Engineering Metal–Organic Frameworks (MOFs) for Controlled Delivery of Physiological Gaseous Transmitters
Abstract
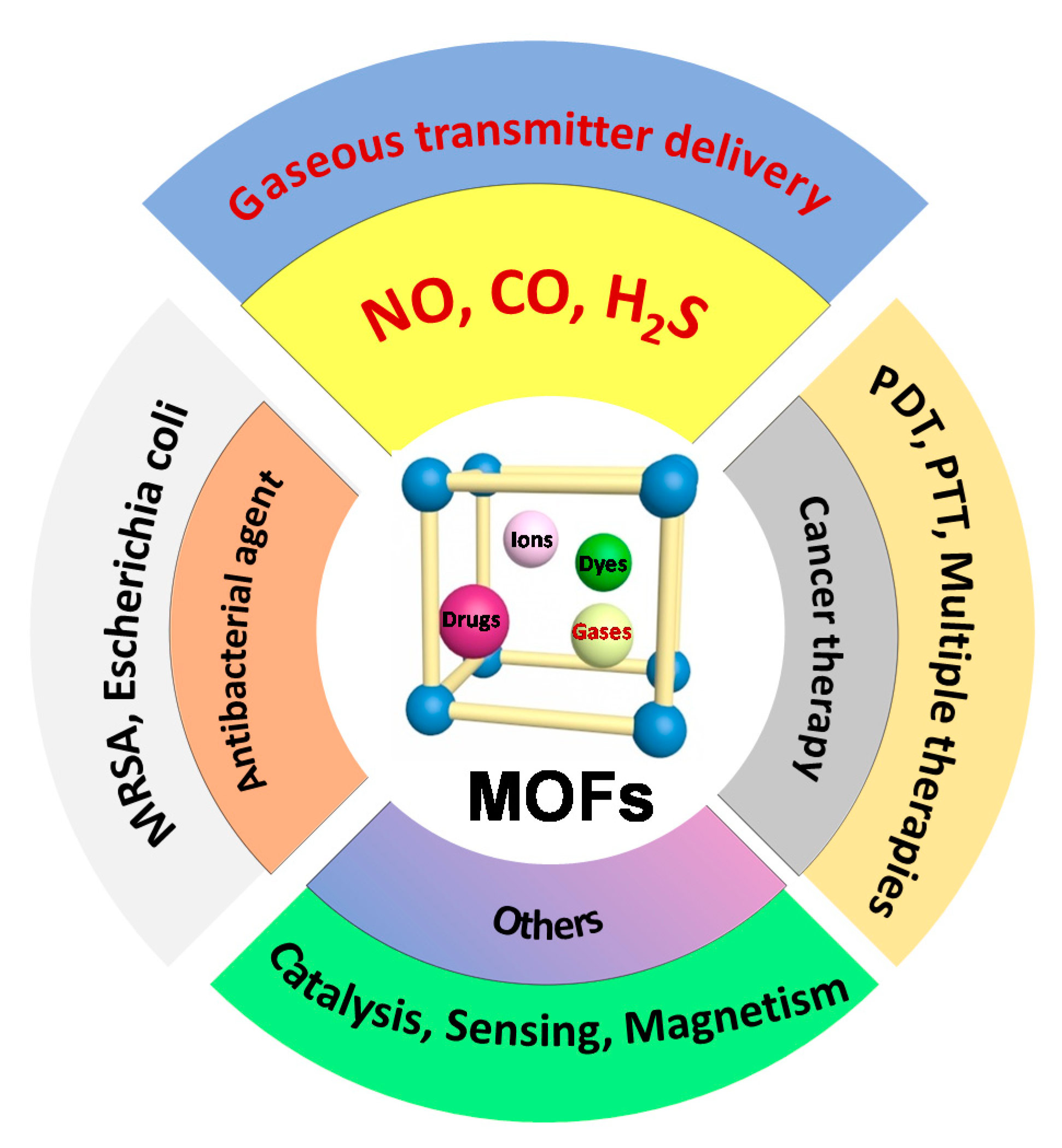
1. Introduction
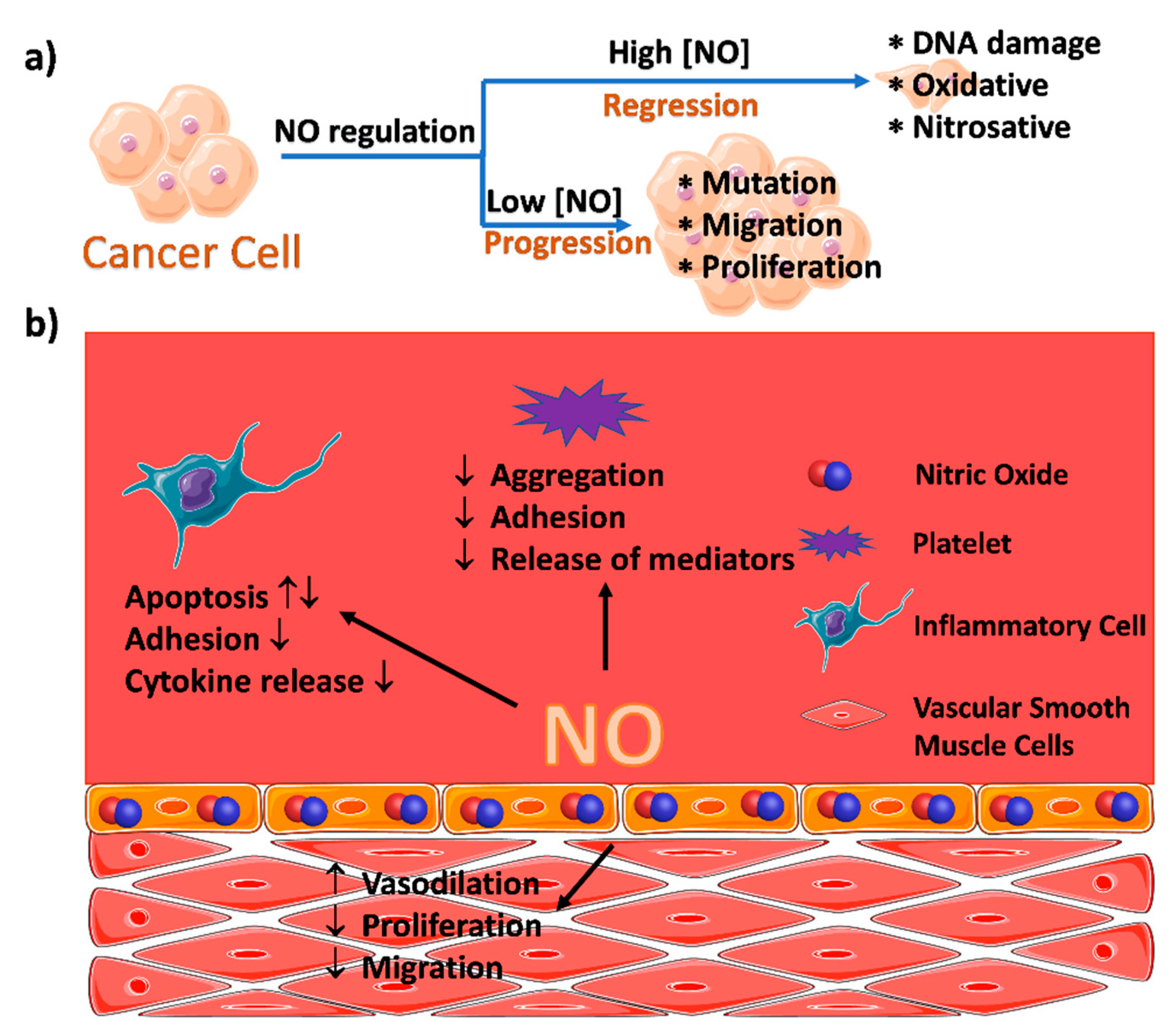
2. MOFs for the Delivery of Nitric Oxide
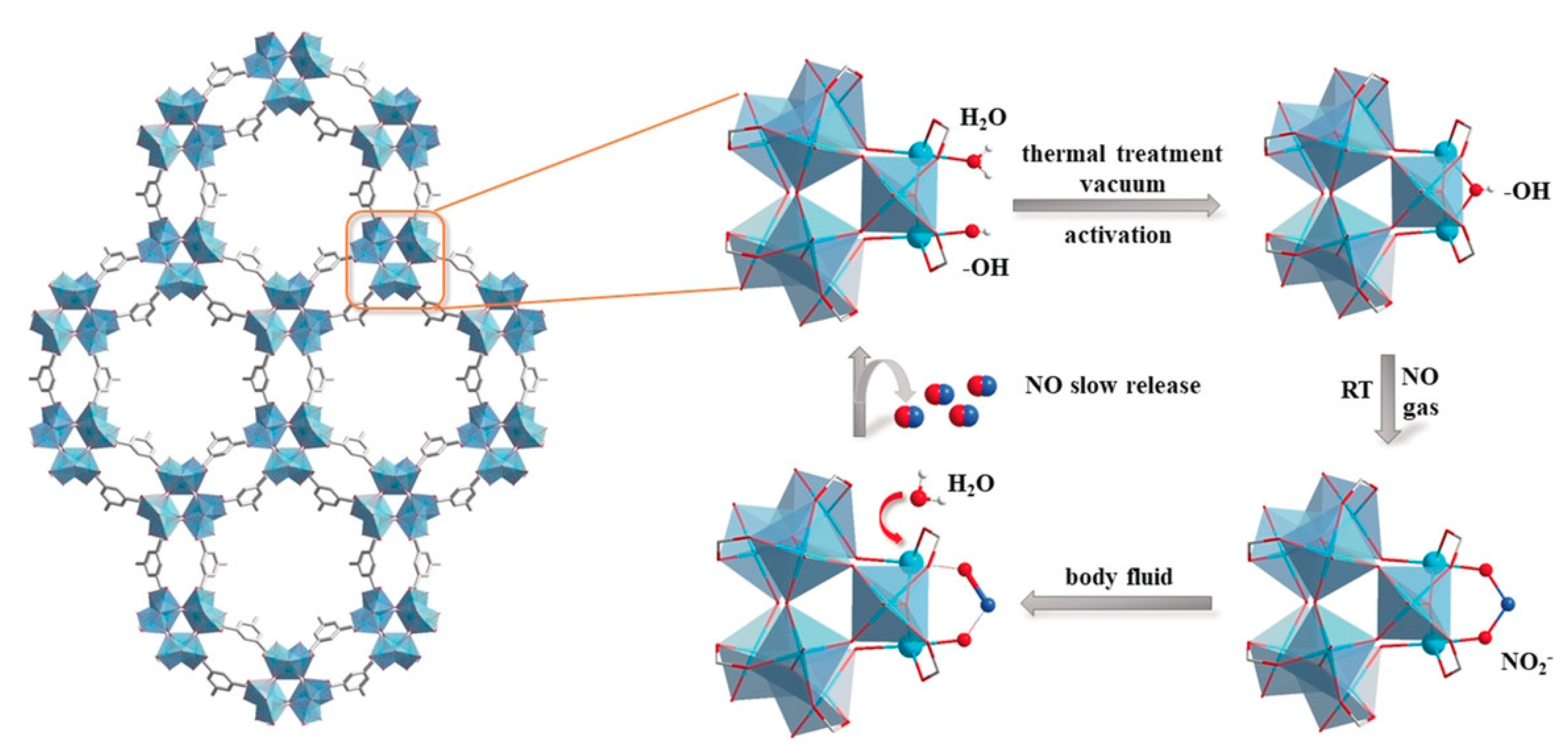
2.1. Coordination of the Metal Ions with NO within MOFs
2.2. Functionalization of the Organic Ligands of MOFs for Controlled NO Release
2.3. MOF-Catalyzed In Situ NO Generation
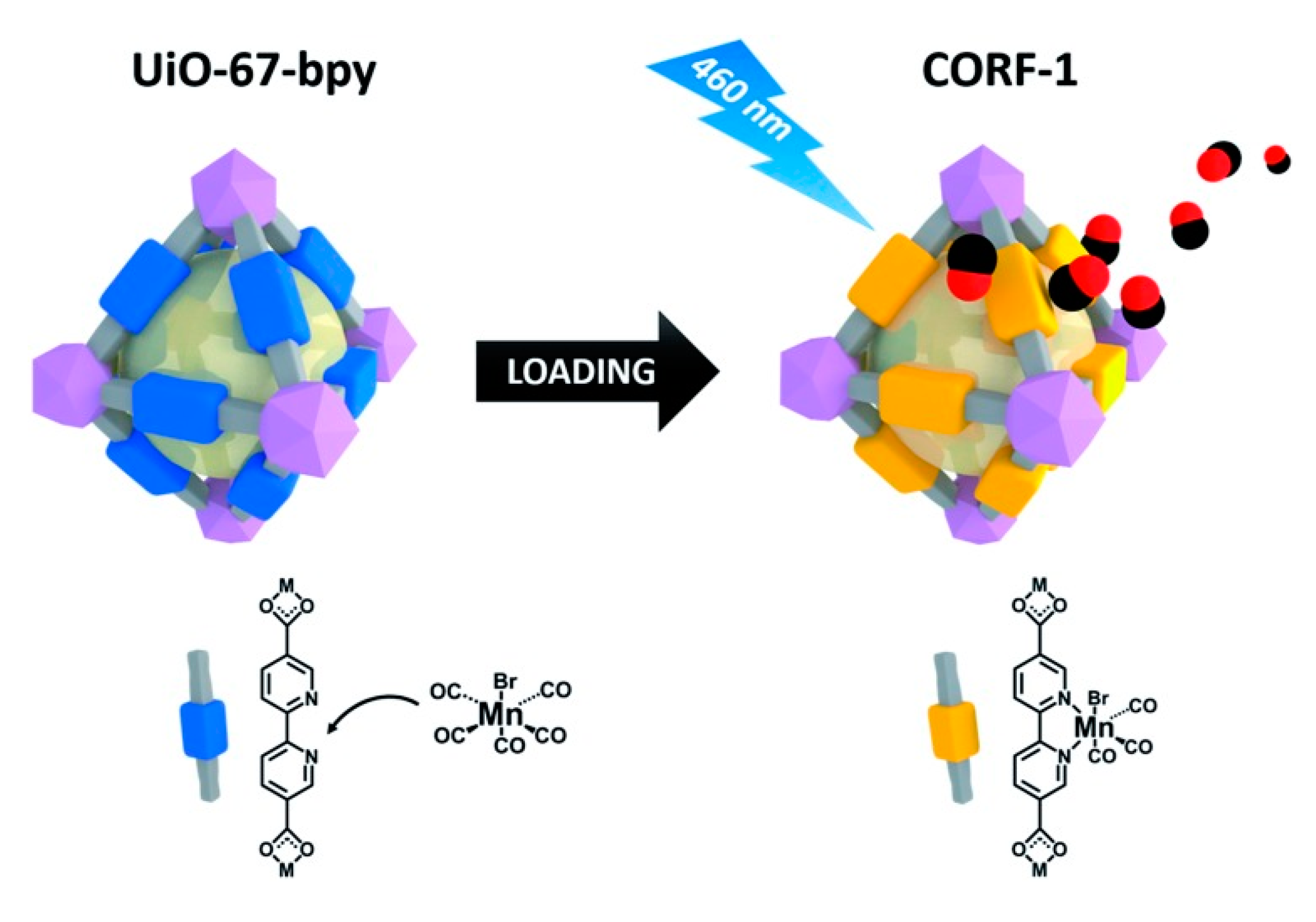
3. MOFs for the Delivery of Carbon Monoxide
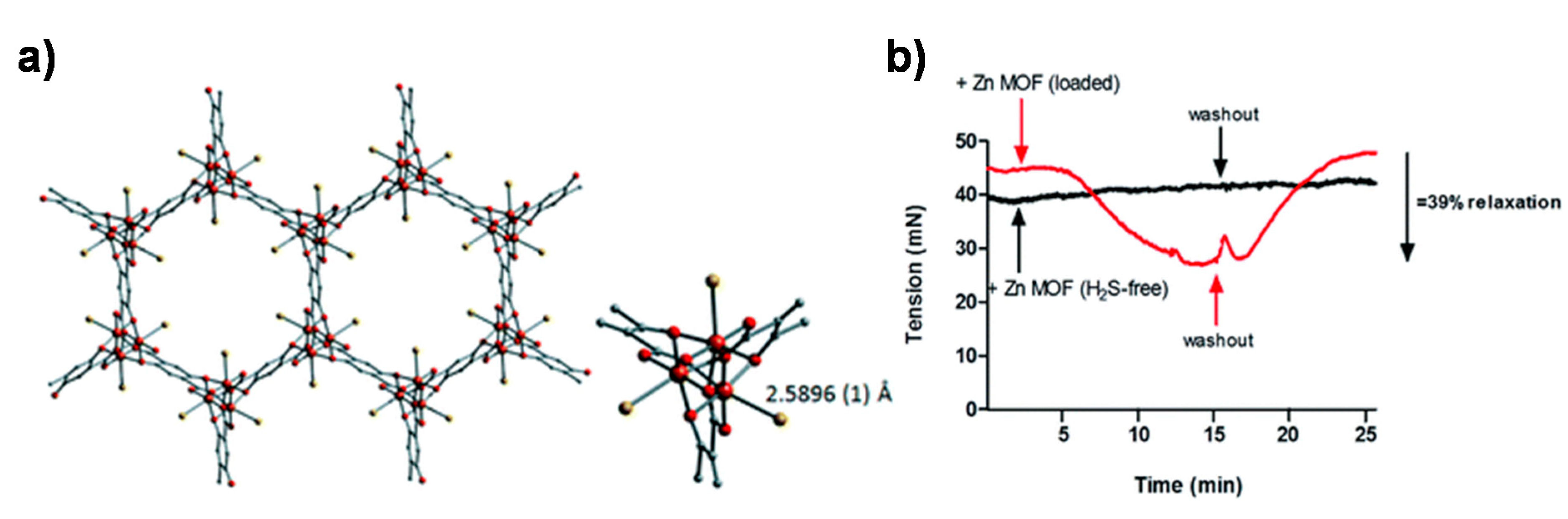
4. MOFs for the Delivery of Hydrogen Sulfide
5. Summary and Outlooks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BDC | 1,4-benzenedicarboxylic acid |
| BNN | bis-N-nitroso |
| BODIPY | Boron dipyrromethene |
| CBS | cystathionine β-synthase |
| CORF | CO-releasing framework |
| CORM | CO-releasing molecules |
| CPO | Coordination Polymer of Oslo |
| CSE | cystathionine γ-lyse |
| HepG2 | A type of human liver cancer cells |
| HKUST | Hong Kong University of Science and Technology |
| IRMOF | Isoreticular Metal-Organic Framework |
| MCF7 | Human breast cancer cells |
| MDR | Multidrug Resistance |
| MIL | Materials of Institute Lavoisier frameworks |
| MIP | Materials of the Institute of Porous Materials of Paris |
| mnIm | 5-methyl-4-nitroimidazole |
| MR | Magnetic Resonance |
| MST | 3-mercaptosulfurtransferase |
| NIR | Near-Infrared |
| NOF | NO framework |
| NONOate | Diazeniumdiolate |
| NOSs | Nitric Oxide Synthases |
| PCL | Polycaprolactone |
| PTT | Photothermal therapy |
| SNO | S-nitrosothiol |
| SNP | Sodium nitroprusside |
| SURMOF | Surface-attached metal-organic framework |
| UiO | University of Oslo |
| UMCM | University of Michigan Crystalline Material |
| ZIF | Zeolitic Imidazolate Framework |
| 2nIm | 2-nitroimidazole |
References
- Dincǎ, M.; Yu, A.F.; Long, J.R. Microporous metal−organic frameworks incorporating 1,4-benzeneditetrazolate: Syntheses, structures, and hydrogen storage properties. J. Am. Chem. Soc. 2006, 128, 8904–8913. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.T. Hydrogen storage in metal−organic frameworks by bridged hydrogen spillover. J. Am. Chem. Soc. 2006, 128, 8136–8137. [Google Scholar] [CrossRef] [PubMed]
- Psofogiannakis, G.M.; Froudakis, G.E. Theoretical explanation of hydrogen spillover in metal−organic frameworks. J. Phys. Chem. C 2011, 115, 4047–4053. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Long, J.R. Metal−organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture. J. Am. Chem. Soc. 2011, 133, 5664–5667. [Google Scholar] [CrossRef] [PubMed]
- Millward, A.R.; Yaghi, O.M. Metal−organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Wheatley, P.S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Gao, Q.; Zhao, M.; Zhang, H.-J.; Zhang, Y.-H.; Chang, Z. Impact of the flexibility of pillar linkers on the structure and CO2 adsorption property of “pillar-layered” MOFs. Chin. Chem. Lett. 2017, 28, 55–59. [Google Scholar] [CrossRef]
- Shultz, A.M.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. A catalytically active, permanently microporous MOF with metalloporphyrin struts. J. Am. Chem. Soc. 2009, 131, 4204–4205. [Google Scholar] [CrossRef]
- Müller, M.; Hermes, S.; Kähler, K.; van den Berg, M.W.E.; Muhler, M.; Fischer, R.A. Loading of MOF-5 with Cu and ZnO nanoparticles by gas-phase infiltration with organometallic precursors: Properties of Cu/ZnO@MOF-5 as catalyst for methanol synthesis. Chem. Mater. 2008, 20, 4576–4587. [Google Scholar] [CrossRef]
- Shu, J.-C.; Yang, X.-Y.; Zhang, X.-R.; Huang, X.-Y.; Cao, M.-S.; Li, L.; Yang, H.-J.; Cao, W.-Q. Tailoring MOF-based materials to tune electromagnetic property for great microwave absorbers and devices. Carbon 2020, 162, 157–171. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, H.G.; Ye, J.H.; Shi, L.Y.; Feng, X. Magnetic CoFe alloy@C nanocomposites derived from ZnCo-MOF for electromagnetic wave absorption. Chem. Eng. J. 2020, 383, 12. [Google Scholar] [CrossRef]
- Li, X.; Cui, E.; Xiang, Z.; Yu, L.; Xiong, J.; Pan, F.; Lu, W. Fe@NPC@CF nanocomposites derived from Fe-MOFs/biomass cotton for lightweight and high-performance electromagnetic wave absorption applications. J. Alloys Compd. 2020, 819, 152952. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Keskin, S.; Kızılel, S. Biomedical applications of metal organic frameworks. Ind. Eng. Chem. Res. 2011, 50, 1799–1812. [Google Scholar] [CrossRef]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Kwon, N.S.; Nathan, C.F.; Griffith, O.W.; Feldman, P.L.; Wiseman, J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J. Biol. Chem. 1991, 266, 6259–6263. [Google Scholar]
- Bo, X.; Wheatley, P.S.; Zhao, X.; Fletcher, A.J.; Fox, S.; Rossi, A.G.; Megson, I.L.; Bordiga, S.; Regli, L.; Thomas, K.M. High-capacity hydrogen and nitric oxide adsorption and storage in a metal–organic framework. J. Am. Chem. Soc. 2007, 129, 1203–1209. [Google Scholar]
- Zhang, Y.C.; Zhou, J.P.; Wu, X.M.; Pan, W.H. Synthesis and antitumor activity of nitric oxide releasing derivatives of AT1 antagonist. Chin. Chem. Lett. 2009, 20, 302–305. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Li, X.; Zhang, G.; Zhang, G.; Hu, J. Light-triggered nitric oxide (NO) release from photoresponsive polymersomes for corneal wound healing. Chem. Sci. 2019, 11, 186–194. [Google Scholar] [CrossRef]
- Cheng, J.; He, K.; Shen, Z.; Zhang, G.; Yu, Y.; Hu, J. Nitric oxide (NO)-releasing macromolecules: Rational design and biomedical applications. Front. Chem. 2019, 7, 530. [Google Scholar] [CrossRef] [PubMed]
- Hinks, N.J.; McKinlay, A.C.; Xiao, B.; Wheatley, P.S.; Morris, R.E. Metal organic frameworks as NO delivery materials for biological applications. Microporous Mesoporous Mater. 2010, 129, 330–334. [Google Scholar] [CrossRef]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.V.; Wang, S.; Tavares, S.R.; Pires, J.; Antunes, F.; Vimont, A.; Clet, G.; Daturi, M.; Maurin, G.; Serre, C.; et al. Tuning cellular biological functions through the controlled release of NO from a porous Ti-MOF. Angew. Chem. Int. Ed. 2020, 59, 5135–5143. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, A.C.; Xiao, B.; Wragg, D.S.; Wheatley, P.S.; Megson, I.L.; Morris, R.E. Exceptional behavior over the whole adsorption−storage−delivery cycle for NO in porous metal organic frameworks. J. Am. Chem. Soc. 2008, 130, 10440–10444. [Google Scholar] [CrossRef]
- Pinto, R.V.; Antunes, F.; Pires, J.; Graça, V.; Brandão, P.; Pinto, M.L. Vitamin B3 metal–organic frameworks as potential delivery vehicles for therapeutic nitric oxide. Acta Biomater. 2017, 51, 66–74. [Google Scholar] [CrossRef]
- Cattaneo, D.; Warrender, S.J.; Duncan, M.J.; Castledine, R.; Parkinson, N.; Haley, I.; Morris, R.E. Water based scale-up of CPO-27 synthesis for nitric oxide delivery. Dalton Trans. 2016, 45, 618–629. [Google Scholar] [CrossRef]
- Cattaneo, D.; Warrender, S.J.; Duncan, M.J.; Kelsall, C.J.; Doherty, M.K.; Whitfield, P.D.; Megson, I.L.; Morris, R.E. Tuning the nitric oxide release from CPO-27 MOFs. RSC Adv. 2016, 6, 14059–14067. [Google Scholar] [CrossRef]
- Ruyra, À.; Yazdi, A.; Espín, J.; Carné-Sánchez, A.; Roher, N.; Lorenzo, J.; Imaz, I.; Maspoch, D. Synthesis, culture medium stability, and in vitro and in vivo zebrafish embryo toxicity of metal–organic framework nanoparticles. Chem. Eur. J. 2015, 21, 2508–2518. [Google Scholar] [CrossRef]
- Schäffer, M.R.; Tantry, U.; Efron, P.A.; Ahrendt, G.M.; Thornton, F.J.; Barbul, A. Diabetes-impaired healing and reduced wound nitric oxide synthesis: A possible pathophysiologic correlation. Surgery 1997, 121, 513–519. [Google Scholar] [CrossRef]
- Witte, M.B.; Kiyama, T.; Barbul, A. Nitric oxide enhances experimental wound healing in diabetes. Br. J. Surg. 2002, 89, 1594–1601. [Google Scholar] [CrossRef]
- Luo, J.-D.; Wang, Y.-Y.; Fu, W.-L.; Wu, J.; Chen Alex, F. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation 2004, 110, 2484–2493. [Google Scholar] [CrossRef]
- Su, C.-H.; Li, W.-P.; Tsao, L.-C.; Wang, L.-C.; Hsu, Y.-P.; Wang, W.-J.; Liao, M.-C.; Lee, C.-L.; Yeh, C.-S. Enhancing microcirculation on multitriggering manner facilitates angiogenesis and collagen deposition on wound healing by photoreleased NO from hemin-derivatized colloids. ACS Nano 2019, 13, 4290–4301. [Google Scholar] [CrossRef] [PubMed]
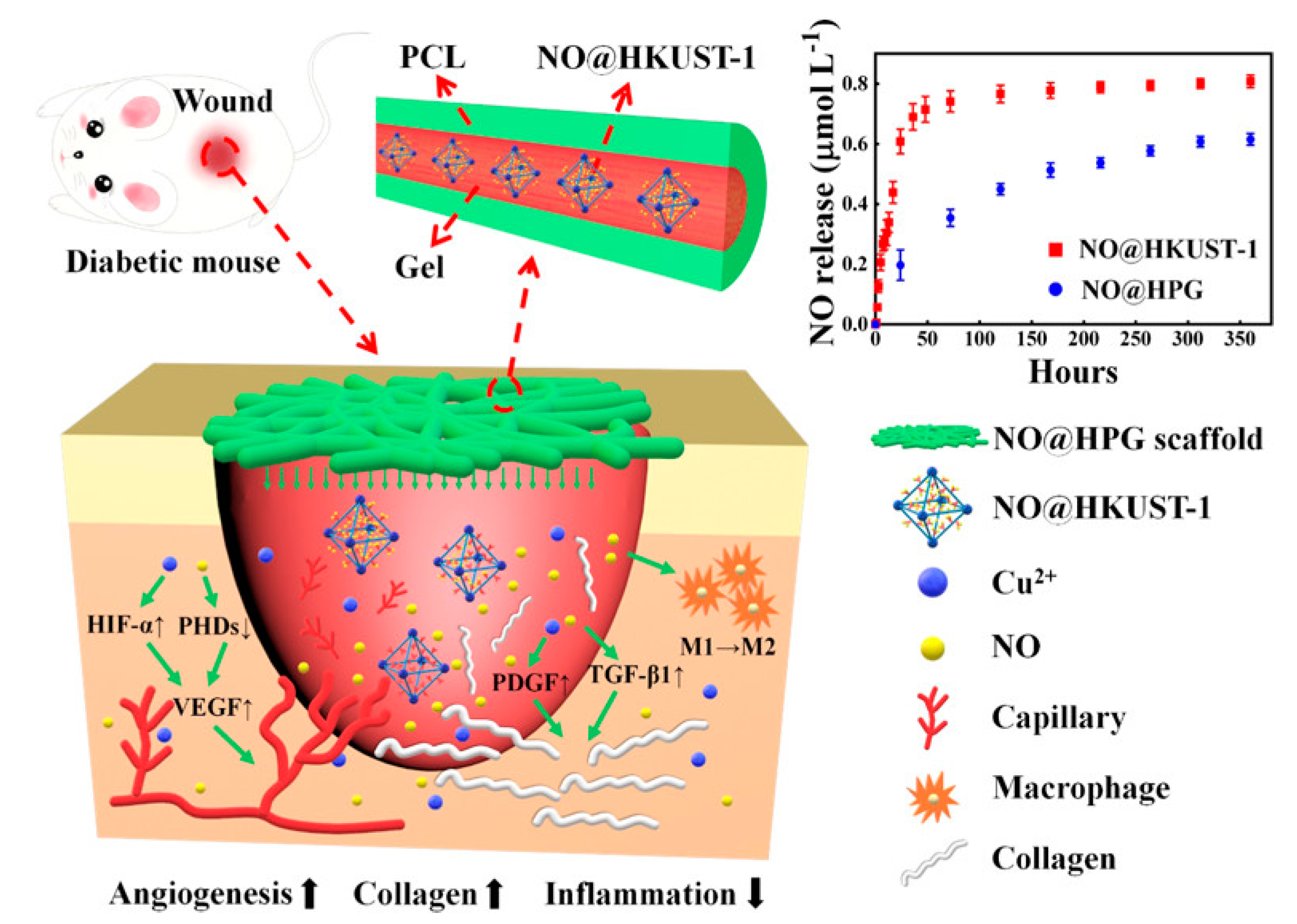
- Zhang, P.; Li, Y.; Tang, Y.; Shen, H.; Li, J.; Yi, Z.; Ke, Q.; Xu, H. Copper-based metal–organic framework as a controllable nitric oxide-releasing vehicle for enhanced diabetic wound healing. ACS Appl. Mater. Interfaces 2020, 12, 18319–18331. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.L.; Rocha, J.; Gomes, J.R.B.; Pires, J. Slow release of NO by microporous titanosilicate ETS-4. J. Am. Chem. Soc. 2011, 133, 6396–6402. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S. Modulating intracellular oxidative stress via engineered nanotherapeutics. J. Control. Release 2020, 319, 333–343. [Google Scholar] [CrossRef]
- Nguyen, J.; Tanabe, K.; Cohen, S. Postsynthetic diazeniumdiolate formation and NO release from MOFs. Crystengcomm 2010, 12, 2335–2338. [Google Scholar] [CrossRef]
- Lowe, A.; Chittajallu, P.; Gong, Q.; Li, J.; Balkus, K.J. Storage and delivery of nitric oxide via diazeniumdiolated metal organic framework. Microporous Mesoporous Mater. 2013, 181, 17–22. [Google Scholar] [CrossRef]
- Diring, S.; Wang, D.O.; Kim, C.; Kondo, M.; Chen, Y.; Kitagawa, S.; Kamei, K.I.; Furukawa, S. Localized cell stimulation by nitric oxide using a photoactive porous coordination polymer platform. Nat. Commun. 2013, 4, 2684. [Google Scholar] [CrossRef]
- Kim, C.; Diring, S.; Furukawa, S.; Kitagawa, S. Light-induced nitric oxide release from physiologically stable porous coordination polymers. Dalton Trans. 2015, 44, 15324–15333. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, X.-T.; Shang, Y.; Li, Y.-H.; Yin, X.-B. Theranostic Mn-porphyrin metal–organic frameworks for magnetic resonance imaging-guided nitric oxide and photothermal synergistic therapy. ACS Appl. Mater. Interfaces 2018, 10, 28390–28398. [Google Scholar] [CrossRef] [PubMed]
- Barton, H.F.; Davis, A.K.; Parsons, G.N. The effect of surface hydroxylation on MOF formation on ALD metal oxides: MOF-525 on TiO2/polypropylene for catalytic hydrolysis of chemical warfare agent simulants. ACS Appl. Mater. Interfaces 2020, 12, 14690–14701. [Google Scholar] [CrossRef]
- Harding, J.L.; Reynolds, M.M. Metal organic frameworks as nitric oxide catalysts. J. Am. Chem. Soc. 2012, 134, 3330–3333. [Google Scholar] [CrossRef]
- Neufeld, M.J.; Ware, B.R.; Lutzke, A.; Khetani, S.R.; Reynolds, M.M. Water-stable metal–organic framework/polymer composites compatible with human hepatocytes. ACS Appl. Mater. Interfaces 2016, 8, 19343–19352. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, M.J.; Harding, J.L.; Reynolds, M.M. Immobilization of metal–organic framework copper(II) benzene-1,3,5-tricarboxylate (CuBTC) onto cotton fabric as a nitric oxide release catalyst. ACS Appl. Mater. Interfaces 2015, 7, 26742–26750. [Google Scholar] [CrossRef] [PubMed]
- Marcilli, R.H.M.; de Oliveira, M.G. Nitric oxide-releasing poly(vinyl alcohol) film for increasing dermal vasodilation. Colloids Surf. B 2014, 116, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Masters, K.S.B.; Leibovich, S.J.; Belem, P.; West, J.L.; Poole-Warren, L.A. Effects of nitric oxide releasing poly(vinyl alcohol) hydrogel dressings on dermal wound healing in diabetic mice. Wound Repair Regen. 2002, 10, 286–294. [Google Scholar] [CrossRef]
- Schanuel, F.S.; Raggio Santos, K.S.; Monte-Alto-Costa, A.; de Oliveira, M.G. Combined nitric oxide-releasing poly(vinyl alcohol) film/F127 hydrogel for accelerating wound healing. Colloids Surf. B 2015, 130, 182–191. [Google Scholar] [CrossRef]
- Neufeld, M.J.; Lutzke, A.; Jones, W.M.; Reynolds, M.M. Nitric oxide generation from endogenous substrates using metal–organic frameworks: Inclusion within Poly(vinyl alcohol) membranes to investigate reactivity and therapeutic potential. ACS Appl. Mater. Interfaces 2017, 9, 35628–35641. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, Y.; Zhang, Y.; Liu, J.; Li, W.; Weng, Y. Copper-based SURMOFs for nitric oxide generation: Hemocompatibility, vascular cell growth, and tissue response. ACS Appl. Mater. Interfaces 2019, 11, 7872–7883. [Google Scholar] [CrossRef]
- Biemmi, E.; Scherb, C.; Bein, T. Oriented growth of the metal organic framework Cu3(BTC)2(H2O)3·xH2O tunable with functionalized self-assembled monolayers. J. Am. Chem. Soc. 2007, 129, 8054–8055. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fischer, R.A. Liquid-phase epitaxy of metal organic framework thin films. Sci. China Chem. 2011, 54, 1851–1866. [Google Scholar] [CrossRef]
- Wang, S.J.; Serre, C. Toward green production of water-stable metal–organic frameworks based on high-valence metals with low toxicities. ACS Sustain. Chem. Eng. 2019, 7, 11911–11927. [Google Scholar] [CrossRef]
- Matsui, T.; Unno, M.; Ikeda-Saito, M. Heme oxygenase reveals its strategy for catalyzing three successive oxygenation reactions. Acc. Chem. Res. 2010, 43, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Kim, H.P.; Nakahira, K.; Zuckerbraun, B.S.; Morse, D.; Choi, A.M.K. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid. Redox Signal. 2007, 9, 2157–2173. [Google Scholar] [CrossRef] [PubMed]
- Riego, G.; Redondo, A.; Leanez, S.; Pol, O. Mechanism implicated in the anti-allodynic and anti-hyperalgesic effects induced by the activation of heme oxygenase 1/carbon monoxide signaling pathway in the central nervous system of mice with neuropathic pain. Biochem. Pharmacol. 2018, 148, 52–63. [Google Scholar] [CrossRef]
- Niesel, J.; Pinto, A.; N’Dongo, H.W.P.; Merz, K.; Ott, I.; Gust, R.; Schatzschneider, U. Photoinduced CO release, cellular uptake and cytotoxicity of a tris(pyrazolyl)methane (tpm) manganese tricarbonyl complex. Chem. Commun. 2008, 15, 1798–1800. [Google Scholar] [CrossRef]
- Motterlini, R.; Clark James, E.; Foresti, R.; Sarathchandra, P.; Mann Brian, E.; Green Colin, J. Carbon monoxide-releasing molecules. Circ. Res. 2002, 90, e17–e24. [Google Scholar] [CrossRef]
- Johnson, T.R.; Mann, B.E.; Clark, J.E.; Foresti, R.; Green, C.J.; Motterlini, R. Metal carbonyls: A new class of pharmaceuticals? Angew. Chem. Int. Ed. 2003, 42, 3722–3729. [Google Scholar] [CrossRef]
- Romanski, S.; Kraus, B.; Schatzschneider, U.; Neudörfl, J.-M.; Amslinger, S.; Schmalz, H.-G. Acyloxybutadiene iron tricarbonyl complexes as enzyme-triggered CO-releasing molecules (ET-CORMs). Angew. Chem. Int. Ed. 2011, 50, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zheng, B.; Cheng, S.; Zhang, G.; Hu, J. Metal-free carbon monoxide-releasing micelles undergo tandem photochemical reactions for cutaneous wound healing. Chem. Sci. 2020, 11, 4499–4507. [Google Scholar] [CrossRef]
- Antony, L.A.P.; Slanina, T.; Sebej, P.; Solomek, T.; Klan, P. Fluorescein analogue xanthene-9-carboxylic acid: A transition-metal-free CO releasing molecule activated by green light. Org. Lett. 2013, 15, 4552–4555. [Google Scholar] [CrossRef] [PubMed]
- Palao, E.; Slanina, T.; Muchova, L.; Solomek, T.; Vitek, L.; Klan, P. Transition-metal-free CO-releasing BODIPY derivatives activatable by visible to NIR light as promising bioactive molecules. J. Am. Chem. Soc. 2016, 138, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Wang, C.M.; Shi, Z.; Johns, V.K.; Ma, L.Y.; Oyer, J.; Copik, A.; Igarashi, R.; Liao, Y. Visible-light activatable organic CO-releasing molecules (PhotoCORMs) that simultaneously generate fluorophores. Org. Biomol. Chem. 2013, 11, 6671–6674. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Ma, M.; Noei, H.; Mienert, B.; Niesel, J.; Bill, E.; Muhler, M.; Fischer, R.A.; Wang, Y.; Schatzschneider, U.; Metzler-Nolte, N. Iron metal–organic frameworks MIL-88B and NH2-MIL-88B for the loading and delivery of the gasotransmitter carbon monoxide. Chem. Eur. J. 2013, 19, 6785–6790. [Google Scholar] [CrossRef]
- Diring, S.; Carné-Sánchez, A.; Zhang, J.; Ikemura, S.; Kim, C.; Inaba, H.; Kitagawa, S.; Furukawa, S. Light responsive metal–organic frameworks as controllable CO-releasing cell culture substrates. Chem. Sci. 2017, 8, 2381–2386. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide: From brain to gut. Antioxid. Redox Signal. 2010, 12, 1111–1123. [Google Scholar] [CrossRef]
- Li, L.; Bhatia, M.; Zhu, Y.Z.; Zhu, Y.C.; Ramnath, R.D.; Wang, Z.J.; Anuar, F.B.M.; Whiteman, M.; Salto-Tellez, M.; Moore, P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005, 19, 1196–1198. [Google Scholar] [CrossRef]
- Dongó, E.; Beliczai-Marosi, G.; Dybvig, A.S.; Kiss, L. The mechanism of action and role of hydrogen sulfide in the control of vascular tone. Nitric Oxide 2018, 81, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Allan, P.K.; Wheatley, P.S.; Aldous, D.; Mohideen, M.I.; Tang, C.; Hriljac, J.A.; Megson, I.L.; Chapman, K.W.; De Weireld, G.; Vaesen, S.; et al. Metal–organic frameworks for the storage and delivery of biologically active hydrogen sulfide. Dalton Trans. 2012, 41, 4060–4066. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Qiao, R.; Hu, J. Engineering Metal–Organic Frameworks (MOFs) for Controlled Delivery of Physiological Gaseous Transmitters. Nanomaterials 2020, 10, 1134. https://doi.org/10.3390/nano10061134
Zhang M, Qiao R, Hu J. Engineering Metal–Organic Frameworks (MOFs) for Controlled Delivery of Physiological Gaseous Transmitters. Nanomaterials. 2020; 10(6):1134. https://doi.org/10.3390/nano10061134
Chicago/Turabian StyleZhang, Mengdan, Ruirui Qiao, and Jinming Hu. 2020. "Engineering Metal–Organic Frameworks (MOFs) for Controlled Delivery of Physiological Gaseous Transmitters" Nanomaterials 10, no. 6: 1134. https://doi.org/10.3390/nano10061134
APA StyleZhang, M., Qiao, R., & Hu, J. (2020). Engineering Metal–Organic Frameworks (MOFs) for Controlled Delivery of Physiological Gaseous Transmitters. Nanomaterials, 10(6), 1134. https://doi.org/10.3390/nano10061134






