Design of Novel Perovskite-Based Polymeric Poly(l-Lactide-Co-Glycolide) Nanofibers with Anti-Microbial Properties for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Perovskite Particles
2.3. Electrospinning of Perovskite-Incorporated Fibers
2.4. Mechanical Characterization of Electrospun Nanofibers
2.5. Anti-Microbial Properties of the Nanofibers
2.6. Culture of Human Dermal Fibroblasts
2.7. Anti-Microbial Properties of the Scaffolds
2.8. Confocal Microscopy of Human Dermal Fibroblast on Nanofibers
3. Results
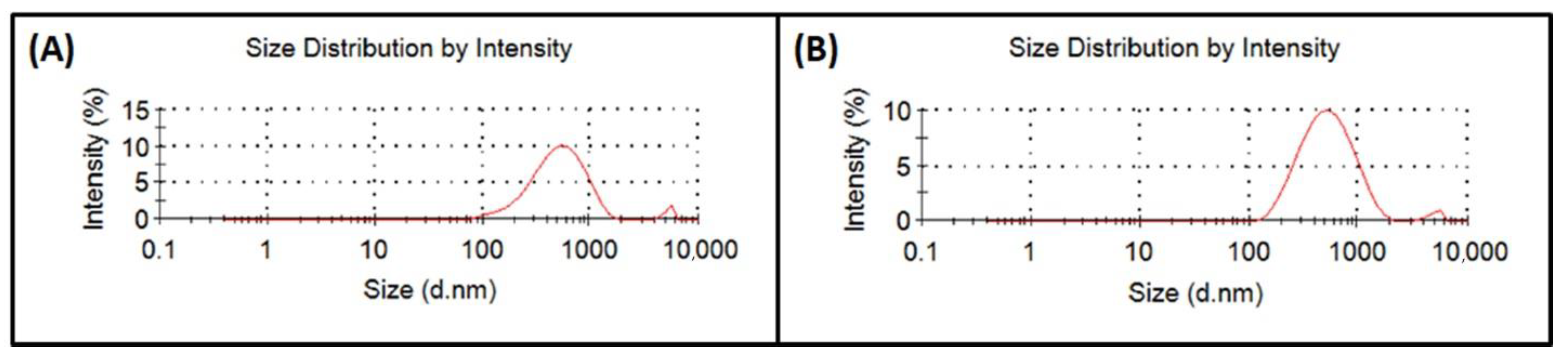
3.1. Analysis of Perovskite Particle Size
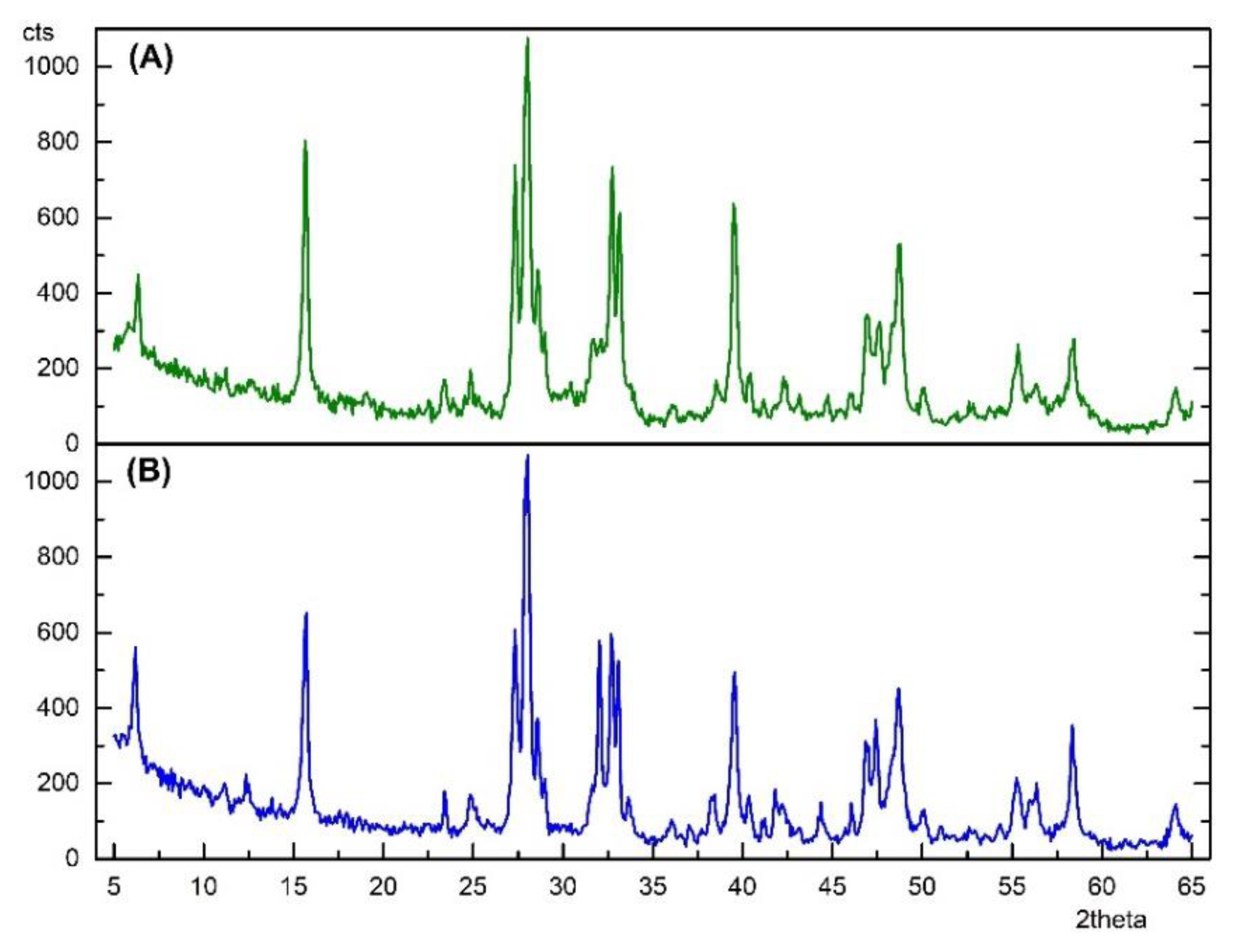
3.2. X-ray Diffraction of Perovskite Particles
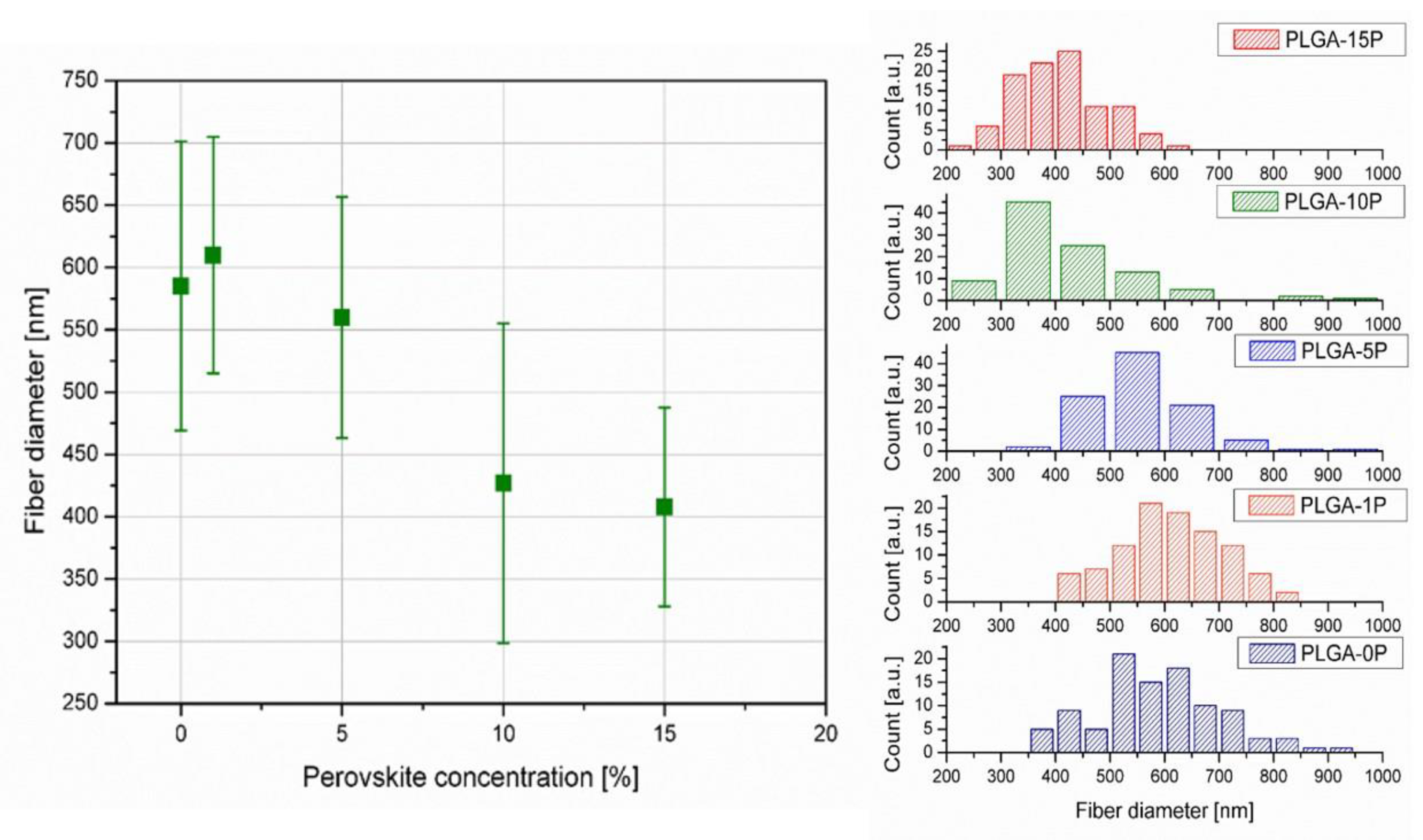
3.3. Electrospinning Process Optimization ad SEM Analysis
3.4. Mechanical Properties and Contact Angle of Electrospun Nanofibers
3.5. FTIR Analysis of Polymeric Perovskite-Incorporated Scaffolds
3.6. Anti-Microbial Characteristic of Synthesized Perovskite and Electrospun Fibers
3.7. Proliferation and Morphology of HDF
3.8. Immunofluorescence of Cellular Cytoskeletal Marker
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and anti-microbial biomaterials: An overview. Apmis 2017, 125, 392–417. [Google Scholar] [CrossRef] [PubMed]
- Peter Vowden, K.V. Keryln Carville, Anti-microbials Made Easy. Wounds Int. 2011, 2, 1–6. [Google Scholar]
- Parsons, D.; Bowler, P.G.; Myles, V.; Jones, S. Anti-microbial Dressings in Wound Management: A Comparison of Antibacterial, Physical, and Chemical Characteristics. Wounds 2005, 17, 222–232. [Google Scholar]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Mitzi, D.B. Synthesis, Structure, and Properties of Organic-Inorganic Perovskites and Related Materials. In Progress in Inorganic Chemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007; pp. 1–121. [Google Scholar] [CrossRef]
- Burn, P.L.; Meredith, P. The rise of the perovskites: The future of low cost solar photovoltaics. NPG Asia Mater. 2014, 6, e79. [Google Scholar] [CrossRef][Green Version]
- Snaith, H.J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Kanhere, P.; Chen, Z. A Review on Visible Light Active Perovskite-Based Photocatalysts. Molecules 2014, 19, 19995–20022. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Jesline, A.; John, N.; Narayanan, P.M.; Vani, C.; Murugan, S. Anti-microbial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant Staphylococcus aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Mishra, A.; Yun, S.I.; Kim, H.C.; Kim, H.Y.; Khil, M.S. Fabrication, characterization and antibacterial effect of novel electrospun TiO2 nanorods on a panel of pathogenic bacteria. J. Biomed. Nanotechnol. 2012, 8, 394–404. [Google Scholar] [CrossRef]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial resistance to silver in wound care. J. Hosp. Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Jin, T.; Behari, J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol. Mech. Methods 2011, 21, 13–24. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Van Der Ven, L.T.; Sleijffers, A.; Park, M.V.; Jansen, E.H.; Van Loveren, H.; Vandebriel, R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef]
- Ma, J.; Lü, X.; Huang, Y. Genomic analysis of cytotoxicity response to nanosilver in human dermal fibroblasts. J. Biomed. Nanotechnol. 2011, 7, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lü, X.; Ma, J. Toxicity of silver nanoparticles to human dermal fibroblasts on microRNA level. J. Biomed. Nanotechnol. 2014, 10, 3304–3317. [Google Scholar] [CrossRef]
- Galandáková, A.; Franková, J.; Ambrožová, N.; Habartová, K.; Pivodová, V.; Zálešák, B.; Šafářová, K.; Smékalová, M.; Ulrichová, J. Effects of silver nanoparticles on human dermal fibroblasts and epidermal keratinocytes. Hum. Exp. Toxicol. 2016, 35, 946–957. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Shah, S.R.; Lam, J.; Bennett, G.N.; Mikos, A.G. A rapid, flexible method for incorporating controlled antibiotic release into porous polymethylmethacrylate space maintainers for craniofacial reconstruction. Biomater. Sci. 2016, 4, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-S.; Park, Y.-B.; Bae, J.-M.; Oh, S. Near-infrared laser-mediated drug release and antibacterial activity of gold nanorod-sputtered titania nanotubes. J. Tissue Eng. 2018, 9, 2041731418790315. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, M.; Pinczowski, P.; Pérez, M.; Giorello, A.; Martínez, M.Á.; Santamaría, J.; Arruebo, M.; Luján, L. A controlled antibiotic release system to prevent orthopedic-implant associated infections: An in vitro study. Eur. J. Pharm. Biopharm. 2015, 96, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, P.Y.; Chow, C.L.; Lim, C.K.; Tan, O.K.; Tse, M.S.; Sze, C.C. Antibacterial activities of mechanochemically synthesized perovskite strontium titanate ferrite metal oxide. Colloids Surf. Physicochem. Eng. Asp. 2014, 456, 169–175. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Xue, X.-X.; Liu, D. Antibacterial Properties of V-doped Titanium-bearing Blast Furnace Slag Prepared at Different Calcination Temperatures. Chin. J. Process Eng. 过程工程学报 2010, 10, 1025–1029. (In Chinese) [Google Scholar]
- Mohseni, S.; Aghayan, M.; Ghorani-Azam, A.; Behdani, M.; Asoodeh, A. Evaluation of antibacterial properties of Barium Zirconate Titanate (BZT) nanoparticle. Braz. J. Microbiol. 2014, 45, 1393–1399. [Google Scholar] [CrossRef]
- Tan, S.Z.; Zhang, L.L.; Xia, L.-y.; Liu, Y.-l.; Li, D.-x. Structure and antibacterial activity of new layered perovskite compounds. Trans. Nonferrous Met. Soc. China 2007, 17, 257–261. [Google Scholar] [CrossRef]
- Tan, S.Z.; Ding, L.C.; Liu, Y.L.; Ouyang, Y.S.; Chen, Y.B. Synthesis and antibacterial activity of new layered perovskite compounds, AgxNa2−xLa2Ti3O10. Chin. Chem. Lett. 2007, 18, 85–88. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. Introduction of Electrospinning. In One-Dimensional Nanostructures; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–13. [Google Scholar]
- Leung, V.; Hartwell, R.; Yang, H.; Ghahary, A.; Ko, F. Bioactive nanofibres for wound healing applications. J. Fiber Bioeng. Inf. 2011, 4, 1–14. [Google Scholar]
- Asencio, I.O.; Mittar, S.; Sherborne, C.; Raza, A.; Claeyssens, F.; MacNeil, S. A methodology for the production of microfabricated electrospun membranes for the creation of new skin regeneration models. J. Tissue Eng. 2018, 9, 2041731418799851. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Malheiro, A.; van Blitterswijk, C.; Mota, C.; Wieringa, P.A.; Moroni, L. Direct Writing Electrospinning of Scaffolds with Multidimensional Fiber Architecture for Hierarchical Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 38187–38200. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X. Structure, morphology and electrochemical properties of LaxSr1−xCo0.1Mn0.9O3−δ perovskite nanofibers prepared by electrospinning method. J. Alloys Compd. 2015, 624, 31–39. [Google Scholar] [CrossRef]
- El-Shazly, A.N.; Rezk, M.Y.; Gameel, K.M.; Allam, N.K. Electrospun Lead-Free All-Inorganic Double Perovskite Nanofibers for Photovoltaic and Optoelectronic Applications. ACS Appl. Nano Mater. 2019, 2, 7085–7094. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Chen, J.-Y.; Ercan, E.; Chueh, C.-C.; Tung, S.-H.; Chen, W.-C. Uniform Luminous Perovskite Nanofibers with Color-Tunability and Improved Stability Prepared by One-Step Core/Shell Electrospinning. Small 2018, 14, 1704379. [Google Scholar] [CrossRef]
- Wang, B.; Gu, S.; Ding, Y.; Chu, Y.; Zhang, Z.; Ba, X.; Zhang, Q.; Li, X. A novel route to prepare LaNiO3 perovskite-type oxide nanofibers by electrospinning for glucose and hydrogen peroxide sensing. Analyst 2013, 138, 362–367. [Google Scholar] [CrossRef]
- Wang, W.; Lin, B.; Zhang, H.; Sun, Y.; Zhang, X.; Yang, H. Synthesis, morphology and electrochemical performances of perovskite-type oxide LaxSr1-xFeO3 nanofibers prepared by electrospinning. J. Phys. Chem. Solids 2019, 124, 144–150. [Google Scholar] [CrossRef]
- Toda, K.; Watanabe, J.; Sato, M. Synthesis and ionic conductivity of new layered perovskite compound, Ag2La2Ti3O10. Solid State Ion. 1996, 90, 15–19. [Google Scholar] [CrossRef]
- Toda, K.; Watanabe, J.; Sato, M. Crystal structure determination of ion-exchangeable layered perovskite compounds, K2La2Ti3O10 and Li2La2Ti3O10. Mater. Res. Bull. 1996, 31, 1427–1435. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Bhat, V. A2Ln2Ti3O10 (A = potassium or rubidium; Ln = lanthanum or rare earth): A new series of layered perovskites exhibiting ion exchange. Inorg. Chem. 1987, 26, 4299–4301. [Google Scholar] [CrossRef]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface 2010, 7, S559–S579. [Google Scholar] [CrossRef]
- Kapuscinski, J. DAPI: A DNA-specific fluorescent probe. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 1995, 70, 220–233. [Google Scholar] [CrossRef]
- Frickmann, H.; Schröpfer, E.; Dobler, G. Actin assessment in addition to specific immuno-fluorescence staining to demonstrate rickettsial growth in cell culture. Eur. J. Microbiol. Immunol. 2013, 3, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Fath, K.; Kelly, T.; Nuckolls, G.; Turner, C. Focal adhesions: Transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 1988, 4, 487–525. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Darzi, S.; Rosamilia, A.; Kadam, V.; Truong, Y.; Werkmeister, J.A.; Gargett, C.E. Blended Nanostructured Degradable Mesh with Endometrial Mesenchymal Stem Cells Promotes Tissue Integration and Anti-Inflammatory Response in Vivo for Pelvic Floor Application. Biomacromolecules 2019, 20, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Gualandi, C.; Focarete, M.L.; Ravichandran, R.; Venugopal, J.R.; Raghunath, M.; Ramakrishna, S. Elastomeric electrospun scaffolds of poly(L-lactide-co-trimethylene carbonate) for myocardial tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 1689–1699. [Google Scholar] [CrossRef]
- Mukherjee, S.; Reddy Venugopal, J.; Ravichandran, R.; Ramakrishna, S.; Raghunath, M. Evaluation of the Biocompatibility of PLACL/Collagen Nanostructured Matrices with Cardiomyocytes as a Model for the Regeneration of Infarcted Myocardium. Adv. Funct. Mater. 2011, 21, 2291–2300. [Google Scholar] [CrossRef]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Poly(Glycerol sebacate)/gelatin core/shell fibrous structure for regeneration of myocardial infarction. Tissue Eng. Part A 2011, 17, 1363–1373. [Google Scholar] [CrossRef]
- Krishnan, R.; Rajeswari, R.; Venugopal, J.; Sundarrajan, S.; Sridhar, R.; Shayanti, M.; Ramakrishna, S. Polysaccharide nanofibrous scaffolds as a model for in vitro skin tissue regeneration. J. Mater. Sci. Mater. Med. 2012, 23, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Advances in polymeric systems for tissue engineering and biomedical applications. Macromol. Biosci. 2012, 12, 286–311. [Google Scholar] [CrossRef] [PubMed]
- Jayarama Reddy, V.; Radhakrishnan, S.; Ravichandran, R.; Mukherjee, S.; Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen 2013, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Yuan, X.-F.; Bayat, A. Electrospun silk fibroin fiber diameter influences in vitro dermal fibroblast behavior and promotes healing of ex vivo wound models. J. Tissue Eng. 2014, 5, 2041731414551661. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, X.; Liu, Y.; Kong, C.; Liu, N.; Wan, Y.; Guo, Q.; Liu, K.; Lu, Z.; Li, M.; et al. Ag nanoparticles decorated PVA-co-PE nanofiber-based membrane with antifouling surface for highly efficient inactivation and interception of bacteria. Appl. Surf. Sci. 2020, 506, 144664. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. One-Step Synthesis of Silver Nanoparticles Embedded Polyurethane Nano-Fiber/Net Structured Membrane as an Effective Antibacterial Medium. Polymers 2019, 11, 1185. [Google Scholar] [CrossRef]
- Agache, P.G.; Monneur, C.; Leveque, J.L.; De Rigal, J. Mechanical properties and Young’s modulus of human skin in vivo. Arch. Derm. Res. 1980, 269, 221–232. [Google Scholar] [CrossRef]
- Jacquemoud, C.; Bruyere-Garnier, K.; Coret, M. Methodology to determine failure characteristics of planar soft tissues using a dynamic tensile test. J. Biomech. 2007, 40, 468–475. [Google Scholar] [CrossRef]
- Góra, A.; Prabhakaran, M.P.; Eunice, G.T.L.; Lakshminarayanan, R.; Ramakrishna, S. Silver nanoparticle incorporated poly(l-lactide-co-glycolide) nanofibers: Evaluation of their biocompatibility and antibacterial properties. J. Appl. Polym. Sci. 2015, 132, 42686. [Google Scholar] [CrossRef]
- Paul, K.; Darzi, S.; McPhee, G.; Del Borgo, M.P.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. 3D bioprinted endometrial stem cells on melt electrospun poly epsilon-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 2019, 97, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Seitz, V.; Reddy Venugopal, J.; Sridhar, R.; Sundarrajan, S.; Mukherjee, S.; Wintermantel, E.; Ramakrishna, S. Mimicking native extracellular matrix with phytic acid-crosslinked protein nanofibers for cardiac tissue engineering. Macromol. Biosci. 2013, 13, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Jeong, L.; Cho, D.; Kwon, O.; Min, B.-M.; Park, W. Cellular response of silk fibroin nanofibers containing silver nanoparticles In vitro. Macromol. Res. 2014, 22, 796–803. [Google Scholar] [CrossRef]


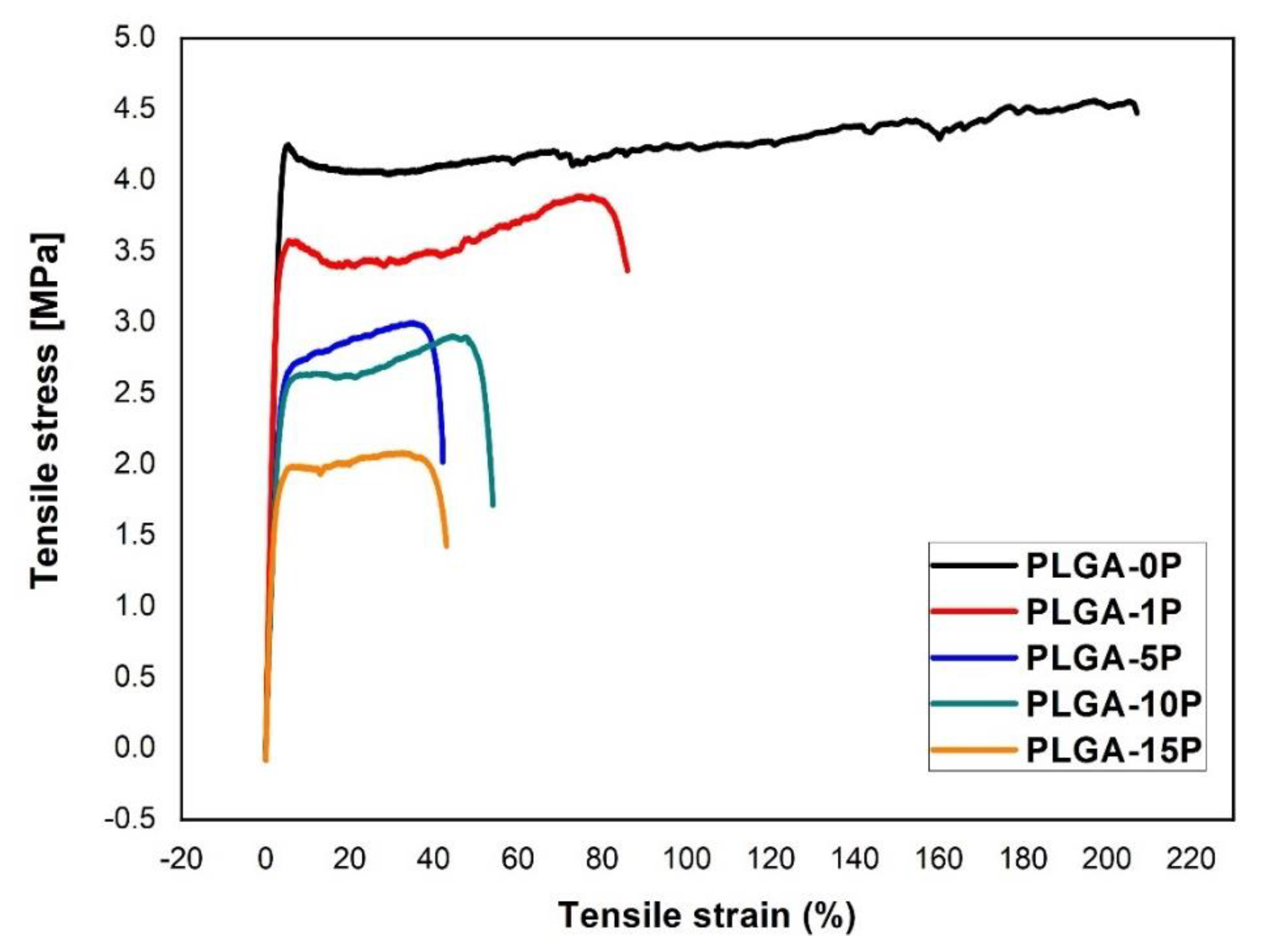
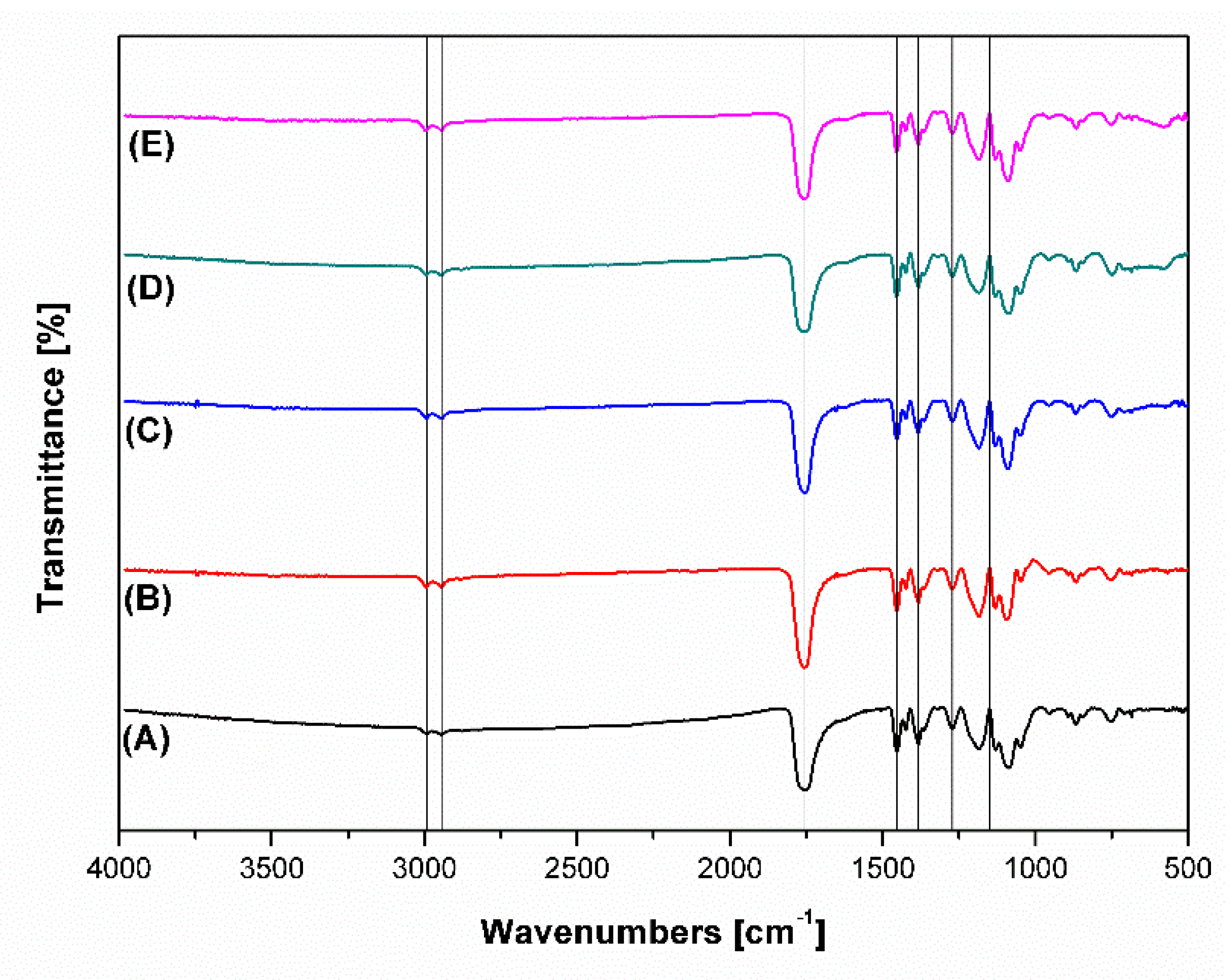

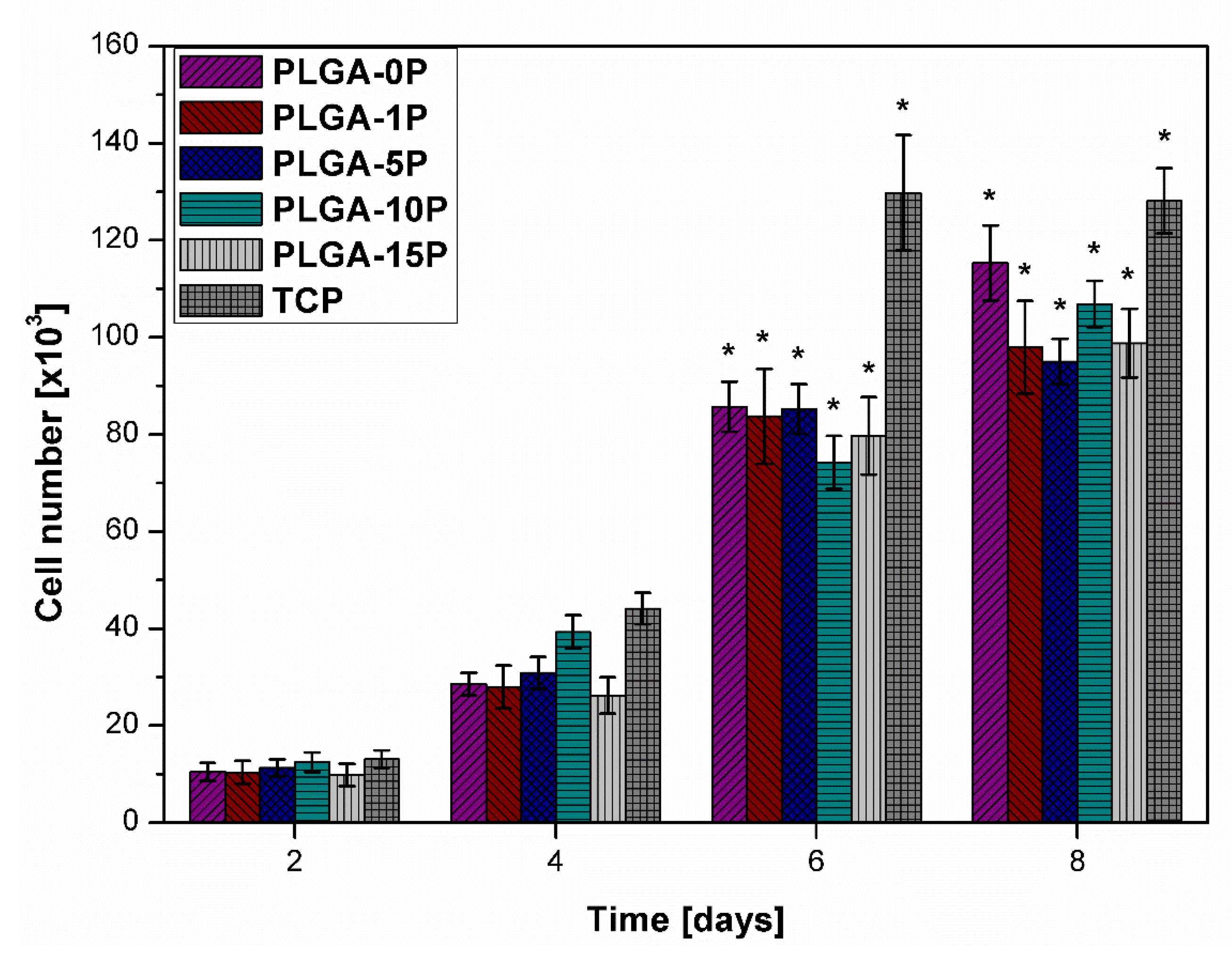

| Scaffold | Young Modulus (MPa) | Ultimate Tensile Stress (MPa) | Ultimate Tensile Strain (%) | Contact Angle (°) |
|---|---|---|---|---|
| PLGA-0P | 130.8 ± 7.5 | 4.5 ± 0.2 | 264.4 ± 53.0 | 134.3 ± 0.7 |
| PLGA-1P | 139.1 ± 7.3 | 3.5 ± 0.4 | 94.8 ± 4.0 | 134.8 ± 0.9 |
| PLGA-5P | 94.5 ± 3.5 | 2.8 ± 0.5 | 44.9 ± 8.6 | 136.9 ± 2.1 |
| PLGA-10P | 83.7 ± 6.6 | 2.8 ± 0.4 | 40.7 ± 6.5 | 138.1 ± 2.4 |
| PLGA-15P | 74.6 ± 7.2 | 2.3 ± 0.5 | 56.6 ± 9.7 | 139.6 ± 1.2 |
| Zone of Inhibition (cm) # | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Material/Scaffold | K. pneumoniae | E. coli | P. aeruginosa | S. saprophyticus | C. albicans | |||||
| 8 | 16027R | PA23155 | PA23376 | SS49907 | SS15305 | SS750 | SS49453 | CA1976 | CA2672 | |
| Perovskite NS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Perovskite S | 1.8–1.9 (0.7) | 1.0–1.1 (0.25) | 0.7–0.8 (0.7) | 0.9(0.8) | 0.8 (0.6) | 0.8–0.9 (1.3) | 0.6–0.8 (1.) | 0.4–0.7 (0.75) | 0.6–0.7 (0.52) | 0.5–0.6 (0.5) |
| PLGA-0P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PLGA-1P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PLGA-5P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PLGA-10P | 0 | 0.4 | 0 | 0.2 (0.0) | 0 | 0 | 0.3 | 0 | 0 | 0 |
| PLGA-15P | 0.35–0.4 (0.0) | 0 (0.7) | 0 (0.4) | 0.25 (0.4) | 0.3 (0.45) | 0 (0.45) | 0.4 (0.) | 0.4 (0.2) | 0 (0.2) | 0 (0.4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góra, A.; Tian, L.; Ramakrishna, S.; Mukherjee, S. Design of Novel Perovskite-Based Polymeric Poly(l-Lactide-Co-Glycolide) Nanofibers with Anti-Microbial Properties for Tissue Engineering. Nanomaterials 2020, 10, 1127. https://doi.org/10.3390/nano10061127
Góra A, Tian L, Ramakrishna S, Mukherjee S. Design of Novel Perovskite-Based Polymeric Poly(l-Lactide-Co-Glycolide) Nanofibers with Anti-Microbial Properties for Tissue Engineering. Nanomaterials. 2020; 10(6):1127. https://doi.org/10.3390/nano10061127
Chicago/Turabian StyleGóra, Aleksander, Lingling Tian, Seeram Ramakrishna, and Shayanti Mukherjee. 2020. "Design of Novel Perovskite-Based Polymeric Poly(l-Lactide-Co-Glycolide) Nanofibers with Anti-Microbial Properties for Tissue Engineering" Nanomaterials 10, no. 6: 1127. https://doi.org/10.3390/nano10061127
APA StyleGóra, A., Tian, L., Ramakrishna, S., & Mukherjee, S. (2020). Design of Novel Perovskite-Based Polymeric Poly(l-Lactide-Co-Glycolide) Nanofibers with Anti-Microbial Properties for Tissue Engineering. Nanomaterials, 10(6), 1127. https://doi.org/10.3390/nano10061127







