Synthesis of Active Graphene with Para-Ester on Cotton Fabrics for Antistatic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphene Oxide
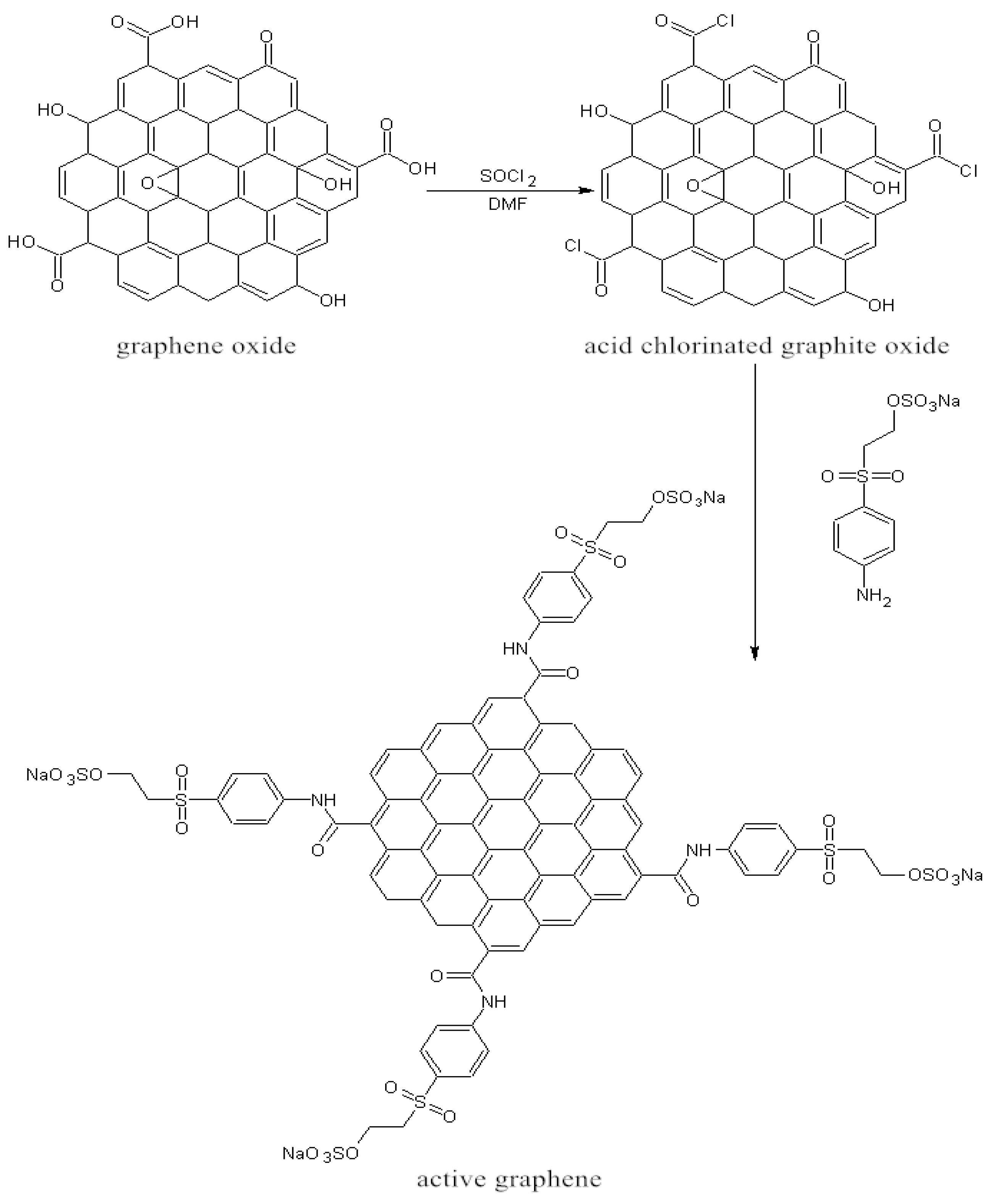
2.3. Preparation of Active Graphene
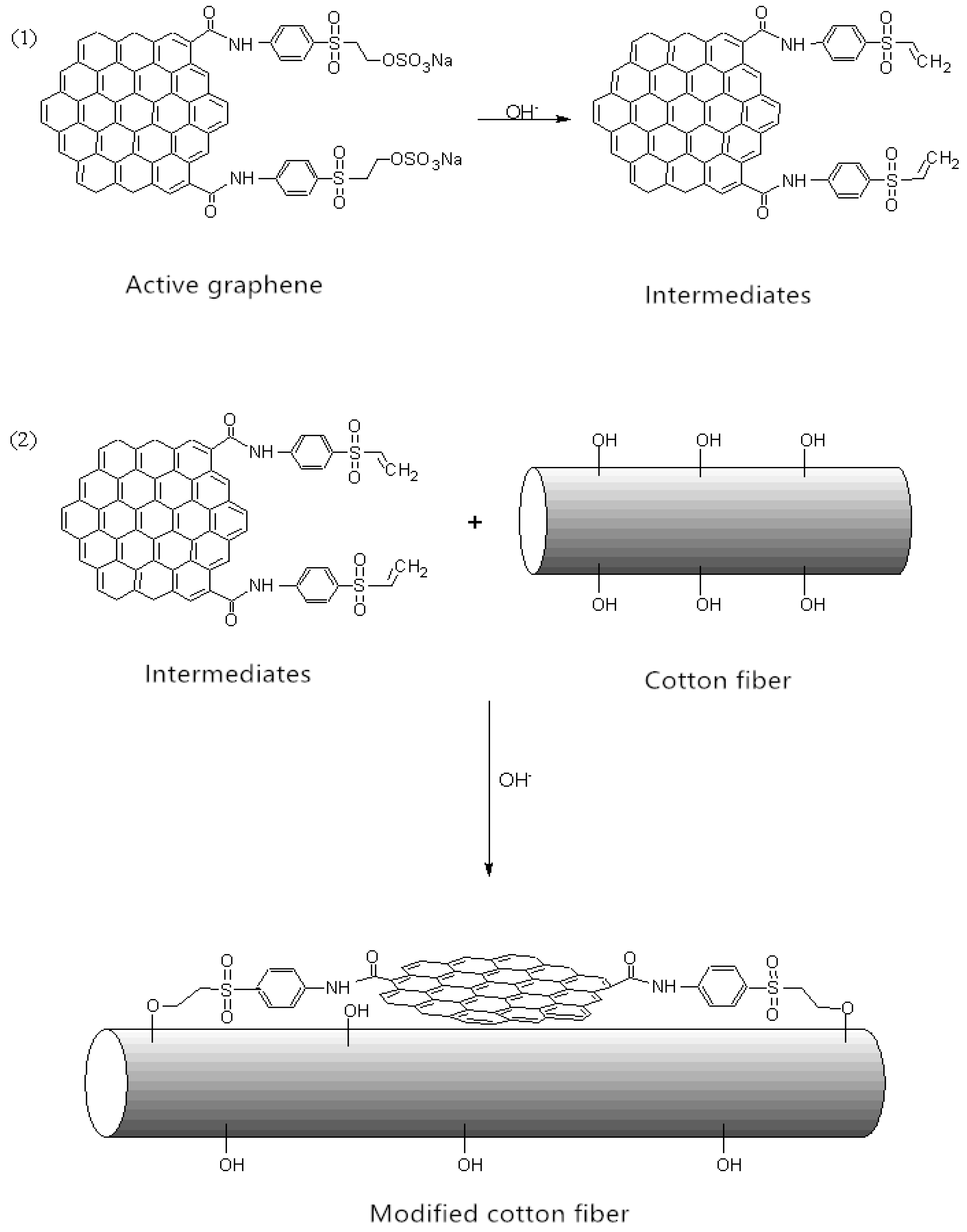
2.4. Active Graphene Modification onto Cotton Fabric
2.5. Characterization of Surface Modification
2.6. Properties of Modified Cotton Fabrics
3. Results and Discussions
3.1. Characterisation of the Synthesized Graphene Oxide (GO) and the Active Graphene (JZGO)
3.1.1. Morphology of the Graphene Oxide and the Active Graphene
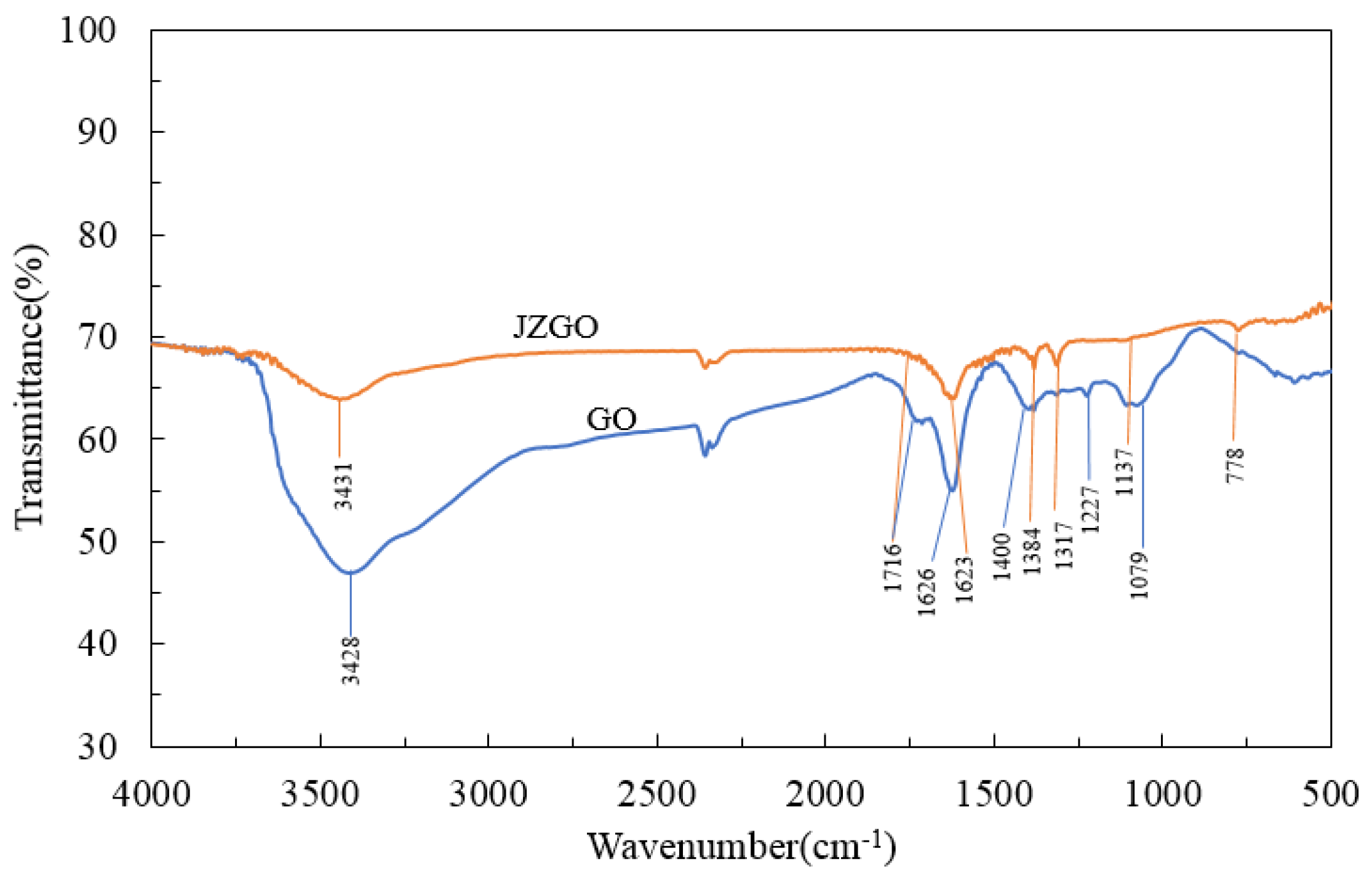
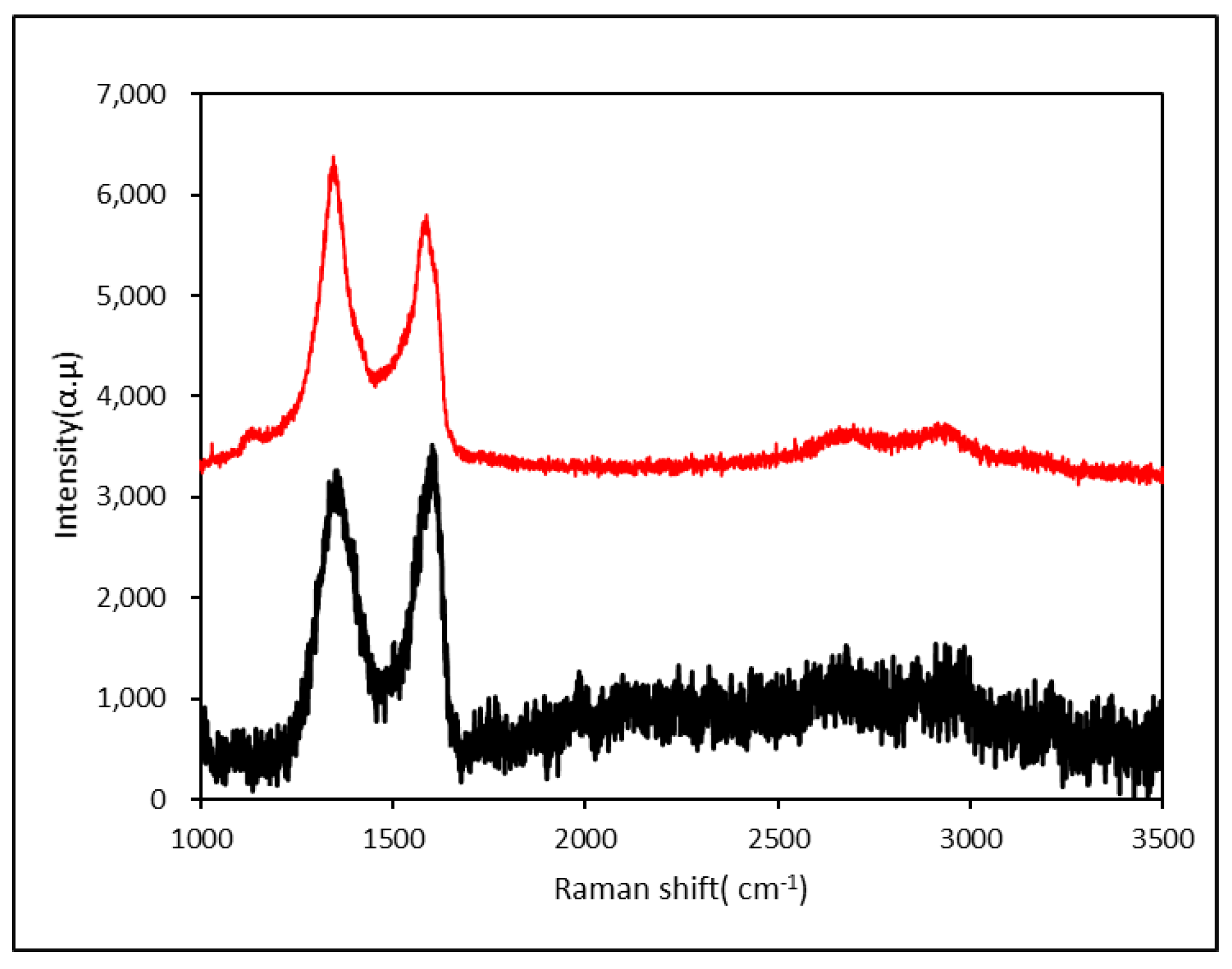
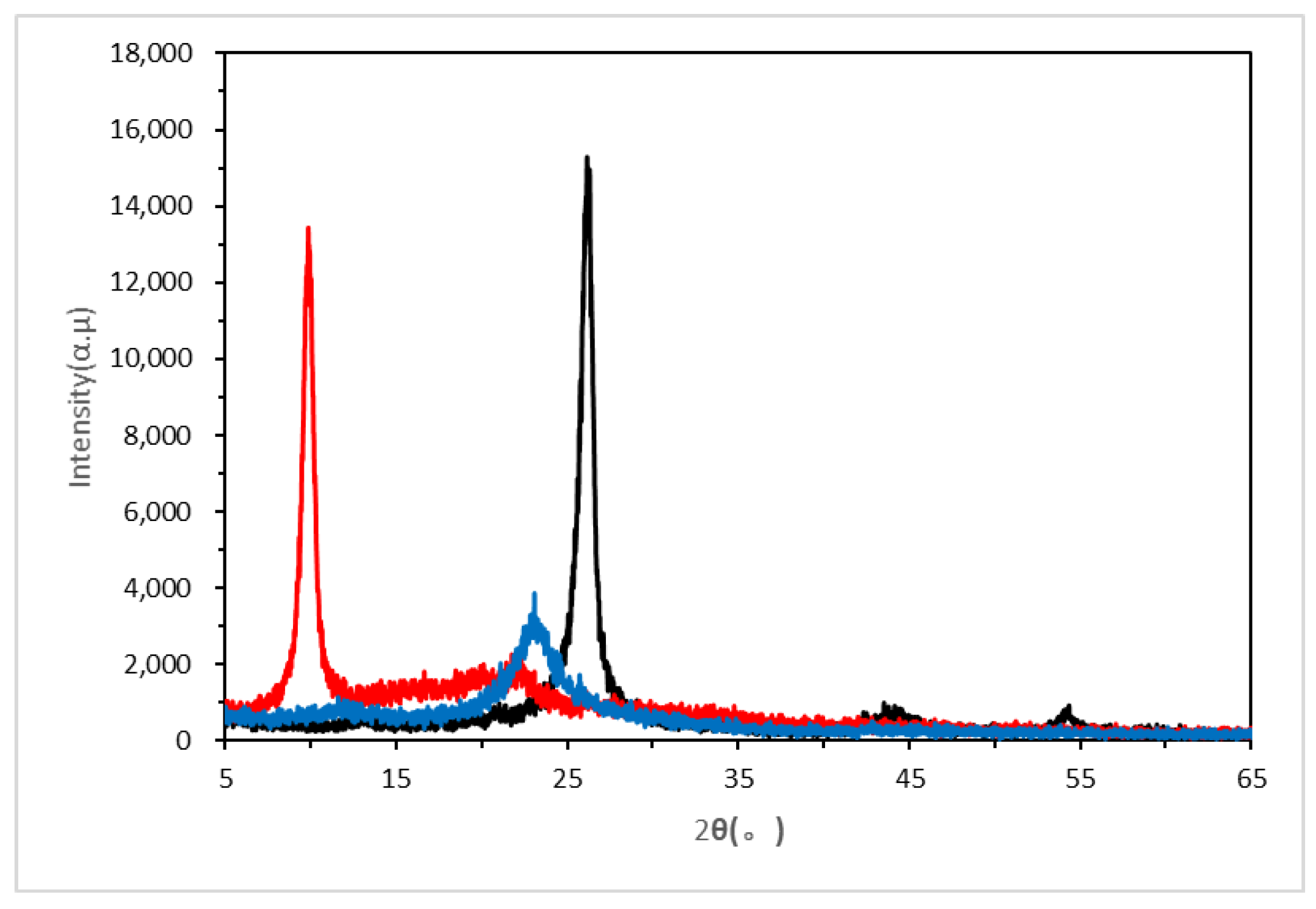
3.1.2. FT-IR, Raman and XRD Characterizations of the Graphene Oxide and the Active Graphene
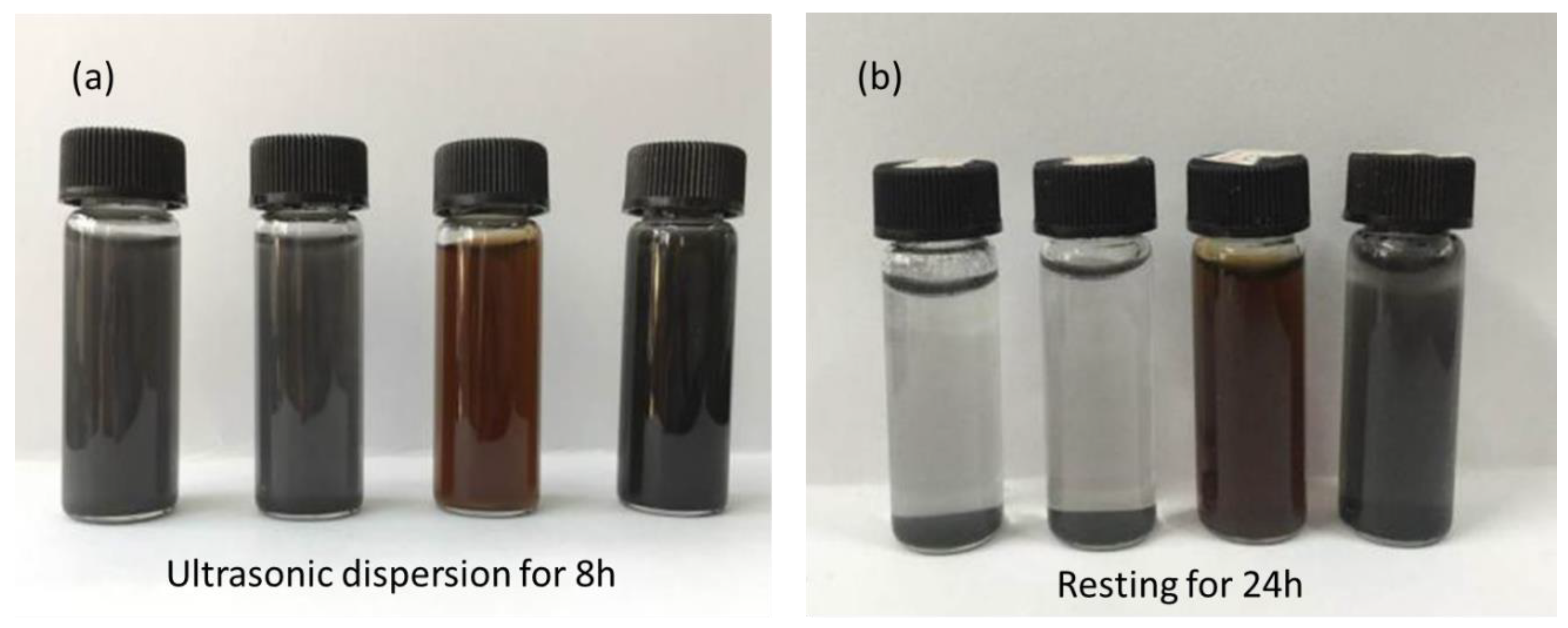
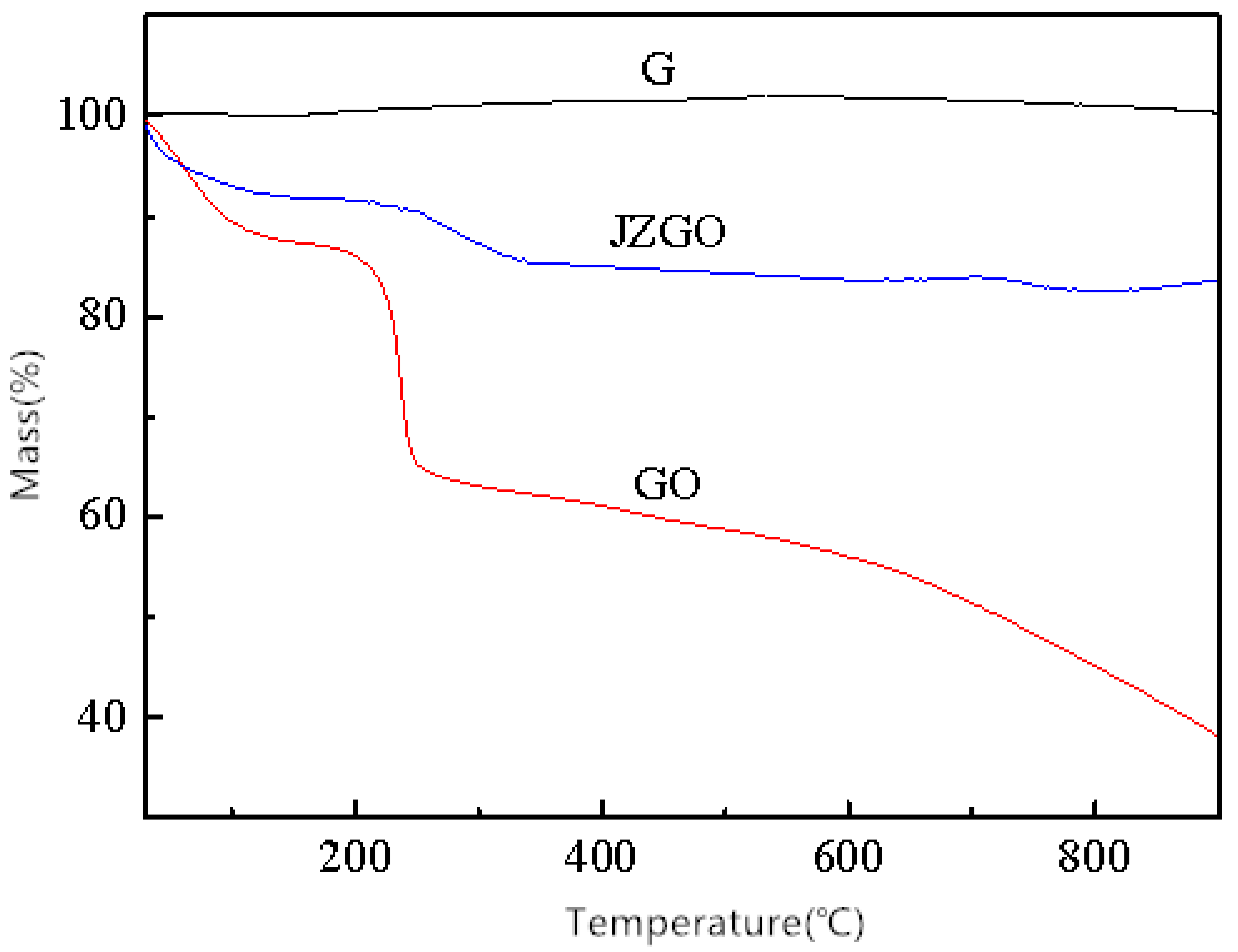
3.1.3. Dispersion and Thermal Stability Characterizations
3.2. Characterisation of the Modified Cotton Fabrics with Active Graphene
3.2.1. Morphology of the Modified Cotton Fabrics
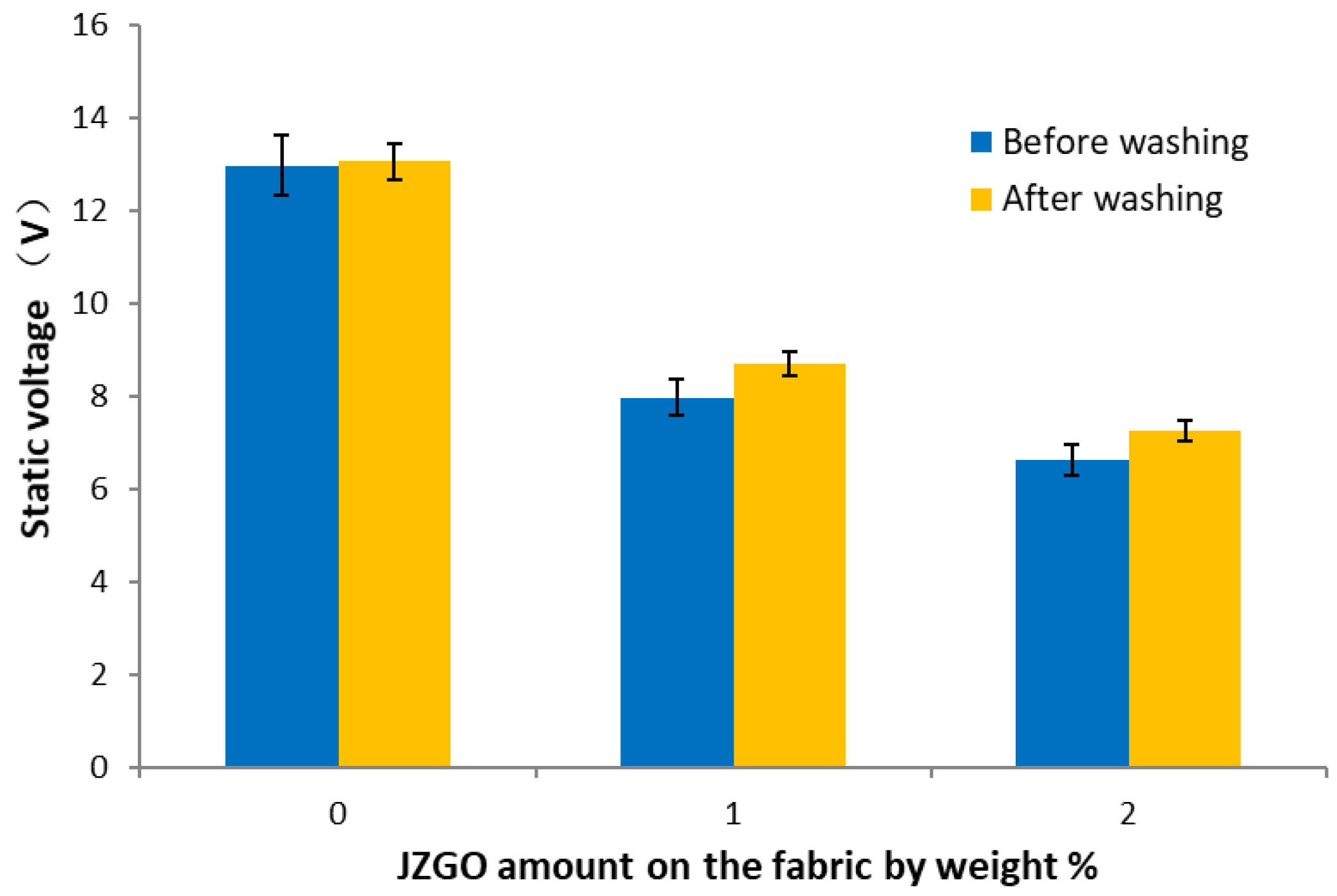
3.2.2. Characterizations of the Antistatic Properties of the Modified Cotton Fabrics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Textor, T.; Mahltig, B. A sol–gel based surface treatment for preparation of water repellent antistatic textiles. Appl. Surf. Sci. 2010, 256, 1668–1674. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Abdel-Mohdy, F.A.; Al-Deyab, S.S.; El-Newehy, M.H. Chitosan and monochlorotriazinyl-β-cyclodextrin finishes improve antistatic properties of cotton/polyester blend and polyester fabrics. Carbohydr. Polym. 2010, 82, 202–208. [Google Scholar] [CrossRef]
- Weiguo, D. Research on Properties of Nano Polypropylene/TiO~ 2 Composite Fiber. Text. Res. J. 2002, 23, 22–23. [Google Scholar]
- Zhang, F.; Yang, J. Preparation of nano-ZnO and its application to the textile on antistatic finishing. Int. J. Chem. 2009, 1, 18. [Google Scholar] [CrossRef]
- Qiaozhen, Y. Influence of nano-particles treatment on the antistatic property of polyester fabric. Text. Res. J. 2007, 12, 19. [Google Scholar]
- Nateghi, M.R.; Shateri-Khalilabad, M. Silver nanowire-functionalized cotton fabric. Carbohydr. Polym. 2015, 117, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar]
- Lotya, M.; King, P.G.; Khan, U.; Dr, S. High-concentration, surfactant-stabilized graphene dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B. Sur le poids atomique du graphite. Ann. Chim. Phys. 1860, 59, e472. [Google Scholar]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber Dtsch Chem Ges 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y., II; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549. [Google Scholar] [CrossRef] [PubMed]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Röhrl, J.; et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat. Mater. 2009, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Yannopoulos, S.N.; Siokou, A.; Nasikas, N.K.; Dracopoulos, V.; Ravani, F.; Papatheodorou, N. CO2-Laser-Induced Growth of Epitaxial Graphene on 6H-SiC (0001). Adv. Funct. Mater. 2012, 22, 113–120. [Google Scholar] [CrossRef]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, M.; Pan, N.; Sun, B.; Li, Z.; Ma, Y.; Zhang, X.; Zhu, S.; Chen, Z.; Qu, L. Structure-tunable graphene oxide fibers via microfluidic spinning route for multifunctional textiles. Carbon 2019, 152, 106–113. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, M.; Sun, X.; Pan, N.; Chen, F.; Zhu, S.; Zhang, X.; Chen, S. Highly sensitive wearable 3D piezoresistive pressure sensors based on graphene coated isotropic non-woven substrate. Comp. Part A Appl. Sci. Manuf. 2019, 117, 202–210. [Google Scholar] [CrossRef]
- Sun, F.; Tian, M.; Sun, X.; Xu, T.; Liu, X.; Zhu, S.; Zhang, X.; Qu, L. Stretchable Conductive Fibers of Ultrahigh Tensile Strain and Stable Conductance Enabled by a Worm-Shaped Graphene Microlayer. Nano Lett. 2019, 19, 6592–6599. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.R.; Myung, C.W.; Yun, J.; Ghassami, A.; Li, N.; Yu, S.U.; Hajibabaei, A.; Park, Y.; Kim, K.S. Graphene and graphene analogs toward optical, electronic, spintronic, green-chemical, energy-material, sensing, and medical applications. ACS Appl. Mater. Interface 2017, 9, 24393–24406. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Fortuniak, W.; Mizerska, U.; Kaminska, I.; Makowski, T.; Brzezinski, S.; Piorkowska, E. Modification of cotton fabric with graphene and reduced graphene oxide using sol–gel method. Cellulose 2017, 24, 4057–4068. [Google Scholar] [CrossRef]
- Molina, J. Graphene-based fabrics and their applications: A review. RSC Adv. 2016, 6, 68261–68291. [Google Scholar] [CrossRef]
- Cai, G.; Xu, Z.; Yang, M.; Tang, B.; Wang, X. Functionalization of cotton fabrics through thermal reduction of graphene oxide. Appl. Surf. Sci. 2017, 393, 441–448. [Google Scholar] [CrossRef]
- Matsumoto, H.; Imaizumi, S.; Konosu, Y.; Ashizawa, M.; Minagawa, M.; Tanioka, A.; Lu, W.; Tour, J.M. Electrospun composite nanofiber yarns containing oriented graphene nanoribbons. ACS Appl. Mater. Interfaces 2013, 5, 6225–6231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, D. Solvated graphenes: An emerging class of functional soft materials. Adv. Mater. 2013, 25, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution properties of graphite and graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Lu, L.; Cai, D.; Zhang, C.; Wang, N.; Zhang, J.; Wu, Z. Adsorption of polycyclic aromatic hydrocarbons (fluoranthene and anthracenemethanol) by functional graphene oxide and removal by pH and temperature-sensitive coagulation. ACS Appl. Mater. Interfaces 2013, 5, 4783–4790. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Parviz, D.; Das, S.; Tanvir Ahmed, H.S.; Irin, F.; Bhattacharia, S.; Green, M.J. Dispersions of non-covalently functionalized graphene with minimal stabilizer. ACS Nano 2012, 6, 8857–8867. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, W.; Liu, X.; Yang, T.; Li, F.; Li, Q.; Cheng, W.; Gao, C.; Jiang, Z.; Jiang, J.; et al. Highly active graphene oxide-supported cobalt single-ion catalyst for chemiluminescence reaction. Anal. Chem. 2017, 89, 13518–13523. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.J.; Leret, S.; Calleja, F.; Stradi, D.; Black, A.; Bernardo-Gavito, R.; Garnica, M.; Granados, D.; Vázquez de Parga, A.L.; Pérez, E.M.; et al. Organic covalent patterning of nanostructured graphene with selectivity at the atomic level. Nano Lett. 2015, 16, 355–361. [Google Scholar] [CrossRef]
- Greenwood, J.; Phan, T.H.; Fujita, Y.; Li, Z.; Ivasenko, O.; Vanderlinden, W.; Van Gorp, H.; Frederickx, W.; Lu, G.; Tahara, K.; et al. Covalent modification of graphene and graphite using diazonium chemistry: Tunable grafting and nanomanipulation. ACS Nano 2015, 9, 5520–5535. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Shao, Q.; Moloney, M.G.; Xu, X.; Zhang, D.; Li, J.; Zhang, C.; Huang, Y. Nondestructive functionalization of graphene by surface-initiated atom transfer radical polymerization: An ideal nanofiller for poly (p-phenylene benzobisoxazole) fibers. Macromolecules 2017, 50, 1422–1429. [Google Scholar] [CrossRef]
- Gorenšek, M. Dye–fibre bond stabilities of some reactive dyes on cotton. Dyes Pigments 1999, 40, 225–233. [Google Scholar] [CrossRef]
- Siddiqua, U.H.; Ali, S.; Iqbal, M.; Hussain, T. Relationship between structure and dyeing properties of reactive dyes for cotton dyeing. J. Mol. Liq. 2017, 241, 839–844. [Google Scholar] [CrossRef]
- Iqbal, M. Textile Dyes; Rehbar Publishers: Karachi, Pakistan, 2008. [Google Scholar]
- Qu, L.; Tian, M.; Hu, X.; Wang, Y.; Zhu, S.; Guo, X.; Han, G.; Zhang, X.; Sun, K.; Tang, X. Functionalization of cotton fabric at low graphene nanoplate content for ultrastrong ultraviolet blocking. Carbon 2014, 80, 565–574. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Chen, X.; Zhang, L.; Min, J. Synthesis of Active Graphene with Para-Ester on Cotton Fabrics for Antistatic Properties. Nanomaterials 2020, 10, 1147. https://doi.org/10.3390/nano10061147
Su M, Chen X, Zhang L, Min J. Synthesis of Active Graphene with Para-Ester on Cotton Fabrics for Antistatic Properties. Nanomaterials. 2020; 10(6):1147. https://doi.org/10.3390/nano10061147
Chicago/Turabian StyleSu, Mengting, Xiaoting Chen, Liyuan Zhang, and Jie Min. 2020. "Synthesis of Active Graphene with Para-Ester on Cotton Fabrics for Antistatic Properties" Nanomaterials 10, no. 6: 1147. https://doi.org/10.3390/nano10061147
APA StyleSu, M., Chen, X., Zhang, L., & Min, J. (2020). Synthesis of Active Graphene with Para-Ester on Cotton Fabrics for Antistatic Properties. Nanomaterials, 10(6), 1147. https://doi.org/10.3390/nano10061147




