neutral pH. Appl. Catal. B Environ. 2015, 166–167, 497–508. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Zheng, X.; Bondarenko, V.; Fava, G.; Ruello, M.L. Paving the Way for A Sustainable and Efficient SiO2/TiO2 Photocatalytic Composite. Environments 2019, 6, 87. [Google Scholar] [CrossRef]
- Haile, H.L.; Abi, T.; Tesfahun, K. Synthesis, characterization and photocatalytic activity of MnO2/Al2O3/Fe2O3 nanocomposite for degradation of malachite green. Afr. J. Pure Appl. Chem. 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Y.; An, T.; Li, G.; Yip, H.Y.; Yu, J.C.; Wong, P.K. Visible-light-driven photocatalytic inactivation of E. coli K-12 by bismuth vanadate nanotubes: Bactericidal performance and mechanism. Environ. Sci. Technol. 2012, 46, 4599–4606. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, A.; Li, Y. Naturally occurring sphalerite as a novel cost-effective photocatalyst for bacterial disinfection under visible light. Environ. Sci. Technol. 2011, 45, 5689–5695. [Google Scholar] [CrossRef]
- Peng, X.; Ng, T.W.; Huang, G.; Wang, W.; An, T.; Wong, P.K. Bacterial disinfection in a sunlight/visible-light-driven photocatalytic reactor by recyclable natural magnetic sphalerite. Chemosphere 2017, 166, 521–527. [Google Scholar] [CrossRef]

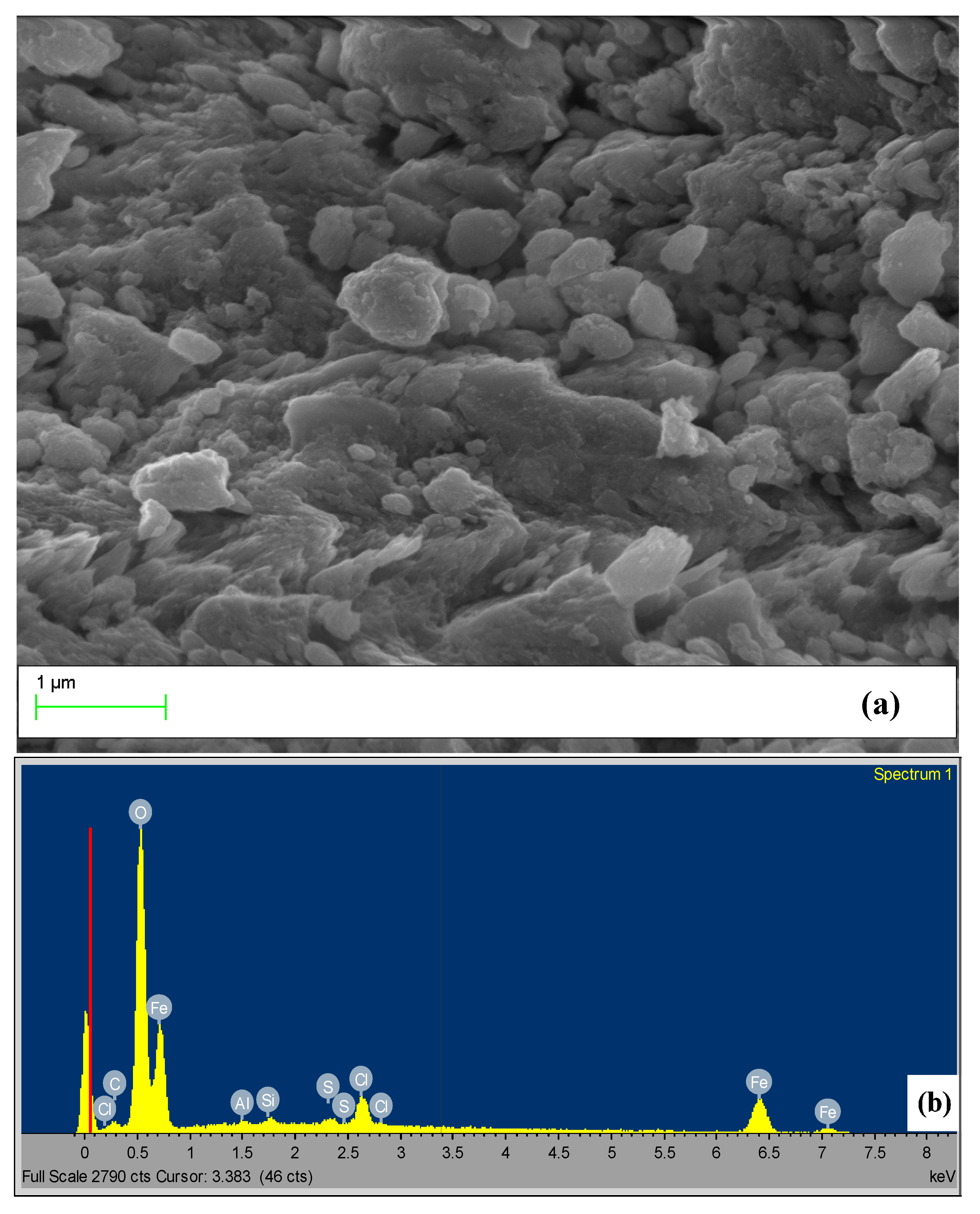

| Element | Weight % | Atomic % |
|---|---|---|
| C | 1.05 ± 0.31 | 2.54 |
| O | 33.38 ± 0.49 | 60.90 |
| Al | 0.30 ± 0.08 | 0.32 |
| Si | 0.61 ± 0.09 | 0.64 |
| S | 0.70 ± 0.11 | 0.64 |
| Cl | 5.11 ± 0.18 | 4.21 |
| Fe | 58.85 ± 0.60 | 30.76 |
| Totals | 100.00 | 100.00 |
| Element | Weight % | Atomic % |
|---|---|---|
| O | 25.21 ± 0.59 | 53.65 |
| Al | 0.35 ± 0.09 | 0.44 |
| Si | 0.58 ± 0.09 | 0.70 |
| S | 0.36 ± 0.10 | 0.38 |
| Cl | 1.60 ± 0.14 | 1.54 |
| Fe | 54.82 ± 1.06 | 33.42 |
| Co | 17.8 ± 1.15 | 9.86 |
| Totals | 100.00 | 100.00 |
| Langmuir Model | MB |
|---|---|
| qm (mg/g) | 2.6 |
| KL (l/mg) | 0.05 |
| R2 | 0.989 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, F.; El Achaby, M.; Aziz, K.; Ouazzani, N.; Mandi, L.; Ghazzal, M.N. Nanocomposite Fiber Based on Natural Material for Water Disinfection under Visible Light Irradiation. Nanomaterials 2020, 10, 1192. https://doi.org/10.3390/nano10061192
Aziz F, El Achaby M, Aziz K, Ouazzani N, Mandi L, Ghazzal MN. Nanocomposite Fiber Based on Natural Material for Water Disinfection under Visible Light Irradiation. Nanomaterials. 2020; 10(6):1192. https://doi.org/10.3390/nano10061192
Chicago/Turabian StyleAziz, Faissal, Mounir El Achaby, Khalid Aziz, Naaila Ouazzani, Laila Mandi, and Mohamed Nawfal Ghazzal. 2020. "Nanocomposite Fiber Based on Natural Material for Water Disinfection under Visible Light Irradiation" Nanomaterials 10, no. 6: 1192. https://doi.org/10.3390/nano10061192
APA StyleAziz, F., El Achaby, M., Aziz, K., Ouazzani, N., Mandi, L., & Ghazzal, M. N. (2020). Nanocomposite Fiber Based on Natural Material for Water Disinfection under Visible Light Irradiation. Nanomaterials, 10(6), 1192. https://doi.org/10.3390/nano10061192









